Influence of the Fly Ash Content on the Fresh and Hardened Properties of Alkali-Activated Slag Pastes with Admixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Alkali-Activated Pastes and Mortars
2.3. Testing Procedure
3. Results and Discussion
3.1. Influence of the Fly Ash Content on the Fresh Properties of Alkali-Activated Pastes with Admixtures
3.1.1. Setting Time
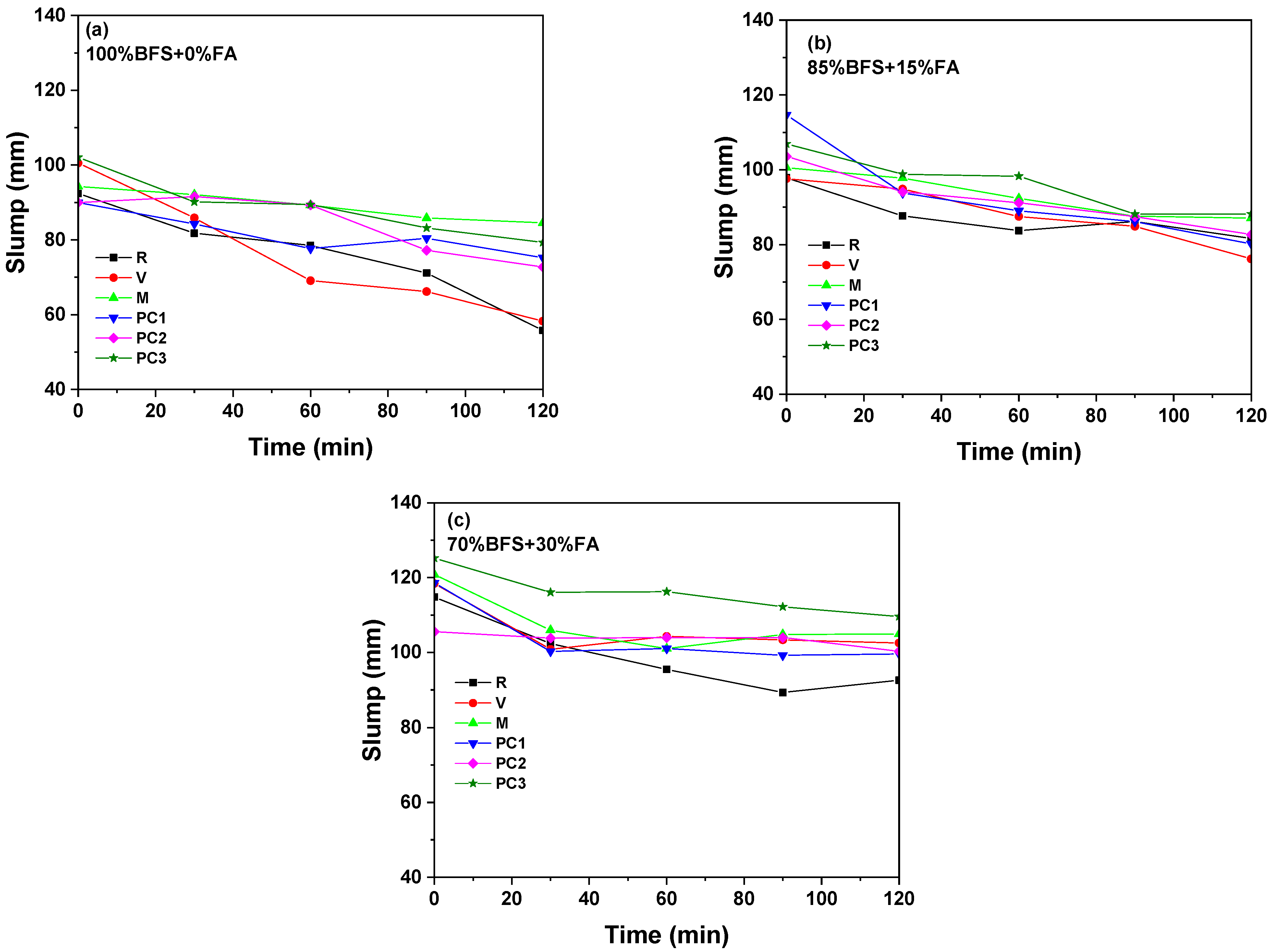
3.1.2. Mini-Slump
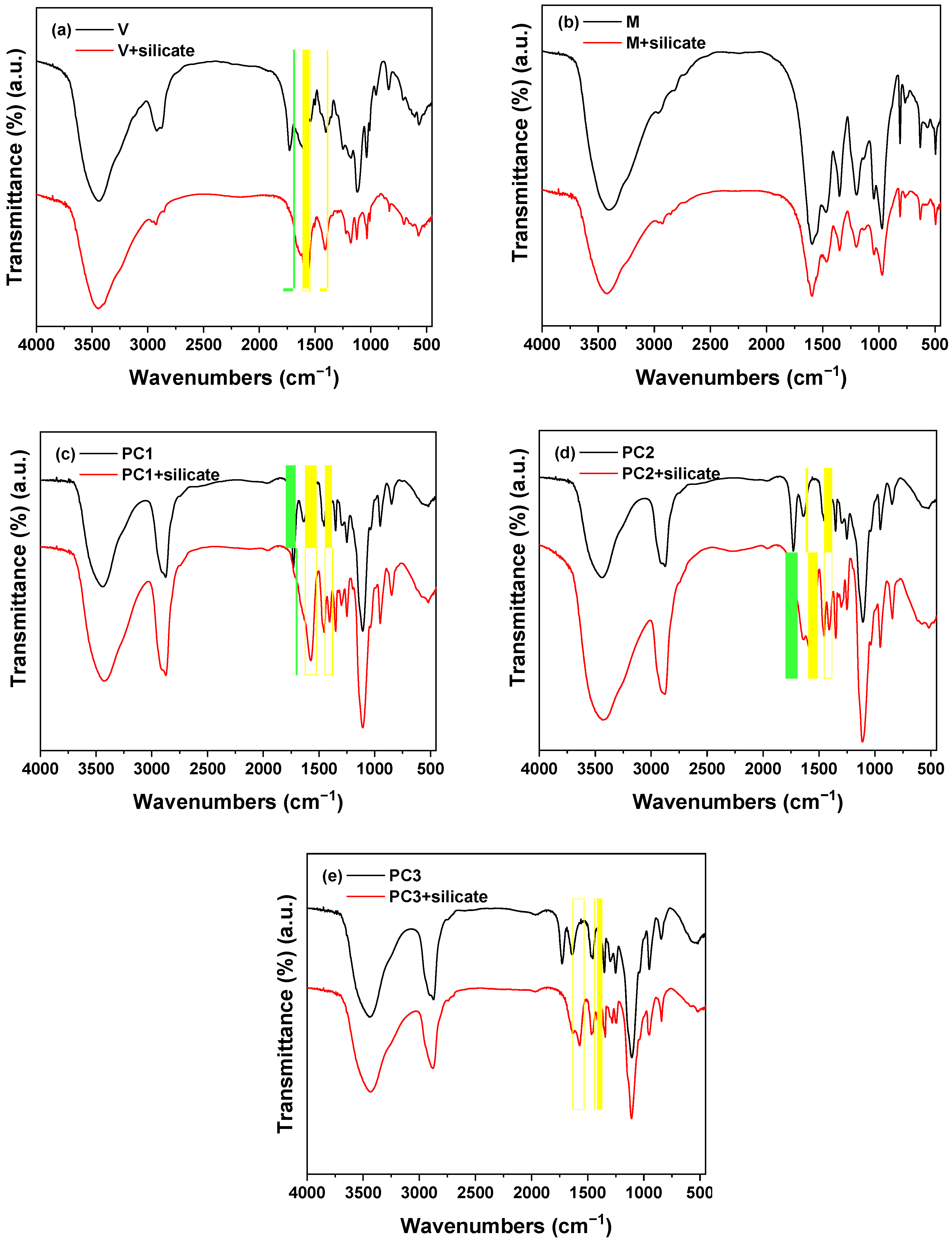
3.1.3. Stability of Admixtures
3.2. Effect of M and PC3 on the Reaction Kinetic and Hardened Properties of Alkali-Activated Slag/Fly Ash Mixtures
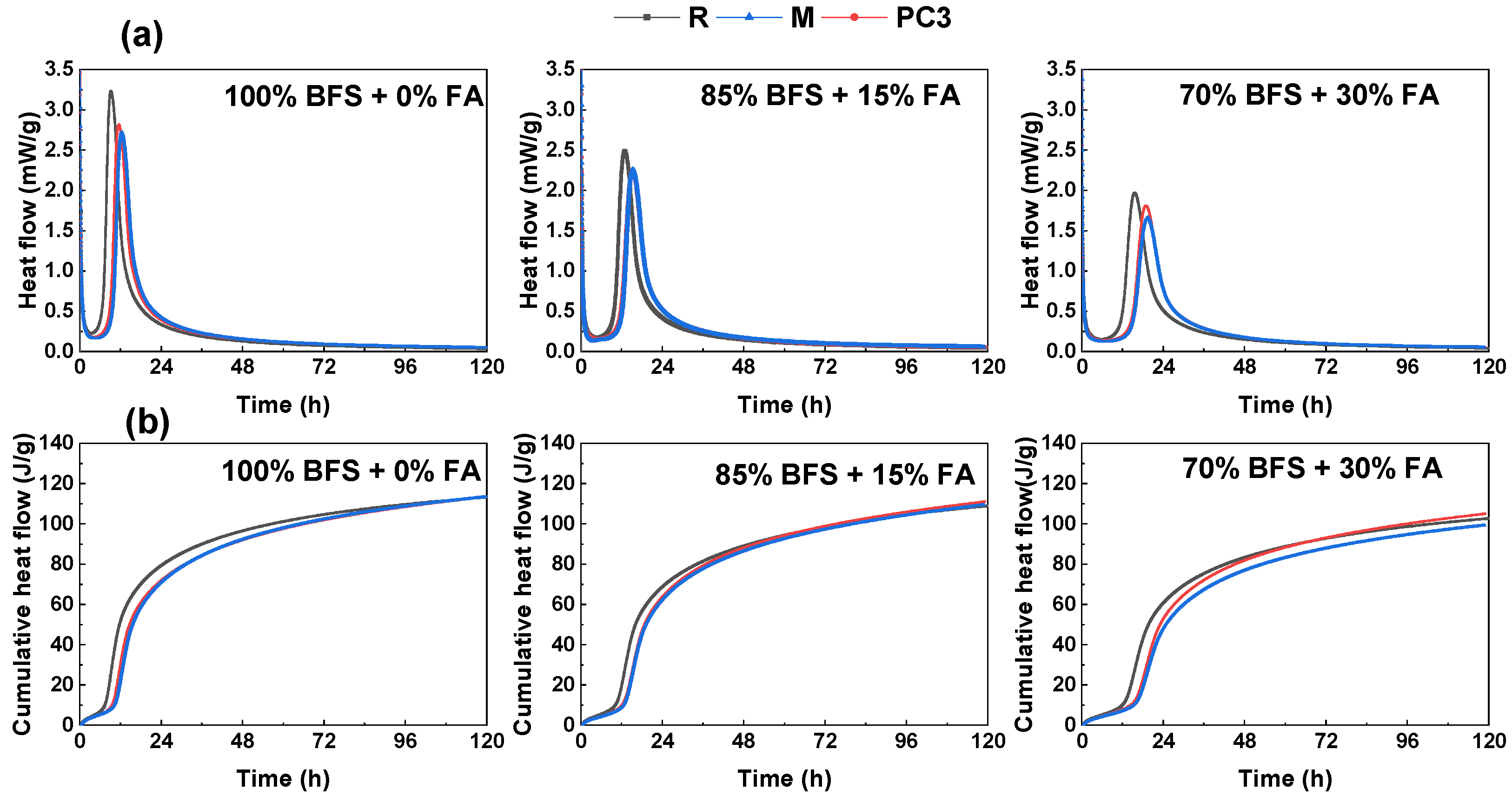
3.2.1. Isothermal Conduction Calorimetry
3.2.2. Mechanical Strengths
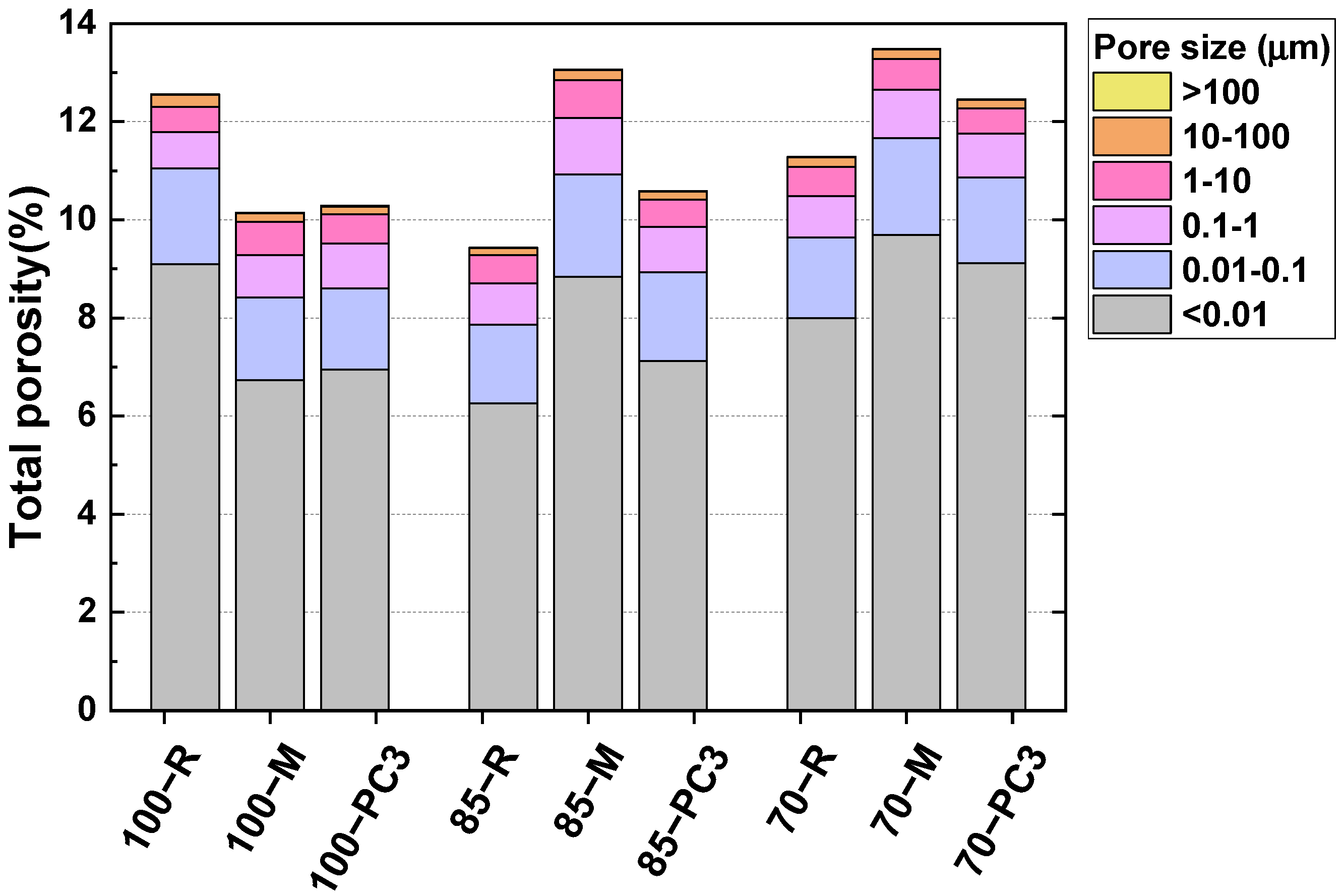
3.2.3. Porosity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Yousefi Oderji, S.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Deir, E.; Gebregziabiher, B.S.; Peethamparan, S. Influence of starting material on the early age hydration kinetics, microstructure and composition of binding gel in alkali activated binder systems. Cem. Concr. Compos. 2014, 48, 108–117. [Google Scholar] [CrossRef]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and mechanical properties of alkali activated Colombian raw materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Roy, D.M.; Jiang, W.; Silsbee, M.R. Chloride diffusion in ordinary, blended, and alkali-activated cement pastes and its relation to other properties. Cem. Concr. Res. 2000, 30, 1879–1884. [Google Scholar] [CrossRef]
- Brough, A.R.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars—Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Karahan, O.; Yakupoglu, A. Resistance of alkali-activated slag mortar to abrasion and fire. Adv. Cem. Res. 2011, 23, 289–297. [Google Scholar] [CrossRef]
- Rostami, H.; Brendley, W. Alkali ash material: A novel fly ash-based cement. Environ. Sci. Technol. 2003, 37, 3454–3457. [Google Scholar] [CrossRef] [PubMed]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Škvára, F.; Jílek, T.; Kopecký, L. Geopolymer materials based on fly ash. Ceram.-Silik 2005, 49, 195–204. [Google Scholar]
- Fernandez-Jimenez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Martin, A.; Pastor, J.Y.; Palomo, A.; Fernández Jiménez, A. Mechanical behaviour at high temperature of alkali-activated aluminosilicates (geopolymers). Constr. Build. Mater. 2015, 93, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Saha, S.; Rajasekaran, C. Enhancement of the properties of fly ash based geopolymer paste by incorporating ground granulated blast furnace slag. Constr. Build. Mater. 2017, 146, 615–620. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; Van Deventer, J.S.J. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Chang, J.J. A study on the setting characteristics of sodium silicate-activated slag pastes. Cem. Concr. Res. 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Blaakmer, J. Diabind: An alkali-activated slag fly ash binder for acid-resistant concrete. Fuel Energy Abstr. 1995, 5, 336. [Google Scholar] [CrossRef]
- Palomo, Á.; Fernández-jiménez, A.; López-hombrados, C.; Lleyda, J.L. Railway sleepers made of alkali activated fly ash concrete. Rev. Ing. Constr. 2007, 22, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Provis, J.L. Demonstration Projects and Applications in Building and Civil Infrastructure. In Alkali Activated Materials. RILEM State-of-the-Art Reports; Provis, J.L., Van Deventer, J.S.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 13, ISBN 9789400776722. [Google Scholar]
- Shojaei, M.; Behfarnia, K.; Mohebi, R. Application of alkali-activated slag concrete in railway sleepers. J. Mater. Des. 2015, 69, 89–95. [Google Scholar] [CrossRef]
- Burgos-Montes, O.; Palacios, M.; Rivilla, P.; Puertas, F. Compatibility between superplasticizer admixtures and cements with mineral additions. Constr. Build. Mater. 2012, 31, 300–309. [Google Scholar] [CrossRef]
- Tong, S.; Yuqi, Z.; Qiang, W. Recent advances in chemical admixtures for improving the workability of alkali-activated slag-based material systems. Constr. Build. Mater. 2021, 272, 121647. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Stability of superplasticiser and shrinkage-reducing admixtures in high basic media. Mater. Constr. 2004, 54, 65–86. [Google Scholar]
- Palacios, M.; Puertas, F. Effect of superplasticizer and shrinkage-reducing admixtures on alkali-activated slag pastes and mortars. Cem. Concr. Res. 2005, 35, 1358–1367. [Google Scholar] [CrossRef]
- Keulen, A.; Yu, Q.L.; Zhang, S.; Grünewald, S. Effect of admixture on the pore structure refinement and enhanced performance of alkali-activated fly ash-slag concrete. Constr. Build. Mater. 2018, 162, 27–36. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Wang, Y.S.; Dai, J.G. The effectiveness of different superplasticizers in ambient cured one-part alkali activated pastes. Cem. Concr. Compos. 2019, 97, 166–174. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, N.K.; Lee, H.K. Fresh and hardened properties of alkali-activated fly ash/slag pastes with superplasticizers. Constr. Build. Mater. 2014, 50, 169–176. [Google Scholar] [CrossRef]
- Laskar, S.M.; Talukdar, S. Preparation and tests for workability, compressive and bond strength of ultra-fine slag based geopolymer as concrete repairing agent. Constr. Build. Mater. 2017, 154, 176–190. [Google Scholar] [CrossRef]
- Namitha, S.; Muhammed Nabil, K.; Mohammed Rafeeque, N.V.; Sundhar, R.; Raju, T.; Ramaswamy, K.P. A study on the setting and flow behaviour of alkali activated slag/fly ash composites in ambient condition. IOP Conf. Ser. Mater. Sci. Eng. 2020, 989, 012004. [Google Scholar] [CrossRef]
- Raju, T.; Ramaswamy, K.P.; Saraswathy, B. Effects of slag and superplasticizers on alkali activated geopolymer paste. IOP Conf. Ser. Earth Environ. Sci. 2020, 491, 012042. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Fundamentals of Adsorption Technology, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 33, ISBN 9780128188057. [Google Scholar]
- Criado, M.; Walkley, B.; Ke, X.; Provis, J.L.; Bernal, S.A. Slag and activator chemistry control the reaction kinetics of sodium metasilicate-activated slag cements. Sustainability 2018, 10, 4709. [Google Scholar] [CrossRef] [Green Version]
- Provis, J.L. Geopolymers Structures Processing Properties and Industrial Applications; Provis, J.L., Van Deventer, J.S.J., Eds.; Wood Publishing: Cambridge, UK, 2009; pp. 50–71. ISBN 9781845692636. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Tan, Z.; Bernal, S.A.; Provis, J.L. Reproducible mini-slump test procedure for measuring the yield stress of cementitious pastes. Mater. Struct. Constr. 2017, 50, 235. [Google Scholar] [CrossRef] [Green Version]
- UNE. EN. 196-3. Métodos de Ensayos de Cementos. Parte 3: Determinación del Tiempo de Fraguado y de la Estabilidad de Volumen; AENOR: Madrid, Spain, 2005. [Google Scholar]
- UNE. EN. 196-1. Métodos de Ensayos de Cementos. Parte 1: Determinación de Resistencias Mecánicas; AENOR: Madrid, Spain, 2005. [Google Scholar]
- Fang, G.; Bahrami, H.; Zhang, M. Mechanisms of autogenous shrinkage of alkali-activated fly ash-slag pastes cured at ambient temperature within 24 h. Constr. Build. Mater. 2018, 171, 377–387. [Google Scholar] [CrossRef]
- Nedunuri, A.S.S.S.; Muhammad, S. Fundamental understanding of the setting behaviour of the alkali activated binders based on ground granulated blast furnace slag and fly ash. Constr. Build. Mater. 2021, 291, 123243. [Google Scholar] [CrossRef]
- Vikas, G.; Rao, T.D.G. Setting Time, Workability and Strength Properties of Alkali Activated Fly Ash and Slag Based Geopolymer Concrete Activated with High Silica Modulus Water Glass. Iran. J. Sci. Technol. Trans. Civ. Eng. 2021, 45, 1483–1492. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernández-Jiménez, A.; Banfill, P.F.G. Alkali activated fly ash: Effect of admixtures on paste rheology. Rheol. Acta 2009, 48, 447–455. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Wang, Y.S.; Dai, J.G. Effect of mixing method on the performance of alkali-activated fly ash/slag pastes along with polycarboxylate admixture. Cem. Concr. Compos. 2021, 117, 103917. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Li, Z.; Gao, Y.; Liu, C. Feasibility study of red mud-blast furnace slag based geopolymeric grouting material: Effect of superplasticizers. Constr. Build. Mater. 2021, 267, 120910. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; de Schutter, G. Influence of water to binder ratio on the rheology and structural Build-up of Alkali-Activated Slag/Fly ash mixtures. Constr. Build. Mater. 2020, 264, 120253. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag-fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Chithiraputhiran, S.; Neithalath, N. Isothermal reaction kinetics and temperature dependence of alkali activation of slag, fly ash and their blends. Constr. Build. Mater. 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Winnefeld, F.; Leemann, A.; Lucuk, M.; Svoboda, P.; Neuroth, M. Assessment of phase formation in alkali activated low and high calcium fly ashes in building materials. Constr. Build. Mater. 2010, 24, 1086–1093. [Google Scholar] [CrossRef]


| Oxides (wt.%) | CaO | SiO2 | Al2O3 | MgO | SO3 | TiO2 | Fe2O3 | K2O | Na2O | Others | L.O.I * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BFS | 45.7 | 32.3 | 9.6 | 7.1 | 1.6 | 0.9 | 0.5 | 0.5 | 0.3 | 0.5 | 0.95 |
| FA | 4.8 | 42.4 | 27.0 | 0.8 | 1.4 | 1.1 | 18.4 | 1.5 | 0.5 | 0.5 | 1.60 |
| Admixtures | V | M | PC1 | PC2 | PC3 |
|---|---|---|---|---|---|
| Structure | Sulphonate groups and ester | Sulphonate groups | Comb-like structure (backbone and short side chain length) | Comb-like structure (backbone and long side chain length) | Structure with very long chain length |
| Action mechanism | Mainly electrostatic repulsion | Electrostatic repulsion | Mainly steric hindrance | Mainly steric hindrance | Mainly steric hindrance |
| Dosage (% binder) | 1.00 | 1.20 | 1.00 | 1.25 | 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Hita, M.J.; Criado, M. Influence of the Fly Ash Content on the Fresh and Hardened Properties of Alkali-Activated Slag Pastes with Admixtures. Materials 2022, 15, 992. https://doi.org/10.3390/ma15030992
de Hita MJ, Criado M. Influence of the Fly Ash Content on the Fresh and Hardened Properties of Alkali-Activated Slag Pastes with Admixtures. Materials. 2022; 15(3):992. https://doi.org/10.3390/ma15030992
Chicago/Turabian Stylede Hita, María Jimena, and María Criado. 2022. "Influence of the Fly Ash Content on the Fresh and Hardened Properties of Alkali-Activated Slag Pastes with Admixtures" Materials 15, no. 3: 992. https://doi.org/10.3390/ma15030992
APA Stylede Hita, M. J., & Criado, M. (2022). Influence of the Fly Ash Content on the Fresh and Hardened Properties of Alkali-Activated Slag Pastes with Admixtures. Materials, 15(3), 992. https://doi.org/10.3390/ma15030992







