Abstract
The paper describes hydrometallurgical methods to recycle wastes of vanadium pentoxide chemical fabrication. Sludges containing a significant amount of V2O5 can be considered as an additional source of raw materials for vanadium production. We studied the one-stage leaching method using various iron-based reductants for converting V5+ to V4+ in a solution allowing to precipitate V when its concentration in the solution is low. As a result of the reduction leaching with further precipitation, we obtained concentrates with V2O5 content of 22–26% and a high amount of harmful impurities. Multistage counterflow leaching can be used to fabricate solutions with vanadium pentoxide concentration suitable for vanadium precipitation by hydrolysis and adding ammonium salts. The solutions with V2O5 content of ≈15 g/L can be obtained from the initial sludge by three-stage counterflow vanadium leaching. A concentrate with a content of 78 wt% V2O5 can be precipitated from these solutions at pH = 2.4 by adding ammonium chloride. Additionally, concentrate with V2O5 content of ≈94 wt% was precipitated from the solution with a concentration of >20 g/L V2O5 obtained from the roasted sludge. The concentrates were purified for increasing the vanadium content to 5–7%. The consumption and technological parameters of the considered processes are presented in the paper.
Keywords:
vanadium; vanadium pentoxide; concentrate; waste; sludge; leaching; reduction; vanadium alloys; precipitation; hydrolysis 1. Introduction
Vanadium is an important strategic metal widely used in various fields of industry. In ferrous metallurgy, V in the form of ferrovanadium is used for steel alloying [1,2,3] and in non-ferrous metallurgy in aluminum-vanadium alloys for alloying titanium-based structural materials used in aerospace engineering (engines, fuselages of high-speed aircraft) [4,5,6,7]. In the chemical industry, vanadium compounds are used as catalysts [8]. Additionally, materials based on vanadium oxides (V2O3, VO2 and V2O5) are used in memristors (resistors with memory), bolometers (thermal infrared detectors), biosensors [9,10]. The application of vanadium in medicine for the manufacture of dental implants is described in [11].
Currently, the main sources for vanadium production are titanomagnetite ores [12,13,14,15]. Additionally, vanadium is extracted from coal [16,17,18], fly ash [19,20], spent catalysts [21,22], and other sources [23]. Titanomagnetite ores are processed to produce vanadium converter slag, from which vanadium pentoxide is obtained by chemical means [7,12,24,25,26,27,28].
Vanadium in converter slags is presented mainly in spinel (FeO·V2O3) practically insoluble in acid solutions. Therefore, in order to convert vanadium into soluble forms, preliminary slag oxidation roasting is carried out [7,12,24,25,26,27,28,29,30,31,32,33].
During the alkaline roasting, the formation of toxic salts of hexavalent chromium CrO42− [7,12,32] is possible. With this technology due to the complexity of disposal, exhausted sodium-containing solutions are thrown into the water basin polluting the environment. Roasting of slag with calcium-containing additives (lime, limestone) is considered more environmentally friendly when vanadium in the spinel is forming acid-soluble phases CaV2O6, Ca2V2O7 and Ca3V2O8 [7,12,33].
Vanadium is precipitated from leaching solutions by hydrolysis, as a result, technical vanadium pentoxide with impurities Mn, Si, Fe, etc., is acquired. To obtain a cleaner vanadium pentoxide, precipitation is carried out with the addition of ammonium salts (for example, NH4SO4, NH4Cl) [7,12,30]. Thus, the concentration of solutions before precipitation is usually higher than 15 g/L V2O5.
A significant amount of vanadium remains after leaching (<4.5 wt% V2O5) [33,34,35,36] in sludges (wastes of hydrometallurgical production of vanadium pentoxide), therefore, it can be considered as a technogenic source of raw materials for vanadium production. Vanadium in sludges similarly to initial vanadium slags exists in the form of spinel (V3+), also part of vanadium is in acid-soluble forms V4+ and V5+ [36]. Consequently, hydrometallurgical methods that are used in the production and research practice of vanadium slags processing should also be effective for this type of vanadium-containing raw materials.
The research was aimed at studies of hydrometallurgical methods of vanadium-containing sludge processing to produce vanadium concentrates.
Previously [34,35] it was shown that multi-stage counterflow leaching is necessary to obtain vanadium concentrates from sludges suitable for further smelting of vanadium alloys. In three stages of leaching, solutions with V2O5 content of ≈10 g/L can be obtained from the initial sludges and more than 20 g/L V2O5 from the roasted sludges in two stages.
It is known that vanadium can exist in aqueous solutions in the form of compounds with oxidation degrees from +2 to +5 [37,38]. All known methods of vanadium precipitation are based on the vanadium extraction into a concentrate in the form V5+. In this work, new methods for vanadium precipitation in the form of V4+ using reagents that are not used in production practice are studied. Additionally, the production of vanadium concentrates by ammonium methods was studied.
2. Materials and Methods
2.1. Materials
The original vanadium-containing sludges were obtained from the EVRAZ Vanadii Tula plant (Tula, Russia). The samples were obtained at various periods and the content of V2O5 somewhat differs.
The chemical analysis was performed with the X-ray fluorescence spectrometer AXIOSmax Advanced (PANalytical, Almelo, The Netherlands) using the method described in [36,39]. Table 1 shows the chemical composition of sludges.

Table 1.
Chemical composition of vanadium-containing sludge samples, wt%.
2.2. Methods
2.2.1. Leaching
The important parameters of the leaching process are the following: concentration of the sulfuric acid solution, leaching temperature and duration, and solid to liquid ratio S/L. To select optimal process parameters the samples were leached by H2SO4 solution with a concentration of 1–20% at 20–80 °C during 5–60 min and S/L = 1/1–1/10 (g/mL).
The leaching process was performed in the 10-L stainless steel reactor with an upper agitator. After the end of leaching, the pulp was filtered under vacuum and washed with water at a ratio of S/L = 1/0.5 (g/mL). The filtrate and washing water were not mixed.
Chemical analysis of leaching solutions before and after vanadium deposition was carried out using an atomic emission spectrometer with inductively coupled plasma Agilent 725 Radial (Agilent Technologies, Santa Clara, CA, USA). Standard solutions from High-Purity Standards were used for calibration.
Leaching rate was calculated as:
where V2O5sol—the mass of V2O5 in solutions after leaching, g; V2O5total—the total mass of V2O5 in the sample of sludge, g.
η(V2O5)sol = m(V2O5sol)/m(V2O5total) × 100, %
2.2.2. Reducing Leaching
Reducing leaching was carried out by two methods using iron sulfate and metallic iron powder as reducing agents.
In the first method, FeSO4·7H2O (99 wt% FeSO4·7H2O) was added to the solution after sulfuric acid leaching of the sludge under optimal conditions and pulp filtration. The filtrate was heated to 80 °C using a heating plate with constant stirring, then brucite Mg(OH)2 was added to increase pH = 2–3 and FeSO4·7H2O was added at the rate of 1 g per 1 g of V2O5 in solution. After that, the pH of the solution was adjusted to ≈5.5 by adding brucite and the solution was kept under constant heating and stirring for 1 h.
In the second method, metallic iron powder (99.7 wt% Fe) was added during the process of vanadium leaching from sludge. Water and concentrated H2SO4 dropwise to pH ≈ 1.6 were added to the sample of sludge (at optimal S/L), the pulp was heated to 80 °C. Metallic iron powder was added at the rate of 2 g per 1 g of V2O5. Stirring and heating were carried out for 1 h. The resulting solution after filtration was heated to 80 °C once more, Mg(OH)2 was added to pH ≈ 5.5 and exposed 1 h.
After filtration and washing the sediment was roasted in a muffle furnace at 550 °C for 2 h, then the content of the main components in the vanadium-containing concentrate was measured.
The chemical composition of brucite, wt% was the following: moisture 0.20; calcination loss 32.0; MgO 64.5; Al2O3 0.06; SiO2 1.00; CaO 2.15; Fe2O3 0.15.
Recovery rate of vanadium into the concentrate from the sludge was calculated as:
where V2O5conc—the mass of V2O5 in the concentrate, g; V2O5total—the total mass of V2O5 in the sample of sludge, g.
η(V2O5)conc = m(V2O5conc)/m(V2O5total) × 100, %
2.2.3. Multistage Counterflow Leaching
To obtain a strong V2O5 solution from the original and roasted sludge (>10 g/L V2O5), multi-stage counterflow leaching was carried out. This method includes the following: the filtrate after leaching with H2SO4 solution in the first stage is used for leaching a new portion of the charge in the second stage; filtrate after the second stage is used similarly for the third stage, and so on until the final solution reaches a concentration V2O5 ≈ 10 g/L.
2.2.4. V2O5 Sedimentation by Ammonium Salt
V2O5 was sedimented from solutions with a content of V2O5 > 10 g/L by adding solid ammonium chloride NH4Cl (99.5 wt% NH4Cl) at a flow rate of 30–45 g/L and pH = 1–8. For this, the solution was preliminarily neutralized by adding NaOH (99 wt% NaOH) in the form of granules permanently monitoring the pH values using a pH-meter.
The recovery rate of V2O5 into the concentrate from the solution was calculated as Equation (2), substituting the value of V2O5 content in the solution into the denominator.
3. Results and Discussion
3.1. Selection of Leaching Conditions
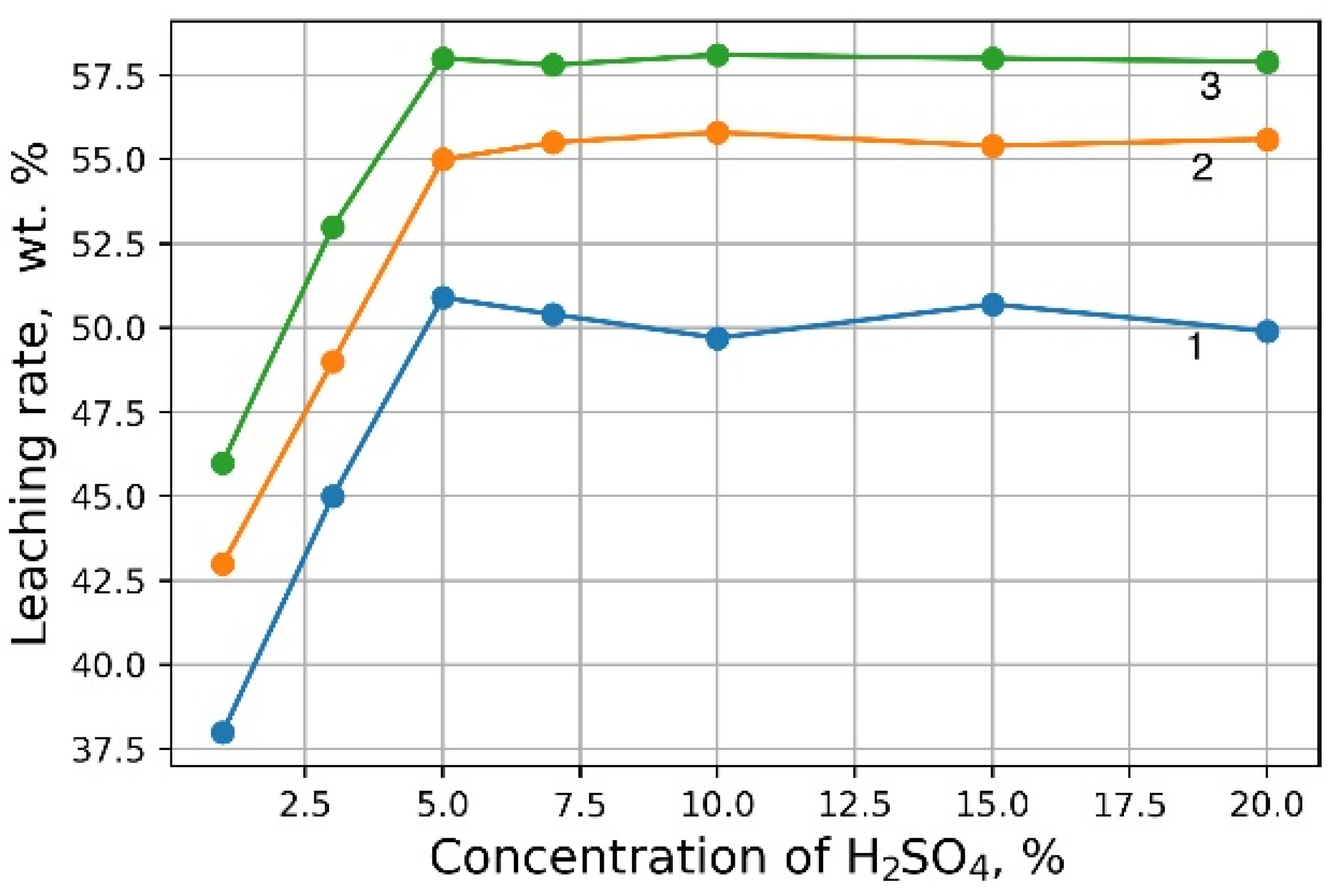
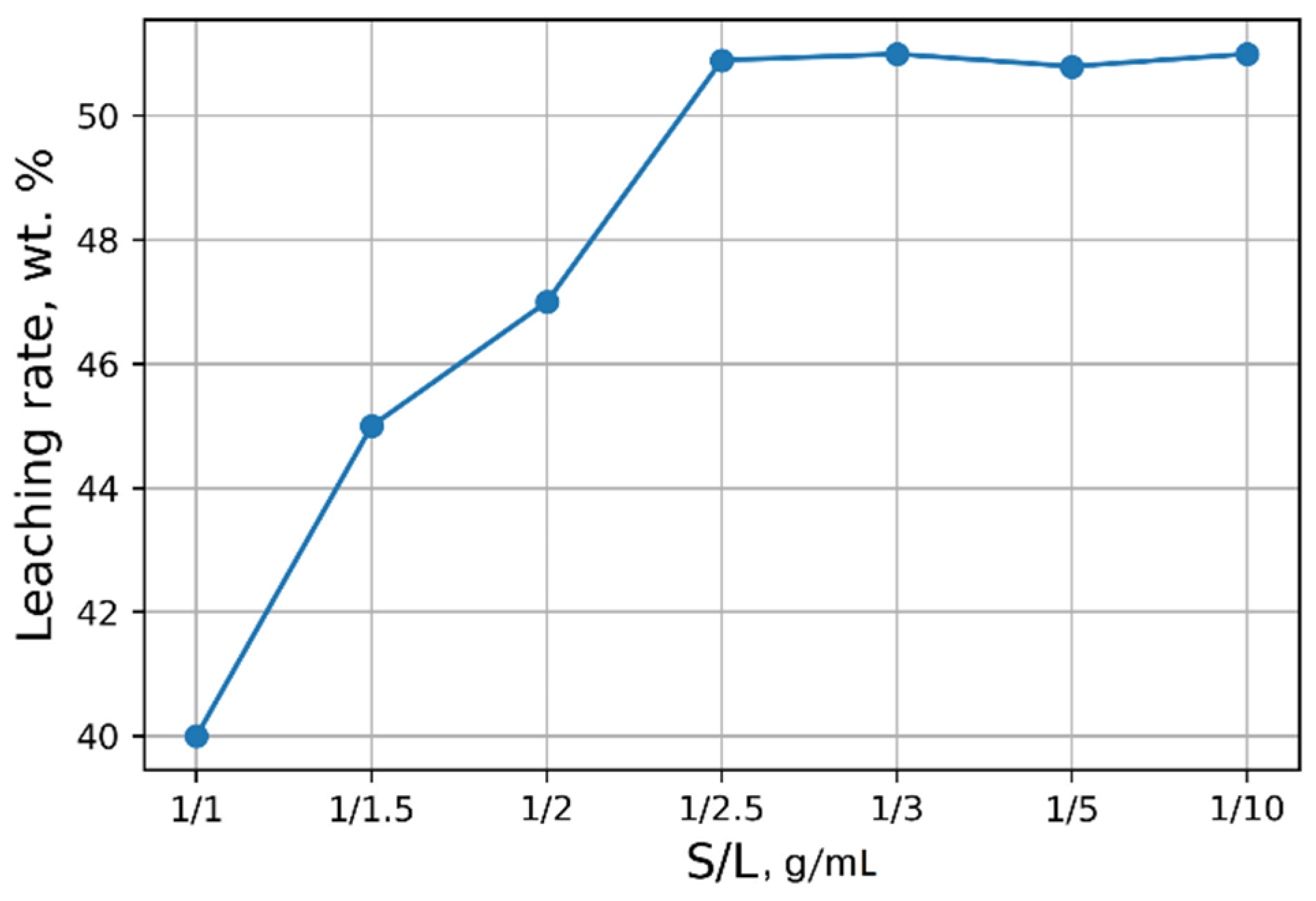
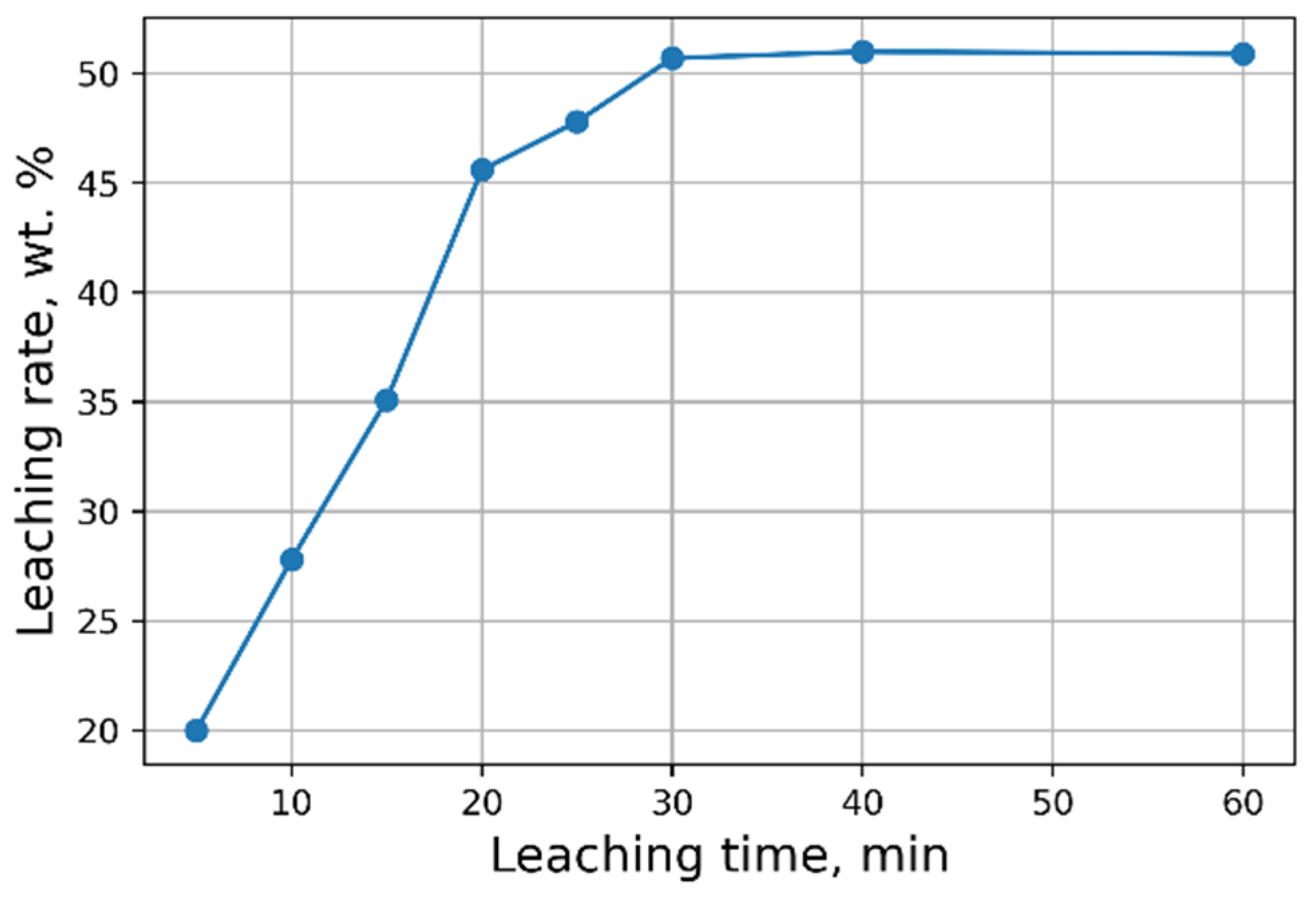
Investigations aimed at the selection of optimal leaching conditions were carried out on a sample of sludge No. 3 (Table 1). Figure 1 demonstrates the outcomes of H2SO4 concentration on the recovery rate of V2O5 into solution from the initial sludge at different temperatures and S/L = 1/5 (g/mL). The maximal recovery rate of V2O5 into solution (58%) is achieved at the leaching temperature of 80 °C and concentration of 5% of H2SO4 solution, without heating, the recovery rate of V2O5 is ≈50%. The leaching process at the temperature of 80 °C has such a disadvantage, as the need for constant monitoring of the level S/L due to significant evaporation of the leaching solution. As can be seen from Figure 1, heating has a small effect on the recovery rate of V2O5, thus it was decided to conduct the experiments without heating at 5% H2SO4 solution, while the concentration of V2O5 in the solution was ≈3 g/L. The optimal S/L ratio was found to be 1/2.5 (g/mL) (Figure 2). The maximum concentration of vanadium in the solution is reached 30 min after the start of the process (Figure 3).
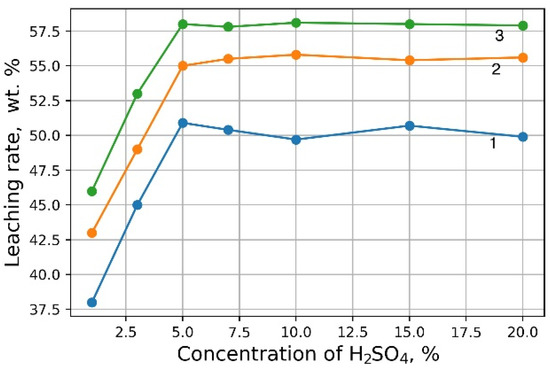
Figure 1.
Effects of H2SO4 concentration on the recovery rate of V2O5 into solution at leaching temperature 20 °C (1), 60 °C (2) and 80 °C (3).
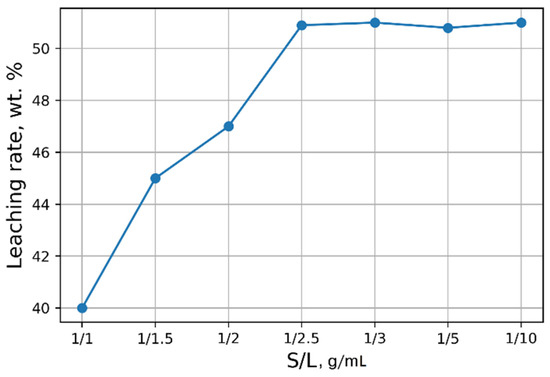
Figure 2.
Effects of S/L on the recovery rate of V2O5 into solution.
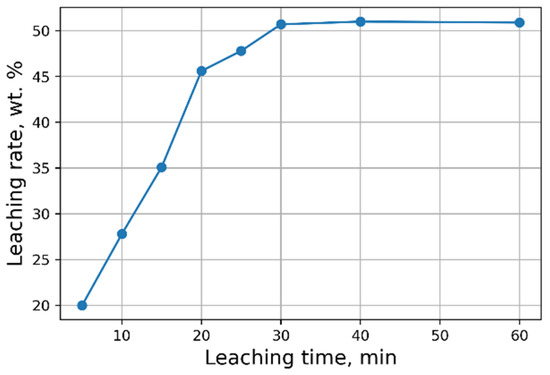
Figure 3.
Effects of leaching time on the recovery rate of V2O5 into solution.
The optimal conditions for two-stage counterflow leaching of roasted sludge with 1 wt% CaCO3 additive were selected in [33]: H2SO4 concentration of the solution is 5–7%, the leaching time is 20 min at each stage of the process; S/L = 1/2.5. These data were used in this work for obtaining vanadium-concentrated solutions with its further precipitation by brucite and NH4Cl.
3.2. Reducing Leaching
For studies of vanadium leaching with its further reduction by FeSO4·7H2O, sludges with V2O5 content of 2.25 wt% were used (Table 1, sample No. 1).
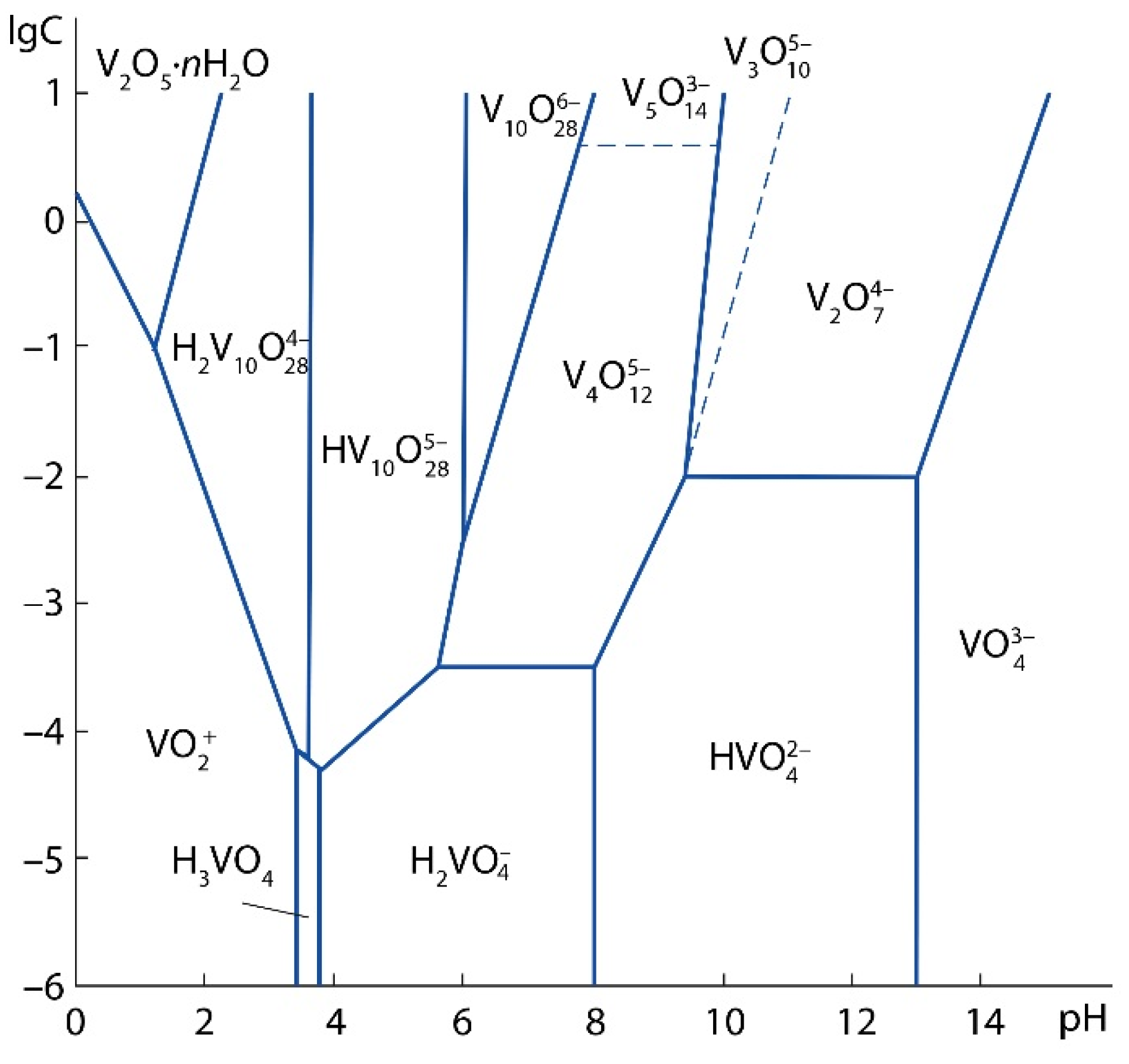
The test sample contains 0.94 wt% V2O5a.s., i.e., this amount of V2O5 can be converted into a solution by leaching with a 7% H2SO4 solution [33]. Vanadium can be represented in the acid-soluble part in the form of salts: orthovanadates (Me3VO4), pyrovanadates (Me4V2O7) and metavanadates (MeVO3), where Me is a monovalent metal ion. Since the sludge under study is a product of vanadium converter slag processing roasted with limestone, the most possible acid-soluble phases in the sludge are Ca3(VO4)2, Ca2V2O7, Ca(VO3)2. As a result of the treatment of calcium vanadates with a solution of sulfuric acid, various vanadium ions can be formed depending on the pH of the solution and the concentration of vanadium, (Figure 4) [38].
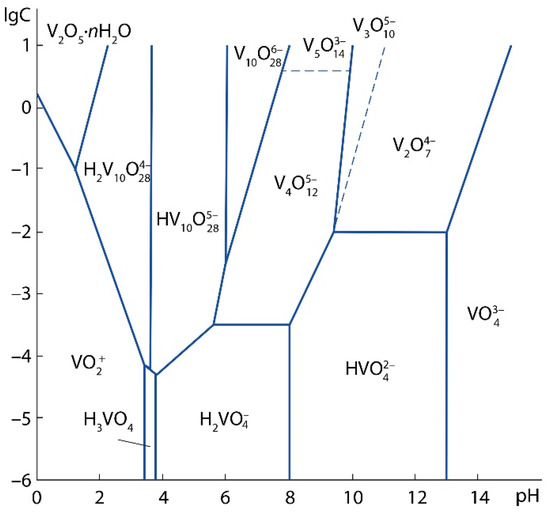
Figure 4.
Diagram of the state of oxo- and hydroxoforms of vanadium (V5+) in aqueous solutions.
When vanadium is leached with a 5% H2SO4 solution, at which the pH of the resulting solution was 0.6–0.8, and the vanadium concentration in the solution after leaching was ≈ 3 g/L, the formation of VO2+ ions is probable by the following reaction:
VO43− + 4H+ → VO2+ + 2H2O.
For example, the reaction could occur as followed:
Ca3(VO4)2 + 4H2SO4 → 3CaSO4 + (VO2)2SO4 + 4H2O
In acidic medium at pH = 2–3 VO2+ is reduced to VO2+ by iron sulphate with the following reactions:
VO2+ + 2H+ + ē → VO2+ + H2O,
Fe2+ − ē → Fe3+.
The leaching solution acquired a blue color after the addition of FeSO4·7H2O crystals indicating the predominance of V4+ in solution.
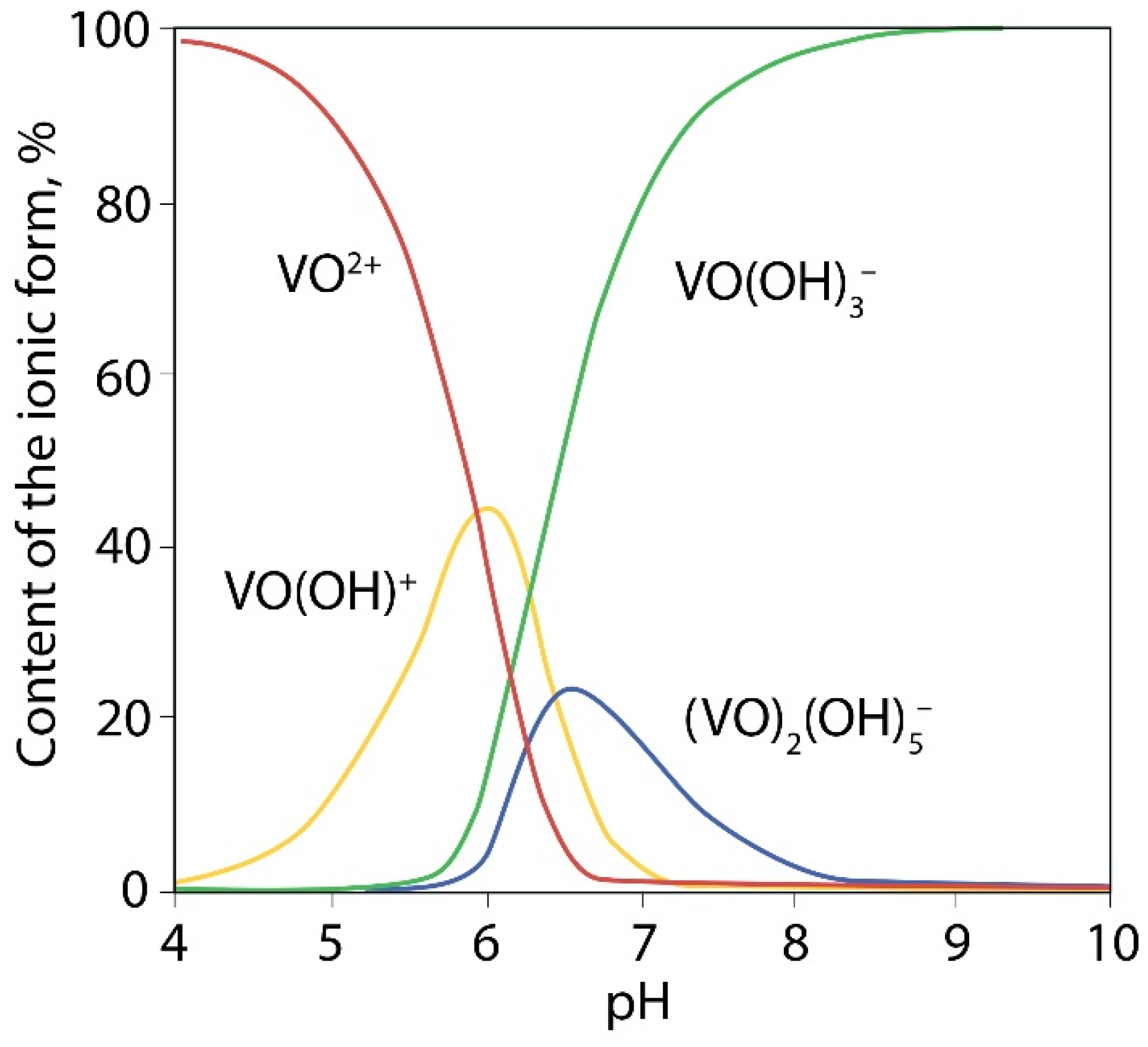
It is known that VO2+ ions in aqueous solutions are existing mainly in the form of [VO(H2O)5]2+ when pH < 3.5 and as [VO(OH)]+ at higher pH values (Figure 5) [40]. At pH > 4 precipitation of VO(OH)2 occurs by reaction:
VO2+ + 2OH− = VO(OH)2↓
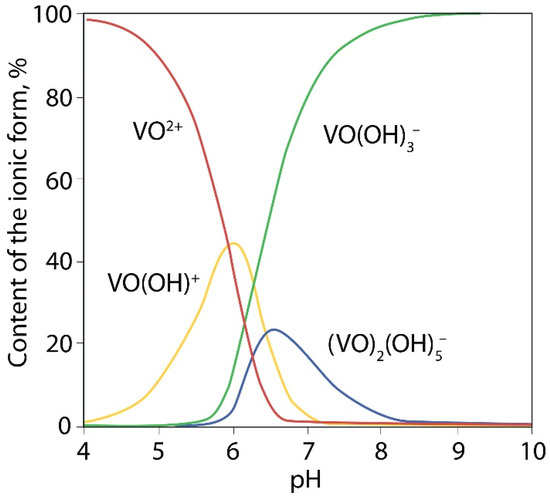
Figure 5.
Distribution diagram effect of pH on of the forms of V4+ in 10 nM solution.
After precipitation by brucite and roasting of the residuum, a light brown concentrate was obtained. As one can see in Table 2, it was possible to obtain a concentrate with V2O5 content of ~22%. This concentrate contains a significant content of impurities, including phosphorus which is a harmful impurity for ferrous metallurgy. Thus, this concentrate requires further processing before obtaining vanadium alloys.

Table 2.
Chemical composition of initial sludges, products and wastes of reducing processes.
Investigations on reduction by metallic iron were carried out using sludge with V2O5 content of 2.78 wt%. (Table 1, sample No. 2). Optimizing the process, we added iron for vanadium reduction during the leaching process and heated the pulp to 80 °C immediately after iron powder addition. As the concentrate obtained by reduction with iron sulfate contains a significant amount of phosphorus, the pH value was increased to ≈ 1.6. Reduction of V5+ passing into solution by metallic iron can proceed by the following reactions:
(VO2)2SO4 + Fe + 2H2SO4 → 2VOSO4 + FeSO4 + 2H2O
2VOSO4 + Fe + 2H2SO4 → V2(SO4)3 + FeSO4 + 2H2O
In this process, solutions with V2O5 content of ≈6.1 g/L were obtained, while the recovery rate of V2O5 into solution was 36.5 wt%. The final concentrate after roasting in the muffle furnace contained 26.5 wt% of V2O5. Thus, metallic iron as a reducing agent allowed to increase V2O5 content slightly and to reduce phosphorus content in the final concentrate (see Table 2).
Methods of one-stage reduction leaching with followed precipitation by brucite are ineffective for processing sludge due to the low vanadium content and high content of harmful impurities in the resulting concentrates as shown by investigations.
The method of reducing leaching with of FeSO4·7H2O additive was investigated for the recovery of vanadium from solutions with a concentration of ~20 g/L obtained under optimal conditions from roasted sludge with 1% CaCO3 additive by two-stage counterflow leaching. The process was carried out as previous studies with FeSO4·7H2O additive, and as a result, a concentrate with content V2O5 of 53.6 wt% was obtained (Table 3). This concentrate is characterized by a significant content of impurities, however, due to the high vanadium content, it may be suitable for smelting vanadium ligatures [40].

Table 3.
Chemical composition of roasted sludge, product and waste of reducing process from solution with concentration V2O5 of ~20 g/L.
Table 4 presents the technological parameters and consumption parameters of the considered processes of reducing leaching and subsequent precipitation in terms of 1 g of the resulting concentrate.

Table 4.
Parameters of reducing leaching.
3.3. V2O5 Precipitation by Ammonium Salt
Investigations of vanadium concentrate production by precipitation with ammonium salt NH4Cl from solutions with a content of V2O5 > 10 g/L were carried out using initial sludge (Table 1, Sample No. 3) and roasted sludge with 1% CaCO3 additive.
A final solution with a concentration of 15 g/L V2O5 and pH = 0.65 was obtained from the initial sludge by three-stage counterflow leaching with 5% H2SO4 solution at the first stage. It is known that vanadium precipitates from an acidic solution at pH = 1.8–3 and at pH = 4–8 [12]. At pH = 1.8–3, vanadium can precipitate in the form of ammonium hexavanadate by reaction:
3V10O286− + 10NH4+ + 8H+ = 5(NH4)2V6O16 + 4H2O
As a result of reaction with NH4Cl ammonium metavanadate is formed from alkaline solutions:
VO3− + NH4+ → NH4VO3
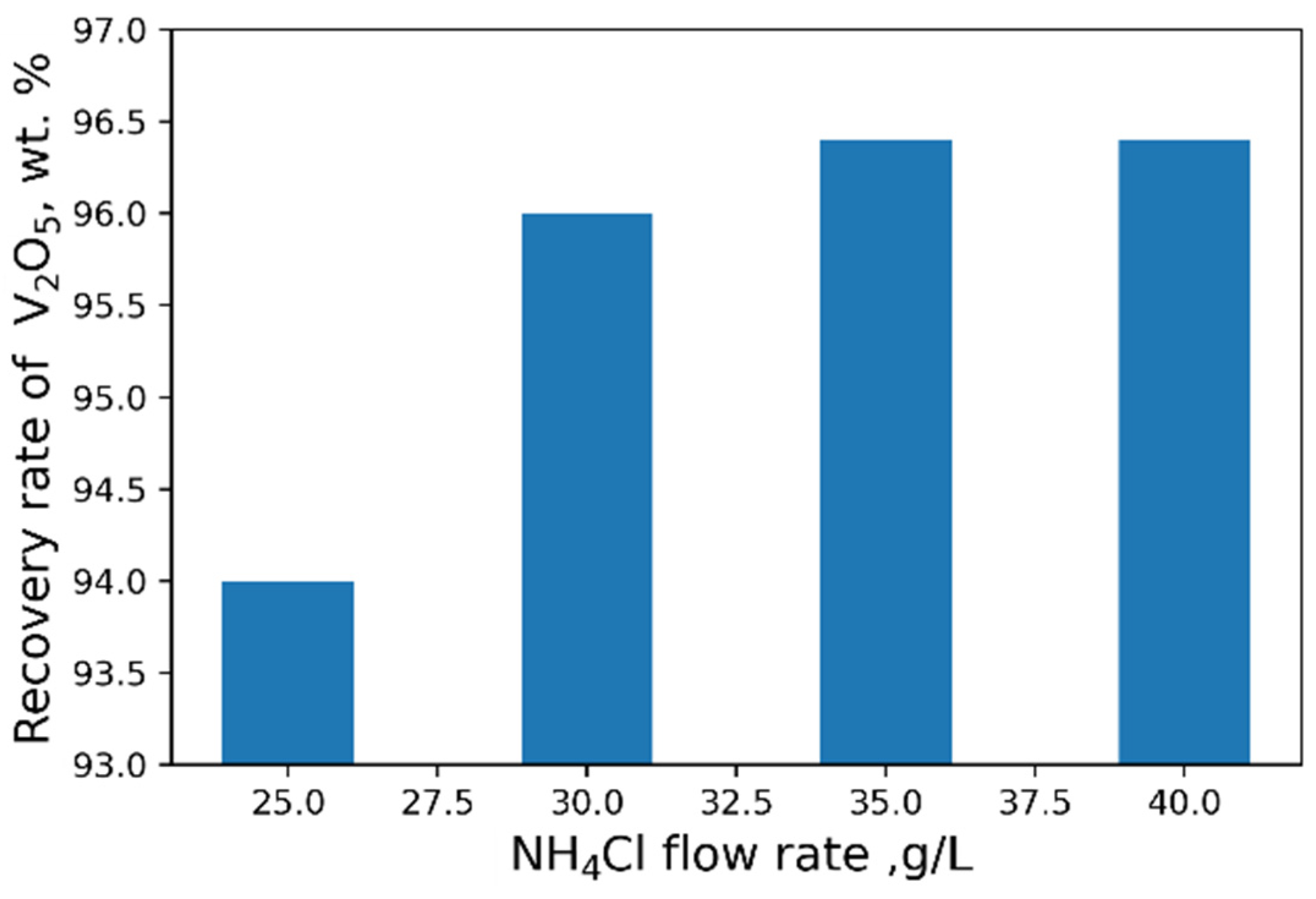
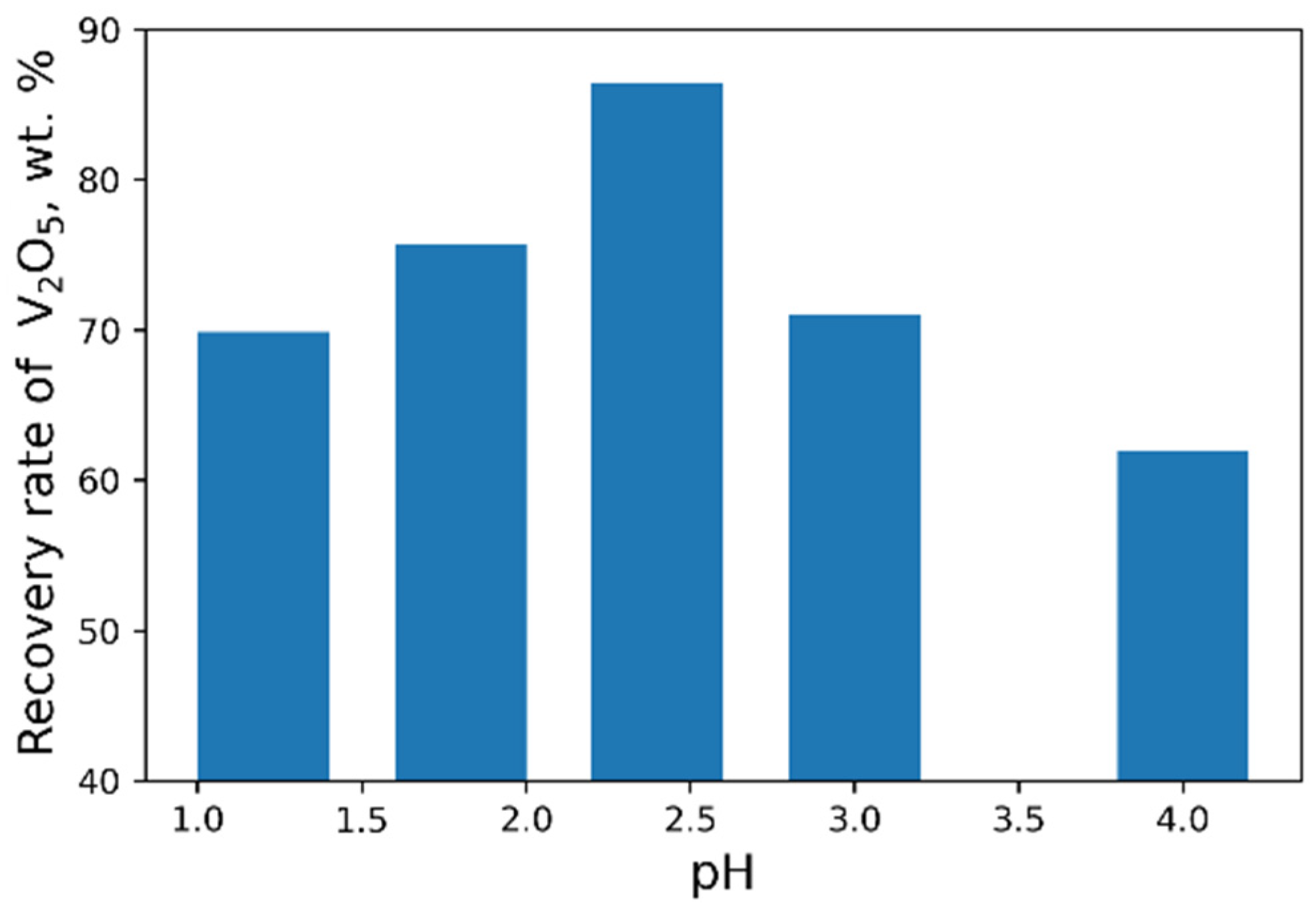
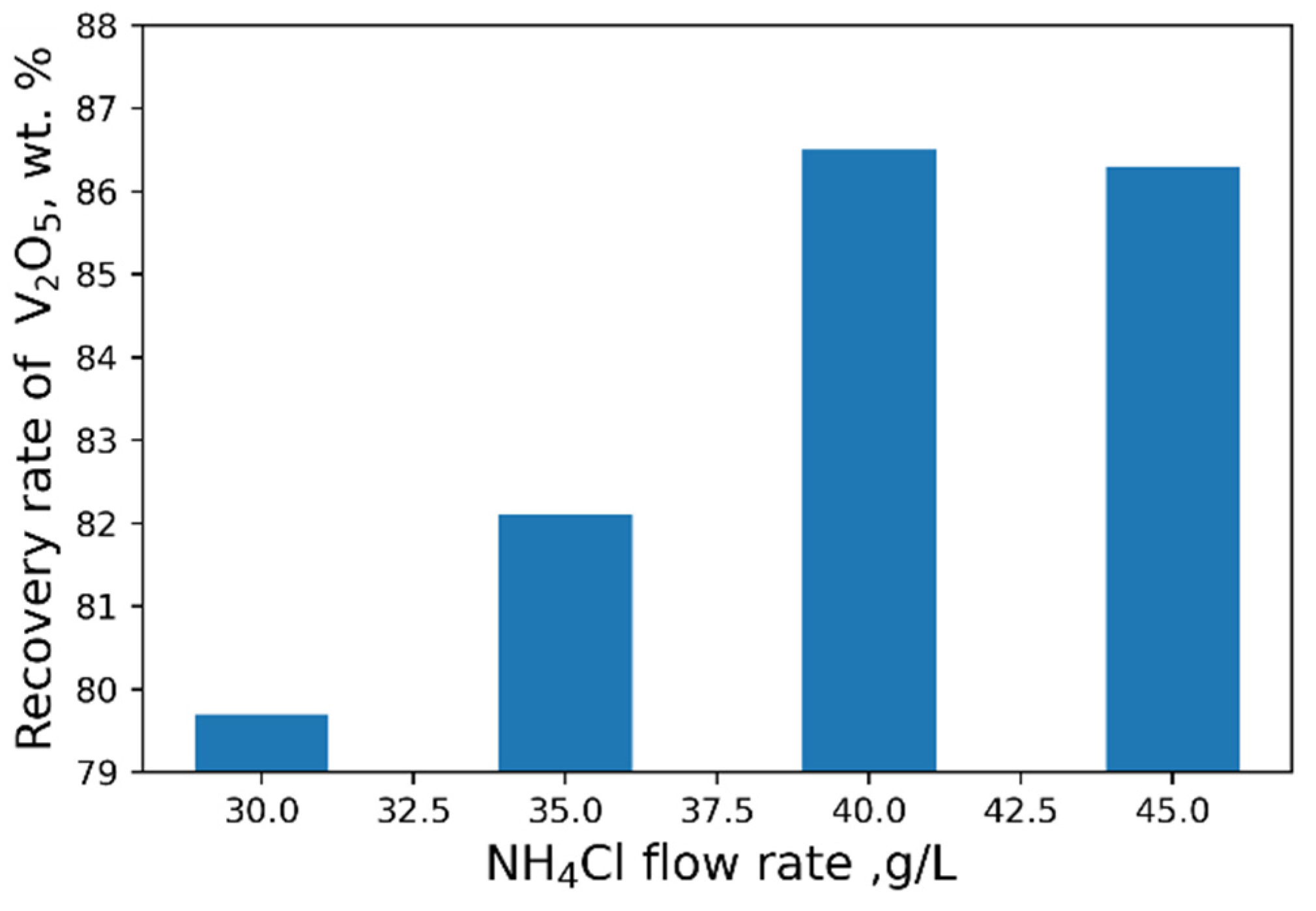
The influence of pH on the recovery rate of V2O5 into the concentrate is shown in Figure 6. Tests at pH < 3 were carried out heating solutions to 95 °C. The maximal recovery rate of 96 % of V2O5 into the concentrate from the solution is achieved at pH = 2.4. An increase in NH4Cl flow rate does not practically affect the yield of V2O5 (Figure 7).
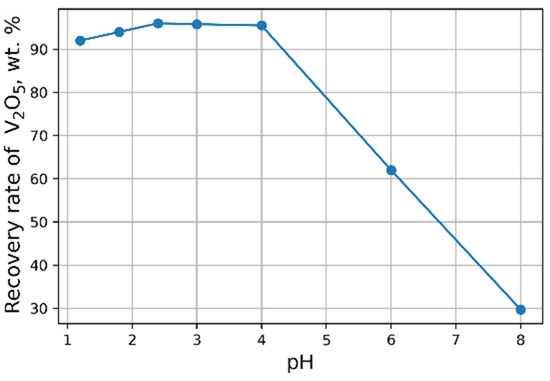
Figure 6.
Effect of pH value on the recovery of V2O5 into the concentrate (30 g/L NH4Cl flow rate).
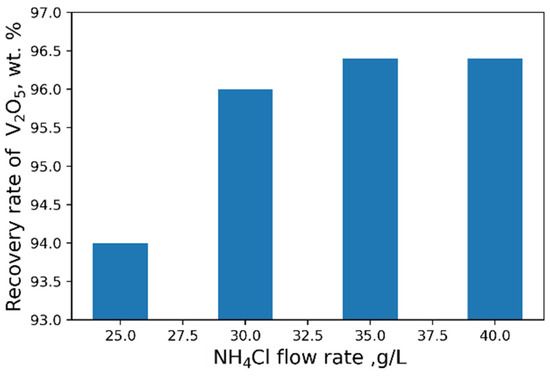
Figure 7.
Effect of NH4Cl flow rate on the recovery of V2O5 into the concentrate.
Compositions of concentrates at different pH values are presented in Table 5. With an increasing pH, the recovery rate of V2O5 into the concentrate decreases, and an increasing amount of impurities is also observed.

Table 5.
Chemical composition of initial sludge and concentrates after precipitation at different pH values.
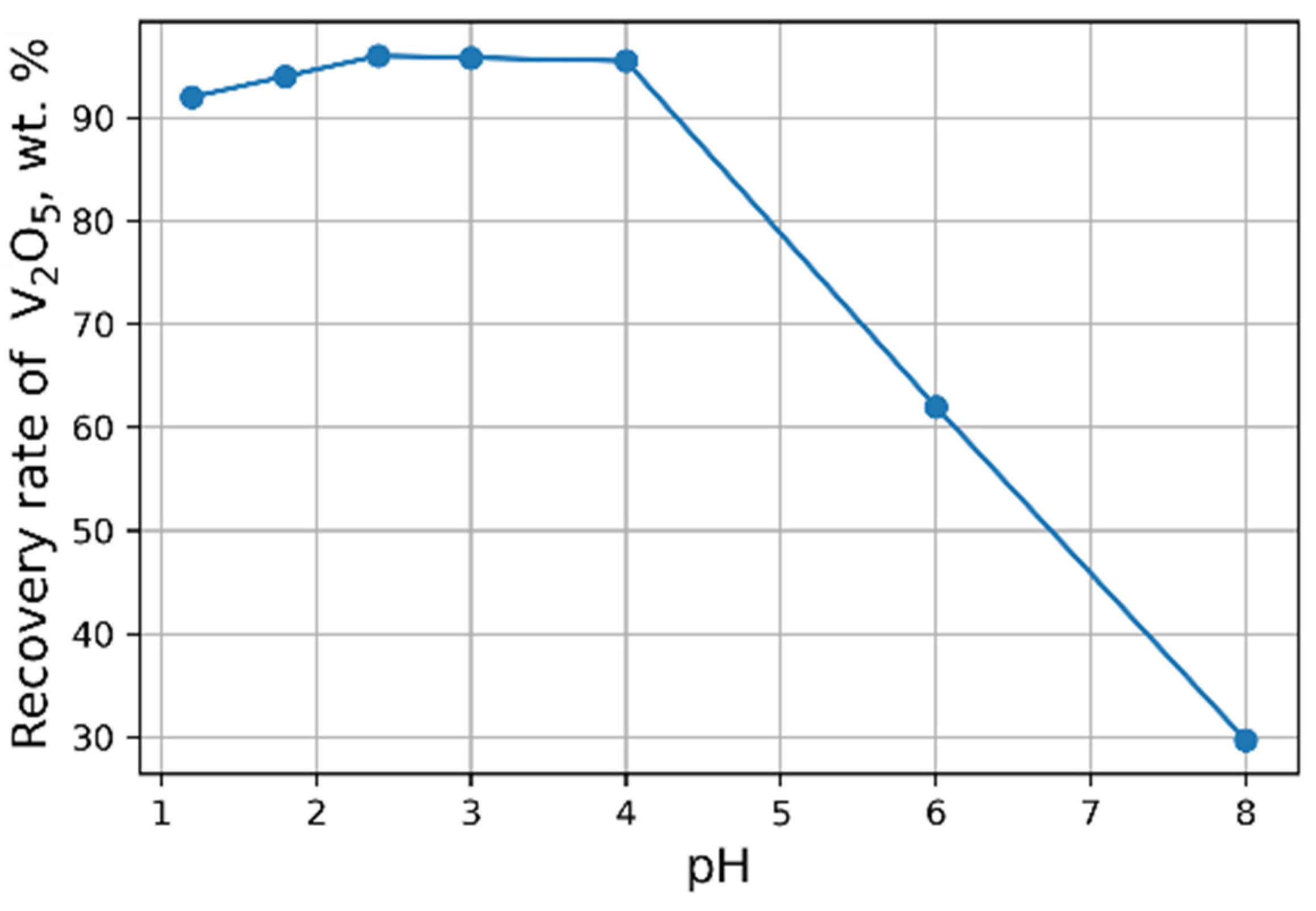
Let us consider the possibility of V2O5 precipitation from a solution with a concentration of V2O5 23 g/L by ammonium salt obtained after two-stage counterflow leaching of roasted sludge with 1% CaCO3 additive.
As a result of our studies, the following optimal parameters were selected: pH = 2.4, 40 g/L NH4Cl flow rate (Figure 8 and Figure 9). At optimal conditions, we obtained the concentrate with V2O5 content of 93.6 wt% (Table 6). However, higher purity vanadium pentoxide requires additional stages of concentrate processing.
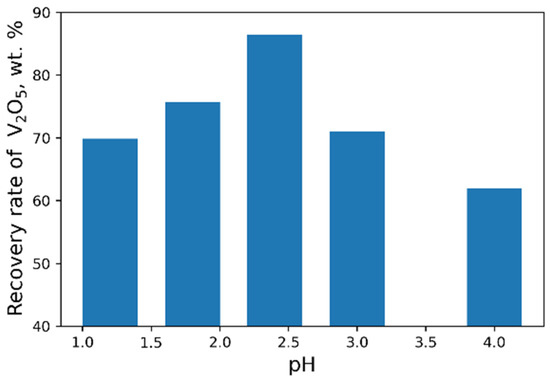
Figure 8.
Effect of pH value on the recovery of V2O5 into the concentrate (45 g/L NH4Cl flow rate).
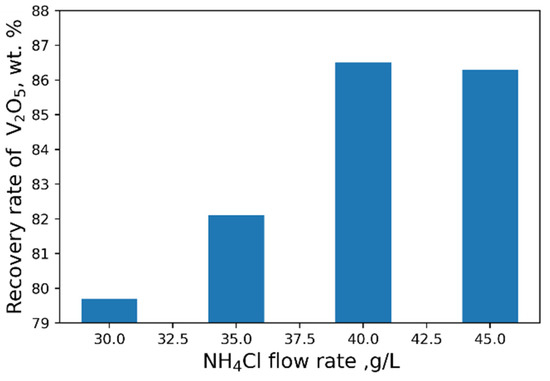
Figure 9.
Effect of NH4Cl flow rate on the recovery of V2O5 into the concentrate.

Table 6.
Chemical composition of initial sludge and concentrate obtained after V2O5 precipitation by NH4Cl.
Reducing the content of impurities in concentrates obtained by precipitation from solutions with V2O5 concentration of 15 and 23 g/L at pH = 2.4 (Table 5 and Table 6) was conducted on their washing by repulpation. Washing was carried out with 1% NH4Cl solution at S/L = 1/10 at a temperature of 95 °C. In this process the removal of soluble sulfates of manganese, iron, titanium, and alkali metals takes place. Due to the low solubility of vanadium compounds in ammonium chloride solution, the losses of V2O5 into washing solutions were less than 0.5%. Washing of the concentrate obtained from the initial sludge allowed to increase V2O5 content to 84.94 wt% (Table 7), which is slightly lower than the requirements of the standard (≥90 wt%). Additionally, the concentrate has an increased phosphorus content unsuitable for smelting high-vanadium alloys, such as FeV60, FeV80. The concentrate obtained from the roasted sludge meeting to the grade of VNO-2 (Table 7). To obtain a cleaner vanadium pentoxide (pure, chemically pure), additional stages of washing from impurities will be required. The parameters of the considered processes are presented in Table 8.

Table 7.
Chemical composition of washing concentrates obtained from initial and roasted sludges by counterflow leaching method.

Table 8.
Parameters of counterflow leaching method.
Parameters of the analyzed processes for obtaining vanadium concentrates from sludges are presented in Table 9. The most effective technology includes preliminary oxidation roasting of the sludge, two-stage leaching of vanadium from the roasted sludge with a sulfuric acid solution and its further precipitation by hydrolysis or ammonium salts.

Table 9.
Comparative table of parameters of various hydrometallurgical processes of sludge processing.
4. Conclusions
Waste from the production of vanadium pentoxide is undoubtedly a promising technogenic source of raw materials for vanadium production. The experimental analysis of various sulfuric acid methods of sludge processing has shown that the most effective method is an oxidation roasting of sludge with further two-stage counterflow leaching of vanadium with 5% H2SO4 solution in the first stage at S/L = 1/2.5 (g/mL). Precipitation from solutions with a high concentration of more than 20 g/L by hydrolysis or ammonium salts allows fabricating concentrates with >90 wt% V2O5 satisfying the requirements of the standard.
Technologies of counterflow leaching of vanadium from the initial sludges can also be used to obtain vanadium concentrates, but in this case, V2O5 content is significantly lower: 72–78 wt% V2O5$ V2O5 content increases after additional purification to 85 wt%, at the same time a significant amount of vanadium remains in the waste sludge. In ferrous metallurgy, such concentrates can be used for the low vanadium ferroalloys (FeV40) and ligatures production.
Methods of reducing leaching with subsequent precipitation of vanadium with brucite have not proven their effectiveness due to the high content of impurities in obtaining concentrates.
Author Contributions
Conceptualization, U.K. and A.V.; investigation, U.K., A.V., I.K. and P.S.; formal analysis, U.K., A.V. and P.S.; visualization, U.K., A.V. and I.K.; writing—Original draft preparation, U.K., A.V., I.K. and D.W.; writing–Review and editing, U.K., A.V. and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research was fulfilled with partial financial support of the RFBR, grant no. 18-29-24074_mk.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gasik, M.; Dashevskii, V.; Bizhanov, A. Ferroalloys: Theory and Practice; Springer: Cham, Switzerland, 2020; p. 53. [Google Scholar] [CrossRef]
- Wan, S.; Li, H.; Tieu, K.; Xue, Q.; Zhu, H. Mechanical and tribological assessments of high-vanadium high-speed steel by the conventional powder metallurgy process. Int. J. Adv. Manuf. Technol. 2019, 103, 943–955. [Google Scholar] [CrossRef]
- Pham, M.K.; Nguyen, D.N.; Hoang, A.T. Influence of Vanadium Content on the Microstructure and Mechanical Properties of High-Manganese Steel. Int. J. Mech. Mechatron. Eng. IJMME-IJENS 2018, 18, 141–147. [Google Scholar]
- Li, L.; Li, B.; Wan, H.; Chen, M.; Yee, S.L. Study of Controlled Aluminum-Vanadium Alloy Prepared by Vacuum Resistance Furnace. Int. J. Photoenergy 2021, 2021, 7055415. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.P.; Deng, C.Y.; Huo, L.; Wang, L.; Fang, R. Novel method to fabricate Ti-Al intermetallic compound coatings on Ti-6Al-4V alloy by combined ultrasonic impact treatment and electrospark deposition. J. Alloys Compd. 2015, 628, 208–212. [Google Scholar] [CrossRef]
- Cheng, C.; Dou, Z.H.; Zhang, T.A.; Zhang, H.J.; Yi, X.; Su, J.M. Synthesis of as-cast Ti-Al-V alloy from titanium-rich material by thermite reduction. JOM 2017, 69, 1818–1823. [Google Scholar] [CrossRef]
- Rappleye, D.; Haun, R. Production of Pure Vanadium: Industry Review and Feasibility Study of Electron Beam Melt Refining of V–Al Alloys. J. Sustain. Metall. 2021, 7, 755–766. [Google Scholar] [CrossRef]
- Xua, J.; Chena, G.; Guo, F.; Xie, J. Development of wide-temperature vanadium-based catalysts for selective catalytic reducing of NOx with ammonia: Review. Chem. Eng. J. 2018, 353, 507–518. [Google Scholar] [CrossRef]
- Monfort, O.; Petrisková, P. Binary and Ternary Vanadium Oxides: General Overview, Physical Properties, and Photochemical Processes for Environmental Applications. Processes 2021, 9, 214. [Google Scholar] [CrossRef]
- Mjejri, I.; Rougier, A.; Gaudon, M. Low-Cost and Facile Synthesis of the Vanadium Oxides V2O3, VO2, and V2O5 and Their Magnetic, Thermochromic and Electrochromic Properties. Inorg. Chem. 2017, 56, 1734–1741. [Google Scholar] [CrossRef] [Green Version]
- Toledano-Serrabona, J.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I. Materials 2021, 14, 6507. [Google Scholar] [CrossRef]
- Peng, H. A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 2019, 7, 103313. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Q.; Lv, X.; Bai, C. Extraction of vanadium from converter slag by two-step sulfuric acid leaching process. J. Clean. Prod. 2018, 170, 1089–1101. [Google Scholar] [CrossRef]
- Gao, F.; Olayiwola, A.U.; Liu, B.; Wang, S.; Du, H.; Li, J.; Wang, X.; Chen, D.; Zhang, Y. Review of Vanadium Production Part I: Primary Resources. Miner. Process. Extr. Metall. Rev. 2021, 15, 1–23. [Google Scholar] [CrossRef]
- Gilligan, R.; Nikoloski, A.N. The extraction of vanadium from titanomagnetites and other sources. Miner. Eng. 2020, 146, 106106. [Google Scholar] [CrossRef]
- Wang, M.; Huang, S.; Chen, B.; Wang, X. A review of processing technologies for vanadium extraction from stone coal. Miner. Process. Extr. Metall. 2018, 129, 290–298. [Google Scholar] [CrossRef]
- Dong, Y.-B.; Liu, Y.; Lin, H.; Liu, C.-J. Improving vanadium extraction from stone coal via combination of blank roasting and bioleaching by ARTP-mutated Bacillus mucilaginosus. Trans. Nonferr. Met. Soc. China 2019, 29, 849–858. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, T.; Zhou, Y.; Xu, W.; Lin, H.; Yan, B. Optimization of Oxidative Leaching for Vanadium Extraction from Low-Grade Stone Coal Using Response Surface Methodology. Processes 2020, 8, 1534. [Google Scholar] [CrossRef]
- Jung, M.; Mishra, B. Vanadium Recovery from Oil Fly Ash by Carbon Removal and Roast-Leach Process. JOM 2018, 70, 168–172. [Google Scholar] [CrossRef]
- Xiao, Y.; Mambote, C.R.; Jalkanen, H.; Yang, Y.; Boom, R. Vanadium Recovery as FeV from Petroleum Fly Ash. In Proceedings of the Twelfth International Ferroalloys Congress Sustainable Future, Helsinki, Finland, 6–9 June 2010; pp. 179–188. [Google Scholar]
- Wu, W.; Wang, C.; Bao, W.; Li, H. Selective reduction leaching of vanadium and iron by oxalic acid from spent V2O5-WO3/TiO2 catalyst. Hydrometallurgy 2018, 179, 52–59. [Google Scholar] [CrossRef]
- Ferella, F. A review on management and recycling of spent selective catalytic reduction catalysts. J. Clean. Prod. 2019, 246, 118990. [Google Scholar] [CrossRef]
- Zou, K.; Xiao, J.; Liang, G.; Huang, W.; Xiong, W. Effective Extraction of Vanadium from Bauxite-Type Vanadium Ore Using Roasting and Leaching. Metals 2021, 11, 1342. [Google Scholar] [CrossRef]
- Wena, J.; Jianga, T.; Zhenga, X.; Wanga, J.; Caoa, J.; Zhoua, M. Efficient separation of chromium and vanadium by calcification roasting–sodium carbonate leaching from high chromium vanadium slag and V2O5 preparation. Sep. Purif. Technol. 2020, 230, 115881. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Aldahrib, T.; Li, C.; Liu, W.; Zhang, G.; Yang, Y.; Luo, D. Direct recovery of low valence vanadium from vanadium slag—Effect of roasting on vanadium leaching. Hydrometallurgy 2020, 191, 10515. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Liu, Y.; Xue, X. Extraction behavior of vanadium and chromium by calcification roasting-acid leaching from high chromium vanadium slag: Optimization using response surface methodology. Miner. Process. Extr. Metall. Rev. 2019, 40, 56–66. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Lin, M.; Guo, Y.; Xie, B. Green one-step roasting method for efficient extraction of vanadium and chromium from vanadium-chromium slag. Powder Technol. 2020, 360, 503–508. [Google Scholar] [CrossRef]
- Li, M.; Liu, B.; Zheng, S.; Wang, S.; Du, H.; Dreisinger, D.B.; Zhang, Y. A cleaner vanadium extraction method featuring non-salt roasting and ammonium bicarbonate leaching. J. Clean. Prod. 2017, 149, 206–217. [Google Scholar] [CrossRef]
- Volkov, A.I.; Kologrieva, U.A.; Kovalev, A.I.; Vainshtein, D.L. Effect of degree of oxidation and element form in vanadium slag on its treatment production capacity. Metallurgist 2019, 63, 813–818. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Zhou, W.; Gao, H.; Xue, X. A cleaner and efficient process for extraction of vanadium from high chromium vanadium slag: Leaching in (NH4)2SO4–H2SO4 synergistic system and NH4+ recycle. Sep. Purif. Technol. 2019, 216, 126–135. [Google Scholar] [CrossRef]
- Li, M.; Zheng, S.; Liu, B.; Wang, S.; Dreisinger, D.; Zhang, Y.; Du, H.; Zhang, Y. A clean and efficient method for recovery of vanadium from vanadium slag: Nonsalt roasting and ammonium carbonate leaching processes. Miner. Process. Extr. Metall. Rev. 2017, 38, 228–237. [Google Scholar] [CrossRef]
- Liu, S.; Xue, W.; Wang, L. Extraction of the Rare Element Vanadium from Vanadium-Containing Materials by Chlorination Method: A Critical Review. Metals 2021, 11, 1301. [Google Scholar] [CrossRef]
- Kologrieva, U.; Volkov, A.; Zinoveev, D.; Krasnyanskaya, I.; Stulov, P.; Wainstein, D. Investigation of Vanadium-Containing Sludge Oxidation Roasting Process for Vanadium Extraction. Metals 2021, 11, 100. [Google Scholar] [CrossRef]
- Kologrieva, U.A.; Volkov, A.I.; Stulov, P.E.; Mirakova, M.G.; Zinoveev, D.V. Counterflow Leaching Technology for Vanadium from Vanadium Pentoxide Hydrometallurgical Production Slurry. Metallurgist 2020, 64, 542–547. [Google Scholar] [CrossRef]
- Kologrieva, U.A.; Volkov, A.I.; Stulov, P.E.; Mirakova, M.G. Influence of Preliminary Heat Treatment of Vanadium Sludges on the Manufacturability of their Processing. Metallurgist 2021, 64, 1179–1187. [Google Scholar] [CrossRef]
- Volkov, A.; Kologrieva, U.; Kovalev, A.; Wainstein, D.; Vakhrushev, V. Vanadium Chemical Compounds forms in Wastes of Vanadium Pentoxide Production. Materials 2020, 13, 4889. [Google Scholar] [CrossRef]
- Tretyakov, Y.D. Inorganic Chemistry: Chemistry of Transition Elements; Academy: Moscow, Russia, 2008; p. 399. (In Russian) [Google Scholar]
- Volkov, A.I.; Osipov, K.B.; Zhdanov, P.A. X-ray fluorescence analysis of vanadium slag after borate melting. Zavod. Lab. Diagnost. Materialov. 2016, 82, 8–15. (In Russian) [Google Scholar]
- Costa Pessoa, J. Thirty years through vanadium chemistry. J. Inorg. Biochem. 2015, 147, 4–24. [Google Scholar] [CrossRef]
- Seregin, A.N. On the problem of the development of the market of vanadium-containing steels. Probl. Chernoy Metall. I Mater. 2010, 2, 92–100. (In Russian) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).