New Horizons for Hydroxyapatite Supported by DXA Assessment—A Preliminary Study
Abstract
1. Introduction
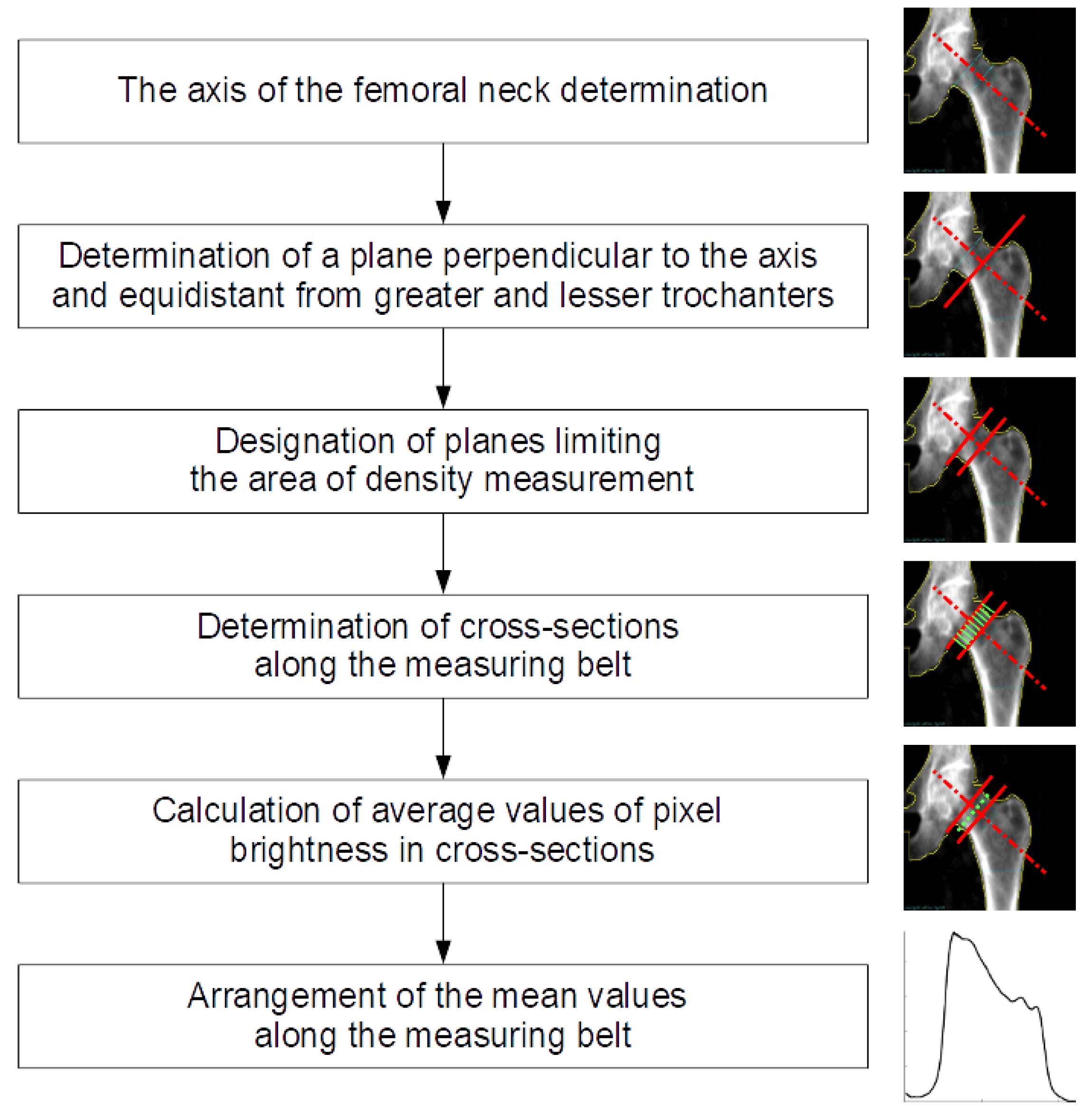
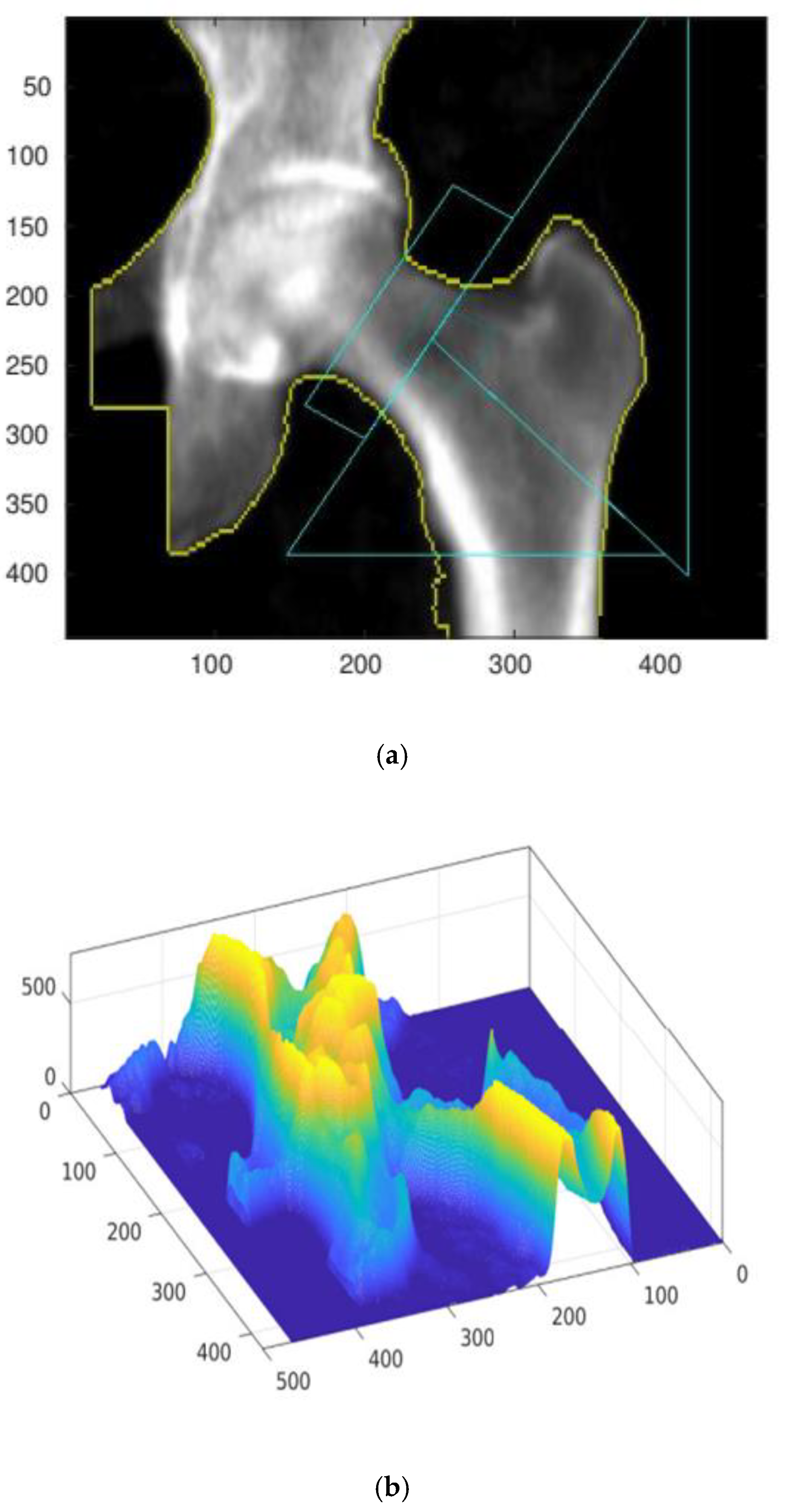
2. Materials and Methods
Numerical Procedure
3. Results
4. Discussion
4.1. Hydroxyapatite Matter
4.2. DXA Matter
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Consensus Development Conference. Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- Cameron, J.R.; Sorenson, G. Measurements of bone mineral in vivo: An improved method. Science 1963, 142, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Briot, K. Current role for bone absorptiometry. Jt. Bone Spine 2017, 84, 35–37. [Google Scholar] [CrossRef] [PubMed]
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis; National Osteoporosis Foundation: Washington, DC, USA, 2010. [Google Scholar]
- Watts, N.B.; Bilezikian, J.P.; Camacho, P.M.; Greenspan, S.L.; Harris, S.T.; Hodgson, S.F.; Kleerekoper, M.; Luckey, M.M.; McClung, M.R.; Pollack, R.P.; et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr. Prac. 2010, 16, 1–37. [Google Scholar] [CrossRef]
- Bonnick, S.L. Bone Densitometry in Clnical Practice; Humana Press: Totowa, NJ, USA, 2004. [Google Scholar]
- Khan, A.A.; Colquhoun, A.; Hanley, D.A.; Jankowski, L.G.; Josse, R.G.; Kendler, D.L.; Lentle, B.; Leslie, W.D.; Lewiecki, E.M.; O’Neill, E.; et al. Standards and guidelines for technologists performing central dual-energy X-ray absorptiometry. J. Clin. Densitom. 2007, 10, 189–195. [Google Scholar] [CrossRef]
- Leslie, W.D. Factors affecting short-term bone density precision assessment and the effect on patient monitoring. J. Bone Miner. Res. 2008, 23, 199–204. [Google Scholar] [CrossRef]
- Leslie, W.D.; Moayyeri, A.; Sadatsafavi, M.; Wang, L. A new approach for quantifying change and test precision in bone densitometry. J. Clin. Densitom. 2007, 10, 365–369. [Google Scholar] [CrossRef]
- Baniak, N.; Grzybowski, S.; Olszynski, W.P. Dual-energy X-ray absorptiometry scan autoanalysis vs manual analysis. J. Clin. Densitom. 2014, 17, 97–103. [Google Scholar] [CrossRef]
- Rosenthall, L. Range of change of measured BMD in the femoral neck and total hip with rotation in women. J. Bone Miner. Metab. 2004, 22, 496–499. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mat. Sci. Eng. R 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Cengiz, B.; Gokce, Y.; Yildiz, N.; Aktas, Z.; Calimli, A. Synthesis and characterization of hydroxyapatite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 29–33. [Google Scholar] [CrossRef]
- Bassi, M.A.; Lopez, M.A.; Confalone, L.; Carinci, F. Hydraulic Sinus Lift Technique in Future Site Development: Clinical and Histomorphometric Analysis of Human Biopsies. Implant. Dent. 2015, 24, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Mann, S. Molecular recognition in biomineralization. Nature 1988, 332, 119–124. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate bioceramics. Ceram. Int. 2015, 41, 13913–13966. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing Methods for Calculating Nano Crystal Size of Natural Hydroxyapatite Using X-Ray Diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Multiphasic calcium orthophosphate (CaPO4) bioceramics and their biomedical applications. Ceram. Int. 2016, 42, 6529–6554. [Google Scholar] [CrossRef]
- Turon, P.; Del Valle, L.; Aleman, C.; Puiggali, J. Biodegradable and Biocompatible Systems Based on Hydroxyapatite Nanoparticles. Appl. Sci. 2017, 7, 60. [Google Scholar] [CrossRef]
- Noor, Z. Nanohydroxyapatite application to osteoporosis management. J. Osteoporos. 2013, 2013, 679025. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Z.; Lin, C. Preparation and characterization of nano-sized hydroxyapatite particles and hydroxyapatite/chitosan nano-composite for use in biomedical materials. Mater. Lett. 2002, 57, 858–861. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, M.; Lu, Y.; Qi, Y.; Liu, Y. Synthesis and microstructure of hydroxyapatite nanofibers synthesized at 37 °C. Mater. Chem. Phys. 2006, 95, 145–149. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, H.; Lee, J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater. 2011, 7, 3813–3828. [Google Scholar] [CrossRef]
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cell Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef]
- Junqueira, L.C.; José, C. Basic Histology, Text & Atlas, 10th ed.; Foltin, J., Lebowitz, H., Boyle, P.J., Eds.; McGraw-Hill Companies: New York, NY, USA, 2003; p. 144. ISBN 978-0-07-137829-1. [Google Scholar]
- Parhi, P.; Ramanan, A.; Ray, A.R. A convenient route for the synthesis of hydroxyapatite through a novel microwave-mediated metathesis reaction. Mater. Lett. 2004, 58, 3610–3612. [Google Scholar] [CrossRef]
- Marchi, J.; Dantas, A.C.S.; Greil, P. Influence of Mg-substitution on the physicochemical properties of calcium-phosphate powders. Mater. Res. Bull. 2007, 42, 1040–1050. [Google Scholar] [CrossRef]
- Olaechea, A.; Mendoza-Azpur, G.; O’valle, F.; Padial-Molina, M.; Martin-Morales, N.; Galindo-Moreno, P. Biphasic hydroxyapatite and ß-tricalcium phosphate biomaterial behavior in a case series of maxillary sinus augmentation in humans. Clin Oral Impl. Res. 2019, 30, 336–343. [Google Scholar] [CrossRef]
- De Godoy, R.F.; Hutchens, S.; Campion, C.; Blunn, G. Silicate-substituted calcium phosphate with enhanced strut porosity stimulates osteogenic differentiation of human mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 5387. [Google Scholar] [CrossRef]
- Kojima, S.; Nakamura, H.; Lee, S.; Nagata, F.; Kato, K. Hydroxyapatite Formation on Self-Assembling Peptides with Differing Secondary Structures and Their Selective Adsorption for Proteins. Int. J. Mol. Sci. 2019, 20, 4650. [Google Scholar] [CrossRef]
- Groppo, M.F.; Caria, P.H.; Freire, A.R.; Figueroba, S.R.; Ribeiro-Neto, W.A.; Bretas, R.E.S.; Prado, F.B.; Haiter-Neto, F.; Aguiar, F.H.B.; Rossi, A.C. The effect of a hydroxyapatite impregnated PCL membrane in rat subcritical calvarial bone defects. Arch Oral Biol. 2017, 82, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Bakhshandeh, B.; Dehghan, M.M.; Mehrnia, M.R.; Khojasteh, A. Functional synergy of anti-mir221 and nanohydroxyapatite scaffold in bone tissue engineering of rat skull. J. Mater Sci. Mater Med. 2016, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.B.; Zhang, Q.-B.; Zhang, Z.-Q.; Chen, D.; Yan, W.-X.; Li, K.-F.; Chen, Y. Performance of coralline hydroxyapatite in sinus floor augmentation: A retrospective study. Clin. Oral Investig. 2013, 17, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Dent, C.E.; Davies, I.J.T. Calcium metabolism in bone disease: Effects of treatment with microcrystalline calcium hydroxyapatite compound and dihydrotachysterol. J. R. Soc. Med. 1980, 73, 780–785. [Google Scholar] [CrossRef]
- Chu, M.; Liu, G. Preparation and characterization of hydroxyapatite/liposome core-shell nanocomposites. Nanotechnology 2005, 16, 1208–1212. [Google Scholar] [CrossRef]
- Grenho, L.; Salgado, C.L.; Fernandes, M.H.F.; Monteiro, F.J.; Ferraz, M.P. Antibacterial activity and biocompatibility of three-dimensional nanostructured porous granules of hydroxyapatite and zinc oxide nanoparticles—An in vitro and in vivo study. Nanotechnology 2015, 26, 315101. [Google Scholar] [CrossRef]
- Salehi, M.; Naseri-Nosar, M.; Ebrahimibarough, S.; Nourani, M.; Vaez, A.; Farzamfar, S.; Ai, J. Regeneration of sciatic nerve crush injury by a hydroxyapatite nanoparticle-containing collagen type I hydrogel. J. Physiol. Sci. 2018, 68, 579–587. [Google Scholar] [CrossRef]
- Chaves, M.D.; Nunes, L.S.D.S.; de Oliveira, R.V.; Holgado, L.A.; Filho, H.N.; Matsumoto, M.A.; Ribeiro, D. Bovine hydroxyapatite (Bio-Oss) induces osteocalcin, RANK-L and osteoprotegerin expression in sinus lift of rabbits. J. Craniomaxillofac. Surg. 2012, 8, e315–e320. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, J.; Kodali, P.; Koh, J.; Ameer, G.A. A citric acid-based hydroxyapatite composite for orthopedic implants. Biomaterials 2006, 27, 5845–5854. [Google Scholar] [CrossRef]
- Jafari, S. Application of hydroxyapatite nanoparticle in the drug delivery systems. J. Mol. Pharm. Org. Process Res. 2015, 3, 1–2. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, J.; Lv, S.; Wen, J.; Chen, J.F. Synthesis and characterization of hydroxyapatite nanoparticles prepared by a high-gravity precipitation method. Ceram. Int. 2015, 41, 14340–14349. [Google Scholar] [CrossRef]
- Song, J.M.; Shin, S.H.; Kim, Y.D.; Lee, J.Y.; Baek, Y.J.; Yoon, S.Y.; Kim, H.S. Comparative study of chitosan/fibroin–hydroxyapatite and collagen membranes for guided bone regeneration in rat calvarial defects: Micro-computed tomography analysis. Int. J. Oral. Sci. 2014, 6, 87–93. [Google Scholar] [CrossRef] [PubMed]
- da Silva Brum, I.; de Carvalho, J.J.; da Silva Pires, J.L.; de Carvalho, M.A.A.; Dos Santos, L.B.F.; Elias, C.N. Nanosized hydroxyapatite and β-tricalcium phosphate composite: Physico-chemical, cytotoxicity, morphological properties and in vivo trial. Sci. Rep. 2019, 9, 19602. [Google Scholar] [CrossRef] [PubMed]
- Faeda, R.S.; Tavares, H.S.; Sartori, R.; Guastaldi, A.C.; Marcantonio, E., Jr. Biological performance of chemical hydroxyapatite coating associated with implant surface modification by laser beam: Biomechanical study in rabbit tibias. J. Oral Maxillofac. Surg. 2009, 6, 1706–1715. [Google Scholar] [CrossRef]
- Govindaraj, D.; Rajan, M.; Munusamy, M.A.; Alarfaj, A.A.; Sadasivuni, K.K.; Kumar, S.S. The synthesis, characterization and in vivo study of mineral substituted hydroxyapatite for prospective bone tissue rejuvenation applications. Nanomedicine 2017, 13, 2661–2669. [Google Scholar] [CrossRef]
- Schroeder, A.; Heller, D.A.; Winslow, M.M.; Dahlman, J.E.; Pratt, G.W.; Langer, R.; Jacks, T.; Anderson, D.G. Treating metastic cancer with nanotechnology. Nat. Rev. Cancer 2012, 12, 39–50. [Google Scholar] [CrossRef]
- Rossi, A.L.; Longuinho, M.; Tanaka, M.N.; Farina, M.; Borojevic, R.; Rossi, A.M. Intracellular pathway and subsequent transformation of hydroxyapatite nanoparticles in the SAOS-2 osteoblast cell line. J. Biomed. Mater. Res. A 2018, 106, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Qayoom, I.; Teotia, A.K.; Kumar, A. Nanohydroxyapatite Based Ceramic Carrier Promotes Bone Formation in a Femoral Neck Canal Defect in Osteoporotic Rats. Biomacromolecules 2019, 21, 328–337. [Google Scholar] [CrossRef]
- Raina, D.B.; Isaksson, H.; Hettwer, W.; Kumar, A.; Lidgren, L.; Tägil, M. A Biphasic Calcium Sulphate/Hydroxyapatite Carrier Containing Bone Morphogenic Protein-2 and Zoledronic Acid Generates Bone. Sci. Rep. 2016, 6, 26033. [Google Scholar] [CrossRef]
- Gao, M.; Gao, W.; Papadimitriou, J.M.; Zhang, C.; Gao, J.; Zheng, M. Exosome the Enigmatic Regulators of Bone Homeostasis. Bone Res. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Teotia, A.K.; Raina, D.B.; Singh, C.; Sinha, N.; Isaksson, H.; Tägil, M.; Lidgren, L.; Kumar, A. Nano-Hydroxyapatite Bone Substitute Functionalized with Bone Active Molecules for Enhanced Cranial Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 6816–6828. [Google Scholar] [CrossRef] [PubMed]
- Sahana, H.; Khajuria, D.K.; Razdan, R.; Mahapatra, D.R.; Bhat, M.R.; Suresh, S.; Rao, R.R.; Mariappan, L. Improvement in Bone Properties by Using Risedronate Adsorbed Hydroxyapatite Novel Nanoparticle Based Formulation in a Rat Model of Osteoporosis. J. Biomed. Nanotechnol. 2013, 9, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, M.; Palevicius, A.; Ebrahimi-Kahrizsangi, R.; Nasiri, S.; Vilkauskas, A.; Janusas, G. New Approach for Preparing In Vitro Bioactive Scaffold Consisted of Ag-Doped Hydroxyapatite + Polyvinyltrimethoxysilane. Polymers 2021, 13, 1695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, L.; Lv, H.; Cao, Y.; Liu, Y.; Xu, Y.; Ye, W.; Wang, J. Repair of rabbit femoral condyle bone defects with injectable nanohydroxyapatite/chitosan composites. J. Mater. Sci. 2012, 23, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Noor, Z.; Sumitro, S.B.; Hidayat, M.; Rahim, A.H.; Sabarudin, A.; Umemura, T. Atomic mineral characteristics of Indonesian osteoporosis by high-resolution inductively coupled plasma mass spectrometry. Sci. World J. 2012, 2012, 372972. [Google Scholar] [CrossRef]
- Yang, F.; Dong, W.-J.; He, F.-M.; Wang, X.-X.; Zhao, S.-F.; Yang, G.-L. Osteoblast response to porous titanium surfaces coated with zinc-substituted hydroxyapatite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2012, 113, 313–318. [Google Scholar] [CrossRef]
- Chang, M.C. Fluoride incorporation in hydroxyapatite/gelatin nanocomposite. J. Mater. Sci. 2008, 19, 2837–2843. [Google Scholar] [CrossRef]
- Li, D.-H.; Lin, J.; Lin, D.-Y.; Wang, X.-X. Synthesized silicon-substituted hydroxyapatite coating on titanium substrate by electrochemical deposition. J. Mater. Sci. 2011, 22, 1205–1211. [Google Scholar] [CrossRef]
- Xie, M.; Olderøy, M.Ø.; Andreassen, J.-P.; Selbach, S.M.; Strand, B.L.; Sikorski, P. Alginate-controlled formation of nanoscale calcium carbonate and hydroxyapatite mineral phase within hydrogel networks. Acta Biomater. 2010, 6, 3665–3675. [Google Scholar] [CrossRef]
- Fabbri, P.; Bondioli, F.; Messori, M.; Bartoli, C.; Dinucci, D.; Chiellini, F. Porous scaffolds of polycaprolactone reinforced with in situ generated hydroxyapatite for bone tissue engineering. J. Mater. Sci. 2010, 21, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.-X.; Huang, G.; Lu, W.W.; Pan, H.-B. The effect of strontium incorporation into hydroxyapatite on their physical and biological propertoes. J. Biomed. Mater. Res. B 2012, 100, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nonami, T.; Yubata, K. Syntheses, structures and photophysical properties of iron containing hydroxyapatite prepared by a modified pseudo-body solution. J. Mater. Sci. 2008, 19, 2663–2667. [Google Scholar] [CrossRef] [PubMed]
- Sözen, T.; Özışık, L.; Basaran, N.C. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar]
- Duncan, E.L.; Brown, M.A. Genetic studies in osteoporosis—The end of the beginning. Arthritis Res. Ter. 2008, 10, 214. [Google Scholar] [CrossRef]
- Song, Y.; Liebschner, M.A.K.; Gunaratne, G.H. A study of age-related architectural changes that are most damaging to bones. Biophys. J. 2004, 87, 3642–3647. [Google Scholar] [CrossRef]
- Walters, S.; Khan, T.; Ong, T.; Sahota, O. Fracture liaison services: Improving outcomes for patients with osteoporosis. Clin. Interv. Aging 2017, 12, 117–127. [Google Scholar] [CrossRef]
- Brandao, C.M.R.; Lima, M.G.; da Silva, A.L.; Silva, G.D.; Guerra, A.A.G., Jr.; De Assis Acurcio, F. Treatment of postmenopausal osteoporosis in women: A systematic review. Cad. De Saude Publica 2008, 24 (Suppl. 4), S592–S606. [Google Scholar] [CrossRef]
- Huang, Q.-Y.; Kung, A.W.C. Genetics of osteoporosis. Mol. Genet. Metab. 2006, 88, 295–306. [Google Scholar] [CrossRef]
- Office of the Surgeon General (US). Bone Health and Osteoporosis; Office of the Surgeon General (US): Rockville, MD, USA, 2004. [Google Scholar]
- Qaseem, A.; Forciea, M.A.; McLean, R.M.; Denberg, T.D. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update from the American College of Physicians. Ann. Intern. Med. 2017, 166, 818. [Google Scholar] [CrossRef]
- Noor, Z. Substitution and Incorporation of Atomic Minerals, Hydroxyapatite Crystale and Microstructure of Osteoporosis Bone. Ph.D. Thesis, Faculty of Medicine, Brawijaya University, East Java Malang, Indonesia, 2011. [Google Scholar]
- Castelo-Branco, C.; Ciria-Recasens, M.; Cancelo-Hidalgo, M.J.; Palacios, S.; Haya-Palazuelos, J.; Carbonell-Abelló, J.; Blanch-Rubió, J.; Martínez-Zapata, M.J.; Manasanch, J.; Pérez-Edo, L. Efficacy of ossein-hydroxyapatite complex compared with calcium carbonate to prevent bone loss: A meta-analysis. Menopause 2009, 16, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Pons, F.; Vicente, J.J.; Sanjuan, A.; Vanrell, J.A. Preventing postmenopausal bone loss with osseinhydroxyapatite compounds: Results of a two-year, prospective trial. J. Reprod. Med. Obstet. Gynecol. 1999, 44, 601–605. [Google Scholar]
- Ciria-Recasens, M.; Blanch-Rubio, J.; Coll-Batet, M.; del Pilar Lisbona-Pérez, M.; Díez-Perez, A.; Carbonell-Abelló, J.; Manasanch, J.; Pérez-Edo, L. Comparison of the effects of ossein-hydroxyapatite complex and calcium carbonate on bone metabolism in women with senile osteoporosis: A randomized, open-label, parallel-group, controlled, prospective study. Clin. Drug Investig. 2011, 31, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.K.; Singh, S.; Kumaresan, R.; Nayar, S.; Sinha, A. Mesenchymal cell response to nanosized biphasic calcium phosphate composites. Colloids Surf. B Biointerfaces 2009, 73, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, S.; Cao, X.; Yuan, L.; Wang, Y.; Yin, Y.; Qiu, T.; Dai, H.; Wang, X. Different inhibitory effect and mechanism of hydroxyapatite nanoparticles on normal cells and cancer cells in vitro and in vivo. Sci. Rep. 2014, 4, 7134. [Google Scholar] [CrossRef] [PubMed]
- Russmueller, G.; Winkler, L.; Lieber, R.; Seemann, R.; Pirklbauer, K.; Perisanidis, C.; Kapeller, B.; Spassova, E.; Halwax, E.; Macfelda, K.; et al. In vitro effects of particulate bone substitute materials on the resorption activity of human osteoclasts. Eur. Cell Mater. 2017, 34, 291–306. [Google Scholar] [CrossRef]
- Yin, M.; Yin, Y.; Han, Y.; Dai, H.; Li, S. Effects of uptake of hydroxyapatite nanoparticles into hepatoma cells on cell adhesion and proliferation. J. Nanomater. 2014, 2014, 731897. [Google Scholar] [CrossRef]
- Liao, C.; Zhou, J. Replica exchange molecular dynamics simulation of basic fibroblast growth factor adsorption on hydroxyapatite. J. Phys. Chem. B 2014, 118, 5843–5852. [Google Scholar] [CrossRef]
- Lee, J.; Yun, H. Hydroxyapatite-containing gelatin/chitosan microspheres for controlled release of lysozyme and enhanced cytocompatibility. J. Mater. Chem. B 2014, 2, 1255. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Yuvakkumar, R.; Hong, S.I.; Oh, T.H. Novel zirconium nitride and hydroxyapatite nanocomposite coating: Detailed analysis and functional properties. ACS Appl. Mater. Interfaces 2014, 6, 9850–9857. [Google Scholar] [CrossRef]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef]
- Johari, B.; Ahmadzadehzarajabad, M.; Azami, M.; Kazemi, M.; Soleimani, M.; Kargozar, S.; Hajighasemlou, S.; Farajollahi, M.M.; Samadikuchaksaraei, A. Repair of rat critical size calvarial defect using osteoblast-like and umbilical vein endothelial cells seeded in gelatin/hydroxyapatite scaffolds. J. Biomed. Mater. Res. A 2016, 104, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P.; Monteiro, F.J.; Serro, A.P.; Saramago, B.; Gibson, I.R.; Santos, J.D. Effect of chemical composition on hydrophobicity and zeta potential of plasma sprayed HA/CaO-P2O5 glass coatings. Biomaterials 2001, 22, 3105–3112. [Google Scholar] [CrossRef]
- Tautzenberger, A.; Lorenz, S.; Kreja, L.; Zeller, A.; Musyanovych, A.; Schrezenmeier, H.; Katharina, L.; Volker, M.; Anita, I. Effect of functionalized fluorescence-labelled nanoparticles on mesenchymal stem cell differentiation. Biomaterials 2010, 31, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Tautzenberger, A.; Kreja, L.; Zeller, A.; Lorenz, S.; Schrezenmeier, H.; Mailänder, V.; Katharina, L.; Anita, I. Direct and indirect effects of functionalized fluorescence-labelled nanoparticles on human osteoclast formation and activity. Biomaterials 2011, 32, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.L.; Cavalcante, C.H.; Souza, M.G.; Ferreira Cde, A.; Rubello, D.; Chondrogiannis, S.; Cardoso, V.N.; Ramaldes, G.A.; Barros, A.L.; Soares, D.C. Hydroxyapatite nanoparticles: Preparation, characterization, and evaluation of their potential use in bone targeting: An animal study. Nucl. Med. Commun. 2016, 37, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.C.A.; Novais, M.V.M.; Teixeira, C.S.; Honorato-Sampaio, K.; Pereira, M.T.P.; Ferreira, L.A.M.; Braga, F.C.; Oliveira, M.C. Preparation, physicochemical characterization, and cell viability evaluation of long-circulating and pH-sensitive liposomes containing ursolic acid. Biomed. Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Uskokovic, V.; Odsinada, R.; Djordjevic, S.; Habelitz, S. Dynamic light scattering and zeta potential of colloidal mixtures of amelogenin and hydroxyapatite in calcium and phosphate rich ionic milieus. Arch. Oral. Biol. 2011, 56, 521–532. [Google Scholar] [CrossRef]
- Solberg, B.D.; Gutow, A.P.; Baumgaertner, M.R. Efficacy of gentamycinimpregnated resorbable hydroxyapatite cement in treating osteomyelitis in a rat model. J. Orthop. Trauma 1999, 13, 102–106. [Google Scholar] [CrossRef]
- Korkusuz, F.; Uchida, A.; Shinto, Y.; Araki, N.; Inoue, K.; Ono, K. Experimental implant-related osteomyelitis treated by antiotic-calcium hydroxyapatite ceramic composites. J. Bone Joint Surg. Br. 1993, 75, 111–114. [Google Scholar] [CrossRef]
- Carcia, C.R.; Scibek, J.S. Causation and management of calcific tendonitis and periarthritis. Curr. Opin. Rheumatol. 2013, 25, 204–209. [Google Scholar] [CrossRef] [PubMed]
- De Barros, A.L.B.; Chacko, A.; Mikitsh, J.L.; Al-Zaki, A.; Saboury, B.; Tsourkas, A.; Alavi, A. Assessment of global cardiac uptake of radiolabeled iron oxide nanoparticles in apolipoprotein-E-deficient mice. Mol. Imag. Biol. 2013, 1, 1. [Google Scholar]
- Carter, D.R.; Bouxsein, M.L.; Marcus, R. New approaches for interpreting projected bone densitometry data. J. Bone Miner. Res. 1992, 7, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Routh, R.H.; Rumancik, S.; Pathak, R.D.; Burshell, A.L.; Nauman, E.A. The relationship between bone mineral density and biomechanics in patients with osteoporosis and scoliosis. Osteoporos. Int. 2005, 16, 1857–1863. [Google Scholar] [CrossRef]
- Rumancik, S.; Routh, R.H.; Pathak, R.D.; Burshell, A.L.; Nauman, E.A. Assessment of bone quantity and distribution in adult lumbar scoliosis: New dual-energy x-ray absorptiometry methodology and analysis. Spine 2005, 30, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Sher, H.L.; Guo, X.; Hung, V.W.; Cheung, A.Y. The effect of vertebral rotation of the lumbar spine on dual energy X-ray absorptiometry measurements: Observational study. Hong Kong Med. J. 2001, 7, 241–245. [Google Scholar] [PubMed]
- Short, D.F.; Zemel, B.S.; Gilsanz, V.; Kalkwarf, H.J.; Lappe, J.M.; Mahboubi, S.; Oberfield, S.E.; Shepherd, J.A.; Winer, K.K.; Hangartner, T.N. Fitting of bone mineral density with consideration of anthropometric parameters. Osteoporos. Int. 2011, 22, 1047–1057. [Google Scholar] [CrossRef][Green Version]
- Whitmarsh, T.; Fritscher, K.D.; Humbert, L.; Barquero, L.M.D.R.; Roth, T.; Kammerlander, C.; Blauth, M.; Schubert, R.; Frangi, A.F. A Statistical Model of Shape and Bone Mineral Density Distribution of the Proximal Femur for Fracture Risk Assessment. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2011; MICCAI 2011, Lecture Notes in Computer Science; Fichtinger, G., Martel, A., Peters, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 6892. [Google Scholar]
- Ito, M.; Wakao, N.; Hida, T.; Matsui, Y.; Abe, Y.; Aoyagi, K.; Uetani, M.; Harada, A. Analysis of hip geometry by clinical CT for the assessment of hip fracture risk in elderly Japanese women. Bone 2010, 46, 453–457. [Google Scholar] [CrossRef]
- Leslie, W.D.; Lix, L.M.; Tsang, J.F.; Caetano, P.A. Manitoba Bone Density Program. Single-Site vs Multisite Bone Density Measurement for Fracture Prediction. Arch. Intern. Med. 2007, 167, 1641–1647. [Google Scholar] [CrossRef]
- Robles-Linares, J.A.; Ramírez-Cedillo, E.; Siller, H.R.; Rodríguez, C.A.; Martínez-López, J.I. Parametric Modeling of Biomimetic Cortical Bone Microstructure for Additive Manufacturing. Materials 2019, 12, 913. [Google Scholar] [CrossRef]
- Kytýř, D.; Zlámal, P.; Koudelka, P.; Fíla, T.; Krčmářová, N.; Kumpová, I.; Vavřík, D.; Gantar, A.; Novakcd, S. Deformation analysis of gellan-gum based bone scaffold using on-the-fly tomography. Mater. Des. 2017, 134, 400–417. [Google Scholar] [CrossRef]
- Pathi, S.P.; Lin, D.D.W.; Dorvee, J.R.; Estroff, L.A.; Fischbach-Teschl, C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials 2011, 32, 5112–5122. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Lowenberg, B.; Shapiro, G.; Davies, J.E. Resorption of sintered synthetic hydroxyapatite by osteoclasts in vitro. Biomaterials 1993, 14, 91–96. [Google Scholar] [CrossRef]
- Redey, S.A.; Razzouk, S.; Rey, C.; Bernache-Assollant, D.; Leroy, G.; Nardin, M.; Cournot, G. Osteoclast adhesion and activity on synthetic hydroxyapatite, carbonated hydroxyapatite, and natural calcium carbonate: Relationship to surface energies. J. Biomed. Mater. 1999, 45, 140–147. [Google Scholar] [CrossRef]
- Raina, D.B.; Larsson, D.; Mrkonjic, F.; Isaksson, H.; Kumar, A.; Lidgren, L.; Tagil, M. Gelatin- Hydroxyapatite- Calcium Sulphate Based Biomaterial for Long Term Sustained Delivery of Bone Morphogenic Protein-2 and Zoledronic Acid for Increased Bone Formation: In-Vitro and in-Vivo Carrier Properties. J. Control. Release 2018, 272, 83–96. [Google Scholar] [CrossRef]

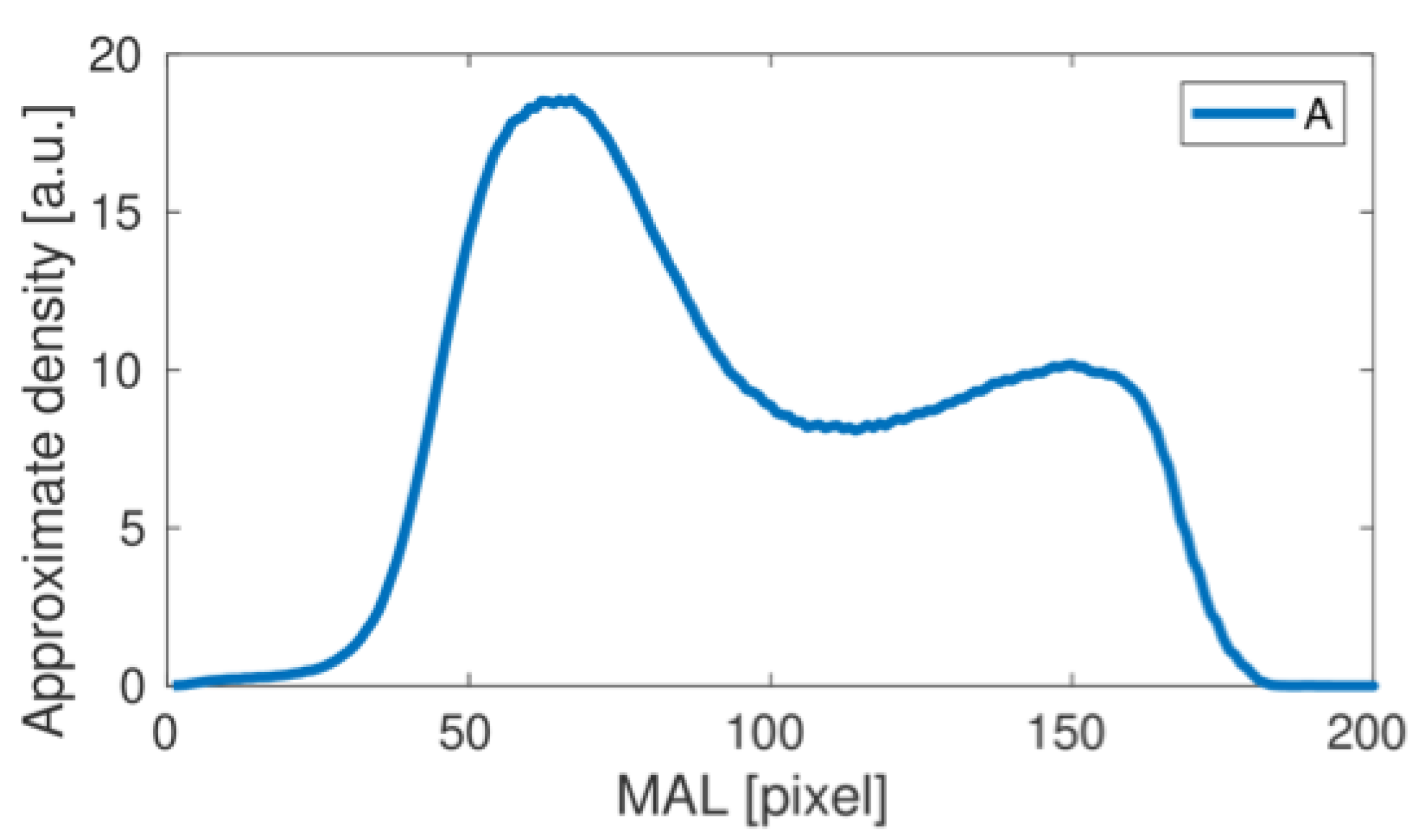


| Curve (Patient) | Maximum 1 | Minimum 1 | Maximum 2 | Relative Decrease | |
|---|---|---|---|---|---|
| Group 1 | A | 18.53 | 10.84 | 12.20 | 11% |
| B | 18.07 | 6.83 | 9.15 | 25% | |
| D | 16.14 | 11.19 | 12.78 | 12% | |
| F | 19.47 | 11.76 | 12.94 | 9% | |
| Group 1 average | 18.05 | 10.16 | 11.77 | 14.5% | |
| Group 1 standard deviation | 1.401 | 2.251 | 1.771 | ||
| Group 2 | C | 16.00 | 9.58 | 9.87 | 3% |
| E | 15.42 | 8.72 | 9.11 | 4% | |
| G | 14.85 | 4.55 | 4.55 | 0% | |
| H | 18.71 | 11.54 | 11.72 | 2% | |
| Group 2 average | 16.25 | 8.60 | 8.81 | 2.2% | |
| Group 2 standard deviation | 1.709 | 2.942 | 3.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litak, J.; Grochowski, C.; Rysak, A.; Mazurek, M.; Blicharski, T.; Kamieniak, P.; Wolszczak, P.; Rahnama-Hezavah, M.; Litak, G. New Horizons for Hydroxyapatite Supported by DXA Assessment—A Preliminary Study. Materials 2022, 15, 942. https://doi.org/10.3390/ma15030942
Litak J, Grochowski C, Rysak A, Mazurek M, Blicharski T, Kamieniak P, Wolszczak P, Rahnama-Hezavah M, Litak G. New Horizons for Hydroxyapatite Supported by DXA Assessment—A Preliminary Study. Materials. 2022; 15(3):942. https://doi.org/10.3390/ma15030942
Chicago/Turabian StyleLitak, Jakub, Cezary Grochowski, Andrzej Rysak, Marek Mazurek, Tomasz Blicharski, Piotr Kamieniak, Piotr Wolszczak, Mansur Rahnama-Hezavah, and Grzegorz Litak. 2022. "New Horizons for Hydroxyapatite Supported by DXA Assessment—A Preliminary Study" Materials 15, no. 3: 942. https://doi.org/10.3390/ma15030942
APA StyleLitak, J., Grochowski, C., Rysak, A., Mazurek, M., Blicharski, T., Kamieniak, P., Wolszczak, P., Rahnama-Hezavah, M., & Litak, G. (2022). New Horizons for Hydroxyapatite Supported by DXA Assessment—A Preliminary Study. Materials, 15(3), 942. https://doi.org/10.3390/ma15030942









