Carbonation Curing on Magnetically Separated Steel Slag for the Preparation of Artificial Reefs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
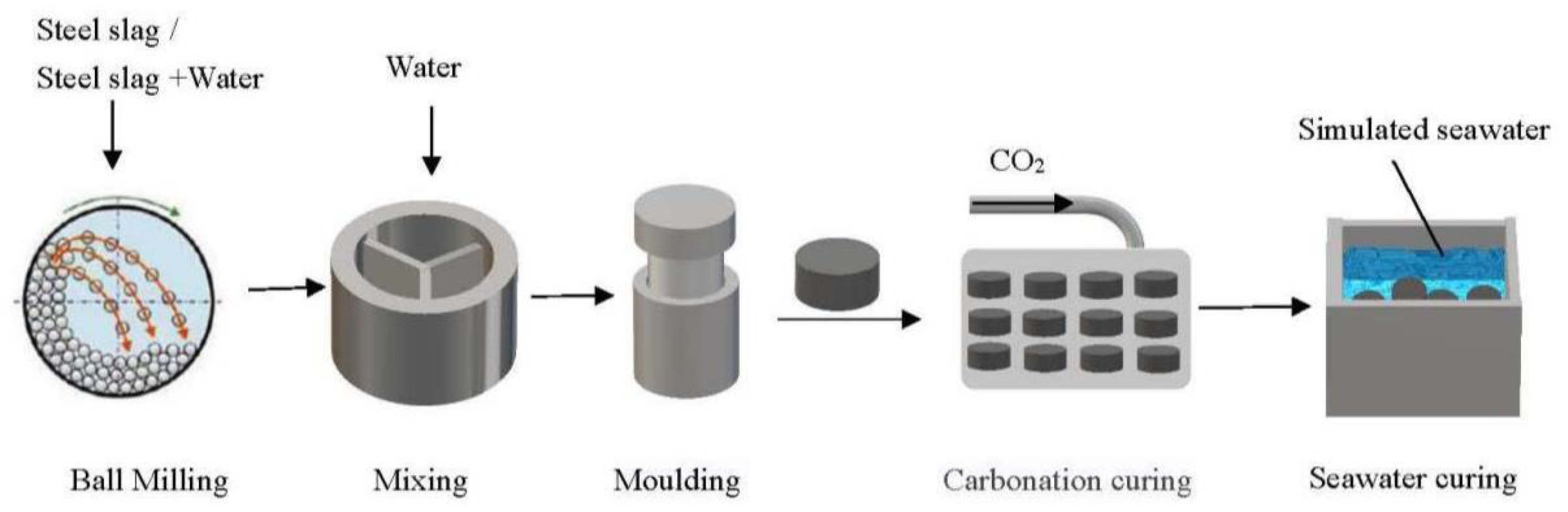
2.2. Experimental Method
2.2.1. Mixing and Moulding
2.2.2. Carbonization Curing
2.2.3. Seawater Curing
2.2.4. Material Characterization
2.2.5. CO2 Uptake Calculation
3. Results and Discussion
3.1. The Effect of Carbonation Time on the Compressive Strength and CO2 Uptake of Compacts
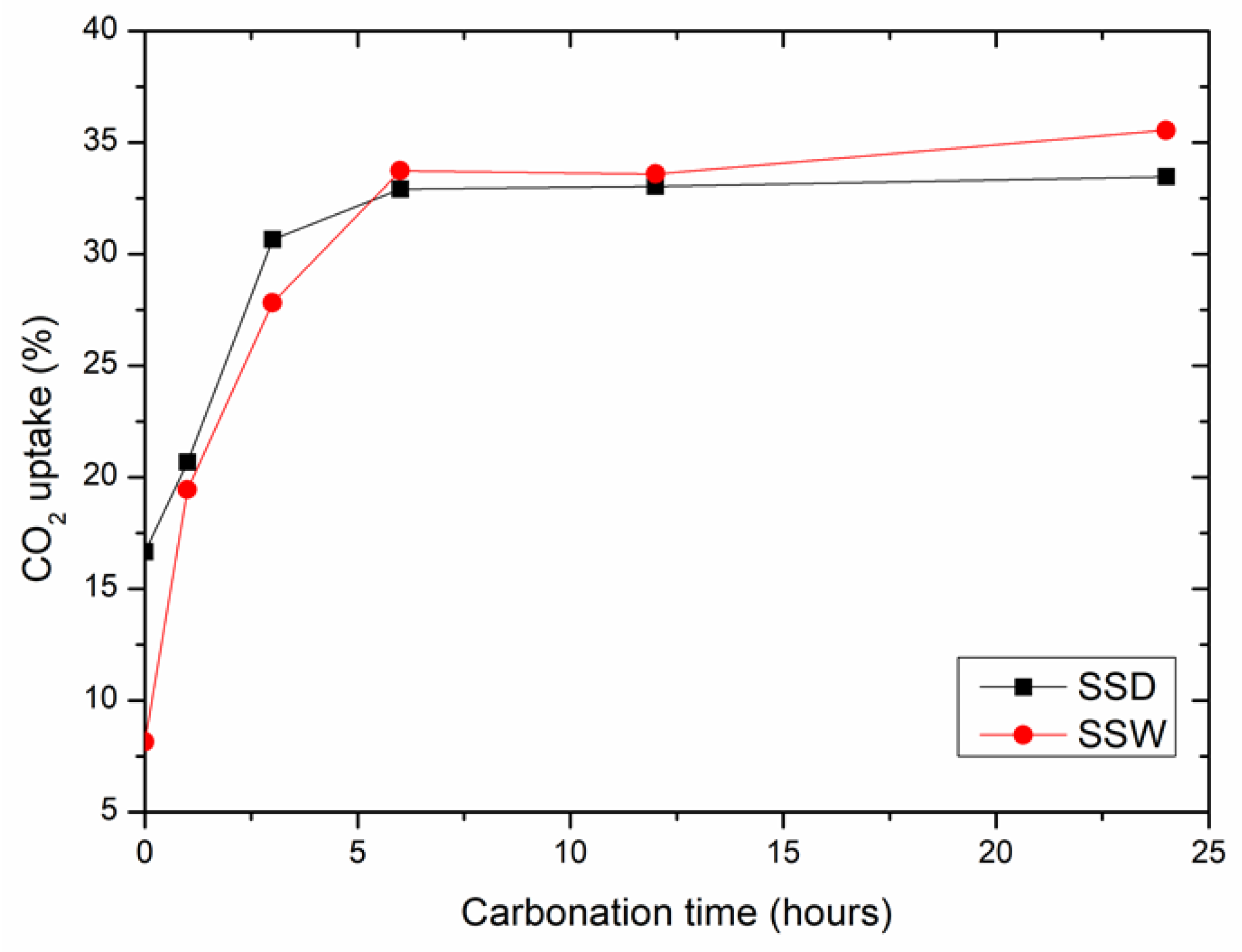
3.1.1. The Changes in Carbonation Conversion
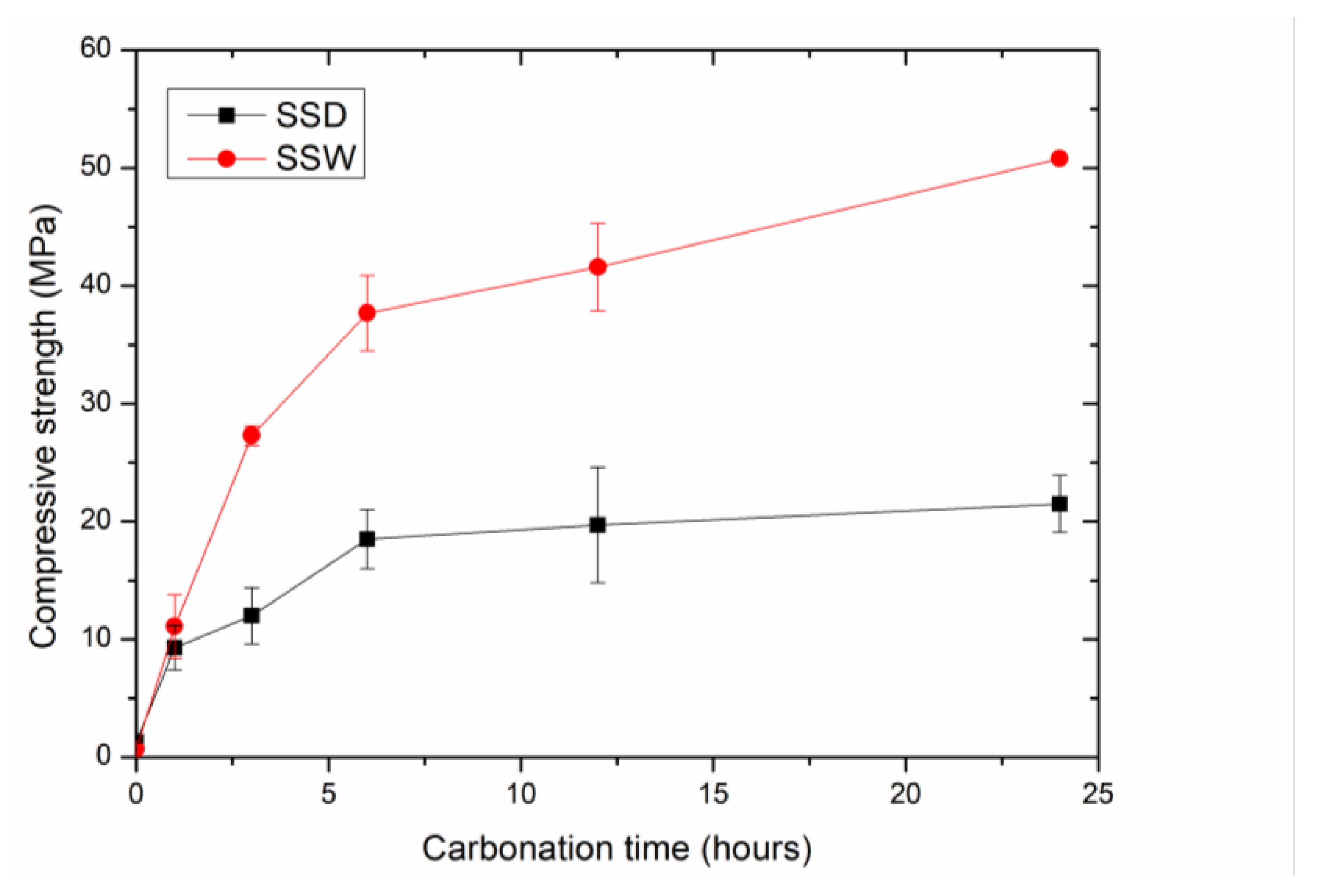
3.1.2. The Changes in Compressive Strength
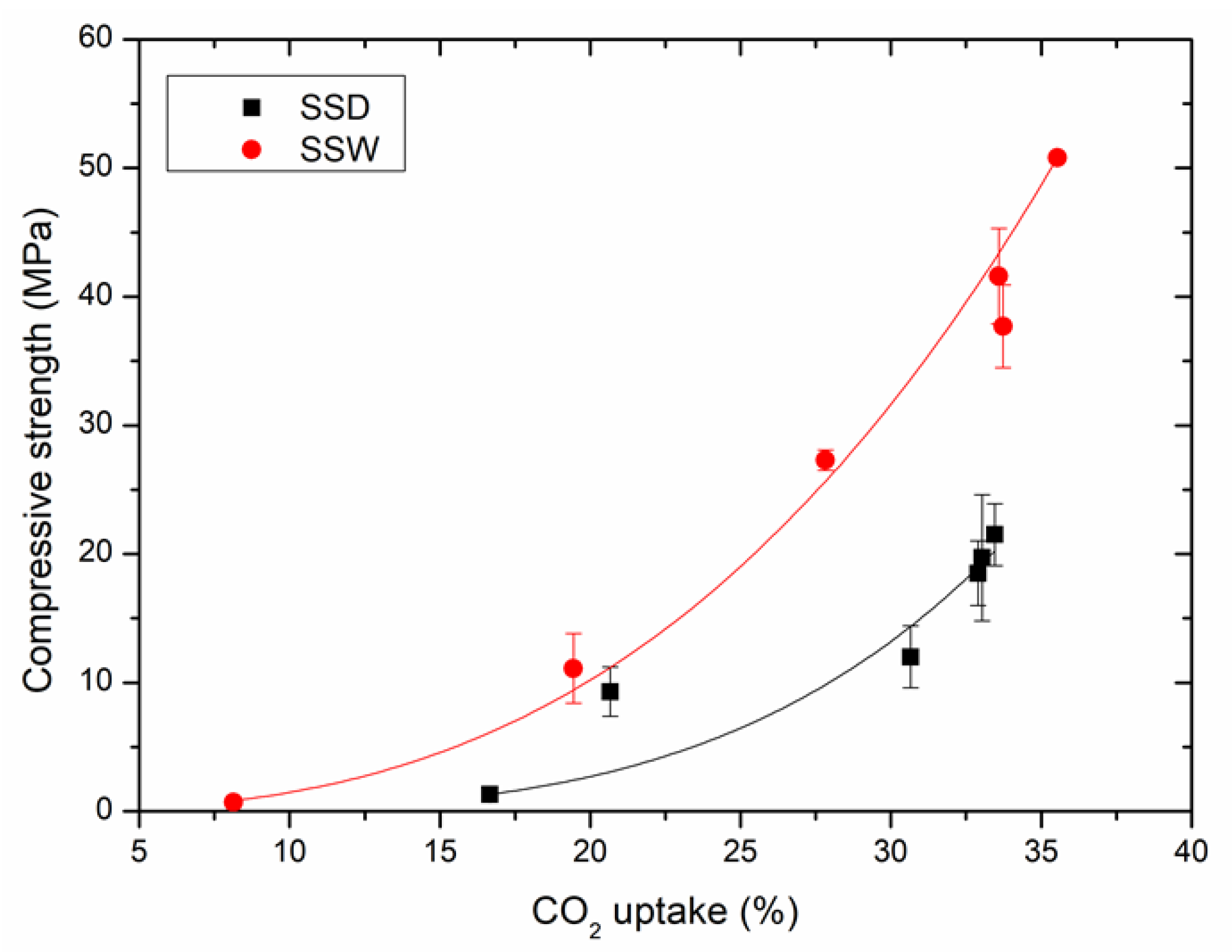
3.1.3. Relationship between the Compressive Strength and Carbonation Conversion
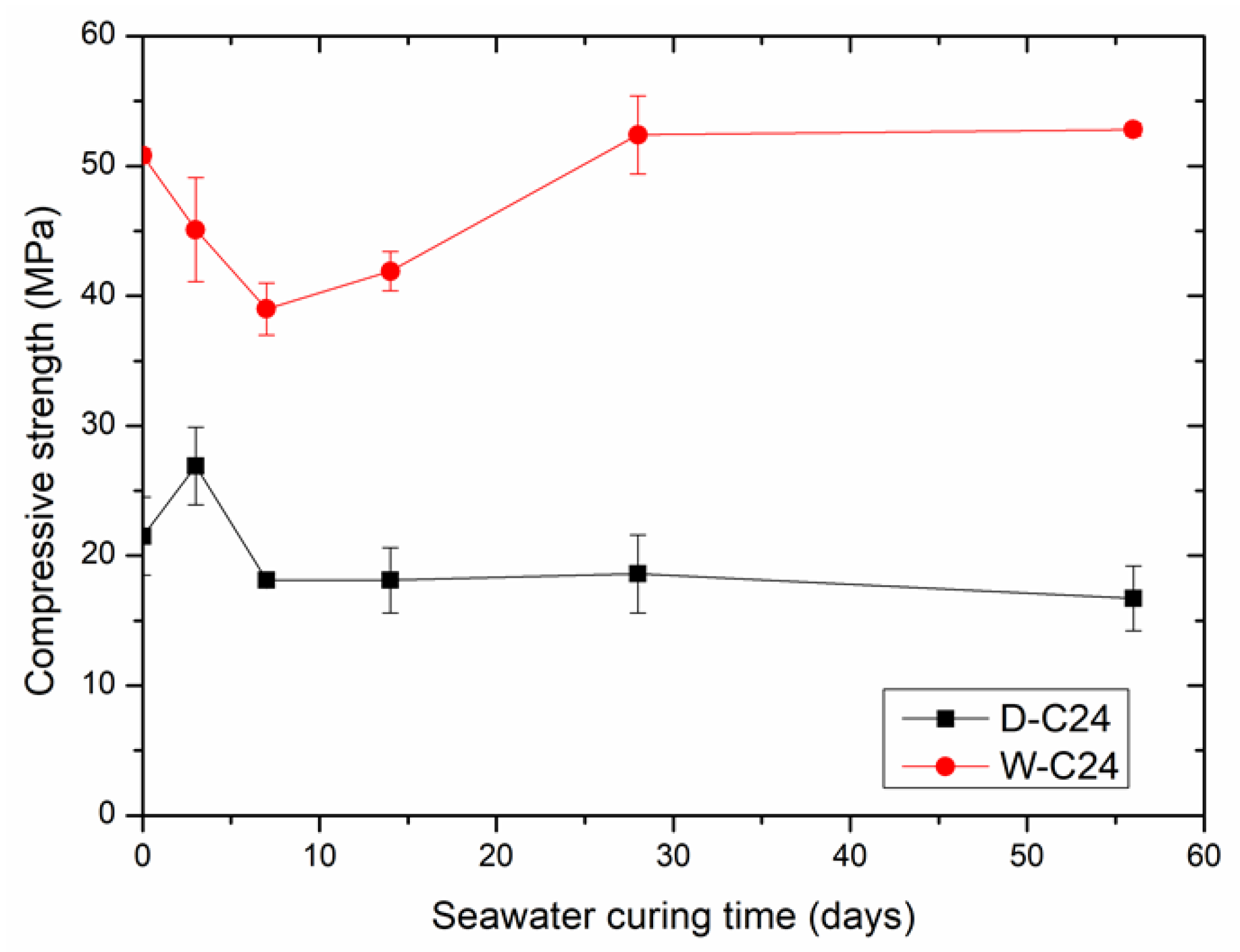
3.2. The Effect of Seawater Curing Time on the Compressive Strength of Carbonated Compacts
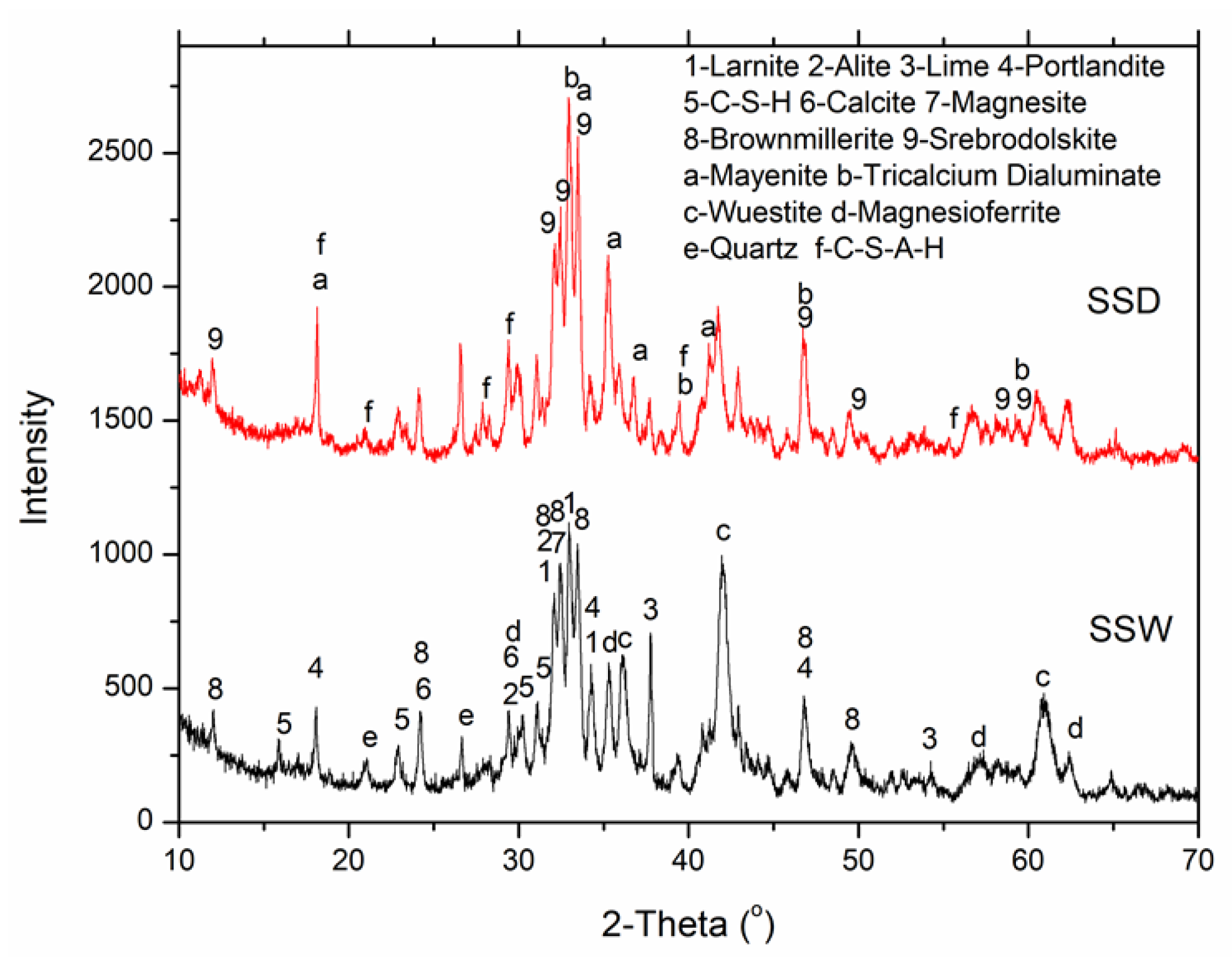
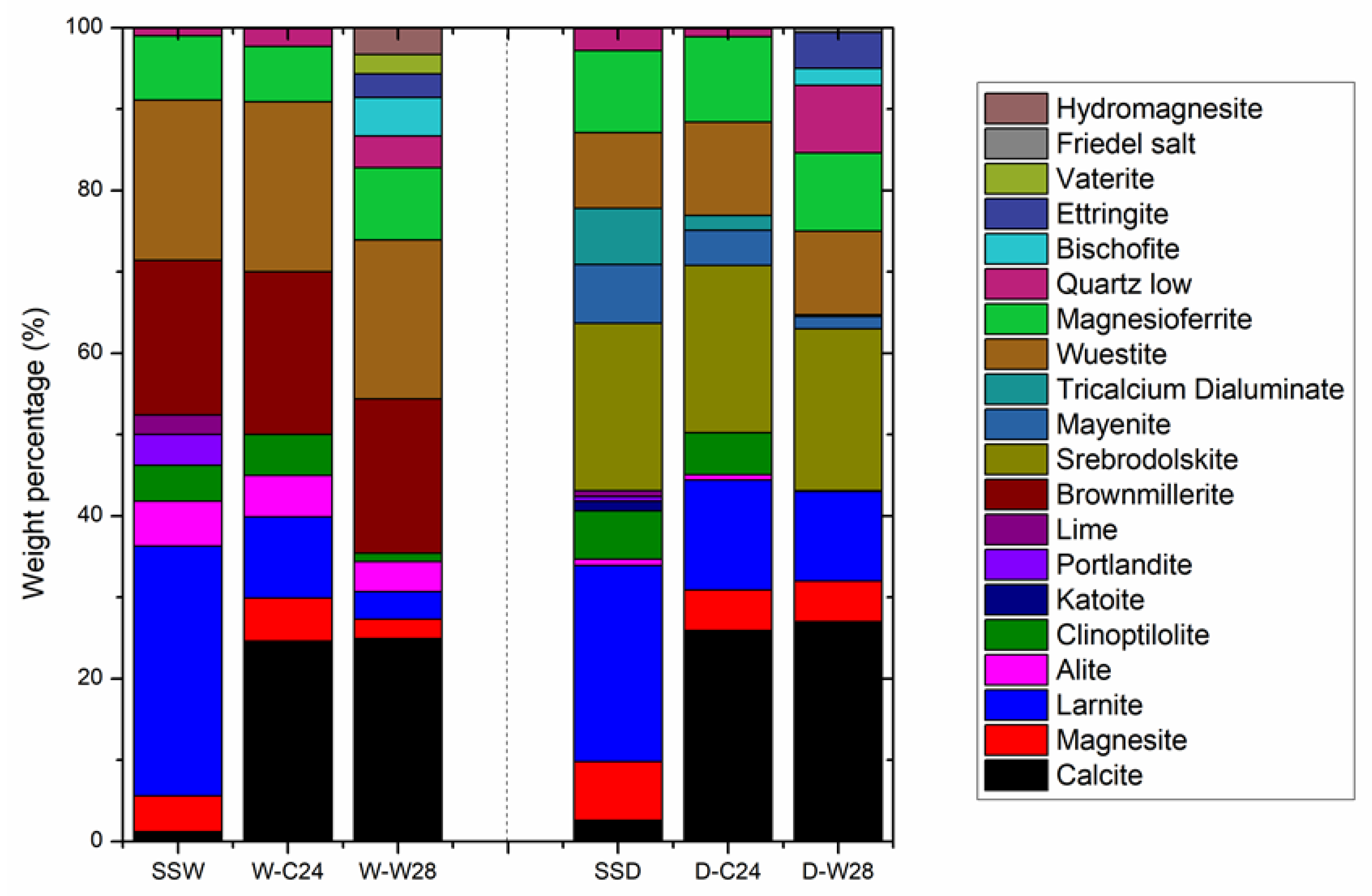
3.3. QXRD Analysis
3.4. TG-DTG Analysis
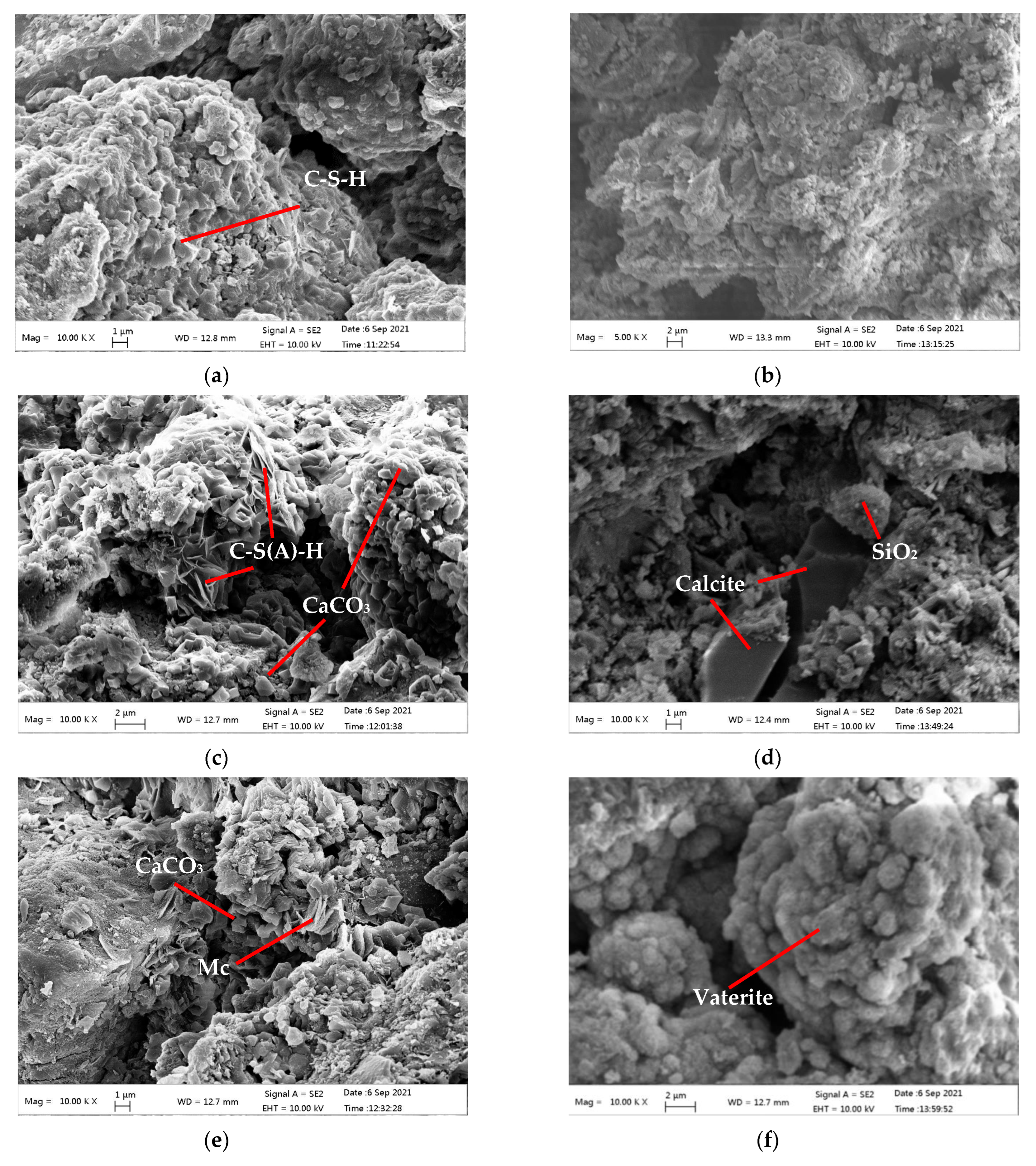
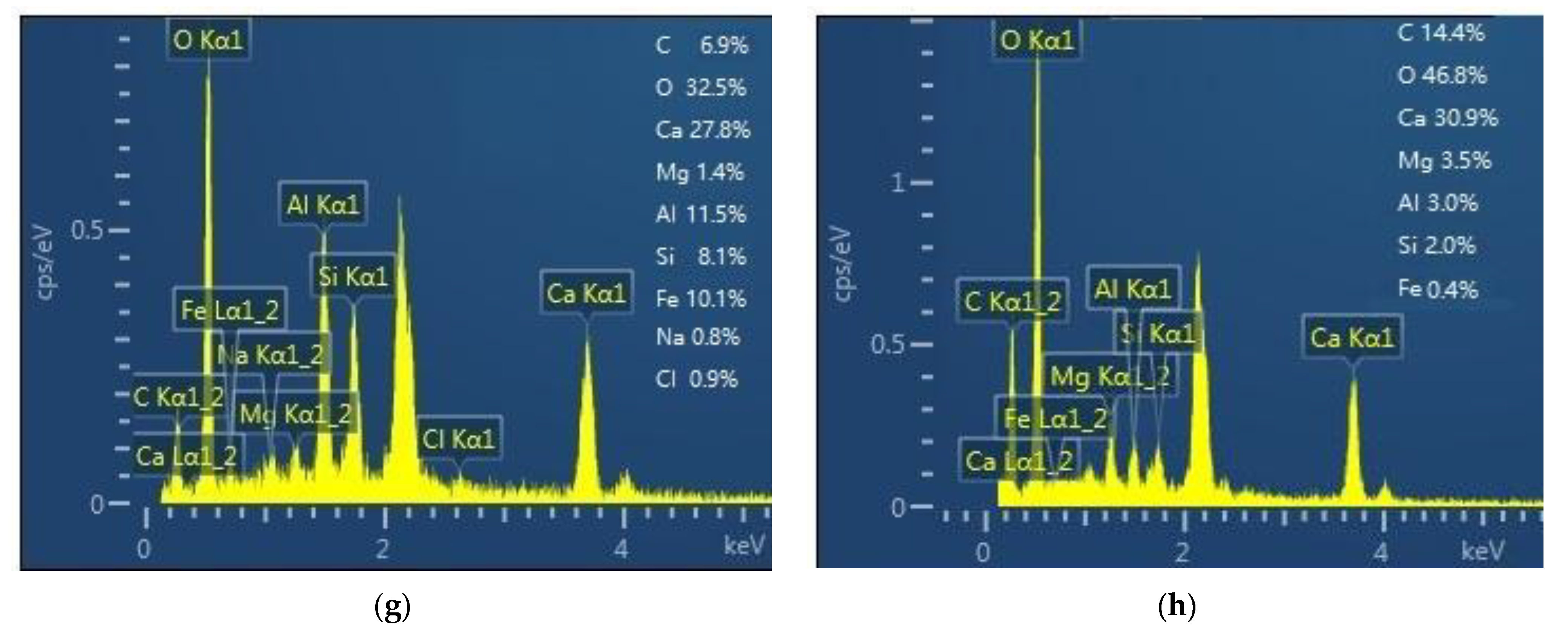
3.5. SEM-EDS Analysis
4. Conclusions
- (1)
- The carbonation consolidation ability of steel slag tailings depends on its CaO content in the carbonation active minerals.
- (2)
- In the process of carbonation curing, both carbonation and hydration occurred in the steel slag powder compacts, while only carbonation occurred in the steel slag mud compacts.
- (3)
- The compressive strength of carbonated steel slag powder compacts increased due to the generation of various hydration products in the early stage of seawater curing. However, their compressive strength decreased due to the decomposition of the newly formed hydration products in the middle stage of seawater curing. Their compressive strength remained unchanged when the generation and decomposition of hydration products reached equilibrium in the late stage of seawater curing.
- (4)
- The compressive strength of carbonated steel slag mud compacts decreased due to the decomposition of their hydration products in the early and middle stages of seawater curing. Their compressive strength increased due to the transformation of calcite to vaterite in the late stage of seawater curing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Adhikari, R.; Chen, Y.H.; Li, P.; Chiang, P.C. Integrated and innovative steel slag utilization for iron reclamation, green material production and CO2 fixation via accelerated carbonation. J. Clean. Prod. 2016, 137, 617–631. [Google Scholar] [CrossRef]
- Ghouleh, Z.; Guthrie, R.I.L.; Shao, Y. Production of carbonate aggregates using steel slag and carbon dioxide for carbon-negative concrete. J. CO2 Util. 2017, 18, 125–138. [Google Scholar] [CrossRef]
- Pan, Z.; Zhou, J.; Jiang, X.; Xu, Y.; Jin, R.; Ma, J.; Zhuang, Y.; Diao, Z.; Zhang, S.; Si, Q.; et al. Investigating the effects of steel slag powder on the properties of self-compacting concrete with recycled aggregates. Constr. Build. Mater. 2019, 200, 570–577. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, C.; Ni, W. Green concrete with ground granulated blast-furnace slag activated by desulfurization gypsum and electric arc furnace reducing slag. J. Clean. Prod. 2020, 269, 122212. [Google Scholar] [CrossRef]
- Geng, B.; Ni, W.; Wu, H.; Huang, X.; Cui, X.; Wang, S.; Zhang, S. On High-Strength Low-Shrinkage Iots-Based Concrete. Int. J. Heat Technol. 2016, 34, 677–686. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Feng, J. A discussion on improving hydration activity of steel slag by altering its mineral compositions. J. Hazard. Mater. 2011, 186, 1070–1075. [Google Scholar] [CrossRef]
- Kriskova, L.; Pontikes, Y.; Zhang, F.; Cizer, Ö.; Tom, P.; Van Balen, K.; Blanpain, B. Influence of mechanical and chemical activation on the hydraulic properties of gamma dicalcium silicate. Cem. Concr. Res. 2014, 55, 59–68. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Shi, C.; Pan, S.Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Sanna, A.; Dri, M.; Hall, M.R.; Maroto-Valer, M. Waste materials for carbon capture and storage by mineralisation (CCSM)—A UK perspective. Appl. Energy 2012, 99, 545–554. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; El-Naas, M.H.; Benamor, A.; Al-Sobhi, S.S.; Zhang, Z. Carbon mineralization by reaction with steel-making waste: A review. Processes 2019, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hitch, M. Mechanical activation of magnesium silicates for mineral carbonation, a review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M.; Power, I.M.; Pan, Y. Integrated mineral carbonation of ultramafic mine, A review. Minerals 2018, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Liu, W.; Wang, L.; Liu, Q.; Chen, F.; Yue, H.; Liang, B.; Lü, L.; Wang, Y.; Zhang, G.; et al. Indirect mineral carbonation of blast furnace slag with (NH4)2SO4 as a recyclable extractant. J. Energy Chem. 2017, 26, 927–935. [Google Scholar] [CrossRef]
- Li, J.; Ni, W.; Wang, X.; Zhu, S.; Wei, X.; Jiang, F.; Zeng, H.; Hitch, M. Mechanical activation of medium basicity steel slag under dry condition for carbonation curing. J. Build. Eng. 2022, 50, 104123. [Google Scholar] [CrossRef]
- Qiu, Q. A state-of-the-art review on the carbonation process in cementitious materials: Fundamentals and characterization techniques. Constr. Build. Mater. 2020, 247, 118503. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Hitch, M.; Pascual, R. Carbonation of steel slag and gypsum for building materials and associated reaction mechanisms. Cem. Concr. Res. 2019, 125, 105893. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Li, K.; Hu, W. Use of CO2 to cure steel slag and gypsum-based material. Energies 2021, 14, 5174. [Google Scholar] [CrossRef]
- Wei, X.; Ni, W.; Zhang, S.; Wang, X.; Li, J.; Du, H. Influence of the key factors on the performance of steel slag-desulphurisation gypsum-based hydration-carbonation materials. J. Build. Eng. 2022, 45, 103591. [Google Scholar] [CrossRef]
- Höllen, D.; Berneder, I.; Capo Tous, F.; Stöllner, M.; Philipp Sedlazeck, K.; Schwarz, T.; Aldrian, A.; Lehner, M. Stepwise treatment of ashes and slags by dissolution, precipitation of iron phases and carbonate precipitation for production of raw materials for industrial applications. Waste Manag. 2018, 78, 750–762. [Google Scholar] [CrossRef]
- Ekolu, S.O. A review on effects of curing, sheltering, and CO2 concentration upon natural carbonation of concrete. Constr. Build. Mater. 2016, 127, 306–320. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, W. Fundamental understanding of carbonation curing and durability of carbonation-cured cement-based composites: A review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Ni, W.; Zhu, S.; Mao, S.; Jiang, F.; Zeng, H.; Sun, X.; Huang, B.; Hitch, M. Orthogonal Test Design for the Optimization of Preparation of Steel Slag-Based Carbonated Building Materials with Ultramafic Tailings as Fine Aggregates. Minerals 2022, 12, 246. [Google Scholar] [CrossRef]
- Duan, S.; Liao, H.; Cheng, F.; Tao, M. Effect of curing condition and carbonization enhancement on mechanical properties of fly ash -desulfurization gypsum-Steel slag blocks. J. CO2 Util. 2020, 38, 282–290. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Qi, L. Effects of temperature and carbonation curing on the mechanical properties of steel slag-cement binding materials. Constr. Build. Mater. 2016, 124, 999–1006. [Google Scholar] [CrossRef]
- Behfarnia, K.; Rostami, M. An assessment on parameters affecting the carbonation of alkali-activated slag concrete. J. Clean. Prod. 2017, 157, 1–9. [Google Scholar] [CrossRef]
- Ma, Z.; Liao, H.; Wang, L.; Cheng, F. Effects of iron/silicon/magnesium/aluminum on CaO carbonation of CO2 in steel slag-based building materials during carbonation curing. Constr. Build. Mater. 2021, 298, 123889. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Li, K. Study on Mineral Compositions of Direct Carbonated Steel Slag by QXRD, TG, FTIR, and XPS. Energies 2021, 14, 4489. [Google Scholar] [CrossRef]
- Prasad, N.T.; Sadhu, S.; Murthy, K.N.V.V.; Pilli, S.R.; Ramesh, S.; Kumar, S.V.S.P.; Dharani, G.; Atmanand, M.A.; Rao, M.B.V.; Dey, T.K.; et al. Carbon-dioxide Fixation by Artificial Reef Development in Marine Environment using Carbonated Slag Material from Steel Plant. In Proceedings of the OCEANS 2014, Taipei, Taiwan, 7–10 April 2014. [Google Scholar]
- Huang, X.; Wang, Z.; Liu, Y.; Hu, W.; Ni, W. On the use of blast furnace slag and steel slag in the preparation of green artificial reef concrete. Constr. Build. Mater. 2016, 112, 241–246. [Google Scholar] [CrossRef]
- Guilbeau, B.P.; Harry, F.P.; Gambrell, R.P.; Knopf, F.C.; Dooley, K.M. Algae attachment on carbonated cements in fresh and brackish waters-Preliminary results. Ecol. Eng. 2003, 20, 309–319. [Google Scholar] [CrossRef]
- Takahashi, T.; Yabuta, K. New Applications for Iron and Steelmaking Slag. NKK Tech. Rev. 2002, 87, 38–44. [Google Scholar]
- Oyamada, K.; Okamoto, M.; Iwata, I. Reproduction technology of coral reefs using “Marine BlockTM”. JFE Tech. Rep. 2014, 19, 46–52. [Google Scholar]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M. Mechanical performance and microstructure of the calcium carbonate binders produced by carbonating steel slag paste under CO2 curing. Cem. Concr. Res. 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Lukens, R.R.; Selberg, C. Guidelines for Marine Artificial Reef Materials; Artificial Reef Subcommittees of the Atalntic and Gulf States Marine Fisheries Commissions, US. 2004. Available online: https://www.gsmfc.org/publications/GSMFC%20Number%20038.pdf (accessed on 10 January 2022).
- Wang, D.; Chang, J. Comparison on accelerated carbonation of β-C2S, Ca(OH)2, and C4AF: Reaction degree, multi-properties, and products. Constr. Build. Mater. 2019, 224, 336–347. [Google Scholar] [CrossRef]
- Chen, Z.; Li, R.; Zheng, X.; Liu, J. Carbon sequestration of steel slag and carbonation for activating RO phase. Cem. Concr. Res. 2021, 139, 106271. [Google Scholar] [CrossRef]
- Richardson, I.G. Tobermorite/jennite- and tobermorite/calcium hydroxide-based models for the structure of C-S-H: Applicability to hardened pastes of tricalcium silicate, β-dicalcium silicate, Portland cement, and blends of Portland cement with blast-furnace slag, metakaolin, or silica fume. Cem. Concr. Res. 2004, 34, 1733–1777. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Zhang, X.; Gu, Y.; Zhang, Y. Effects of pozzolanic reaction on carbonation degree and strength of steel slag compacts containing zeolite. Constr. Build. Mater. 2021, 277, 122334. [Google Scholar] [CrossRef]
- Wang, Y.; Karasev, A.; Park, J.H.; Jönsson, P.G. Non-metallic Inclusions in Different Ferroalloys and Their Effect on the Steel Quality: A Review. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2021, 52, 2892–2925. [Google Scholar] [CrossRef]
- Li, F.; Li, H.; Zheng, S.; You, J.; Han, K.; Zhai, Q. Impacts of modification of alloying method on inclusion evolution in RH refining of silicon steel. Materials 2017, 10, 1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, W.; Chen, Y.; Cang, D. A statistical study of inclusions in medium-grade non-oriented silicon steel. Metall. Res. Technol. 2019, 116, 207. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.P.; Chang, H.H.; Yang, S.Y.; Huang, S.J.; Tsai, Y.J.; Huang, J.J.T.; Chan, J.C. Study of binding interaction between Pif80 protein fragment and aragonite. Sci. Rep. 2016, 6, 10. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J.; Sahu, S. Phase evolution and strength development during carbonation of low-lime calcium silicate cement (CSC). Constr. Build. Mater. 2019, 210, 473–482. [Google Scholar] [CrossRef]
- Ševčík, R.; Šašek, P.; Viani, A. Physical and nanomechanical properties of the synthetic anhydrous crystalline CaCO3 polymorphs: Vaterite, aragonite and calcite. J. Mater. Sci. 2018, 53, 4022–4033. [Google Scholar] [CrossRef]

| Oxides | SSW | SSD | Minerals | Chemical Formula | SSW | SSD |
|---|---|---|---|---|---|---|
| CaO | 37.57 | 40.42 | Larnite | Ca2SiO4 | 30.7 | 24.2 |
| Fe2O3 | 27.75 | 24.48 | Alite | Ca3SiO5 | 5.5 | 0.8 |
| SiO2 | 16.35 | 14.13 | Lime | CaO | 3.5 | 0.7 |
| MgO | 6.95 | 4.51 | Portlandite | Ca(OH)2 | 3.8 | 0.6 |
| MnO | 3.56 | 4.12 | Clinoptilolite | C-S-H | 4.4 | 5.9 |
| Al2O3 | 3.21 | 7.02 | Katoite | C-S-A-H | - | 1.2 |
| P2O5 | 2.69 | 1.86 | Calcite | CaCO3 | 1.2 | 2.6 |
| TiO2 | 0.84 | 1.04 | Magnesite | MgCO3 | 4.4 | 7.2 |
| SO3 | 0.30 | 0.56 | Brownmillerite | Ca2(Fe,Al)O5 | 19 | - |
| V2O5 | 0.27 | 0.26 | Srebrodolskite | Ca2Fe2O5 | - | 20.6 |
| Na2O | 0.13 | 0.23 | Mayenite | Ca12Al14 O33 | - | 7.2 |
| Cr2O3 | 0.11 | 0.63 | Tricalcium Dialuminate | Ca3Al2O6 | - | 6.9 |
| K2O | 0.08 | 0.36 | Wuestite | (Fe, Mg, Mn)O | 19.7 | 9.3 |
| Cl | 0.04 | 0.29 | Magnesioferrite | (Mg, Fe)2 O3 | 7.9 | 10.1 |
| Others | 0.14 | 0.10 | Quartz | SiO2 | 1 | 2.7 |
| Total C | 0.33 | 0.63 | ||||
| LOI | 1.03 | 1.63 |
| Compound | NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | Total |
|---|---|---|---|---|---|---|---|---|
| Weight (g) | 23.497 | 4.981 | 3.917 | 1.102 | 0.664 | 0.192 | 0.096 | 34.449 |
| 50–240 °C C-S-H Gel Ettringite | 240–500 °C Ca(OH)2 Monocarboaluminate | 500–800 °C Carbonates | 800–1000 °C | LOI | |
|---|---|---|---|---|---|
| SSW | 1.6 | 0.2 | 0.1 | −0.5 | 99.0 |
| SSD | 0.9 | 0.5 | 0.5 | −0.2 | 98.4 |
| W-C24 | 1.7 | 0.7 | 6.8 | 0.0 | 90.8 |
| D-C24 | 2.5 | 1.2 | 6.6 | 0.2 | 89.5 |
| W-W28 | 1.5 | 1.4 | 6.6 | 0.5 | 89.9 |
| D-W28 | 1.5 | 1.8 | 6.6 | 0.5 | 89.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhao, S.; Song, X.; Ni, W.; Mao, S.; Du, H.; Zhu, S.; Jiang, F.; Zeng, H.; Deng, X.; et al. Carbonation Curing on Magnetically Separated Steel Slag for the Preparation of Artificial Reefs. Materials 2022, 15, 2055. https://doi.org/10.3390/ma15062055
Li J, Zhao S, Song X, Ni W, Mao S, Du H, Zhu S, Jiang F, Zeng H, Deng X, et al. Carbonation Curing on Magnetically Separated Steel Slag for the Preparation of Artificial Reefs. Materials. 2022; 15(6):2055. https://doi.org/10.3390/ma15062055
Chicago/Turabian StyleLi, Jiajie, Shaowei Zhao, Xiaoqian Song, Wen Ni, Shilong Mao, Huihui Du, Sitao Zhu, Fuxing Jiang, Hui Zeng, Xuejie Deng, and et al. 2022. "Carbonation Curing on Magnetically Separated Steel Slag for the Preparation of Artificial Reefs" Materials 15, no. 6: 2055. https://doi.org/10.3390/ma15062055
APA StyleLi, J., Zhao, S., Song, X., Ni, W., Mao, S., Du, H., Zhu, S., Jiang, F., Zeng, H., Deng, X., & Hitch, M. (2022). Carbonation Curing on Magnetically Separated Steel Slag for the Preparation of Artificial Reefs. Materials, 15(6), 2055. https://doi.org/10.3390/ma15062055











