Hydration of Hybrid Cements at Low Temperatures: A Study on Portland Cement-Blast Furnace Slag—Na2SO4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Isothermal Calorimetry
2.4. Hydration Stoppage
2.5. TGA
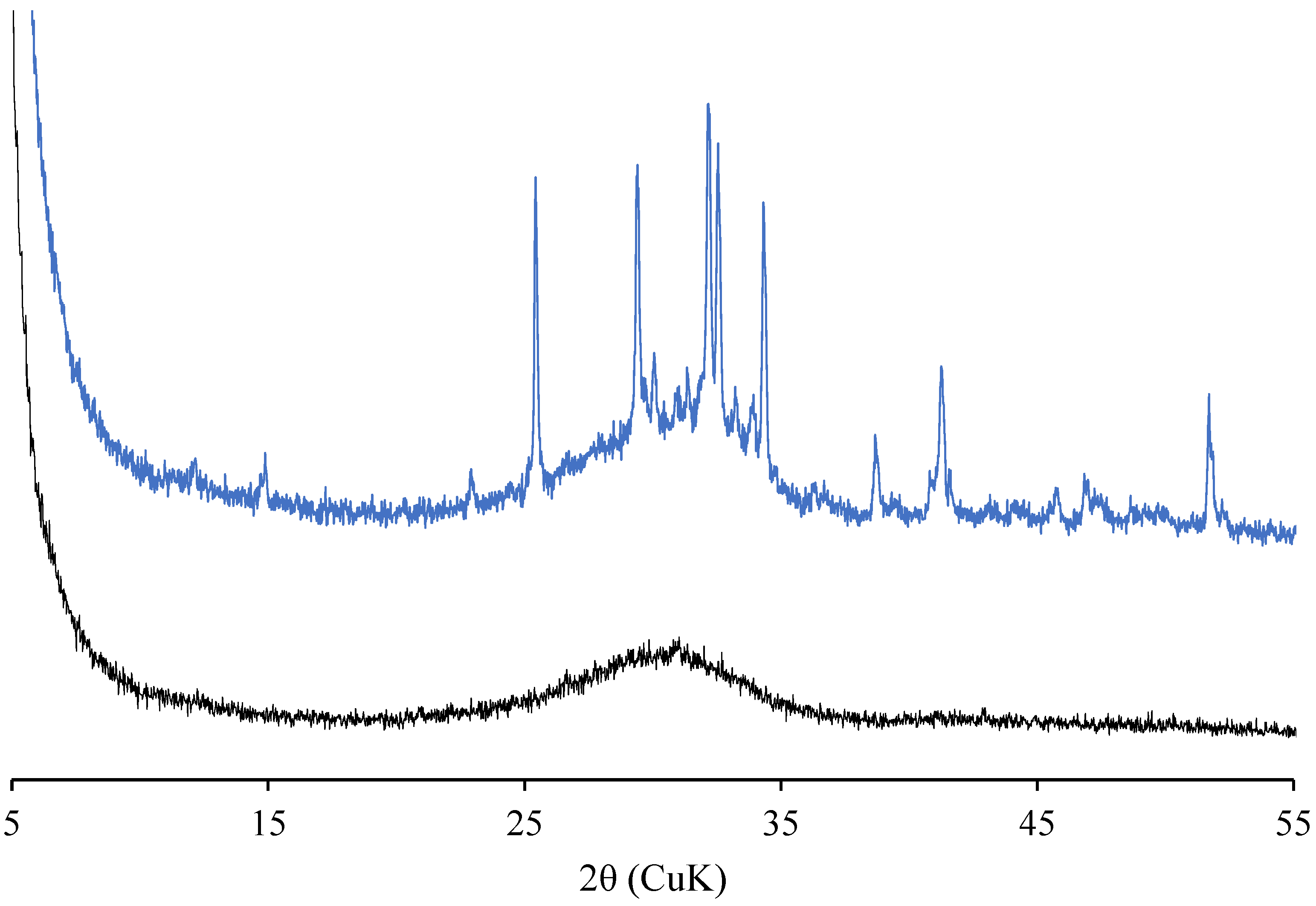
2.6. XRD Measurement
2.7. Rietveld-PONKCS Analysis
2.8. Electron Microscopy
2.9. MIP
2.10. Selective Dissolution
3. Results
3.1. Characterisation of Cement
3.2. Compressive Strength
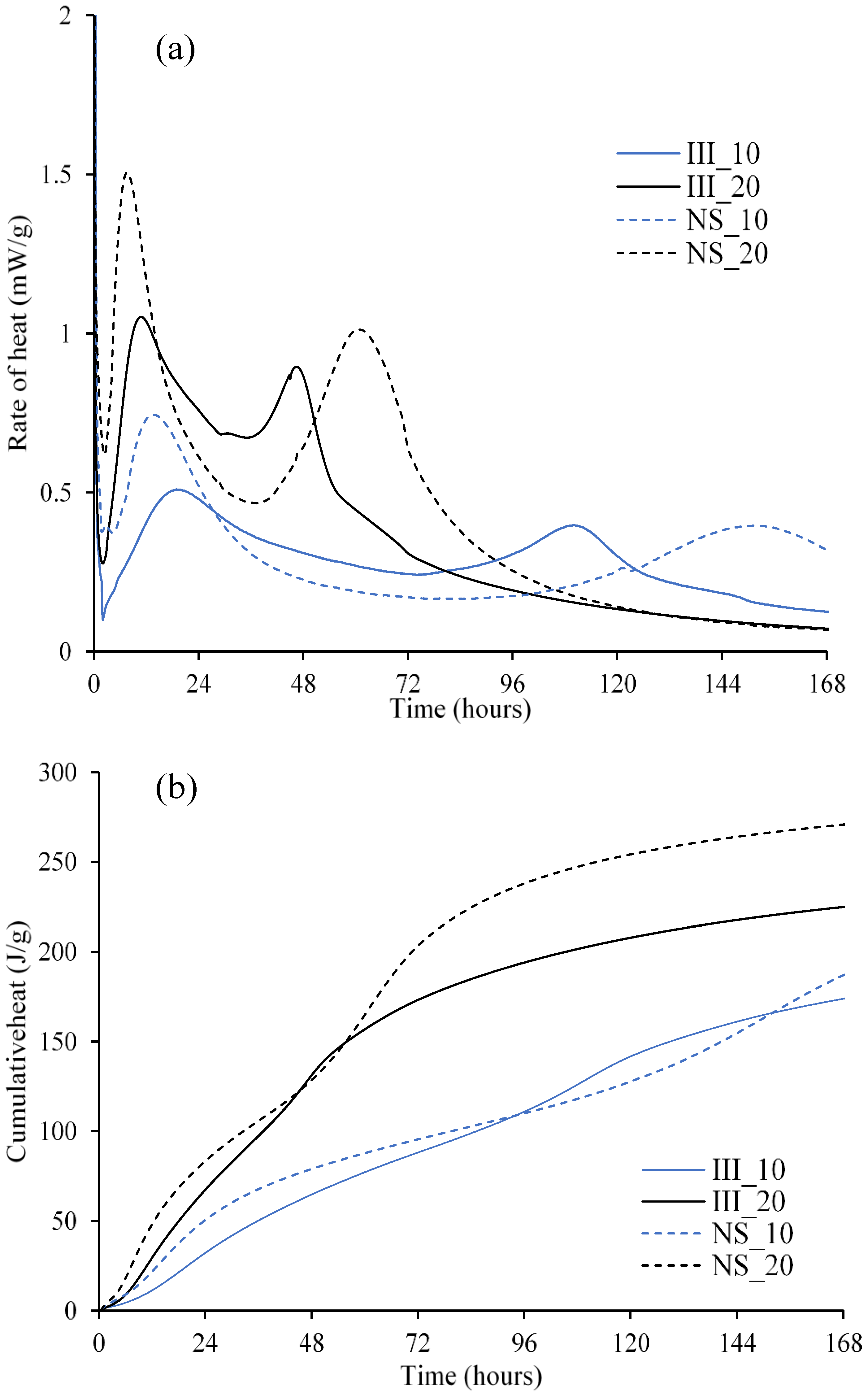
3.3. Hydration Kinetics
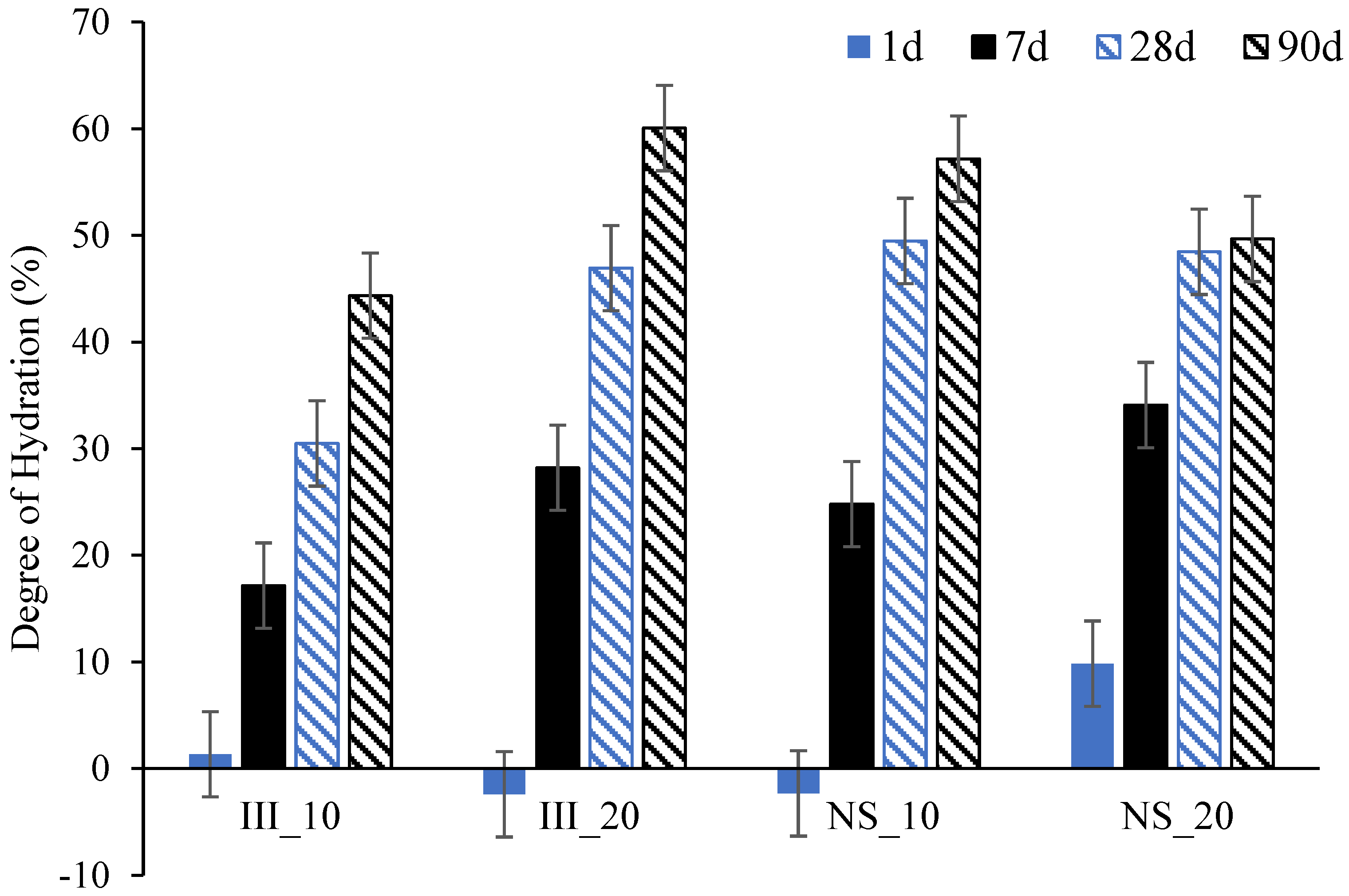
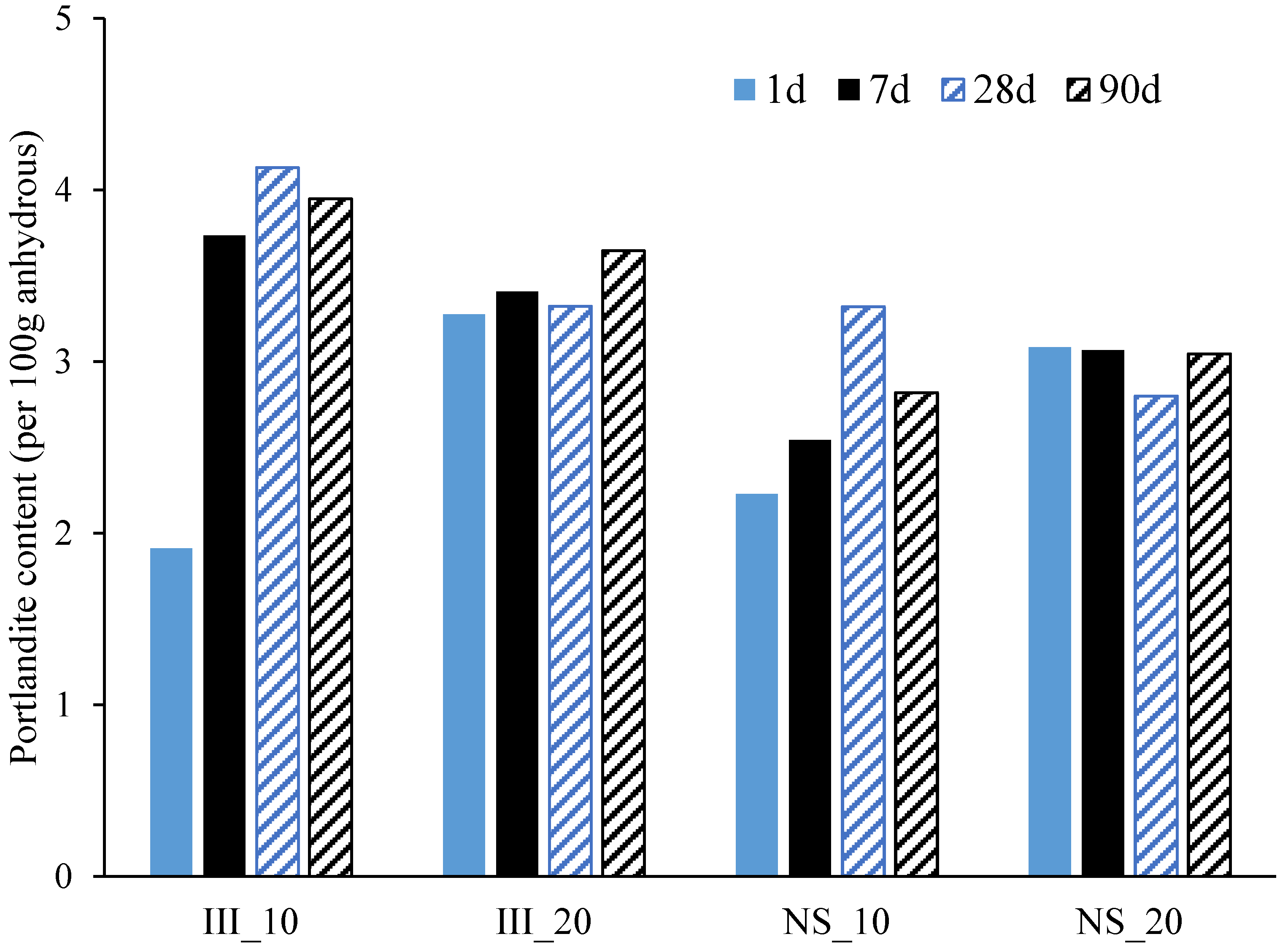
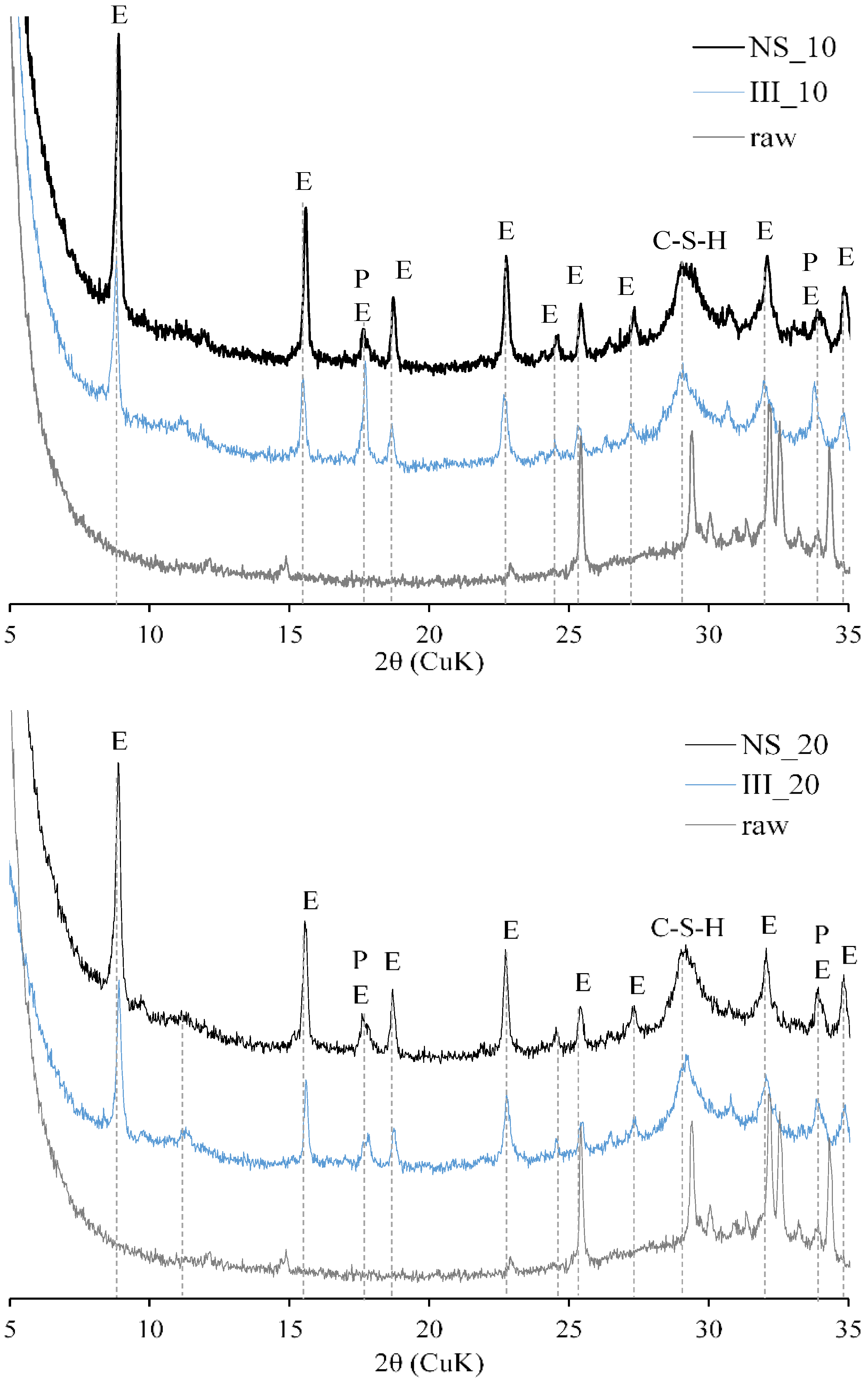
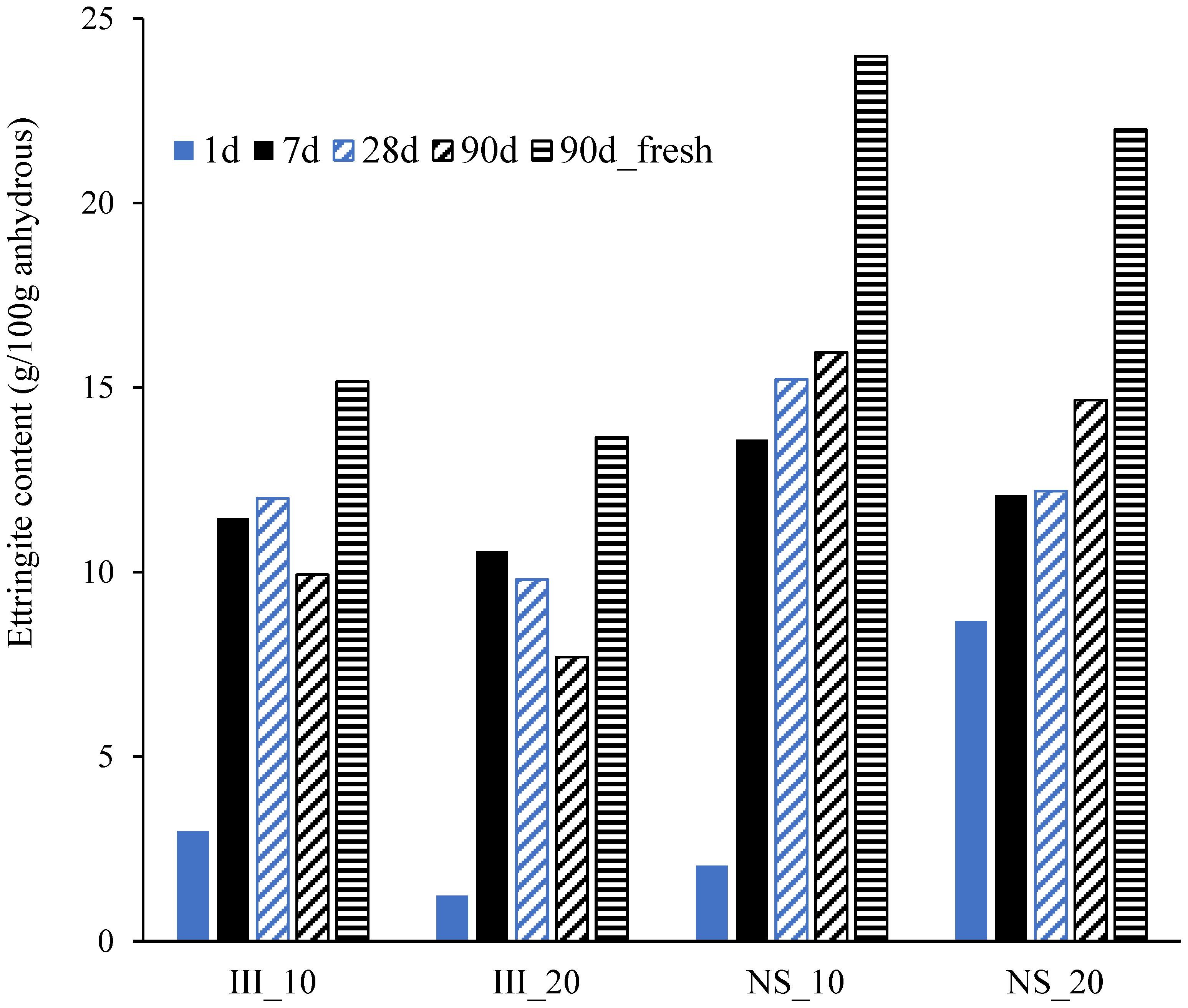
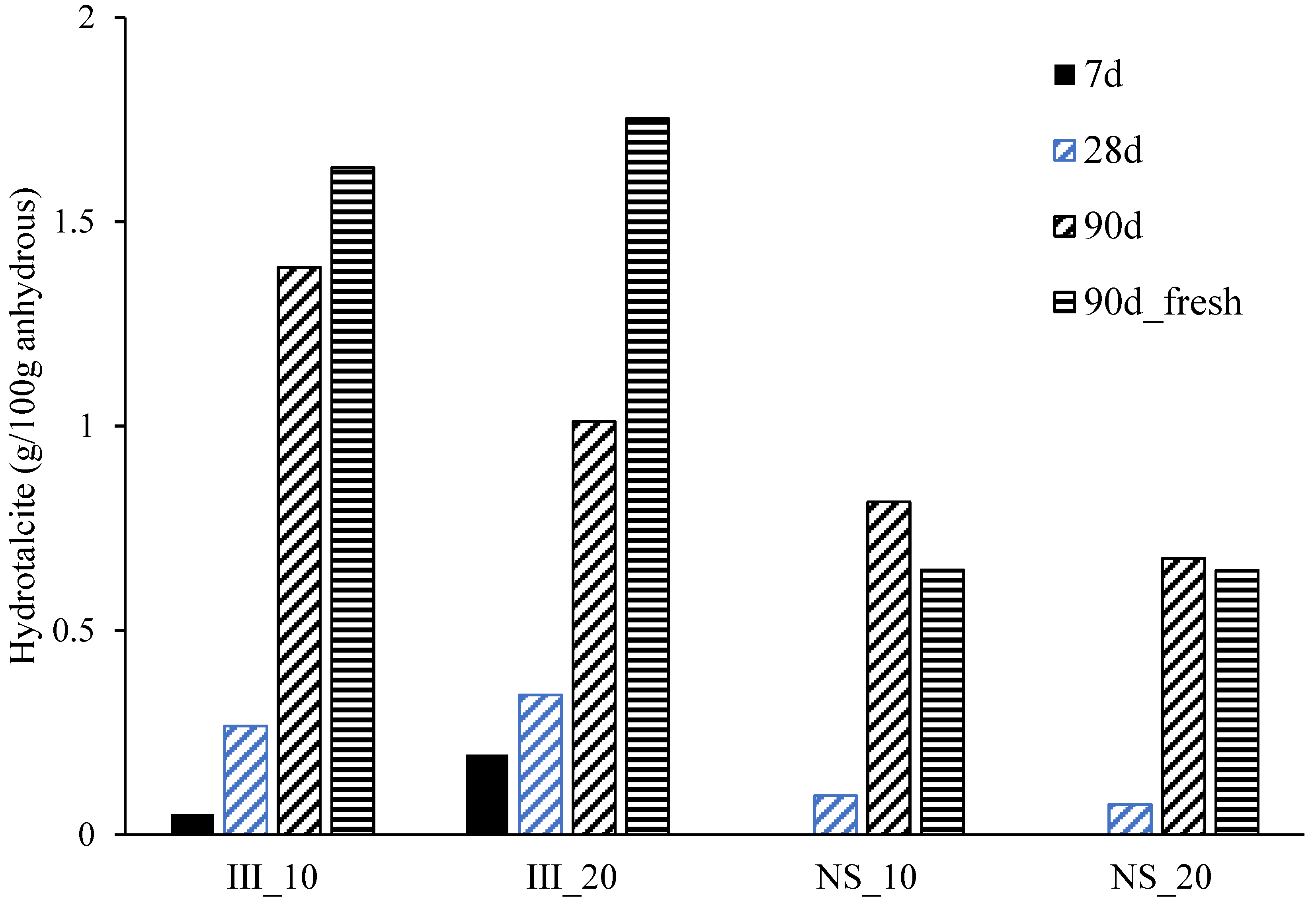
3.4. Phase Assemblage
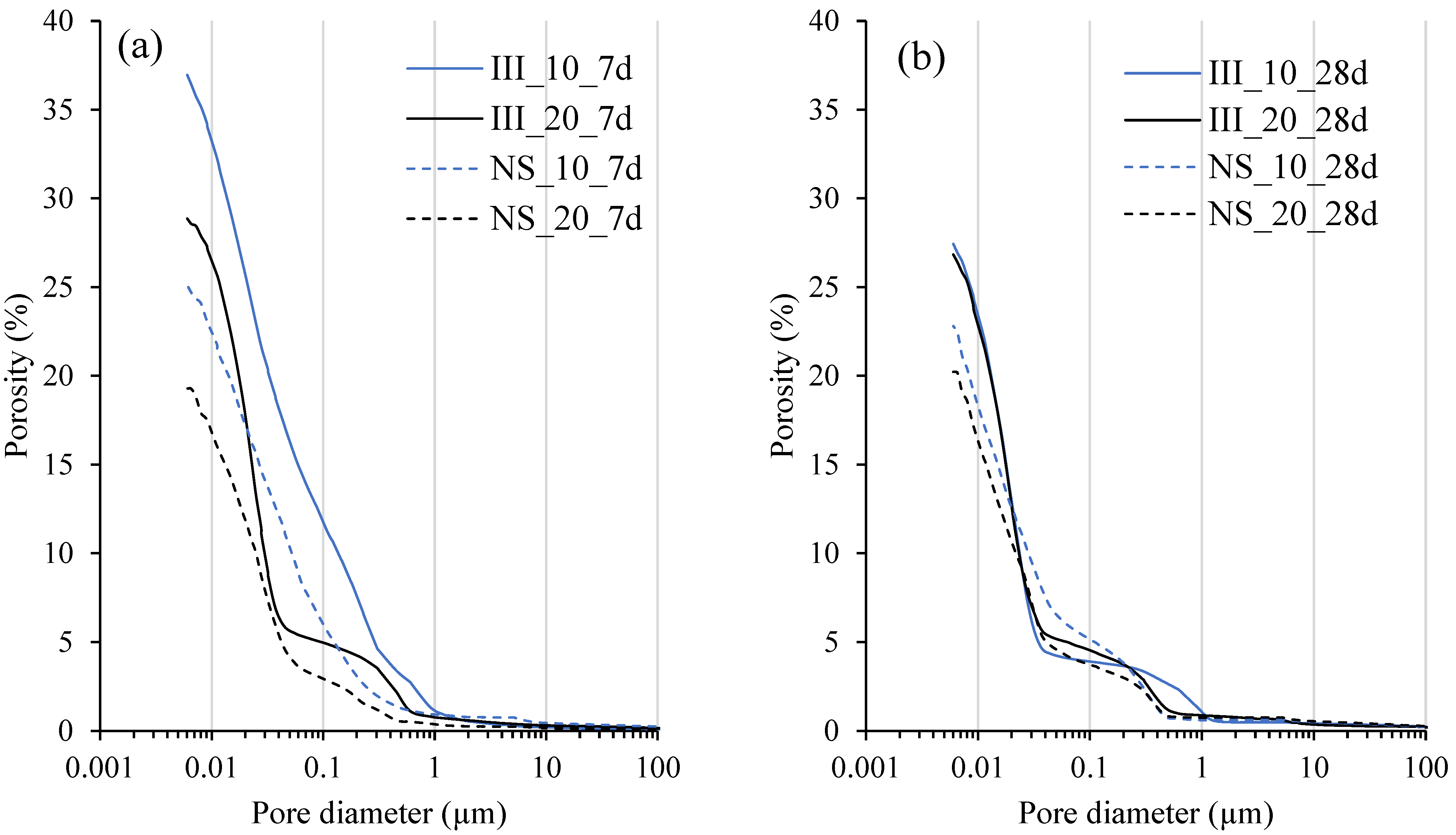
3.5. Microstructure
4. Discussion
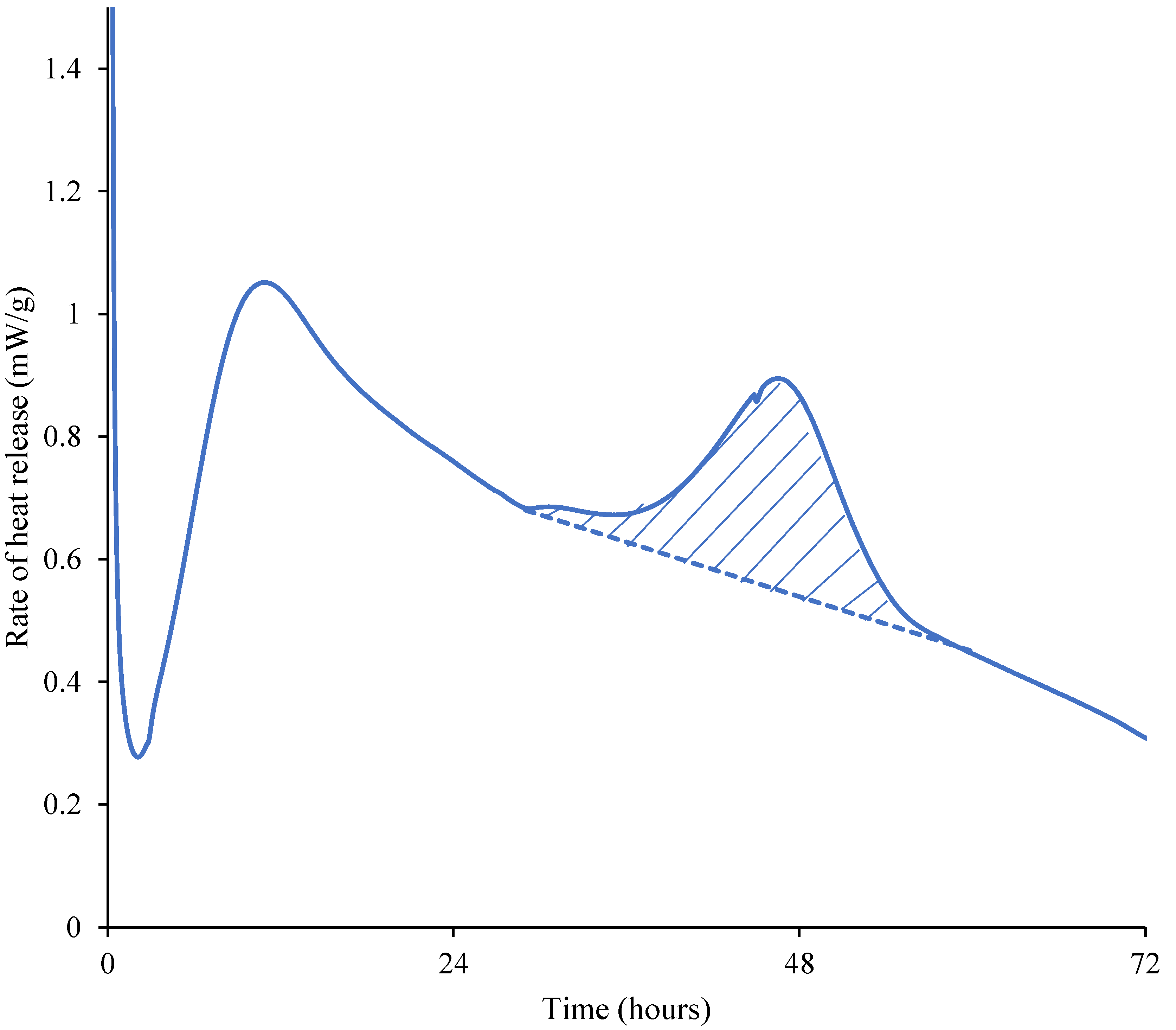
4.1. Second Peak in Isothermal Calorimetry
4.2. Enthalpy of Slag Hydration
4.3. Strength Development
5. Conclusions
- Compressive strength is enhanced with the addition of Na2SO4 at all studied ages (1, 7, 28 and 90 days) and curing temperatures.
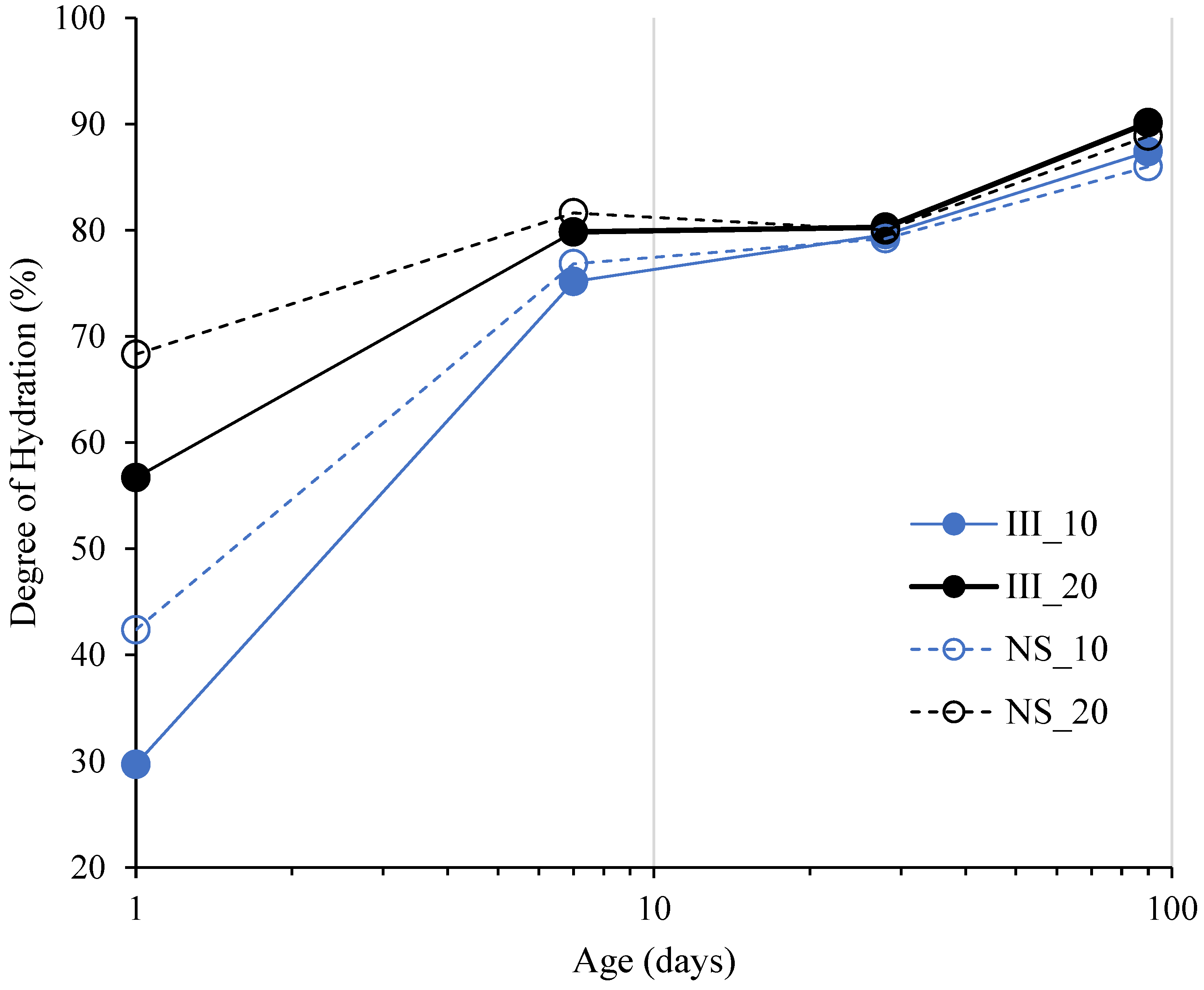
- There is a significant improvement in the degree of hydration of alite after 1 day of hydration with Na2SO4, but the overall degree of reaction of clinker is very similar at later ages.
- Addition of Na2SO4 boosts the degree of hydration of slag at early ages (up to 7 days) at both 10 and 20 °C, but in the later ages, higher degree of hydration with Na2SO4 was only found for the mixture cured at 10 °C.
- Without the addition of Na2SO4, a clear peak for C3A hydration is visible, but with its addition, although a peak for C3A is not present, there is a clear hydration peak of slag.
- The enthalpy of slag hydration was found to be 498–510 J/g-slag and 587–625 J/g-slag with and without Na2SO4 addition respectively. The higher enthalpy with Na2SO4 should be due to the higher enthalpy of formation of ettringite.
- Ettringite and C-S-H are the major hydrate phases in these systems with and without Na2SO4, while a significant increase in the amount of ettringite was found for the Na2SO4 systems.
- Microstructure is densified and the overall porosity decreases with the addition of Na2SO4
- The improvement of strength with the addition of Na2SO4 is attributed to the increased reaction of cement phases and slag at early ages, while at later ages, it is due to the higher ettringite content.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Lang, E. Blast Furnace Cements. In Structure and Performance of Cements; Bensted, J., Barnes, P., Eds.; Spon Press: London, UK, 2002; pp. 310–325. [Google Scholar]
- Regourd, M. Cements made from blast furnace slag. In Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2001; pp. 637–678. [Google Scholar]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary cementitious materials. Rev. Mineral. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Skibsted, J.; Snellings, R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, Understanding and Unlocking Supplementary Cementitious Materials. RILEM Technol. Lett. 2016, 1, 50. [Google Scholar] [CrossRef] [Green Version]
- CEMBUREAU. 2019 Activity Report, Brussels. 2019. Available online: https://cembureau.eu/media/clkdda45/activity-report-2019.pdf (accessed on 23 January 2022).
- Berodier, E.; Scrivener, K. Understanding the filler effect on the nucleation and growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Gutteridge, W.A.; Dalziel, J.A. Filler cement: The effect of the secondary component on the hydration of Portland cement. Cem. Concr. Res. 1990, 20, 778–782. [Google Scholar] [CrossRef]
- Cyr, M.; Lawrence, P.; Ringot, E. Mineral admixtures in mortars: Quantification of the physical effects of inert materials on short-term hydration. Cem. Concr. Res. 2005, 35, 719–730. [Google Scholar] [CrossRef]
- Joseph, S.; Bishnoi, S.; Van Balen, K.; Cizer, Ö. Modeling the effect of fineness and filler in early-age hydration of tricalcium silicate. J. Am. Ceram. Soc. 2017, 100, 1178–1194. [Google Scholar] [CrossRef]
- Osmanovic, Z.; Haračić, N.; Zelić, J. Properties of blastfurnace cements (CEM III/A, B, C) based on Portland cement clinker, blastfurnace slag and cement kiln dusts. Cem. Concr. Compos. 2018, 91, 189–197. [Google Scholar] [CrossRef]
- Demirboǧa, R.; Türkmen, I.; Karakoç, M.B. Relationship between ultrasonic velocity and compressive strength for high-volume mineral-admixtured concrete. Cem. Concr. Res. 2004, 34, 2329–2336. [Google Scholar] [CrossRef]
- Ganesh Babu, K.; Sree Rama Kumar, V. Efficiency of GGBS in concrete. Cem. Concr. Res. 2000, 30, 1031–1036. [Google Scholar] [CrossRef]
- Escalante, J.I.; Gómez, L.Y.; Johal, K.K.; Mendoza, G.; Mancha, H.; Méndez, J. Reactivity of blast-furnace slag in Portland cement blends hydrated under different conditions. Cem. Concr. Res. 2001, 31, 1403–1409. [Google Scholar] [CrossRef]
- Barnett, S.J.; Soutsos, M.N.; Millard, S.G.; Bungey, J.H. Strength development of mortars containing ground granulated blast-furnace slag: Effect of curing temperature and determination of apparent activation energies. Cem. Concr. Res. 2006, 36, 434–440. [Google Scholar] [CrossRef]
- Miura, T.; Iwaki, I. Strength Development of Concrete Incorporating High Levels of Ground Granulated Blast-Furnace Slag at Low Temperatures. ACI Mater. J. 2000, 97, 66–70. [Google Scholar]
- Escalante-García, J.I.; Sharp, J.H. The microstructure and mechanical properties of blended cements hydrated at various temperatures. Cem. Concr. Res. 2001, 31, 695–702. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Joseph, S.; Uppalapati, S.; Cizer, Ö. Instantaneous activation energy of alkali activated materials. RILEM Technol. Lett. 2018, 3, 121–123. [Google Scholar] [CrossRef]
- Thomas, J.J. The instantaneous apparent activation energy of cement hydration measured using a novel calorimetry-based method. J. Am. Ceram. Soc. 2012, 95, 3291–3296. [Google Scholar] [CrossRef]
- Thomas, J.J.; Ghazizadeh, S.; Masoero, E. Kinetic mechanisms and activation energies for hydration of standard and highly reactive forms of β-dicalcium silicate (C2S). Cem. Concr. Res. 2017, 100, 322–328. [Google Scholar] [CrossRef] [Green Version]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash-portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernandez-Jimenez, A.; Palomo, A. Hydration kinetics in hybrid binders: Early reaction stages. Cem. Concr. Compos. 2013, 39, 82–92. [Google Scholar] [CrossRef]
- Mota-Gasso, B.; Scrivener, T.M.K. Impact of Alkali Salts on the Kinetics and Microstructural Development of Cementitious Systems. L’Ingénieur. Ph.D. Thesis, EPFL, Lausanne, Switzerland, 2015. Available online: https://infoscience.epfl.ch/record/214540 (accessed on 23 January 2022).
- Smaoui, N.; Bérubé, M.A.; Fournier, B.; Bissonnette, B.; Durand, B. Effects of alkali addition on the mechanical properties and durability of concrete. Cem. Concr. Res. 2005, 35, 203–212. [Google Scholar] [CrossRef]
- Hubert, N.; Bouroukba, M.; Schuffenecker, L. Aqueous solution of sodium sulfate. Determination of the dissolution enthalpy at 25, 27.5 and 45 °C. Thermochim. Acta 1995, 259, 41–48. [Google Scholar] [CrossRef]
- Mota, B.; Matschei, T.; Scrivener, K. Impact of sodium gluconate on white cement-slag systems with Na2SO4. Cem. Concr. Res. 2019, 122, 59–71. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, W.; Roy, D.M. Early activation and properties of slag cement. Cem. Concr. Res. 1990, 20, 961–974. [Google Scholar] [CrossRef]
- Fu, X.; Hou, W.; Yang, C.; Li, D.; Wu, X. Studies on Portland cement with large amount of slag. Cem. Concr. Res. 2000, 30, 645–649. [Google Scholar] [CrossRef]
- Fu, J.; Jones, A.M.; Bligh, M.W.; Holt, C.; Keyte, L.M.; Moghaddam, F.; Foster, S.J.; Waite, T.D. Mechanisms of enhancement in early hydration by sodium sulfate in a slag-cement blend—Insights from pore solution chemistry. Cem. Concr. Res. 2020, 135, 106110. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, G.; Wang, C.; Zhang, Y.; Wang, P.; Yan, N. Activation effects and micro quantitative characterization of high-volume ground granulated blast furnace slag in cement-based composites. Cem. Concr. Compos. 2020, 109, 103556. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Xing, J.; Sun, X. Chemical activation of binary slag cement with low carbon footprint. J. Clean. Prod. 2020, 267, 121455. [Google Scholar] [CrossRef]
- Singh, N.; Sarita, R.; Singh, N.B. Effect of sodium sulphate on the hydration of granulated blast furnace slag blended portland cement. Indian J. Eng. Mater. Sci. 2001, 8, 110–113. [Google Scholar]
- Joseph, S.; Snellings, R.; Cizer, Ö. Activation of Portland cement blended with high volume of fly ash using Na2SO4. Cem. Concr. Compos. 2019, 104, 103417. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Lothenbach, B.; Durdzinski, P.; De Weerdt, K. Thermogravimetric analysis. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K.L., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 177–212. [Google Scholar]
- Cheary, R.W.; Coelho, A. Fundamental parameters approach to X-ray line-profile fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Snellings, R. X-ray powder diffraction applied to cement. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K.L., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 107–176. [Google Scholar]
- Scarlett, N.V.Y.; Madsen, I.C. Quantification of phases with partial or no known crystal structures. Powder Diffr. 2006, 21, 278–284. [Google Scholar] [CrossRef]
- Snellings, R.; Salze, A.; Scrivener, K.L. Use of X-ray diffraction to quantify amorphous supplementary cementitious materials in anhydrous and hydrated blended cements. Cem. Concr. Res. 2014, 64, 89–98. [Google Scholar] [CrossRef]
- Jansen, D.; Goetz-Neunhoeffer, F.; Stabler, C.; Neubauer, J. A remastered external standard method applied to the quantification of early OPC hydration. Cem. Concr. Res. 2011, 41, 602–608. [Google Scholar] [CrossRef]
- Villagrán-Zaccardi, Y.A.; Vollpracht, A.; Gruyaert, E.; De Belie, N. Recommendation of RILEM TC 238-SCM: Determination of the degree of reaction of siliceous fly ash and slag in hydrated cement paste by the selective dissolution method. Mater. Struct. Constr. 2018, 51, 27. [Google Scholar] [CrossRef]
- Snellings, R.; Bazzoni, A.; Scrivener, K. The existence of amorphous phase in Portland cements: Physical factors affecting Rietveld quantitative phase analysis. Cem. Concr. Res. 2014, 59, 139–146. [Google Scholar] [CrossRef]
- Blotevogel, S.; Ehrenberg, A.; Steger, L.; Doussang, L.; Kaknics, J.; Patapy, C.; Cyr, M. Ability of the R3 test to evaluate differences in early age reactivity of 16 industrial ground granulated blast furnace slags (GGBS). Cem. Concr. Res. 2020, 130, 105998. [Google Scholar] [CrossRef]
- Qian, J.; Shi, C.; Wang, Z. Activation of blended cements containing fly ash. Cem. Concr. Res. 2001, 31, 1121–1127. [Google Scholar] [CrossRef]
- Velandia, D.F.; Lynsdale, C.J.; Provis, J.L.; Ramirez, F.; Gomez, A.C. Evaluation of activated high volume fly ash systems using Na2SO4, lime and quicklime in mortars with high loss on ignition fly ashes. Constr. Build. Mater. 2016, 128, 248–255. [Google Scholar] [CrossRef]
- Fu, J.; Bligh, M.W.; Shikhov, I.; Jones, A.M.; Holt, C.; Keyte, L.M.; Moghaddam, F.; Arns, C.H.; Foster, S.J.; Waite, T.D. A microstructural investigation of a Na2SO4 activated cement-slag blend. Cem. Concr. Res. 2021, 150, 106609. [Google Scholar] [CrossRef]
- Joseph, S.; Skibsted, J.; Cizer, Ö. Hydration of polyphase Ca3SiO5-Ca3Al2O6 in the presence of gypsum and Na2SO4. J. Am. Ceram. Soc. 2020, 103, 6461–6474. [Google Scholar] [CrossRef]
- Mota, B.; Matschei, T.; Scrivener, K. Impact of NaOH and Na2SO4 on the kinetics and microstructural development of white cement hydration. Cem. Concr. Res. 2018, 108, 172–185. [Google Scholar] [CrossRef]
- Collier, N.C. Transition and decomposition temperatures of cement phases—A collection of thermal analysis data. Ceram. Silik. 2016, 60, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Le Bescop, P.; Moranville, M. Expansion mechanism associated with the secondary formation of the U phase in cement-based systems containing high amounts of Na2SO4. Cem. Concr. Res. 1996, 26, 195–201. [Google Scholar] [CrossRef]
- Li, G.; Le Bescop, P.; Moranville, M. The U phase formation in cement-based systems containing high amounts of Na2SO4. Cem. Concr. Res. 1996, 26, 27–33. [Google Scholar] [CrossRef]
- Snellings, R.; Chwast, J.; Cizer, Ö.; De Belie, N.; Dhandapani, Y.; Durdzinski, P.; Elsen, J.; Haufe, J.; Hooton, D.; Patapy, C.; et al. Report of TC 238-SCM: Hydration stoppage methods for phase assemblage studies of blended cements—Results of a round robin test. Mater. Struct. Constr. 2018, 51, 111. [Google Scholar] [CrossRef]
- Knapen, E.; Cizer, O.; Van Balen, K.; Van Gemert, D. Effect of free water removal from early-age hydrated cement pastes on thermal analysis. Constr. Build. Mater. 2009, 23, 3431–3438. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Critical examination of drying damage to cement pastes. Adv. Cem. Res. 2000, 12, 79–88. [Google Scholar] [CrossRef]
- Joseph, S.; Skibsted, J.; Cizer, Ö. A quantitative study of the C3A hydration. Cem. Concr. Res. 2019, 115, 145–159. [Google Scholar] [CrossRef]
- Hadley, D. The Nature of the Paste-Aggregate Interface; Purdue University: West Lafayette, IN, USA, 1972. [Google Scholar]
- Kjellsen, K.O.; Lagerblad, B. Microstructure of tricalcium silicate and Portland cement systems at middle periods of hydration-development of Hadley grains. Cem. Concr. Res. 2007, 37, 13–20. [Google Scholar] [CrossRef]
- Richardson, I.G.; Wilding, C.R.; Dickson, M.J. The hydration of blastfurnace slag cements. Adv. Cem. Res. 1989, 2, 147–157. [Google Scholar] [CrossRef]
- Minard, H.; Garrault, S.; Regnaud, L.; Nonat, A. Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem. Concr. Res. 2007, 37, 1418–1426. [Google Scholar] [CrossRef]
- Quennoz, K.L. Scrivener, Hydration of C 3A-gypsum systems. Cem. Concr. Res. 2012, 42, 1032–1041. [Google Scholar] [CrossRef]
- Kocaba, V.; Gallucci, E.; Scrivener, K.L. Methods for determination of degree of reaction of slag in blended cement pastes. Cem. Concr. Res. 2012, 42, 511–525. [Google Scholar] [CrossRef]
- Maekawa, K.; Ishida, T.; Kishi, T. Multi-Scale Modelling of Structural Concrete; Taylor & Francis: London, UK, 2009. [Google Scholar]
- Han, F.; He, X.; Zhang, Z.; Liu, J. Hydration heat of slag or fly ash in the composite binder at different temperatures. Thermochim. Acta 2017, 655, 202–210. [Google Scholar] [CrossRef]



| MIX ID | CEM III (g) | Sand (g) | Solution (g) | [Na2SO4] | w/c Ratio | S/c Ratio | Mortar Flow (mm) |
|---|---|---|---|---|---|---|---|
| III | 450 | 1350 | 225 | - | 0.5 | 0.5 | 130 ± 2 |
| NS | 450 | 1350 | 249 | 0.75 M | 0.5 | 0.55 | 146 ± 3 |
| Chemical Composition | Mineralogical Composition | Physical Properties | |||
|---|---|---|---|---|---|
| CaO | 48.5 | C3S | 18.0 | Blaine | 5024 cm2/g |
| SiO2 | 29.4 | C2S | 2.3 | Specific gravity | 2.99 g/cm3 |
| Al2O3 | 8.4 | C3A | 2.0 | ||
| MgO | 5.4 | C4AF | 2.0 | Selective dissolution residue | 69.4% |
| SO3 | 5.2 | Bassanite | 1.2 | ||
| Fe2O3 | 1.0 | Anhydrite | 1.9 | ||
| K2O | 0.6 | Quartz | 0.3 | Particle size | |
| TiO2 | 0.6 | Calcite | 1.0 | d10 (µm) | 1.4 |
| Na2O | 0.3 | Arcanite | 1.2 | d50 (µm) | 8.8 |
| MnO | 0.2 | Syngenite | 0.9 | d90 (µm) | 21.0 |
| P2O5 | 0.1 | Amorphous | 69.1 | ||
| Elemental Ratios (wt%) | Oxide Composition (wt%) | |||||
|---|---|---|---|---|---|---|
| Si/Ca | Al/Si | Mg/Si | CaO | SiO2 | Al2O3 | MgO |
| 0.52 ± 0.07 | 0.35 ± 0.03 | 0.26 ± 0.04 | 43.3 ± 3.5 | 34.5 ± 3.7 | 10.5 ± 1.0 | 6.6 ± 1.3 |
| Age (Days) | C3S | C2S | C3A | C4AF | |
|---|---|---|---|---|---|
| CEM III/B 42.5N | 0 | 0 (18.0) | 0 (2.3) | 0 (2.0) | 0 (2.0) |
| III_10 | 1 | 37.4 (11.3) | 18.4 (1.9) | 10.6 (1.8) | −7.7 (2.2) |
| 7 | 92.5 (1.3) | −11.9 (2.6) | 75.0 (0.5) | 19.3 (1.6) | |
| 28 | 93.8 (1.1) | 4.9 (2.2) | 100 (0) | 17.1 (1.7) | |
| 90 | 96.3 (0.7) | 28.4 (1.6) | 100 (0) | 62.7 (0.7) | |
| III_20 | 1 | 70.3 (5.3) | 0.6 (2.3) | 17.0 (1.7) | 38.6 (1.2) |
| 7 | 94.7 (1.0) | −14.7 (2.6) | 94.4 (0.1) | 40.4 (1.2) | |
| 28 | 93.3 (1.2) | −13.3 (2.6) | 100 (0) | 51.0 (1.0) | |
| 90 | 98.0 (0.7) | 35.7 (1.5) | 100 (0) | 71.6 (0.6) | |
| NS_10 | 1 | 58.7 (7.4) | −13.9 (2.6) | 30.2 (1.4) | 6.9 (1.9) |
| 7 | 95.5 (0.8) | −19.0 (2.7) | 78.5 (0.4) | 17.6 (1.6) | |
| 28 | 96.8 (0.6) | −2.0 (2.3) | 65.6 (0.7) | 27.5 (1.5) | |
| 90 | 97.8 (0.4) | 29.8 (1.6) | 80.2 (0.4) | 49.8 (1.0) | |
| NS_20 | 1 | 83.0 (3.1) | −4.4 (2.4) | 53.1 (0.9) | 35.1 (1.3) |
| 7 | 95.1 (0.9) | 2.5 (2.2) | 87.6 (0.2) | 45.0 (1.1) | |
| 28 | 95.2 (0.9) | 4.6 (2.2) | 76.1 (0.5) | 33.6 (1.3) | |
| 90 | 96.3 (0.7) | 42.8 (1.3) | 100 (0) | 63.9 (0.7) |
| III_10 | III_20 | NS_10 | NS_20 | |
|---|---|---|---|---|
| Si/Ca | 0.54 ± 0.08 | 0.54 ± 0.13 | 0.59 ± 0.06 | 0.59 ± 0.07 |
| Al/Ca | 0.17 ± 0.03 | 0.18 ± 0.05 | 0.17 ± 0.02 | 0.17 ± 0.03 |
| S/Ca | 0.08 ± 0.03 | 0.06 ± 0.03 | 0.11 ± 0.03 | 0.11 ± 0.02 |
| Al/Si | 0.31 ± 0.05 | 0.32 ± 0.06 | 0.31 ± 0.05 | 0.30 ± 0.05 |
| Mg/Si | 0.18 ± 0.07 | 0.16 ± 0.09 | 0.23 ± 0.09 | 0.23 ± 0.12 |
| Na/Ca | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.12 ± 0.05 | 0.08 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, S.; Cizer, Ö. Hydration of Hybrid Cements at Low Temperatures: A Study on Portland Cement-Blast Furnace Slag—Na2SO4. Materials 2022, 15, 1914. https://doi.org/10.3390/ma15051914
Joseph S, Cizer Ö. Hydration of Hybrid Cements at Low Temperatures: A Study on Portland Cement-Blast Furnace Slag—Na2SO4. Materials. 2022; 15(5):1914. https://doi.org/10.3390/ma15051914
Chicago/Turabian StyleJoseph, Shiju, and Özlem Cizer. 2022. "Hydration of Hybrid Cements at Low Temperatures: A Study on Portland Cement-Blast Furnace Slag—Na2SO4" Materials 15, no. 5: 1914. https://doi.org/10.3390/ma15051914
APA StyleJoseph, S., & Cizer, Ö. (2022). Hydration of Hybrid Cements at Low Temperatures: A Study on Portland Cement-Blast Furnace Slag—Na2SO4. Materials, 15(5), 1914. https://doi.org/10.3390/ma15051914






