Histological Processing of CAD/CAM Titanium Scaffold after Long-Term Failure in Cranioplasty
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient History and CAD/CAM Titanium Scaffold
2.2. Histological Assessment
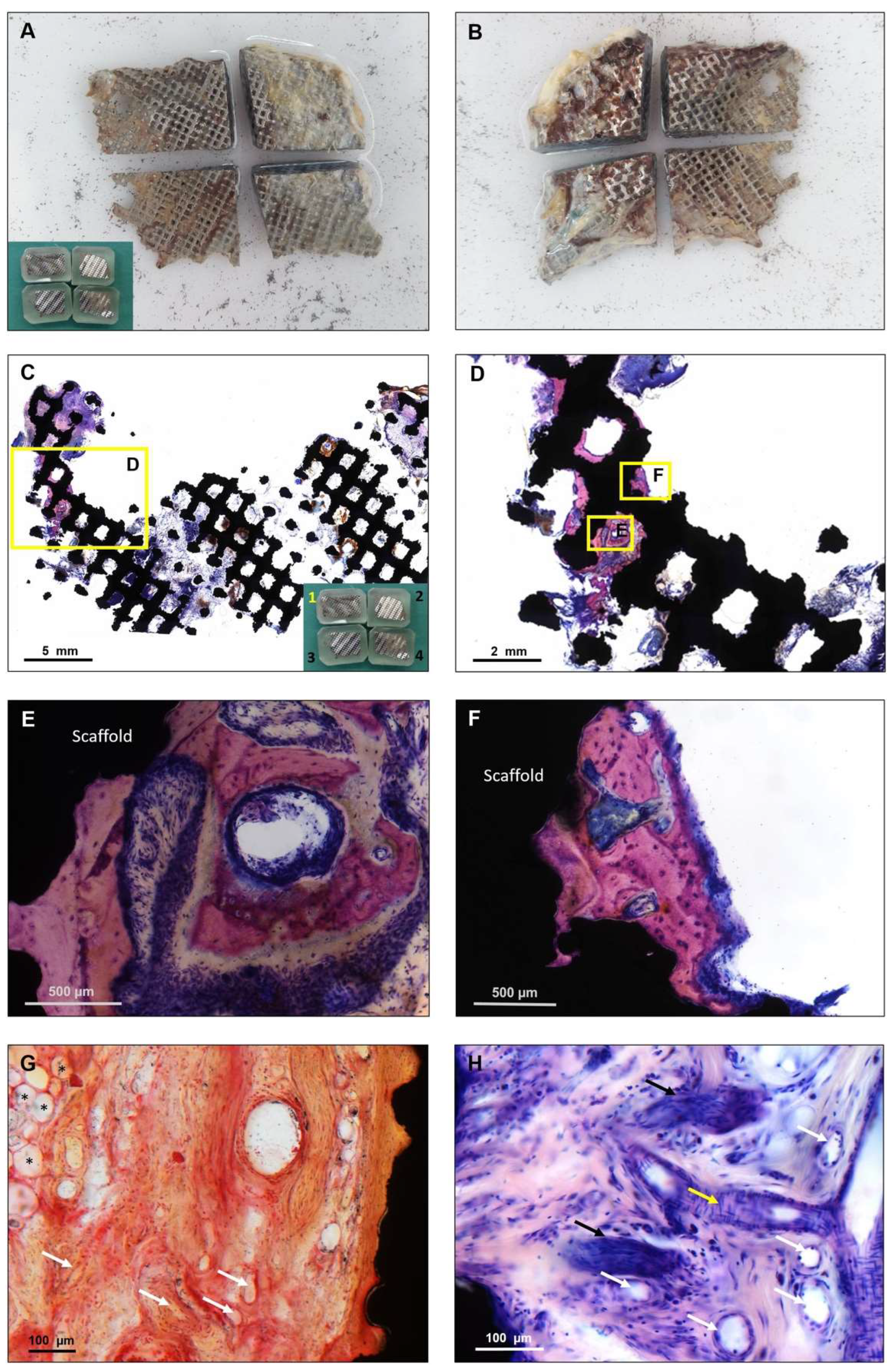
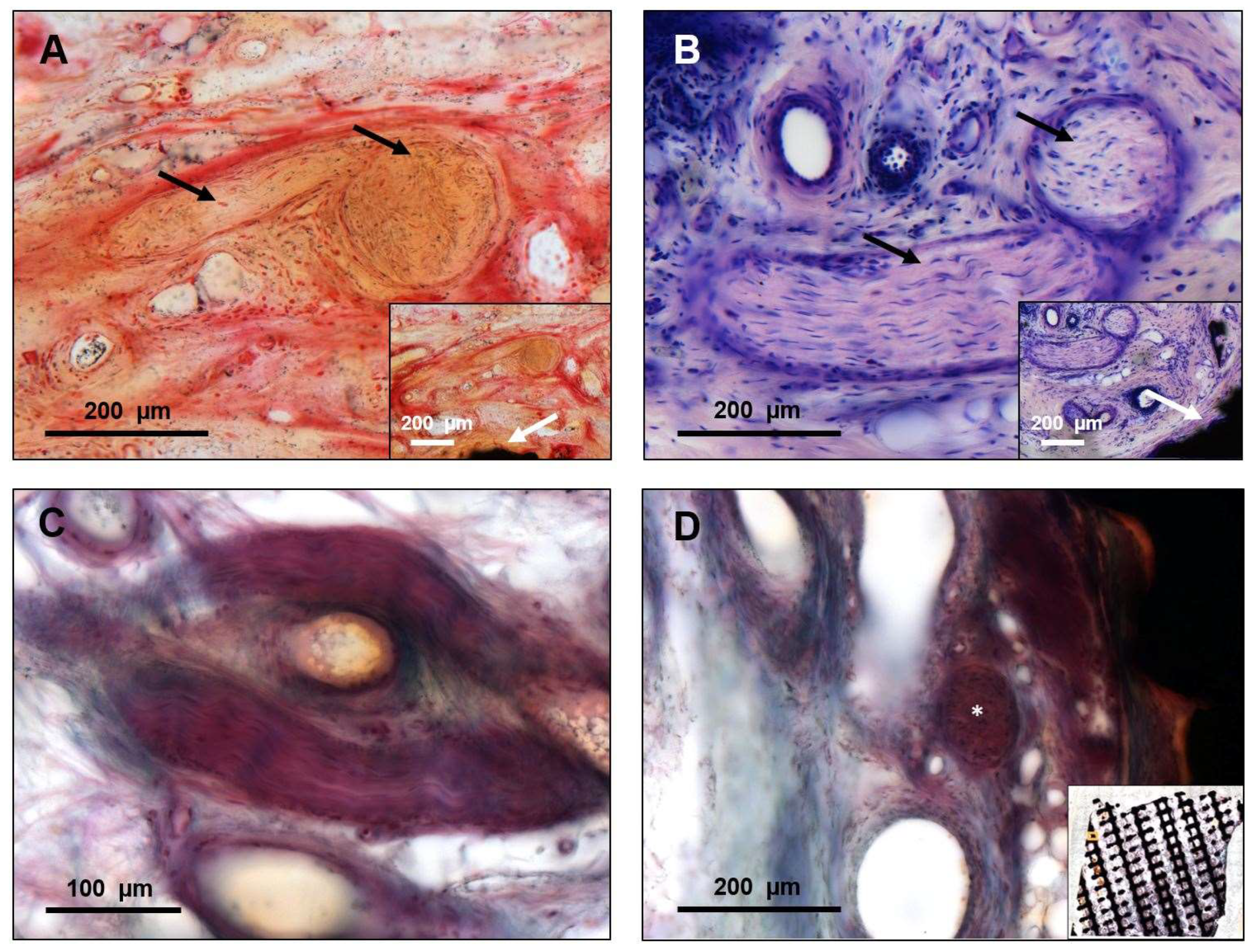
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kurland, D.B.; Khaladj-Ghom, A.; Stokum, J.A.; Carusillo, B.; Karimy, J.K.; Gerzanich, V.; Sahuquillo, J.; Simard, J.M. Complications associated with decompressive craniectomy: A systematic review. Neurocrit. Care 2015, 23, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, B.; Zingaretti, N.; Verlicchi, A.; Robiony, M.; Alfieri, A.; Parodi, P.C. Cranioplasty: Review of materials. J. Craniofac. Surg. 2016, 27, 2061–2072. [Google Scholar] [CrossRef] [PubMed]
- Lethaus, B.; Bloebaum, M.; Koper, D.; Poort-Ter Laak, M.; Kessler, P. Interval cranioplasty with patient-specific implants and autogenous bone grafts—Success and cost analysis. J. Craniomaxillofac. Surg. 2014, 42, 1948–1951. [Google Scholar] [CrossRef] [PubMed]
- van de Vijfeijken, S.; Münker, T.; Spijker, R.; Karssemakers, L.H.E.; Vandertop, W.P.; Becking, A.G.; Ubbink, D.T. Autologous bone is inferior to alloplastic cranioplasties: Safety of autograft and allograft materials for cranioplasties, a systematic review. World Neurosurg. 2018, 117, 443–452.e8. [Google Scholar] [CrossRef]
- Aydin, S.; Kucukyuruk, B.; Abuzayed, B.; Aydin, S.; Sanus, G.Z. Cranioplasty: Review of materials and techniques. J. Neurosci. Rural Pract. 2011, 2, 162–167. [Google Scholar] [CrossRef]
- Cabraja, M.; Klein, M.; Lehmann, T.N. Long-term results following titanium cranioplasty of large skull defects. Neurosurg. Focus 2009, 26, E10. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.R.; Fan, K.F.; Bentley, R.P. Custom-made titanium cranioplasty: Early and late complications of 151 cranioplasties and review of the literature. Int. J. Oral Maxillofac. Surg. 2015, 44, 599–608. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Lin, F.; Chen, Z.; Jiang, X.; Zhang, J.; Wang, J. Complications following titanium cranioplasty compared with nontitanium implants cranioplasty: A systematic review and meta-analysis. J. Clin. Neurosci. 2021, 84, 66–74. [Google Scholar] [CrossRef]
- Tamaddon, M.; Samizadeh, S.; Wang, L.; Blunn, G.; Liu, C. Intrinsic osteoinductivity of porous titanium scaffold for bone tissue engineering. Int. J. Biomater. 2017, 2017, 5093063. [Google Scholar] [CrossRef]
- Crovace, A.M.; Lacitignola, L.; Forleo, D.M.; Staffieri, F.; Francioso, E.; di Meo, A.; Becerra, J.; Crovace, A.; Santos-Ruiz, L. 3D biomimetic porous titanium (Ti6Al4V ELI) scaffolds for large bone critical defect reconstruction: An experimental study in sheep. Animals 2020, 10, 1389. [Google Scholar] [CrossRef]
- van den Brink, W.; Lamerigts, N. Complete osseointegration of a retrieved 3-D printed porous titanium cervical cage. Front. Surg. 2020, 7, 526020. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, G.J.; Rueda, S.; Couto, R.A.; Hashem, A.; Nagel, S.; Schwarz, G.S.; Zins, J.E.; Gastman, B.R. Long-term outcomes of cranioplasty: Titanium mesh is not a long-term solution in high-risk patients. Ann. Plast. Surg. 2018, 81, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Miyata, K.; Komatsu, K.; Yamashita, K.; Wanibuchi, M.; Mikuni, N. Exposure of titanium implants after cranioplasty: A matter of long-term consequences. Interdiscip. Neurosurg. 2017, 8, 64–67. [Google Scholar] [CrossRef]
- Barcia, J.J. The Giemsa stain: Its history and applications. Int. J. Surg. Pathol. 2007, 15, 292–296. [Google Scholar] [CrossRef] [PubMed]
- de Leon, E.R.; Armstrong, R. Modified giemsa stain for polymethyl methacrylate embedded undecalcified bone and bone marrow specimens. J. Histotechnol. 1991, 14, 85–88. [Google Scholar] [CrossRef]
- Malcolm, J.G.; Mahmooth, Z.; Rindler, R.S.; Allen, J.W.; Grossberg, J.A.; Pradilla, G.; Ahmad, F.U. Autologous cranioplasty is associated with increased reoperation rate: A systematic review and meta-analysis. World Neurosurg. 2018, 116, 60–68. [Google Scholar] [CrossRef]
- Oliver, J.D.; Banuelos, J.; Abu-Ghname, A.; Vyas, K.S.; Sharaf, B. Alloplastic cranioplasty reconstruction: A systematic review comparing outcomes with titanium mesh, polymethyl methacrylate, polyether ether ketone, and norian implants in 3591 adult patients. Ann. Plast. Surg. 2019, 82, S289–s294. [Google Scholar] [CrossRef]
- Rosinski, C.L.; Patel, S.; Geever, B.; Chiu, R.G.; Chaker, A.N.; Zakrzewski, J.; Rosenberg, D.M.; Parola, R.; Shah, K.; Behbahani, M.; et al. A retrospective comparative analysis of titanium mesh and custom implants for cranioplasty. Neurosurgery 2020, 86, E15–E22. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [Green Version]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- Weinans, H.; Prendergast, P.J. Tissue adaptation as a dynamical process far from equilibrium. Bone 1996, 19, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Pobloth, A.M.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Schmidt-Bleek, K.; Windolf, M.; Tatai, A.A.; Roth, C.P.; Schaser, K.D.; et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018, 10, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar] [PubMed]
- Yeap, M.-C.; Tu, P.-H.; Liu, Z.-H.; Hsieh, P.-C.; Liu, Y.-T.; Lee, C.-Y.; Lai, H.-Y.; Chen, C.-T.; Huang, Y.-C.; Wei, K.-C.; et al. Long-term complications of cranioplasty using stored autologous bone graft, three-dimensional polymethyl methacrylate, or titanium mesh after decompressive craniectomy: A single-center experience after 596 procedures. World Neurosurg. 2019, 128, e841–e850. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Gonzalez, A.; Jackson, I.T.; Miyawaki, T.; Barakat, K.; DiNick, V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J. Craniofacial Surg. 2003, 14, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G.; Hobgood, C.D.; Webb, L.X.; Myrvik, Q.N. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials 1987, 8, 423–426. [Google Scholar] [CrossRef]
- Zins, J.E.; Moreira-Gonzalez, A.; Parikh, A.; Arslan, E.; Bauer, T.; Siemionow, M. Biomechanical and histologic evaluation of the Norian craniofacial repair system and Norian Craniofacial Repair System Fast Set Putty in the long-term reconstruction of full-thickness skull defects in a sheep model. Plast. Reconstr. Surg. 2008, 121, 271e–282e. [Google Scholar] [CrossRef]
- Zins, J.E.; Langevin, C.J.; Nasir, S. Controversies in skull reconstruction. J. Craniofacial Surg. 2010, 21, 1755–1760. [Google Scholar] [CrossRef]
- Shah, A.M.; Jung, H.; Skirboll, S. Materials used in cranioplasty: A history and analysis. Neurosurg. Focus 2014, 36, E19. [Google Scholar] [CrossRef]
- Punchak, M.; Chung, L.K.; Lagman, C.; Bui, T.T.; Lazareff, J.; Rezzadeh, K.; Jarrahy, R.; Yang, I. Outcomes following polyetheretherketone (PEEK) cranioplasty: Systematic review and meta-analysis. J. Clin. Neurosci. 2017, 41, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, T.; Yuan, Y.; Li, X.; Yu, H.; Guan, J. Evaluation of titanium cranioplasty and polyetheretherketone cranioplasty after decompressive craniectomy for traumatic brain injury: A prospective, multicenter, non-randomized controlled trial. Medicine 2020, 99, e21251. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.; Guerrero, C.; Moody, R. Cerebrospinal fluid fistula from fractured acrylic cranioplasty plate. Case report. J. Neurosurg. 1976, 45, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Spetzger, U.; Vougioukas, V.; Schipper, J. Materials and techniques for osseous skull reconstruction. Minim. Invasive Ther. Allied Technol. 2010, 19, 110–121. [Google Scholar] [CrossRef]
- Matsuno, A.; Tanaka, H.; Iwamuro, H.; Takanashi, S.; Miyawaki, S.; Nakashima, M.; Nakaguchi, H.; Nagashima, T. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir. 2006, 148, 535–540. [Google Scholar] [CrossRef]
- Salam, A.A.; Ibbett, I.; Thani, N. Paediatric cranioplasty: A review. Interdiscip. Neurosurg. 2018, 13, 59–65. [Google Scholar] [CrossRef]
- Fiaschi, P.; Pavanello, M.; Imperato, A.; Dallolio, V.; Accogli, A.; Capra, V.; Consales, A.; Cama, A.; Piatelli, G. Surgical results of cranioplasty with a polymethylmethacrylate customized cranial implant in pediatric patients: A single-center experience. J. Neurosurg. Pediatr. 2016, 17, 705. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.S.; Schneider, S.J.; Rosenthal, A.D. Methyl methacrylate cranioplasty in children: Long-term results. Pediatric Neurosurg. 1997, 26, 33–35. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.; Jain, K.; Walia, B. Cranioplasty following decompressive craniectomy—Analysis of complication rates and neurological outcomes: A single center study. Surg. Neurol. Int. 2019, 10, 142. [Google Scholar] [CrossRef]
- Aitasalo, K.M.; Piitulainen, J.M.; Rekola, J.; Vallittu, P.K. Craniofacial bone reconstruction with bioactive fiber-reinforced composite implant. Head Neck 2014, 36, 722–728. [Google Scholar] [CrossRef]
- Eppley, B.L. Craniofacial reconstruction with computer-generated HTR patient-matched implants: Use in primary bony tumor excision. J. Craniofacial Surg. 2002, 13, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Wurm, G.; Tomancok, B.; Holl, K.; Trenkler, J. Prospective study on cranioplasty with individual carbon fiber reinforced polymer (CFRP) implants produced by means of stereolithography. Surg. Neurol. 2004, 62, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Kihlström Burenstam Linder, L.; Birgersson, U.; Lundgren, K.; Illies, C.; Engstrand, T. Patient-specific titanium-reinforced calcium phosphate implant for the repair and healing of complex cranial defects. World Neurosurg. 2019, 122, e399–e407. [Google Scholar] [CrossRef] [PubMed]
- Sundblom, J.; Gallinetti, S.; Birgersson, U.; Engqvist, H.; Kihlström, L. Gentamicin loading of calcium phosphate implants: Implications for cranioplasty. Acta Neurochir. 2019, 161, 1255–1259. [Google Scholar] [CrossRef] [Green Version]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, H.; Steffen, C.; Schmidt-Bleek, K.; Duda, G.N.; Heiland, M.; Rendenbach, C.; Raguse, J.-D. Histological Processing of CAD/CAM Titanium Scaffold after Long-Term Failure in Cranioplasty. Materials 2022, 15, 982. https://doi.org/10.3390/ma15030982
Fischer H, Steffen C, Schmidt-Bleek K, Duda GN, Heiland M, Rendenbach C, Raguse J-D. Histological Processing of CAD/CAM Titanium Scaffold after Long-Term Failure in Cranioplasty. Materials. 2022; 15(3):982. https://doi.org/10.3390/ma15030982
Chicago/Turabian StyleFischer, Heilwig, Claudius Steffen, Katharina Schmidt-Bleek, Georg N. Duda, Max Heiland, Carsten Rendenbach, and Jan-Dirk Raguse. 2022. "Histological Processing of CAD/CAM Titanium Scaffold after Long-Term Failure in Cranioplasty" Materials 15, no. 3: 982. https://doi.org/10.3390/ma15030982






