The Effect of Hf Addition on the Boronizing and Siliciding Behavior of CoCrFeNi High Entropy Alloys
Abstract
:1. Introduction
2. Experimental
3. Results
4. Discussion
5. Conclusions
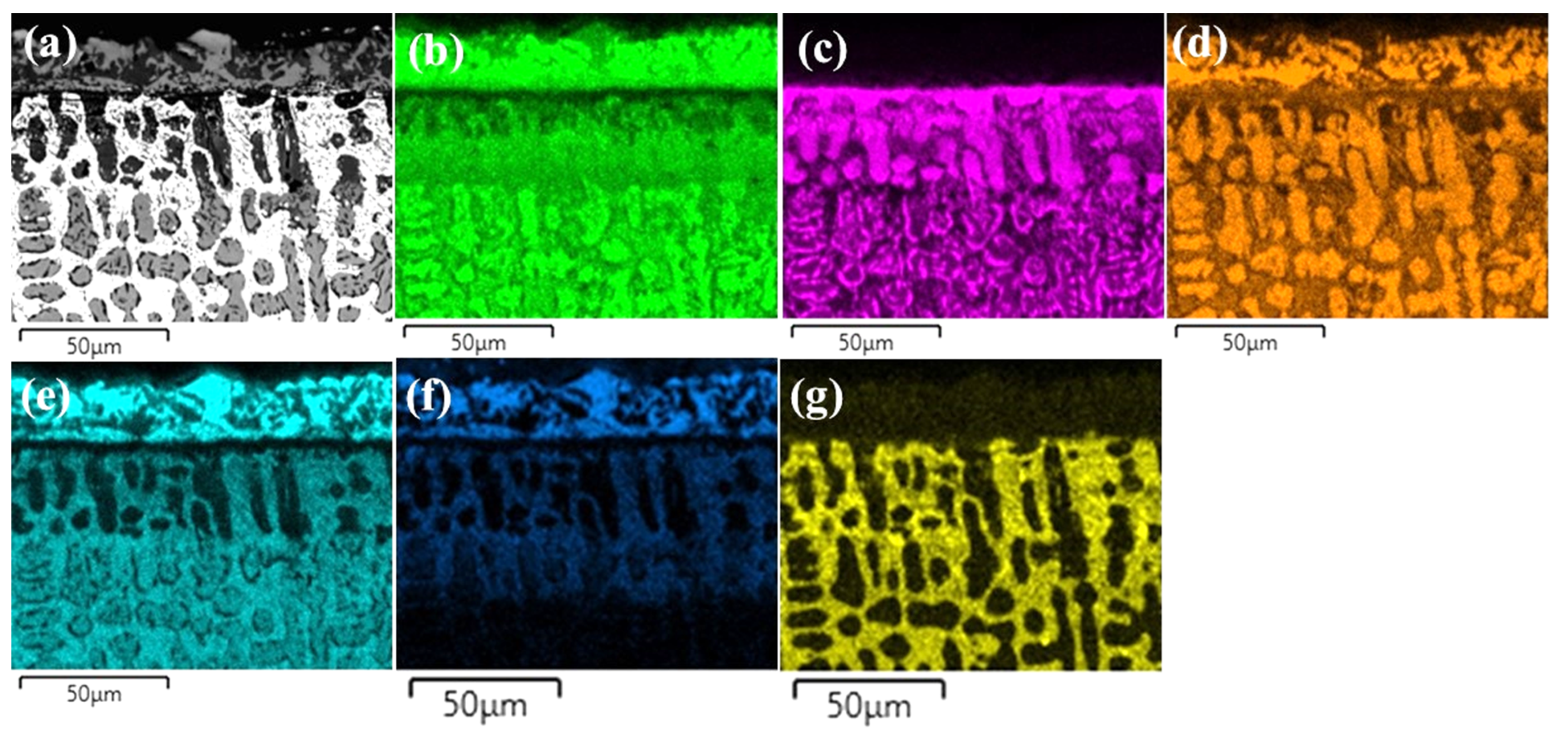
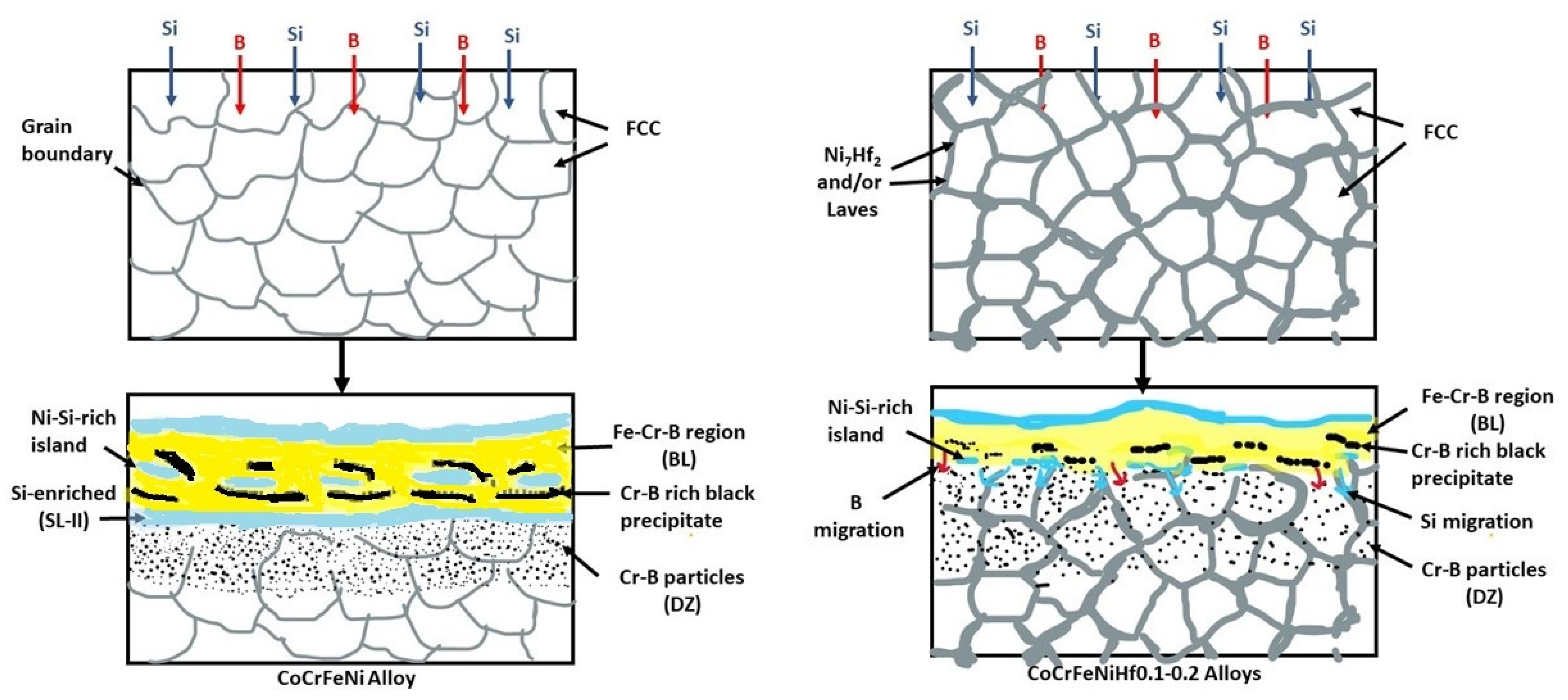
- Si atoms were accumulated close to the BL and they were not transferred into the substrates, because the BL prevented the transition of Si atoms for CoCrFeNi alloy. With the addition of Hf, Si atoms were diffusing through the Ni/Ni-Hf rich phase into the substrates.
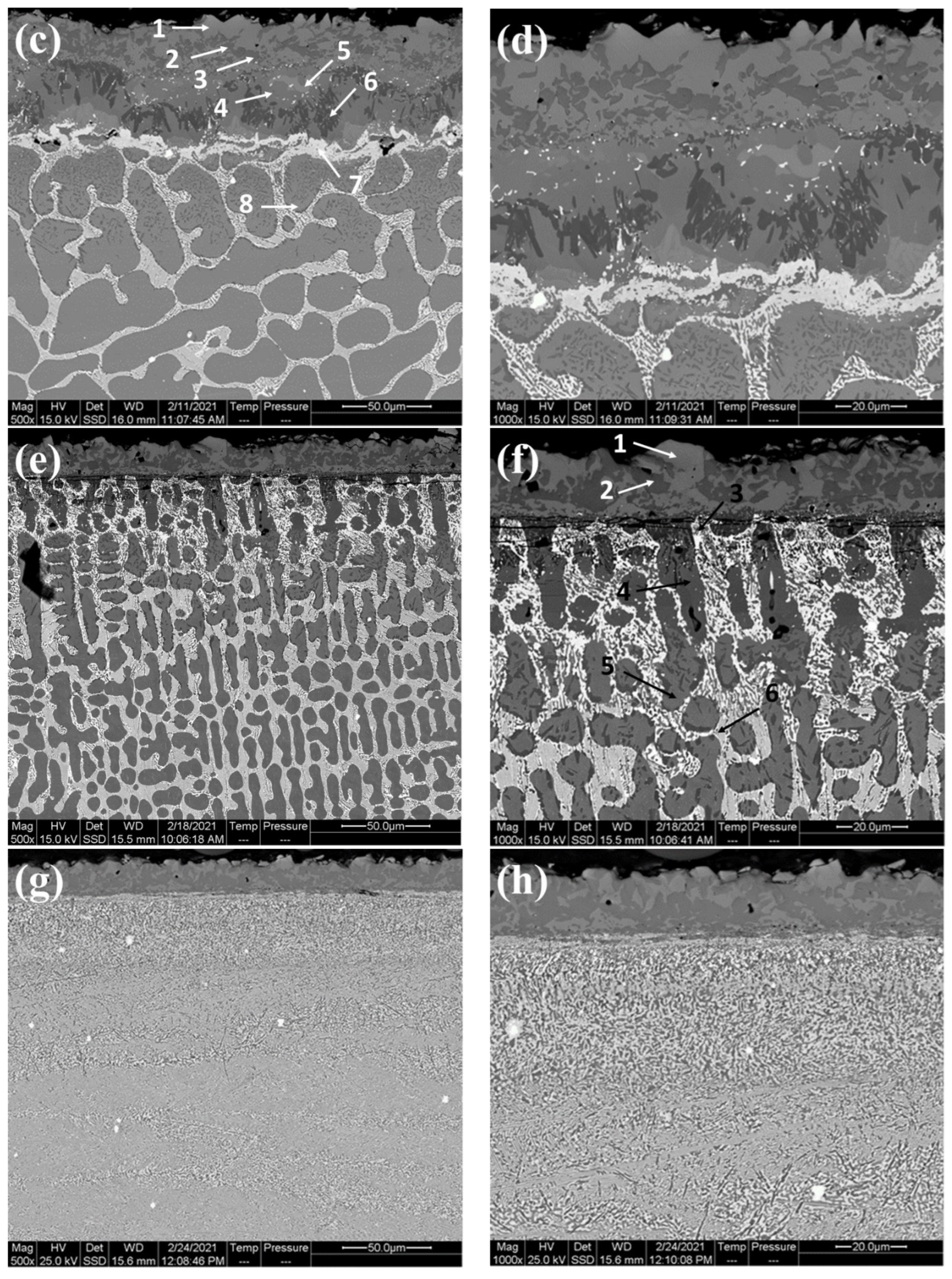
- The thickness of the SL-1 + BL gradually decreased with the addition of Hf, whereas the thickness of the DZ increased.
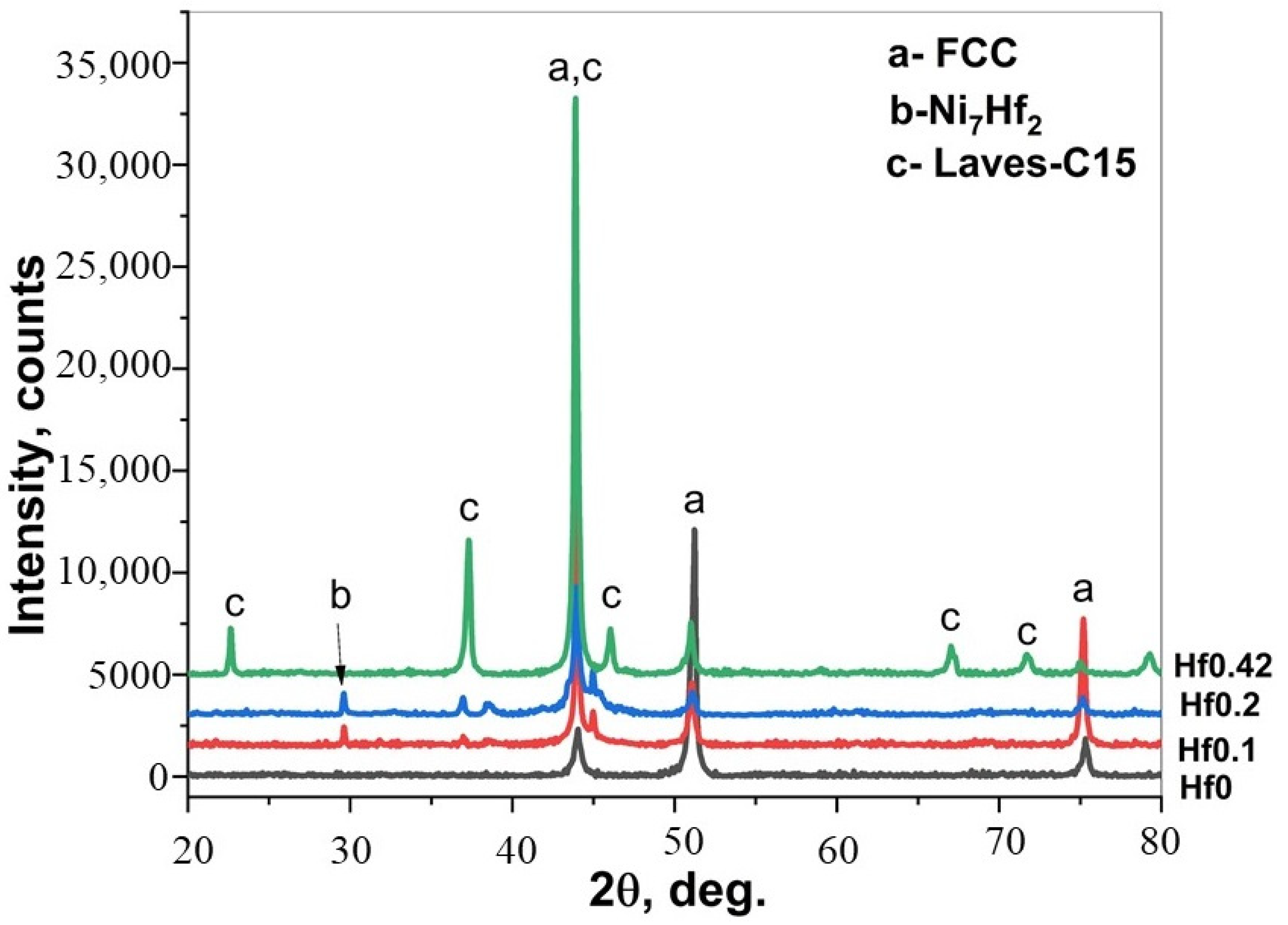
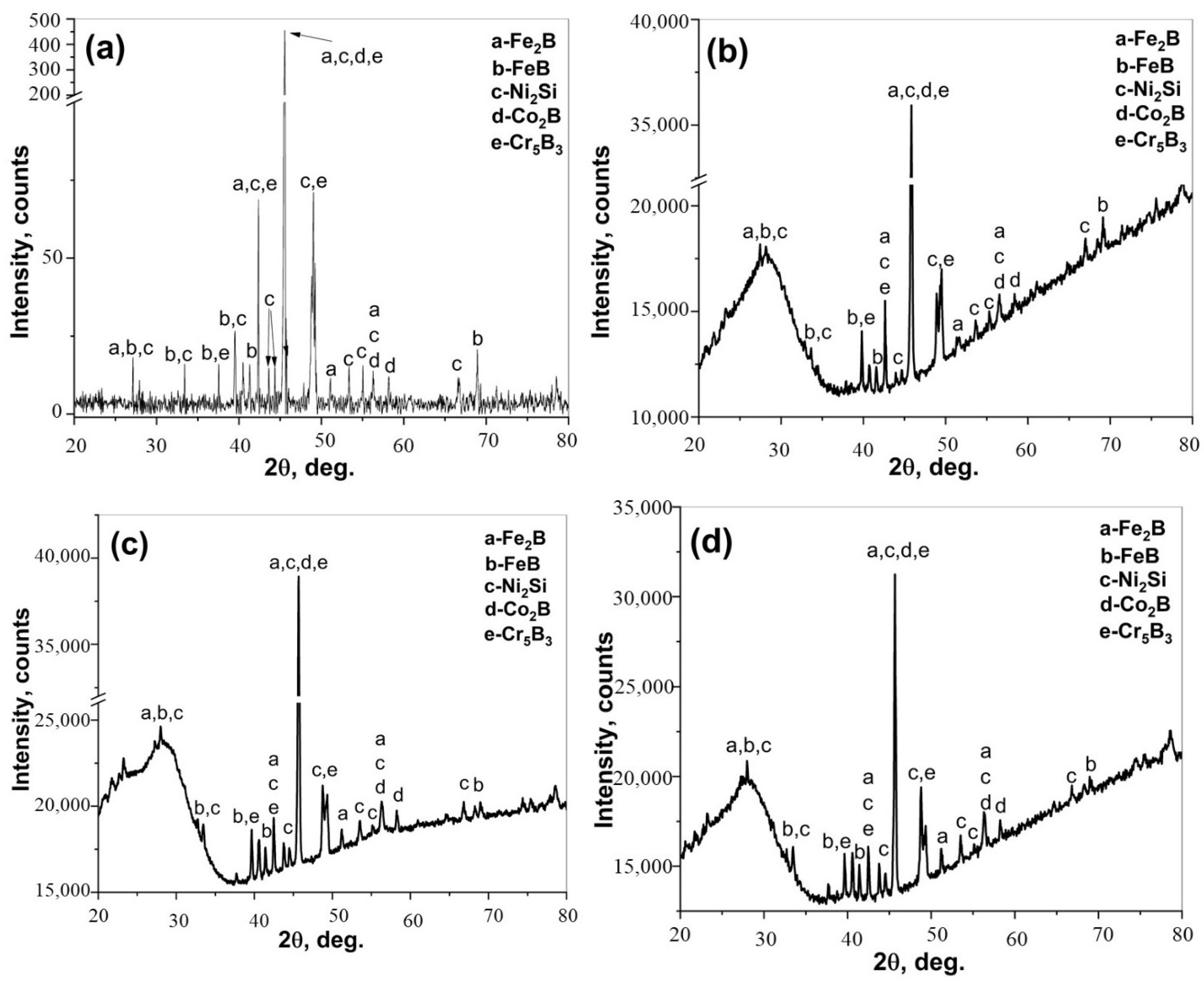
- Ni2Si, FeB, Fe2B, Co2B, and Cr5B3 phases were detected in the CoCrFeNi and CoCrFeNiHfx alloys.
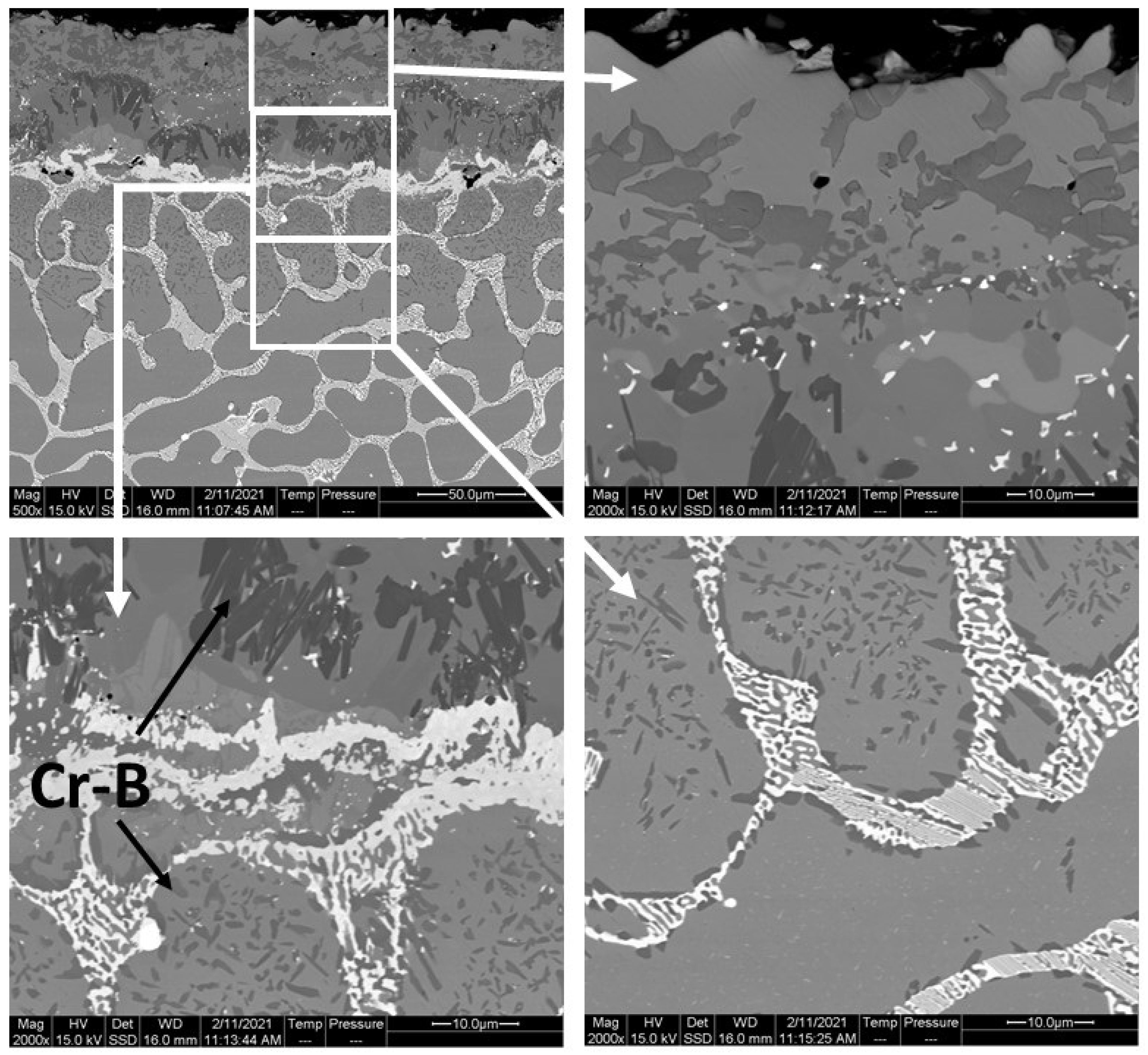
- Cr-B precipitates were observed in the BL and DZ of CoCrFeNi and CoCrFeNiHf0.1. In CoCrFeNiHf0.2 and CoCrFeNiHf0.42, Cr-B precipitates were only detected in the DZ. A Cr-B layer had formed between the region of the substrate and the coating for CoCrFeNiHf0.2 and CoCrFeNiHf0.42. The Cr-B phase was predominantly found in FCC regions of CoCrFeNiHf0.1–0.2.
- CoCrFeNiHfx alloys are multiphase systems, which have fine lamellar and eutectic structures. The eutectic structures are dominant when large amounts of Hf are added and the diffusion of B atoms is fast during the boronizing process.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; He, J.; Huang, H.; Wang, H.; Lu, Z.; Liu, C.T. Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Ma, H.; Shek, C.H. Effects of Hf on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. J. Alloys Compd. 2020, 827, 154159. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, Z.; Xia, G.; Wang, M.; Xie, T.; Cheng, X. Microstructures and mechanical properties of CoCrFeNiHfx high-entropy alloys. Mater. Sci. Eng. A 2020, 792, 139820. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, L.; Zhang, C.; Jiang, H.; Lu, Y.; Li, T. Effect of carbon addition on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. Sci. China Technol. Sci. 2017, 61, 117–123. [Google Scholar] [CrossRef]
- Xie, T.; Xiong, Z.; Xu, Z.; Liu, Z.; Cheng, X. Another eutectic point of Co–Cr–Fe–Ni-M (Hf, Ta, Nb) high-entropy system determined using a simple mixture method correlated with mixing enthalpy. Mater. Sci. Eng. A 2020, 802, 140634. [Google Scholar] [CrossRef]
- Liu, W.; Yang, T.; Liu, C. Precipitation hardening in CoCrFeNi-based high entropy alloys. Mater. Chem. Phys. 2018, 210, 2–11. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Cheng, P.; Wang, Q.; Li, J.; Dang, Y.; Wang, J.; Liu, C. Designing eutectic high entropy alloys of CoCrFeNiNb x. J. Alloys Compd. 2016, 656, 284–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Shi, P.; Ren, W.; Zheng, T.; Ren, Z.; Hou, X.; Peng, J.; Hu, P.; Gao, Y.; Zhong, Y.; Liaw, P.K. Enhanced strength–ductility synergy in ultrafine-grained eutectic high-entropy alloys by inheriting microstructural lamellae. Nat. Commun. 2019, 10, 489. [Google Scholar] [CrossRef]
- Muangtong, P.; Rodchanarowan, A.; Chaysuwan, D.; Chanlek, N.; Goodall, R. The corrosion behaviour of CoCrFeNi-x (x = Cu, Al, Sn) high entropy alloy systems in chloride solution. Corros. Sci. 2020, 172, 108740. [Google Scholar] [CrossRef]
- Huo, W.; Zhou, H.; Fang, F.; Xie, Z.; Jiang, J. Microstructure and mechanical properties of CoCrFeNiZrx eutectic high-entropy alloys. Mater. Des. 2017, 134, 226–233. [Google Scholar] [CrossRef]
- Stein, F.; Palm, M.; Sauthoff, G. Structure and stability of Laves phases. Part I. Critical assessment of factors controlling Laves phase stability. Intermetallics 2004, 12, 713–720. [Google Scholar] [CrossRef]
- Anton, D.; Shah, D. High temperature evaluation of topologically close packed intermetallics. Mater. Sci. Eng. A 1992, 153, 410–415. [Google Scholar] [CrossRef]
- Scudino, S.; Donnadieu, P.; Surreddi, K.; Nikolowski, K.; Stoica, M.; Eckert, J. Microstructure and mechanical properties of Laves phase-reinforced Fe–Zr–Cr alloys. Intermetallics 2009, 17, 532–539. [Google Scholar] [CrossRef]
- Murty, B.; Yeh, J.; Ranganathan, S.; Bhattacharjee, P. High-Entropy Alloys. 2019. Available online: https://doi.org/10.1016/c2017-0-03317-7 (accessed on 15 November 2021).
- Cengiz, S. Effect of refractory elements on boronizing properties of the CoCrFeNi high entropy alloy. Int. J. Refract. Met. Hard Mater. 2020, 95, 105418. [Google Scholar] [CrossRef]
- Erdogan, A.; Günen, A.; Gök, M.S.; Zeytin, S. Microstructure and mechanical properties of borided CoCrFeNiAl0.25Ti0.5 high entropy alloy produced by powder metallurgy. Vacuum 2020, 183, 109820. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, M.; Yang, H.; Qiao, J.; Wu, Y. Surface strengthening in Al0.25CoCrFeNi high-entropy alloy by boronizing. Mater. Lett. 2019, 238, 258–260. [Google Scholar] [CrossRef]
- Lindner, T.; Löbel, M.; Sattler, B.; Lampke, T. Surface hardening of FCC phase high-entropy alloy system by powder-pack boriding. Surf. Coat. Technol. 2018, 371, 389–394. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, H.; Guo, R.; Wang, X.; Shi, X.; Liaw, P.; Qiao, J. Tribological behavior of boronized Al0.1CoCrFeNi high-entropy alloys under dry and lubricated conditions. Wear 2020, 460–461, 203452. [Google Scholar] [CrossRef]
- Nishimoto, A.; Fukube, T.; Maruyama, T. Microstructural, mechanical, and corrosion properties of plasma-nitrided CoCrFeMnNi high-entropy alloys. Surf. Coat. Technol. 2018, 376, 52–58. [Google Scholar] [CrossRef]
- Huang, D.; Lu, J.; Zhuang, Y.; Tian, C.; Li, Y. The role of Nb on the high temperature oxidation behavior of CoCrFeMnNbxNi high-entropy alloys. Corros. Sci. 2019, 158, 108088. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Zhang, H.; Ni, N.; Li, L.; He, L.; Mu, R.; Zhao, X.; Guo, F. Y/Hf-doped AlCoCrFeNi high-entropy alloy with ultra oxidation and spallation resistance. Corros. Sci. 2020, 166, 108426. [Google Scholar] [CrossRef]
- Qiu, L. Role of Hafnium (HF) in the Oxidation Behaviour of Nickel Aluminide (β-NiAl) in Thermal Barrier Coatings. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2019. [Google Scholar]
- Peng, Y.; Gong, J.; Christiansen, T.L.; Somers, M.A. Surface modification of CoCrFeNi high entropy alloy by low-temperature gaseous carburization. Mater. Lett. 2020, 283, 128896. [Google Scholar] [CrossRef]
- Gunduz, K.O.; Gencer, Y.; Tarakci, M.; Calik, A. The effect of vanadium on the boronizing properties of pure iron. Surf. Coat. Technol. 2013, 221, 104–110. [Google Scholar] [CrossRef]
- Brakman, C.M.; Gommers, A.W.J.; Mittemeijer, E.J. Boriding of Fe and Fe–C, Fe–Cr, and Fe–Ni alloys; Boride-layer growth kinetics. J. Mater. Res. 1989, 4, 1354–1370. [Google Scholar] [CrossRef]
- Sinha, A.K. Boriding (Boronizing) of Steels. In ASM Metals Handbook Volume 4 Heat Treating; ASM International: Novelty, OH, USA, 1991. [Google Scholar]
- Azakli, Y.; Cengiz, S.; Tarakci, M.; Gencer, Y. Characterisation of boride layer formed on Fe–Mo binary alloys. Surf. Eng. 2016, 32, 589–595. [Google Scholar] [CrossRef]
- Gencer, Y.; Tarakci, M.; Calik, A. Effect of titanium on the boronizing behaviour of pure iron. Surf. Coat. Technol. 2008, 203, 9–14. [Google Scholar] [CrossRef]
- Dybkov, V.I.; Goncharuk, L.V.; Khoruzha, V.G.; Samelyuk, A.V.; Sidorko, V.R. Growth kinetics and abrasive wear resistance of boride layers on Fe–15Cr alloy. Mater. Sci. Technol. 2011, 27, 1502–1512. [Google Scholar] [CrossRef]
- Cengiz, S.; Gençer, Y.; Tarakçi, M.; Azakli, Y. Influence of copper amount on the pack boronizing behaviour of Fe-Cu binary alloys. J. Fac. Eng. Archit. Gazi Univ. 2015, 30, 339–349. [Google Scholar]
- Gencer, Y. Influence of manganese on pack boriding behaviour of pure iron. Surf. Eng. 2011, 27, 634–638. [Google Scholar] [CrossRef]
- Azakli, Y.; Tarakci, M. Microstructural characterisation of borided binary Fe–W alloys. Surf. Eng. 2016, 34, 226–234. [Google Scholar] [CrossRef]
- Günen, A. Tribocorrosion behavior of boronized Co1.19Cr1.86Fe1.30Mn1.39Ni1.05Al0.17B0.04 high entropy alloy. Surf. Coat. Technol. 2021, 421, 127426. [Google Scholar] [CrossRef]
- Dong, J.; Wu, H.; Chen, Y.; Zhang, Y.; Wu, Y.; Yin, S.; Du, Y.; Hua, K.; Wang, H. Study on self-lubricating properties of AlCoCrFeNi2.1 eutectic high entropy alloy with electrochemical boronizing. Surf. Coat. Technol. 2022, 433, 128082. [Google Scholar] [CrossRef]
- Guo, X.; Jin, X.; Shi, X.; Yang, H.; Zhang, M.; Qiao, J. Tribological Behavior of Boronized Fe40Mn20Cr20Ni20 High-Entropy Alloys. Metals 2021, 11, 1561. [Google Scholar] [CrossRef]
- Parthasarathy, T.; Rapp, R.; Opeka, M.; Kerans, R. A model for the oxidation of ZrB2, HfB2 and TiB2. Acta Mater. 2007, 55, 5999–6010. [Google Scholar] [CrossRef]
- Levine, S.R.; Opila, E.J.; Halbig, M.C.; Kiser, J.D.; Singh, M.; A Salem, J. Evaluation of ultra-high temperature ceramics foraeropropulsion use. J. Eur. Ceram. Soc. 2002, 22, 2757–2767. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G. Oxidation of ultra-high temperature transition metal diboride ceramics. Int. Mater. Rev. 2012, 57, 61–72. [Google Scholar] [CrossRef]
- Liu, X.B.; Yu, L.G.; Wang, H.M. Synthesis of a nickel silicide-base composite coating on austenitic steel by laser cladding. J. Mater. Sci. Lett. 2001, 20, 1489–1492. [Google Scholar] [CrossRef]
- Mitra, R. Mechanical behaviour and oxidation resistance of structural silicides. Int. Mater. Rev. 2006, 51, 13–64. [Google Scholar] [CrossRef]
- Mu, D.; Shen, B.-L.; Yang, C.; Zhao, X. Microstructure analysis of boronized pure nickel using boronizing powders with SiC as diluent. Vacuum 2009, 83, 1481–1484. [Google Scholar] [CrossRef]
- Lou, D.; Akselsen, O.; Solberg, J.; Onsoien, M.; Berget, J.; Dahl, N. Silicon-boronising of Nimonic 90 superalloy. Surf. Coat. Technol. 2006, 200, 3582–3589. [Google Scholar] [CrossRef]
- Dzyadykevich, Y.V.; Kytskay, L.I. Improving the oxidation protection of niobium and tantalum by the use of multilayer coatings. JOM 1997, 49, 30–31. [Google Scholar] [CrossRef]
- Hernández-Sánchez, E.; Domínguez-Galicia, Y.M.; Orozco-Álvarez, C.; Carrera-Espinoza, R.; Herrera-Hernández, H.; Velazquez, J.C. A Study on the Effect of the Boron Potential on the Mechanical Properties of the Borided Layers Obtained by Boron Diffusion at the Surface of AISI 316L Steel. Adv. Mater. Sci. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hunger, H.; Trute, G. Successful Boronizing of Nickel-Based Alloys. Mater. Sci. Forum 1994, 163-165, 341–348. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Fu, Y. High-entropy alloys: Emerging materials for advanced functional applications. J. Mater. Chem. A 2020, 9, 663–701. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Thermodynamics and Phase Diagrams. In Phase Transformations in Metals and Alloys; CRC Press: Boca Raton, FL, USA, 2009; pp. 17–80. [Google Scholar] [CrossRef]
- Stevenson, R. Engineered Materials Handbook Volume 4: Ceramics and Glasses. In Engineered Materials Handbook; ASM International: Novelty, OH, USA, 1991; Volume 4. [Google Scholar]
- Kummamuru, R.K.; Hu, L.; Efremov, M.Y.; Green, M.L.; Lavan, D.A.; De La Rama, L.; Vaudin, M.D.; Allen, L.H. Measurement of heat capacity and enthalpy of formation of nickel silicide using nanocalorimetry. Appl. Phys. Lett. 2009, 95, 181911. [Google Scholar] [CrossRef] [Green Version]





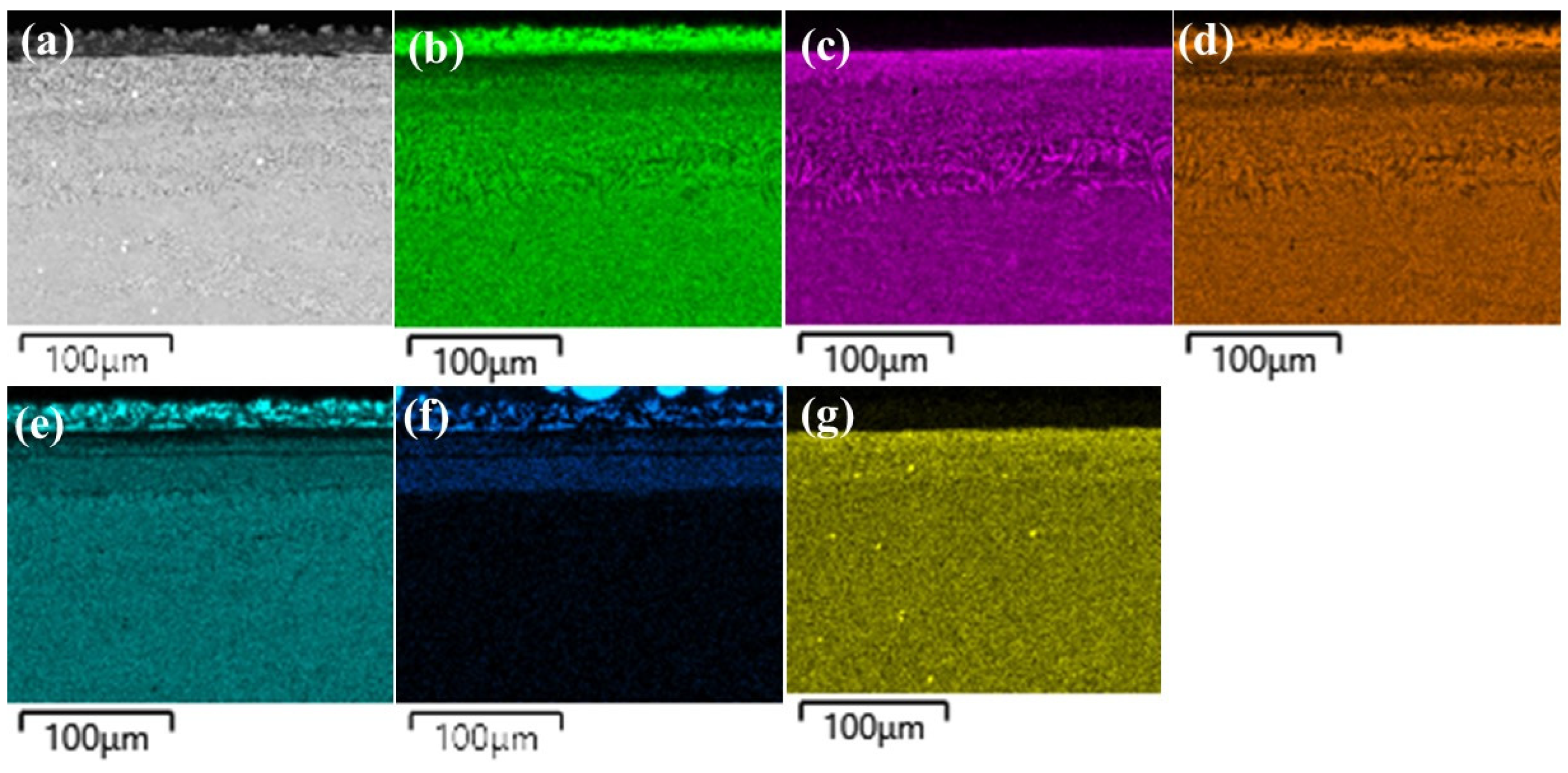
| Alloys | Points/At. % | B K | Si K | Cr K | Fe K | Co K | Ni K | Hf L |
|---|---|---|---|---|---|---|---|---|
| CrCoFeNi | 1 | 55 | 12.8 | 0.2 | 1.4 | 6.3 | 24.8 | - |
| 2 | 67 | - | 0.7 | 15.6 | 13.3 | 2.6 | - | |
| 3 | 63 | - | 14.6 | 13.2 | 7.8 | 1.4 | - | |
| 4 | 66 | - | 31.1 | 2.1 | 0.4 | 0.2 | - | |
| 5 | 54 | 11 | 2 | 6.2 | 10 | 16.5 | - | |
| 6 | 65 | - | 17.1 | 11.1 | 6.1 | 1 | - | |
| 7 | 61 | - | 30.6 | 4.8 | 2.7 | 1 | - | |
| CrCoFeNiHf0.1 | 1 | 30 | 20.1 | - | 2 | 11 | 37.4 | - |
| 2 | 20 | - | 0.3 | 33 | 37 | 9.7 | - | |
| 3 | 46 | 0.3 | 0.7 | 28 | 21 | 3.6 | - | |
| 4 | 46 | 0.1 | 8.3 | 23.2 | 17.5 | 5 | - | |
| 5 | - | 26.7 | 2 | 13.2 | 22 | 36.1 | - | |
| 6 | 60 | - | 36 | 2.8 | 1 | 0.2 | - | |
| 7 | - | - | 3.5 | 5.5 | 24.4 | 45 | 21.6 | |
| 8 | - | 10 | 25 | 24 | 23 | 18 | - | |
| CrCoFeNiHf0.2 | 1 | - | 38 | 0.1 | 2.90 | 12.5 | 46.5 | - |
| 2 | 50 | 0.1 | 1.30 | 23.5 | 20.3 | 4.8 | - | |
| 3 | - | 26.4 | 18.2 | 9 | 14 | 22.2 | 10.2 | |
| 4 | 21 | 0.3 | 27.9 | 28 | 18.7 | 4.5 | 0.1 | |
| 5 | - | 5.8 | 11 | 30.6 | 28 | 24.5 | 0.1 | |
| 6 | - | - | 9.5 | 19.8 | 24.8 | 35.5 | 10.4 |
| Phase | Enthalpy of Formation, ΔHf (kJ/mol) | Gibbs Free Energy of Formation, ΔGf (kJ/mol) | Ref. |
|---|---|---|---|
| Co2B | −128.7 (at 1000 K) | −118.4 (at 1000 K) | [54] |
| CrB2 | −94.0 (at 1000 K) | −85.5 (at 1000 K) | [54] |
| FeB | −73.0 (at 1000 K) | −68.0 (at 1000 K) | [54] |
| Fe2B | −77.8 (at 1000 K) | −68.2 (at 1000 K) | [54] |
| HfB2 | −334.9 (at 1000 K) | −324.5 (at 1000 K) | [54] |
| NiB | −102.4 (at 1000 K) | −93.2 (at 1000 K) | [54] |
| Ni4B3 | −318.8 (at 1000 K) | −283.5 (at 1000 K) | [54] |
| Ni2Si | −46.9 (at 1123 K) | - | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cengiz, S.; Thuvander, M. The Effect of Hf Addition on the Boronizing and Siliciding Behavior of CoCrFeNi High Entropy Alloys. Materials 2022, 15, 2282. https://doi.org/10.3390/ma15062282
Cengiz S, Thuvander M. The Effect of Hf Addition on the Boronizing and Siliciding Behavior of CoCrFeNi High Entropy Alloys. Materials. 2022; 15(6):2282. https://doi.org/10.3390/ma15062282
Chicago/Turabian StyleCengiz, Sezgin, and Mattias Thuvander. 2022. "The Effect of Hf Addition on the Boronizing and Siliciding Behavior of CoCrFeNi High Entropy Alloys" Materials 15, no. 6: 2282. https://doi.org/10.3390/ma15062282







