Study of the Optimal Composition and Storage Conditions of the Fricke–XO–Pluronic F–127 Radiochromic Dosimeter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Dosimeter
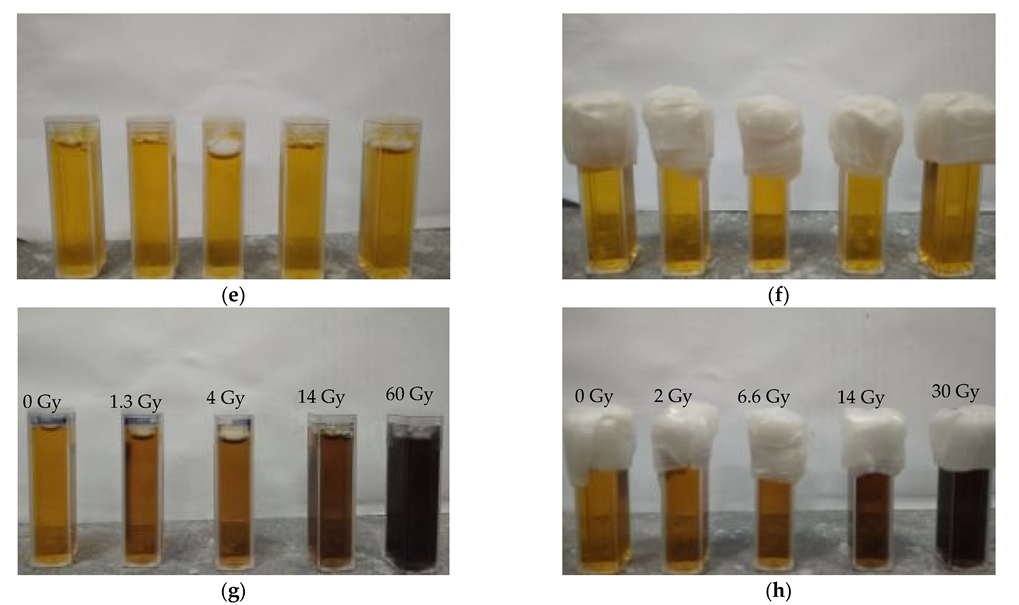
2.2. Storage Conditions of Fricke–XO–Pluronic F–127
2.3. Irradiation of Samples
2.4. Stability of Samples after Irradiation
2.5. UV-Vis Measurements
3. Results and Discussion
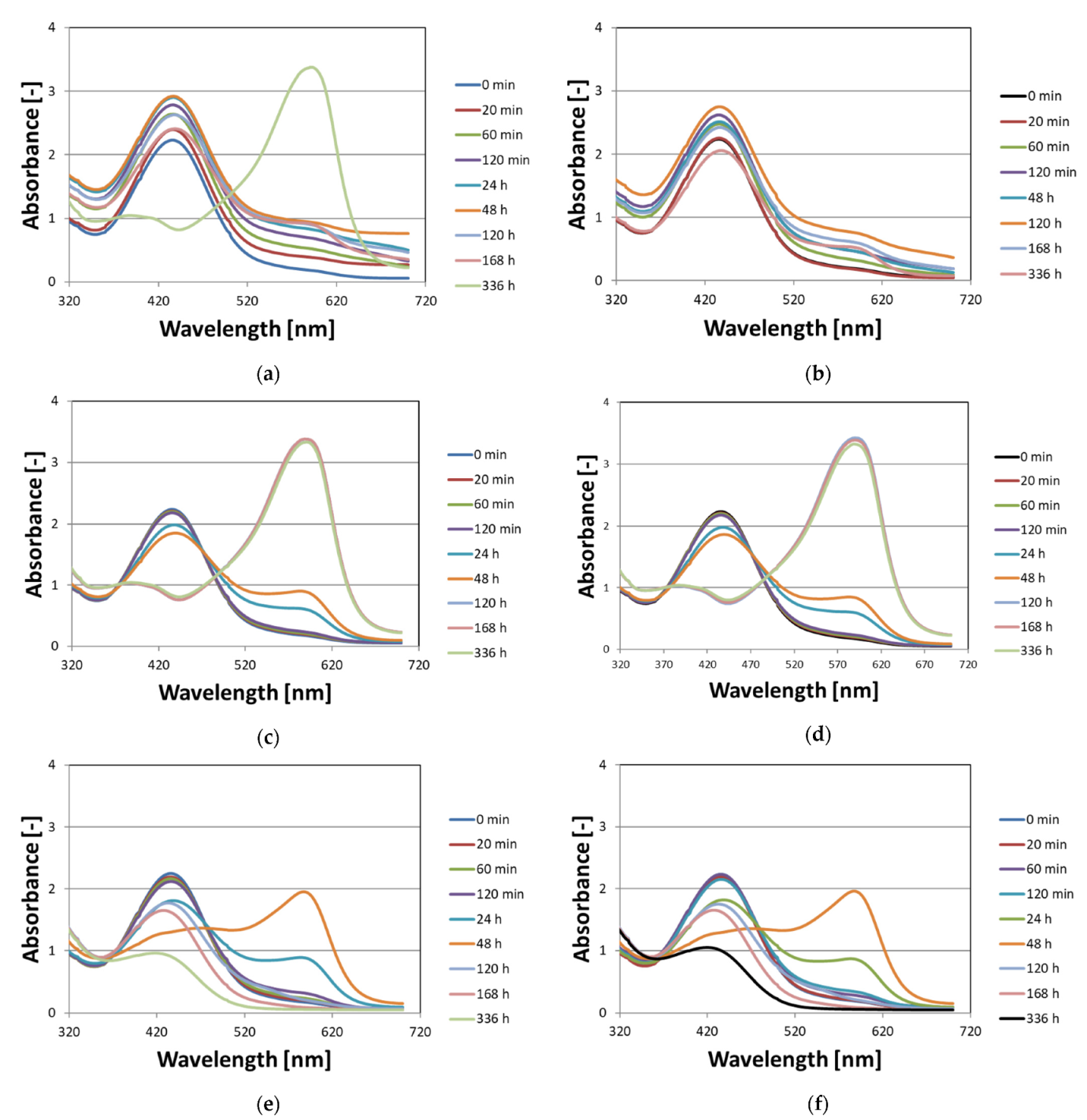
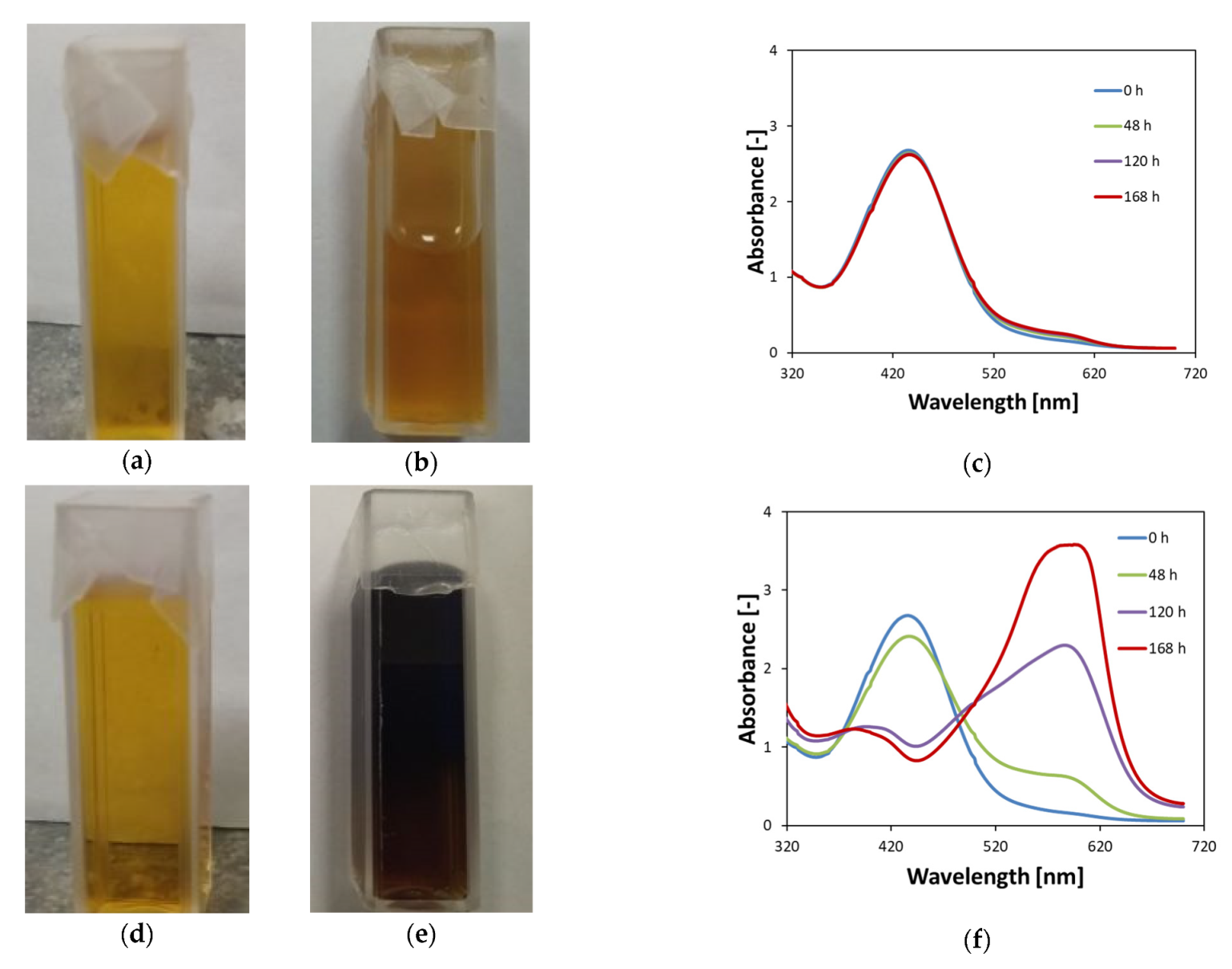
3.1. Stability of Non-Irradiated Fricke–XO–Pluronic F–127
3.2. Stability of Irradiated Fricke–XO–Pluronic F–127
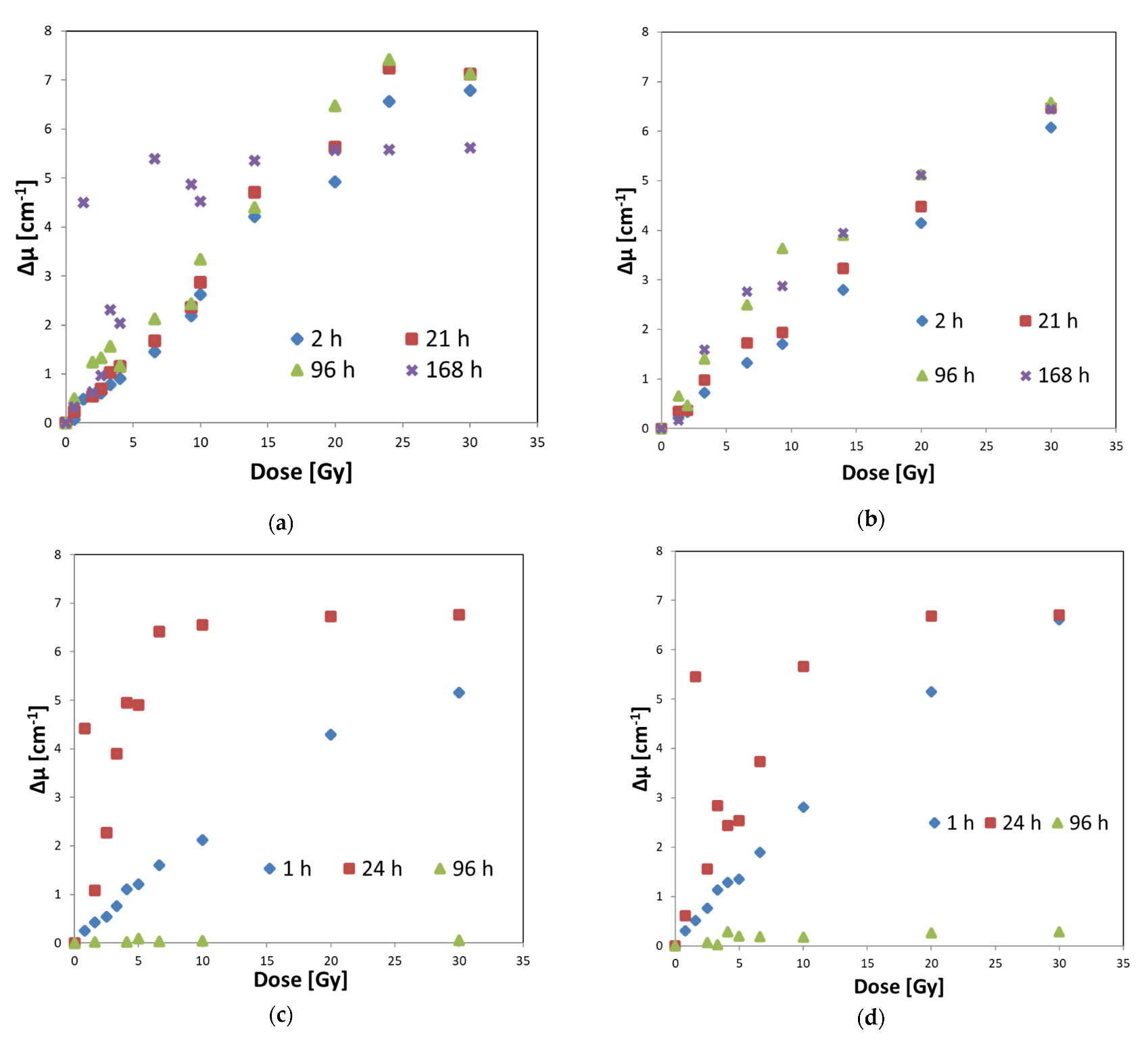
3.3. Optimal Composition of Fricke–XO–Pluronic F–127
3.4. Calibration of Fricke–XO–Pluronic F–127 with a Medical Accelerator
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gore, J.C.; Kang, Y.S.; Schultz, R.J. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys. Med. Biol. 1984, 29, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.C.; Kang, Y.S.; Schultz, R.J. Measurement of radiation dose distributions by NMR imaging. Magn. Reson. Imaging 1984, 2, 244. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, L.J. Review of Fricke gel dosimeters. J. Phys. Conf. Ser. 2004, 3, 9–21. [Google Scholar] [CrossRef]
- Liosi, G.M.; Dondi, D.; Vander Griend, D.A.; Lazzaroni, S.; D’Agostino, G.; Mariani, M. Fricke-gel dosimeter: Overview of Xylenol Orange chemical behavior. Radiat. Phys. Chem. 2017, 140, 74–77. [Google Scholar] [CrossRef]
- d’Errico, F.; Lazzeri, L.; Dondi, D.; Mariani, M.; Marrale, M.; Souza, S.O.; Gambarini, S. Novel GTA-PVA Fricke gels for three-dimensional dose mapping in radiotherapy. Radiat. Meas. 2017, 106, 612–617. [Google Scholar] [CrossRef]
- Penev, K.; Mequanint, K. Controlling sensitivity and stability of ferrous–xylenol orange–gelatin 3D gel dosimeters by doping with phenanthroline-type ligands and glyoxal. Phys. Med. Biol. 2013, 58, 1823–1838. [Google Scholar] [CrossRef]
- Pappas, E.P.; Peppa, V.; Hourdakis, C.J.; Karaiskos, P.; Papagiannis, P. On the use of a novel Ferrous Xylenol-orange gelatin dosimeter for HDR brachytherapy commissioning and quality assurance testing. Phys. Med. 2018, 45, 162–169. [Google Scholar] [CrossRef]
- Cavinato, C.C.; Campos, L.L. Study of the stability, reproducibility and dose rate dependence of the Fricke gel dosimeter developed at IPEN. In Proceedings of the 2007 International Nuclear Atlantic Conference-INAC, Santos, Brazil, 30 September–5 October 2007; Associação Brasileira De Energia Nuclear-ABEN: Rio de Janeiro, Brasil, 2007. ISBN 978-85-99141-02-1. [Google Scholar]
- Marrale, M.; d’Errico, F. Hydrogels for Three-Dimensional Ionizing-Radiation Dosimetry. Gels 2021, 7, 74. [Google Scholar] [CrossRef]
- Mizukami, S.; Watanabe, Y.; Mizoguchi, T.; Gomi, T.; Hara, H.; Takei, H.; Fukunishi, N.; Ishikawa, K.L.; Fukuda, S.; Maeyama, T. Whole three-dimensional dosimetry of carbon ion beams with an MRI-based nanocomposite Fricke gel dosimeter using rapid T1 mapping method. Gels 2021, 7, 233. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Zeng, Y.; Hu, X.; Zhang, X.; Chang, S.; Zhang, H. Low-diffusion Fricke gel dosimeters with core-shell structure based on spatial confinement. Materials 2021, 14, 3932. [Google Scholar] [CrossRef] [PubMed]
- Appleby, A.; Christman, E.A.; Leghrouz, A. Imaging of spatial radiation dose distribution in agarose gels using magnetic resonance. Med. Phys. 1987, 14, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Appleby, A.; Leghrouz, A.; Christman, E.A. Radiation chemical and magnetic resonance studies of aqueous agarose gels containing ferrous ions. Radiat. Phys. Chem. 1988, 32, 241–244. [Google Scholar] [CrossRef]
- Olsson, L.E.; Appleby, A.; Sommer, I. A new dosimeter based on ferrous sulphate solution and agarose gel. Appl. Radiat. Isot. 1991, 42, 1081–1086. [Google Scholar] [CrossRef]
- Olsson, L.E.; Petersson, L.; Ahlgren, L.; Mattsson, S. Ferrous sulphate gels for determination of absorbed dose distributions using MRI technique: Basic studies. Phys. Med. Biol. 1989, 34, 43–52. [Google Scholar] [CrossRef]
- Schulz, R.I.; de Guzman, A.F.; Nguyen, D.B.; Gore, J.C. Dose-response curves for Fricke infused agarose gels as obtained by nuclear magnetic resonance. Phys. Med. Biol. 1990, 35, 1611–1622. [Google Scholar] [CrossRef]
- Gambarini, U.; Arrigoni, S. Dose-response curve slope improvement and result reproducibility of ferrous sulphate-doped gels analysed by NMR imaging. Phys. Med. Biol. 1994, 39, 703–717. [Google Scholar] [CrossRef]
- Jordan, K.; Sekimoto, M. Development of a radiochromic ferric oligomer hydrogel. J. Phys. Conf. Ser. 2010, 250, 012030. [Google Scholar] [CrossRef]
- Jordan, K.; Sekimoto, M. Effects of adding glycerol and sucrose to ferrous xylenol orange hydrogel. J. Phys. Conf. Ser. 2010, 250, 012048. [Google Scholar] [CrossRef]
- Jordan, K.; Battista, J. Dose response of ferrous- xylenol orange gels: The effects of gel substrate, gelation time and dose fractionation. J. Phys. Conf. Ser. 2004, 3, 232–235. [Google Scholar] [CrossRef]
- Babu, S.E.S.; Rafic, K.M.; Peace, B.S.T.; Raj, I.J.S.; Ravindran, B.P. Fricke xylenol glicyne gel dosimeter for in vivo dosimetry at extendent treatment distance. Appl. Radiat. Isot. 2019, 145, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.T.; Boase, N.R.B.; Masters, K.-S.; Hosokawa, K.; Asena, A.; Crowe, S.B.; Kairn, T.; Trapp, J.V. A very low diffusion Fricke gel dosimeter with functionalised xylenol orange-PVA (XOPVA). Phys. Med. Biol. 2019, 64, 205017. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.C.; Jordan, K.J.; Battista, J.J.; Van Dyk, J.; Rutt, B.K. Polyvinyl alcohol-Fricke hydrogel and cryogel: Two new gel dosimetry systems with low Fe3+ diffusion. Phys. Med. Biol. 2000, 45, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Kwiatos, K.; Kadlubowski, S.; Dudek, M. TTC-Pluronic 3D radiochromic gel dosimetry of ionizing radiation. Phys. Med. Biol. 2017, 62, 5668–5690. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. Pluronic F–127 and Pluronic lecithin organogel (PLO): Main features and their applications in topical and transdermal administration of drugs. J. Pharm. Pharm. Sci. 2012, 15, 592–605. [Google Scholar] [CrossRef] [Green Version]
- Diniz, I.M.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F–127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef] [Green Version]
- Dantas Lopes dos Santos, D.; Besegato, J.F.; de Melo, P.B.G.; Oshiro Junior, J.A.; Chorilli, M.; Deng, D.; Bagnato, V.S.; de Souza Rastelli, A.N. Curcumin-loaded Pluronic® F-127 micelles as a drug delivery system for curcumin-mediated photodynamic therapy for oral application. Photochem. Photobiol. 2021, 97, 1072–1088. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Catalano, G.; Arrigo, R.; Morici, E.; Cavallaro, G.; Lazzara, G.; Bruno, M. Pluronic nanoparticles as anti-oxidant carriers for polymers. Polym. Degrad. Stab. 2016, 134, 194–201. [Google Scholar] [CrossRef]
- Kozicki, M.; Kwiatos, K.; Dudek, M.; Stempien, Z. Radiochromic gels for UV radiation measurements in 3D. J. Photochem. Photobiol. A 2018, 351, 197–207. [Google Scholar] [CrossRef]
- Kwiatos, K.; Maras, P.; Kadlubowski, S.; Stempień, Z.; Dudek, M.; Kozicki, M. Tetrazolium salts-Pluronic F–127 gels for 3D radiotherapy dosimetry. Phys. Med. Biol. 2018, 63, 095012. [Google Scholar] [CrossRef]
- Jaszczak, M.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Substituting gelatine with Pluronic F–127 matrix in 3D polymer gel dosimeters can improve nuclear magnetic resonance, thermal and optical properties. Phys. Med. Biol. 2018, 63, 175010. [Google Scholar] [CrossRef] [PubMed]
- Kouvati, K.; Jaszczak, M.; Papagiannis, P.; Kadlubowski, S.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Leuco crystal violet-Pluronic F–127 3D radiochromic gel dosimeter. Phys. Med. Biol. 2019, 64, 175017. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Jaszczak, M.; Kwiatos, K.; Maras, P.; Kadlubowski, S.; Wach, R.; Dudek, M. Three-dimensional radiochromic and polymer gel dosimeters with Pluronic F–127 matrix—A review of current research. J. Phys. Conf. Ser. 2019, 1305, 012035. [Google Scholar] [CrossRef] [Green Version]
- Jaszczak, M.; Maras, P.; Kozicki, M. Characterization of a new N-vinylpyrrolidone-containing polymer gel dosimeter with Pluronic F–127 gel matrix. Radiat. Phys. Chem. 2020, 177, 109125. [Google Scholar] [CrossRef]
- Jaszczak, M.; Sasiadek, M.; Kadlubowski, S.; Dudek, M.; Kozicki, M. Preliminary study on a new 3D radiochromic KI-Pluronic F–127 gel dosimeter for radiotherapy. Radiat. Phys. Chem. 2021, 185, 109507. [Google Scholar] [CrossRef]
- Kozicki, M.; Bartosiak, M.; Dudek, M.; Kadlubowski, S. LCV-Pluronic F–127 dosimeter for UV light dose distribution measurements. J. Photochem. Photobiol. A 2021, 405, 112930. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Naglik, R.; Dudek, M.; Kadlubowski, S.; Wach, R. Preliminary study on a 3D lung mimicking dosimeter based on Pluronic F–127 matrix. Radiat. Phys. Chem. 2021, 185, 109479. [Google Scholar] [CrossRef]
- Dudek, M.; Piotrowski, M.; Maras, P.; Jaszczak, M.; Kozicki, M. Anisotropic diffusion of Fe ions in Fricke–XO–Pluronic F–127 and Fricke-XO-gelatine 3D radiotherapy dosimeters. Phys. Med. Biol. 2021, 66, 155005. [Google Scholar] [CrossRef]
- Otomoto, M. Composition of the Xylenol Orange complexes of iron (III) and their application to the determination of iron or Xylenol Orange. Bunseki Kagaku 1965, 14, 677–682. [Google Scholar] [CrossRef] [Green Version]
- O, T.S.; Lee, S.H.; Park, J.H. Spetrophotometric study of the complex formation of ferric ion with xylenol orange. J. Korean Chem. Soc. 1978, 22, 311–316. [Google Scholar]
- Babic, S.; Battista, J.; Jordan, K. An apparent treshold dose response in ferrous-xylenol orange gel dosimeters when scanned with a yellow light source. Phys. Med. Biol. 2008, 53, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Collura, G.; Gallo, S.; Tranchina, L.; Abbate, B.F.; Bartolotta, A.; D’Errico, F.; Marrale, M. Analysis of the response of PVA-GTA Fricke-gel dosimeters with clinical magnetic resonance imaging. Nucl. Instrum. Methods Phys. 2018, 414, 146–153. [Google Scholar] [CrossRef]
- Liosi, G.M.; Giacobbo, F.; Pignoli, E.; Carrara, M.; Gambarini, G.; Mariani, M. Effects of the pre-irradiation storage procedure on the dose response of a Fricke xylenol orange gel dosimeter. Nukleonika 2015, 60, 603–607. [Google Scholar] [CrossRef] [Green Version]


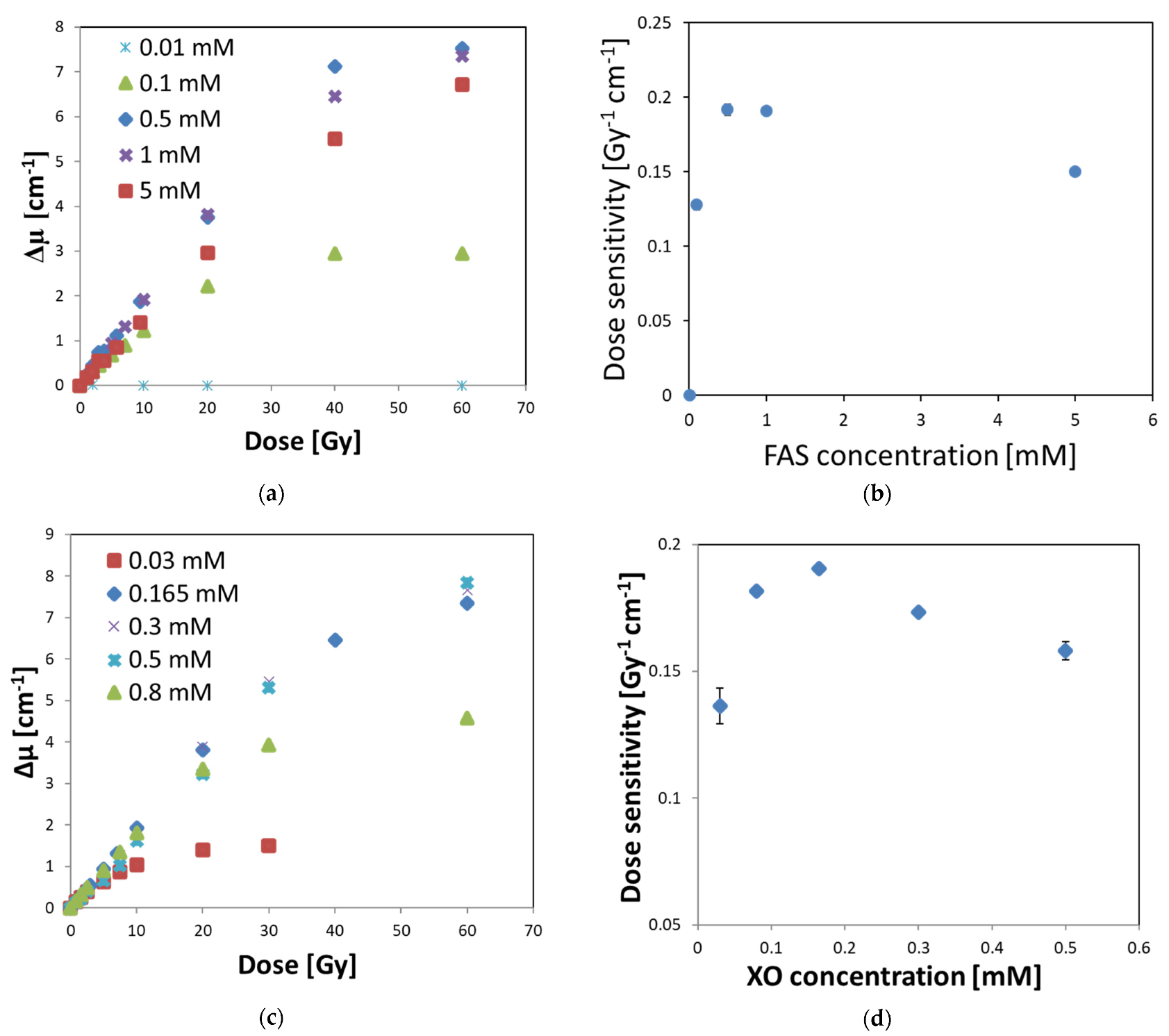
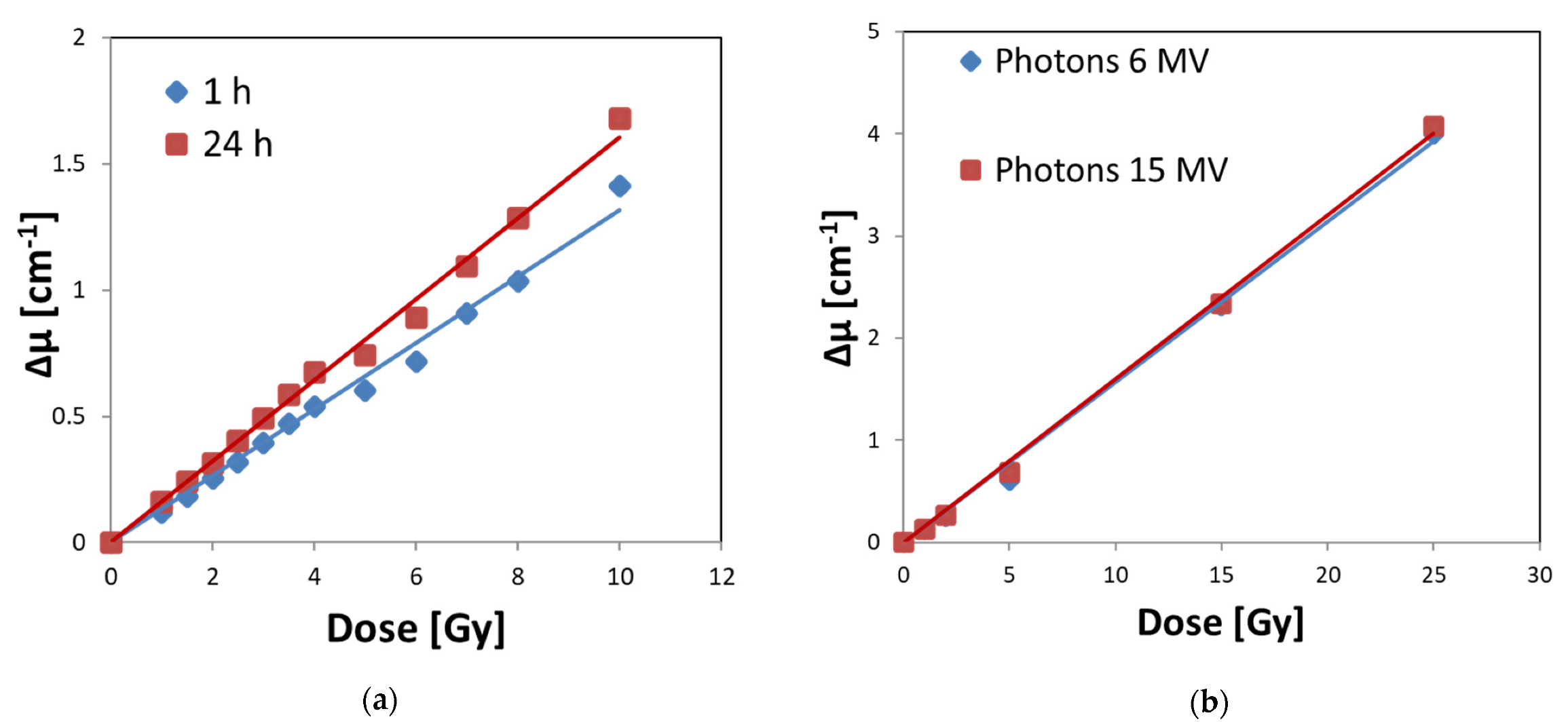
| Oxygen (Air) Access | Dose Sensitivity [Gy−1 cm−1] | R2 [-] | Quasi-Linear Dose Range [Gy] | Dynamic Dose Range [Gy] |
|---|---|---|---|---|
| Samples stored in the dark in a refrigerator (4 °C) | ||||
| No oxygen access | 0.221 ± 0.004 | 0.997 | 20 | ~35 Gy or higher |
| Hindered oxygen access | 0.291 ± 0.009 | 0.990 | 20 | |
| Samples stored in the dark in a cabinet (21 °C) | ||||
| No oxygen access | 0.268 ± 0.006 | 0.995 | 20 | ~35 Gy or higher |
| Hindered oxygen access | 0.219 ± 0.004 | 0.996 | 20 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowski, M.; Maras, P.; Kadłubowski, S.; Kozicki, M. Study of the Optimal Composition and Storage Conditions of the Fricke–XO–Pluronic F–127 Radiochromic Dosimeter. Materials 2022, 15, 984. https://doi.org/10.3390/ma15030984
Piotrowski M, Maras P, Kadłubowski S, Kozicki M. Study of the Optimal Composition and Storage Conditions of the Fricke–XO–Pluronic F–127 Radiochromic Dosimeter. Materials. 2022; 15(3):984. https://doi.org/10.3390/ma15030984
Chicago/Turabian StylePiotrowski, Michał, Piotr Maras, Sławomir Kadłubowski, and Marek Kozicki. 2022. "Study of the Optimal Composition and Storage Conditions of the Fricke–XO–Pluronic F–127 Radiochromic Dosimeter" Materials 15, no. 3: 984. https://doi.org/10.3390/ma15030984







