Interfacial Polarization of Thin Alq3, Gaq3, and Erq3 Films on GaN(0001)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flack, T.J.; Pushpakaran, B.N.; Bayne, S.B. GaN Technology for Power Electronic Applications: A Review. J. Electron. Mater. 2016, 45, 2673–2682. [Google Scholar] [CrossRef]
- Lidow, A.; Strydom, J.; de Rooij, M.; Reusch, D. (Eds.) GaN Technology Overview. In GaN Transistors for Efficient Power Conversion; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 1–18. ISBN 978-1-118-84477-9. [Google Scholar] [CrossRef]
- Krieg, L.; Meierhofer, F.; Gorny, S.; Leis, S.; Splith, D.; Zhang, Z.; von Wenckstern, H.; Grundmann, M.; Wang, X.; Hartmann, J.; et al. Toward Three-Dimensional Hybrid Inorganic/Organic Optoelectronics Based on GaN/OCVD-PEDOT Structures. Nat. Commun. 2020, 11, 5092. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X. Group III Nitride Nanomaterials for Biosensing. Nanoscale 2017, 9, 7320–7341. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.W.; Vanslyke, S.A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Xu, Y.; Hofmann, O.T.; Schlesinger, R.; Winkler, S.; Frisch, J.; Niederhausen, J.; Vollmer, A.; Blumstengel, S.; Henneberger, F.; Koch, N.; et al. Space-Charge Transfer in Hybrid Inorganic-Organic Systems. Phys. Rev. Lett. 2013, 111, 226802. [Google Scholar] [CrossRef] [Green Version]
- Schultz, T.; Schlesinger, R.; Niederhausen, J.; Henneberger, F.; Sadofev, S.; Blumstengel, S.; Vollmer, A.; Bussolotti, F.; Yang, J.P.; Kera, S.; et al. Tuning the Work Function of GaN with Organic Molecular Acceptors. Phys. Rev. B 2016, 93, 125309. [Google Scholar] [CrossRef]
- Schlesinger, R.; Bianchi, F.; Blumstengel, S.; Christodoulou, C.; Ovsyannikov, R.; Kobin, B.; Moudgil, K.; Barlow, S.; Hecht, S.; Marder, S.R.; et al. Efficient Light Emission from Inorganic and Organic Semiconductor Hybrid Structures by Energy-Level Tuning. Nat. Commun. 2015, 6, 6754. [Google Scholar] [CrossRef] [Green Version]
- Le, S.; Jiang, Q.; Pan, H. Synthesis of a Crystal Violet-Cadmium Hydroxyquinoline Iodine Nanocomposite for the Photoelectrochemical Sensing of Ascorbic Acid. Int. J. Electrochem. Sci. 2018, 13, 8960–8969. [Google Scholar] [CrossRef]
- Zampetti, A.; Minotto, A.; Cacialli, F. Near-Infrared (NIR) Organic Light-Emitting Diodes (OLEDs): Challenges and Opportunities. Adv. Funct. Mater. 2019, 29, 1807623. [Google Scholar] [CrossRef]
- Nagata, R.; Nakanotani, H.; Potscavage, W.J., Jr.; Adachi, C. Exploiting Singlet Fission in Organic Light-Emitting Diodes. Adv. Mater. 2018, 30, 1801484. [Google Scholar] [CrossRef]
- Muhammad, F.F.; Ketuly, K.A.; Yahya, M.Y. Effect of Thermal Annealing on a Ternary Organic Solar Cell Incorporating Gaq3 Organometallic as a Boosting Acceptor. J. Inorg. Organomet. Polym. Mater. 2018, 28, 102–109. [Google Scholar] [CrossRef]
- Üngördü, A. Charge Transfer Properties of Gaq3 and Its Derivatives: An OLED Study. Chem. Phys. Lett. 2019, 733, 136696. [Google Scholar] [CrossRef]
- Alzahrani, H.; Sulaiman, K.; Mahmoud, A.Y.; Bahabry, R.R. Study of Organic Visible-Blind Photodetector Based on Alq3:NPD Blend for Application in near-Ultraviolet Detection. Opt. Mater. 2020, 110, 110490. [Google Scholar] [CrossRef]
- Meierhofer, F.; Krieg, L.; Tobias, V. GaN Meets Organic: Technologies and Devices Based on Gallium-Nitride/Organic Hybrid Structures. Semicond. Sci. Technol. 2018, 33, 083001. [Google Scholar] [CrossRef]
- Lindell, L.; Çakr, D.; Brocks, G.; Fahlman, M.; Braun, S. Role of Intrinsic Molecular Dipole in Energy Level Alignment at Organic Interfaces. Appl. Phys. Lett. 2013, 102, 223301. [Google Scholar] [CrossRef] [Green Version]
- Jäger, L.; Schmidt, T.D.; Brütting, W. Manipulation and Control of the Interfacial Polarization in Organic Light-Emitting Diodes by Dipolar Doping. AIP Adv. 2016, 6, 095220. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Morikawa, Y. Theoretical Investigation on the Electronic Structure of the Tris-(8-Hydroxyquinolinato) Aluminum/Aluminum Interface. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2006, 45, 413–416. [Google Scholar] [CrossRef]
- Droghetti, A.; Steil, S.; Großmann, N.; Haag, N.; Zhang, H.; Willis, M.; Gillin, W.P.; Drew, A.J.; Aeschlimann, M.; Sanvito, S.; et al. Electronic and Magnetic Properties of the Interface between Metal-Quinoline Molecules and Cobalt. Phys. Rev. B-Condens. Matter Mater. Phys. 2014, 89, 094412. [Google Scholar] [CrossRef] [Green Version]
- Curry, R.J.; Gillin, W.P. 1.54 Μm Electroluminescence from Erbium (III) Tris(8-Hydroxyquinoline) (ErQ)-Based Organic Light-Emitting Diodes. Appl. Phys. Lett. 1999, 75, 1380–1382. [Google Scholar] [CrossRef] [Green Version]
- Barraud, C.; Seneor, P.; Mattana, R.; Fusil, S.; Bouzehouane, K.; Deranlot, C.; Graziosi, P.; Hueso, L.; Bergenti, I.; Dediu, V.; et al. Unravelling the Role of the Interface for Spin Injection into Organic Semiconductors. Nat. Phys. 2010, 6, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular Semiconductors in Organic Photovoltaic Cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef] [PubMed]
- Steil, S.; Großmann, N.; Laux, M.; Ruffing, A.; Steil, D.; Wiesenmayer, M.; Mathias, S.; Monti, O.L.A.; Cinchetti, M.; Aeschlimann, M. Spin-Dependent Trapping of Electrons at Spinterfaces. Nat. Phys. 2013, 9, 242–247. [Google Scholar] [CrossRef]
- Kim, H.; Dang, C.; Song, Y.K.; Zhang, Q.; Patterson, W.; Nurmikko, A.V.; Kim, K.K.; Song, S.Y.; Han, J. Nitride-Organic Semiconductor Hybrid Heterostructures for Optoelectronic Devices. Phys. Status Solidi (C) Curr. Top. Solid State Phys. 2007, 4, 2411–2414. [Google Scholar] [CrossRef]
- Hill, J.M.; Royce, D.G.; Fadley, C.S.; Wagner, L.F.; Grunthaner, F.J. Properties of Oxidized Silicon as Determined by Angular-Dependent X-Ray Photoelectron Spectroscopy. Chem. Phys. Lett. 1976, 44, 225–231. [Google Scholar] [CrossRef]
- Cumpson, P.J. The Thickogram: A Method for Easy Film Thickness Measurement in XPS. Surf. Interface Anal. 2000, 29, 403–406. [Google Scholar] [CrossRef]
- Blyth, R.I.R.; Thompson, J.; Zou, Y.; Fink, R.; Umbach, E.; Gigli, G.; Cingolani, R. Characterisation of Thin Films of the Organic Infra-Red Emitters Yb- and Er-Tris(8-Hydroxyquinoline) by X-Ray Photoemission Spectroscopy. Synth. Met. 2003, 139, 207–213. [Google Scholar] [CrossRef]
- Grodzicki, M.; Mazur, P.; Krupski, A.; Ciszewski, A. Studies of Early Stages of Mn/GaN(0001) Interface Formation Using Surface-Sensitive Techniques. Vacuum 2018, 153, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Powell, C.J.; Jablonski, A. NIST Electron Inelastic-Mean-Free-Path Database, version 1.2; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010.
- Grodzicki, M.; Moszak, K.; Hommel, D.; Bell, G.R. Bistable Fermi Level Pinning and Surface Photovoltage in GaN. Appl. Surf. Sci. 2020, 533, 147416. [Google Scholar] [CrossRef]
- Grodzicki, M.; Mazur, P.; Pers, J.; Zuber, S.; Ciszewski, A. Sb Layers on P-GaN: UPS, XPS and LEED Study. Acta Phys. Pol. A 2014, 126, 1128–1130. [Google Scholar] [CrossRef]
- Segev, D.; Van De Walle, C.G. Origins of Fermi-Level Pinning on GaN and InN Polar and Nonpolar Surfaces. Europhys. Lett. 2006, 76, 305–311. [Google Scholar] [CrossRef]
- Van De Walle, C.G.; Segev, D. Microscopic Origins of Surface States on Nitride Surfaces. J. Appl. Phys. 2007, 101, 081704. [Google Scholar] [CrossRef] [Green Version]
- Long, J.P.; Bermudez, V.M. Band Bending and Photoemission-Induced Surface Photovoltages on Clean n- and p-GaN (0001) Surfaces. Phys. Rev. B-Condens. Matter Mater. Phys. 2002, 66, 121308. [Google Scholar] [CrossRef] [Green Version]
- Grodzicki, M.; Mazur, P.; Ciszewski, A. Changes of Electronic Properties of P-GaN(0 0 0 1) Surface after Low-Energy N + -Ion Bombardment. Appl. Surf. Sci. 2018, 440, 547–552. [Google Scholar] [CrossRef]
- Majchrzak, D.; Grodzicki, M.; Ciechanowicz, P.; Rousset, J.G.; Piskorska-Hommel, E.; Hommel, D. The Influence of Oxygen and Carbon Contaminants on the Valence Band of P-GaN(0001). Acta Phys. Pol. A 2019, 136, 585–588. [Google Scholar] [CrossRef]
- Wasielewski, R.; Mazur, P.; Grodzicki, M.; Ciszewski, A. TiO Thin Films on GaN(0001). Phys. Status Solidi (B) Basic Res. 2015, 252, 1001–1005. [Google Scholar] [CrossRef]
- Grodzicki, M. Properties of Bare and Thin-Film-Covered Gan(0001) Surfaces. Coatings 2021, 11, 145. [Google Scholar] [CrossRef]
- Grodzicki, M.; Rousset, J.G.; Ciechanowicz, P.; Piskorska-Hommel, E.; Hommel, D. Surface Studies of Physicochemical Properties of As Films on GaN(0001). Appl. Surf. Sci. 2019, 493, 384–388. [Google Scholar] [CrossRef]
- Grodzicki, M.; Mazur, P.; Brona, J.; Ciszewski, A. MnGa and (Mn,Ga)N-like Alloy Formation during Annealing of Mn/GaN(0001) Interface. Appl. Surf. Sci. 2019, 481, 790–794. [Google Scholar] [CrossRef]
- Duvenhage, M.M.; Ntwaeaborwa, M.; Visser, H.G.; Swarts, P.J.; Swarts, J.C.; Swart, H.C. Determination of the Optical Band Gap of Alq3 and Its Derivatives for the Use in Two-Layer OLEDs. Opt. Mater. 2015, 42, 193–198. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Taveira, R.J.S.; Lima, C.F.R.A.C.; Mendes, A.; Santos, L.M.N.B.F. Optical Band Gaps of Organic Semiconductor Materials. Opt. Mater. 2016, 58, 51–60. [Google Scholar] [CrossRef]
- Bisti, F.; Stroppa, A.; Donarelli, M.; Anemone, G.; Perrozzi, F.; Picozzi, S.; Ottaviano, L. Unravelling the Role of the Central Metal Ion in the Electronic Structure of Tris(8-Hydroxyquinoline) Metal Chelates: Photoemission Spectroscopy and Hybrid Functional Calculations. J. Phys. Chem. A 2012, 116, 11548–11552. [Google Scholar] [CrossRef]
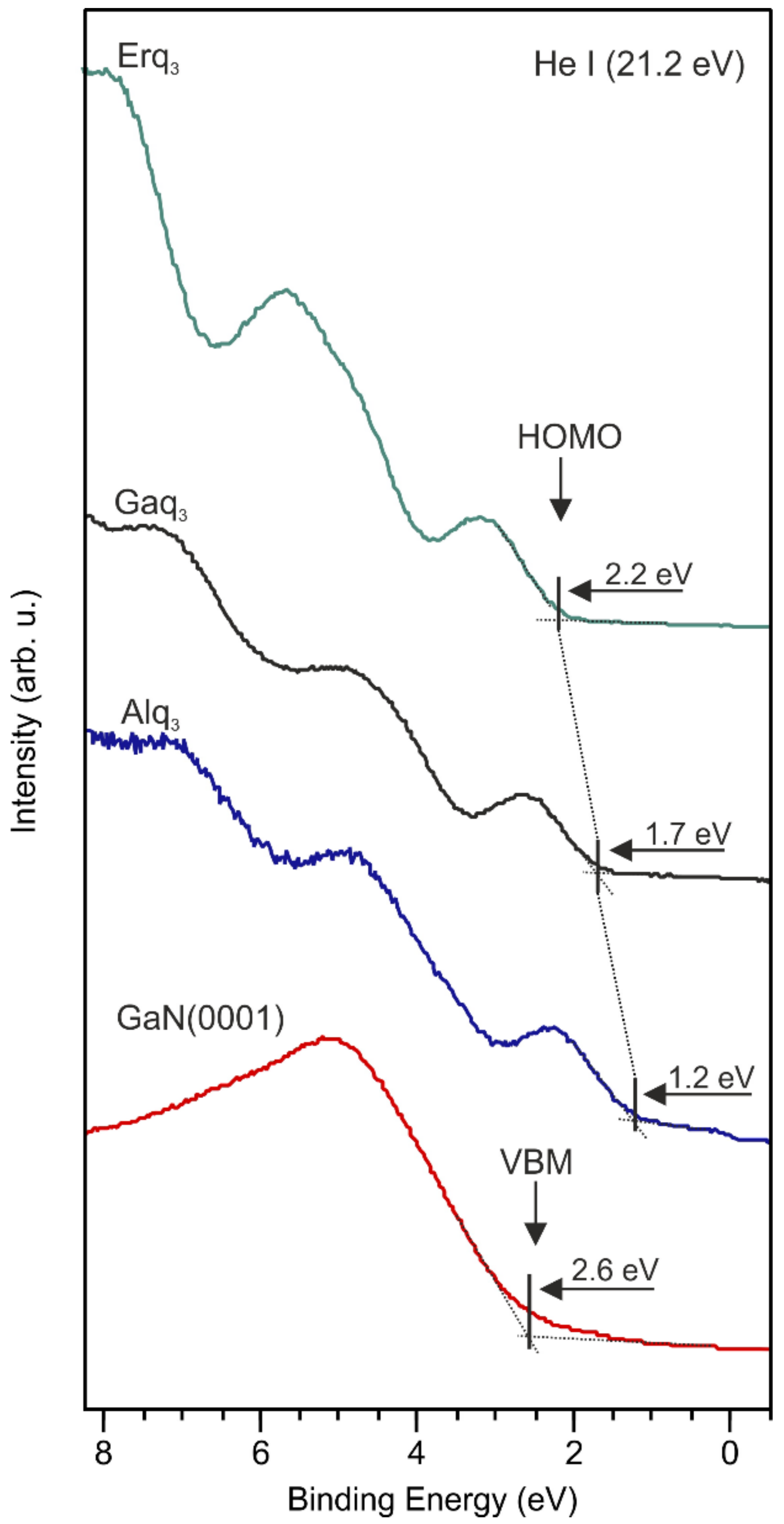
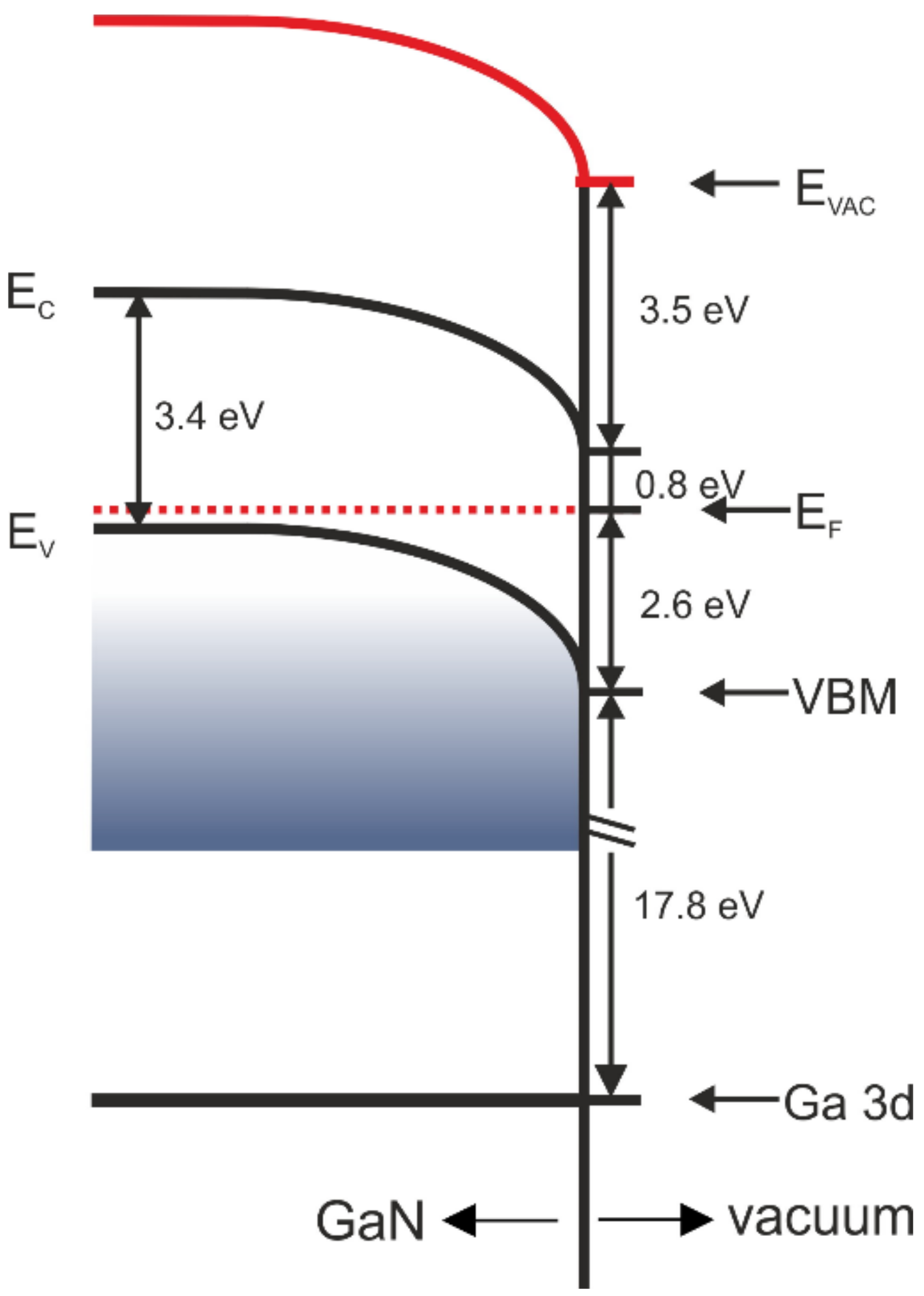
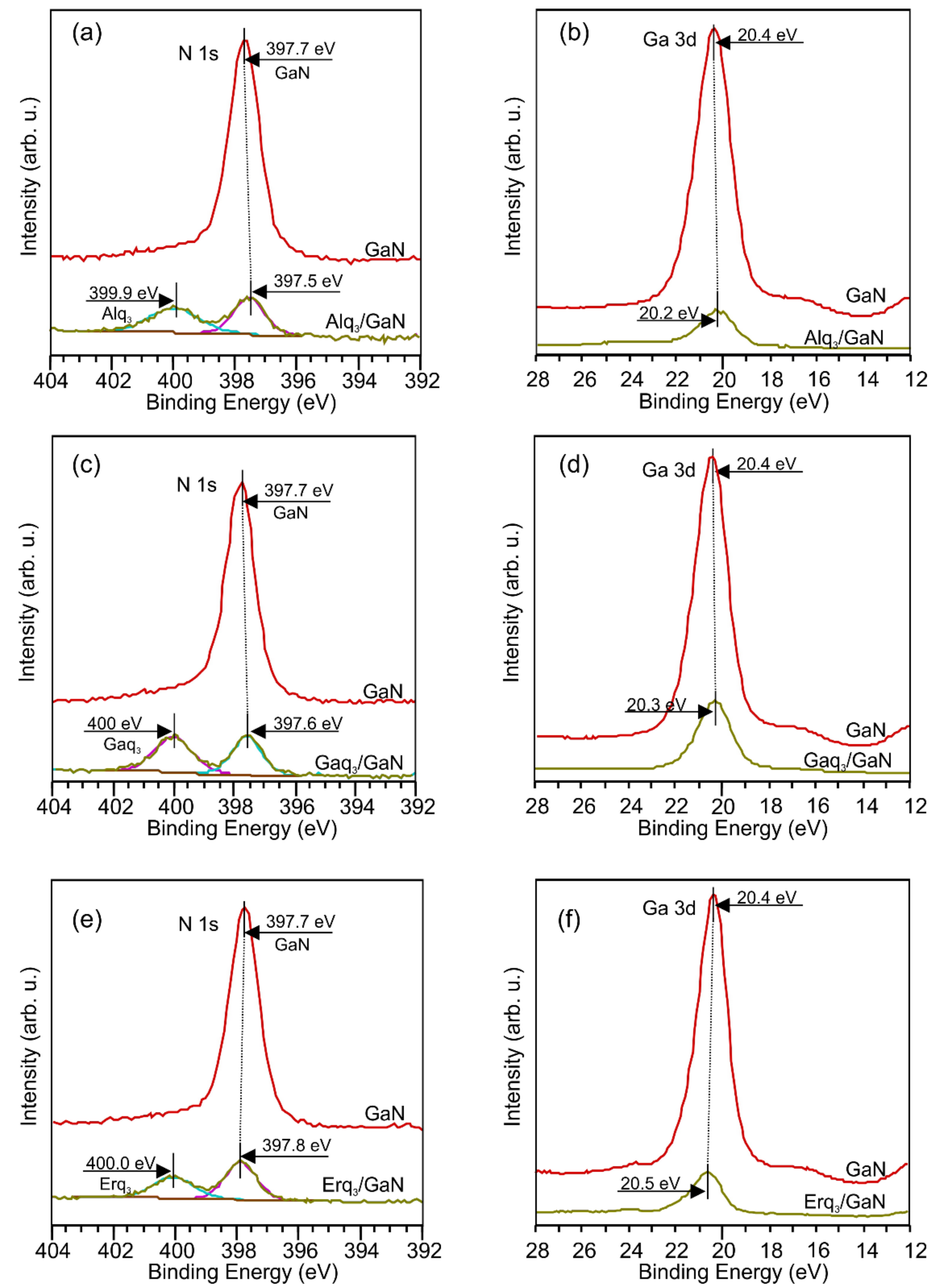
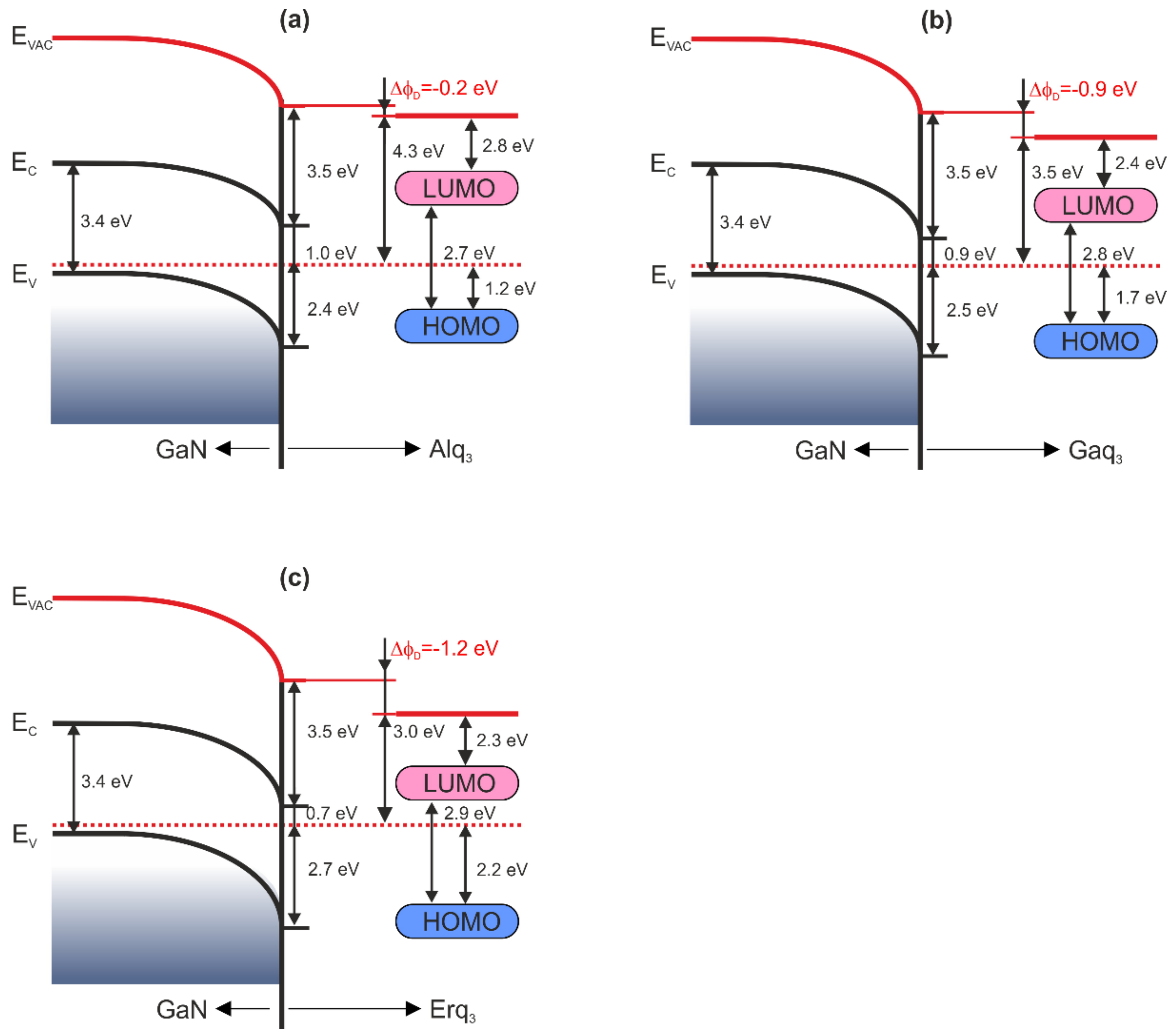
| Organic Layer | Δϕ (eV) | ΔϕBB (eV) | ΔϕD (eV) |
|---|---|---|---|
| Alq3 | 0 | −0.2 | −0.2 |
| Gaq3 | −0.8 | −0.1 | −0.9 |
| Erq3 | −1.3 | 0.1 | −1.2 |
| Organic Layer | HOMO (eV) | ΔCV (eV) | ΔEV (eV) |
|---|---|---|---|
| Alq3 | 1.2 | 0.5 | 1.2 |
| Gaq3 | 1.7 | 0.2 | 0.8 |
| Erq3 | 2.2 | 0 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grodzicki, M.; Sito, J.; Lewandków, R.; Mazur, P.; Ciszewski, A. Interfacial Polarization of Thin Alq3, Gaq3, and Erq3 Films on GaN(0001). Materials 2022, 15, 1671. https://doi.org/10.3390/ma15051671
Grodzicki M, Sito J, Lewandków R, Mazur P, Ciszewski A. Interfacial Polarization of Thin Alq3, Gaq3, and Erq3 Films on GaN(0001). Materials. 2022; 15(5):1671. https://doi.org/10.3390/ma15051671
Chicago/Turabian StyleGrodzicki, Miłosz, Jakub Sito, Rafał Lewandków, Piotr Mazur, and Antoni Ciszewski. 2022. "Interfacial Polarization of Thin Alq3, Gaq3, and Erq3 Films on GaN(0001)" Materials 15, no. 5: 1671. https://doi.org/10.3390/ma15051671






