Quo Vadis, Nanothermite? A Review of Recent Progress
Abstract
:1. Introduction
2. Preparation of Nanothermites
3. Characterization Techniques
3.1. Sensitivity
3.1.1. Sensitivity to Mechanical Stimuli
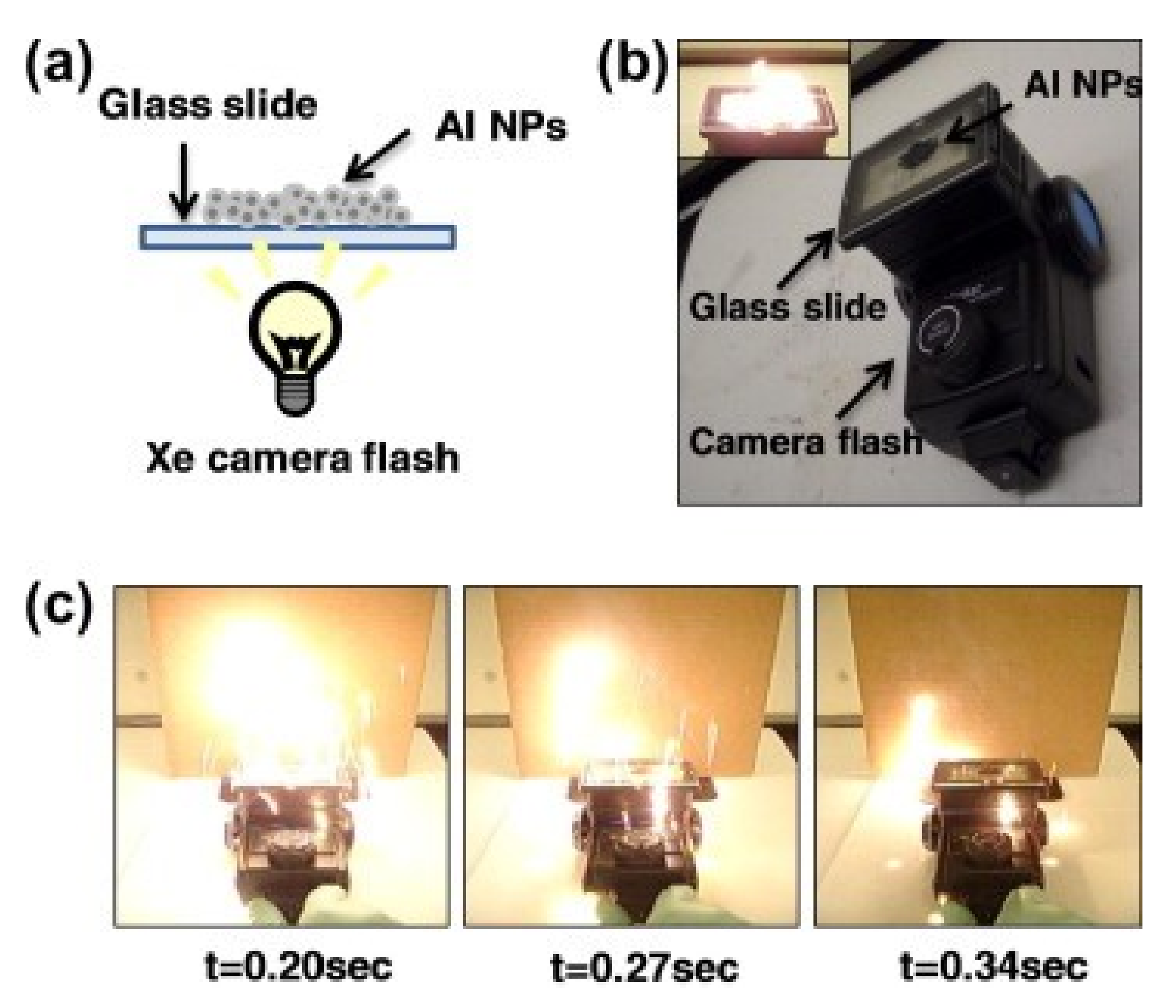
3.1.2. Sensitivity to Electromagnetic Radiation
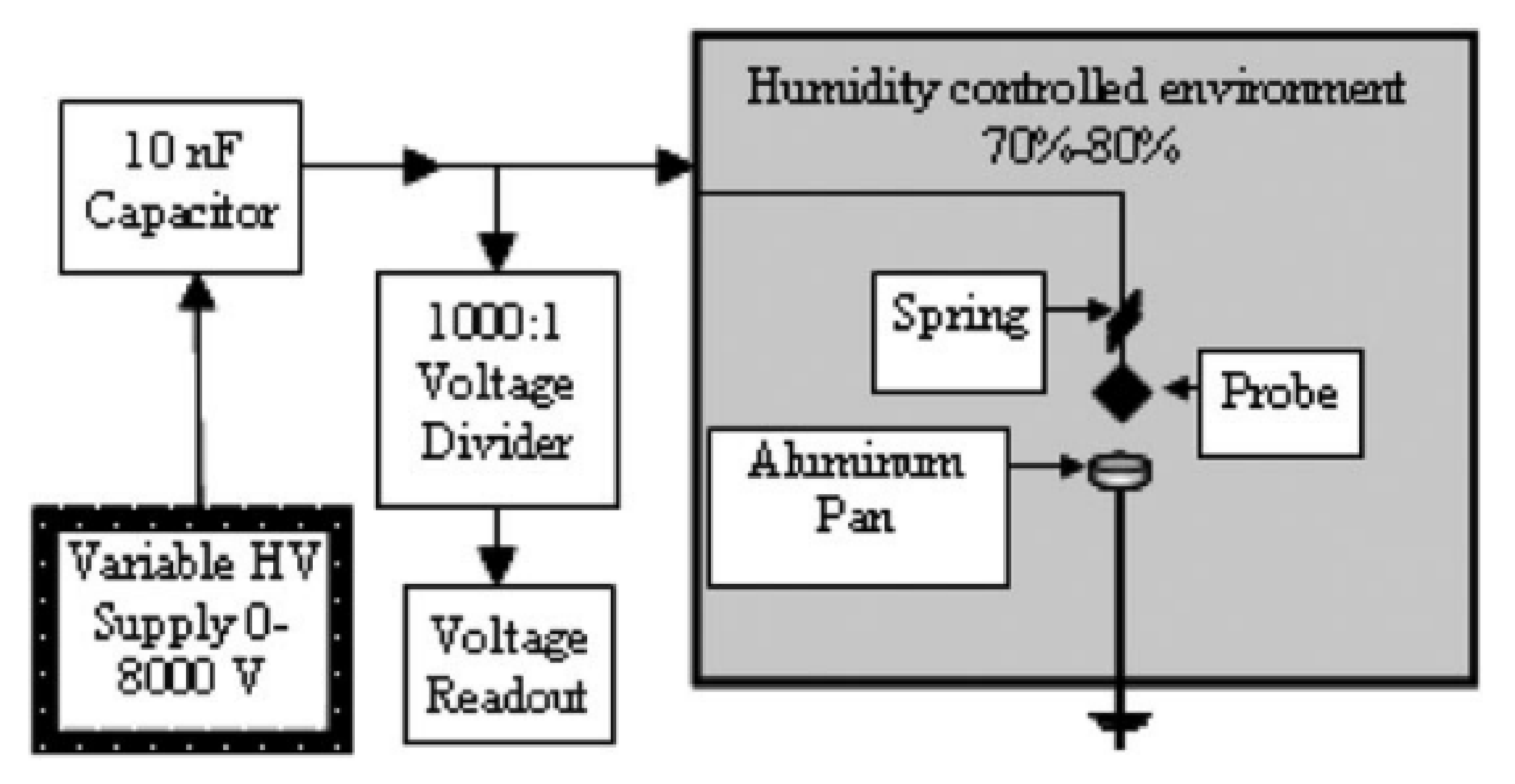
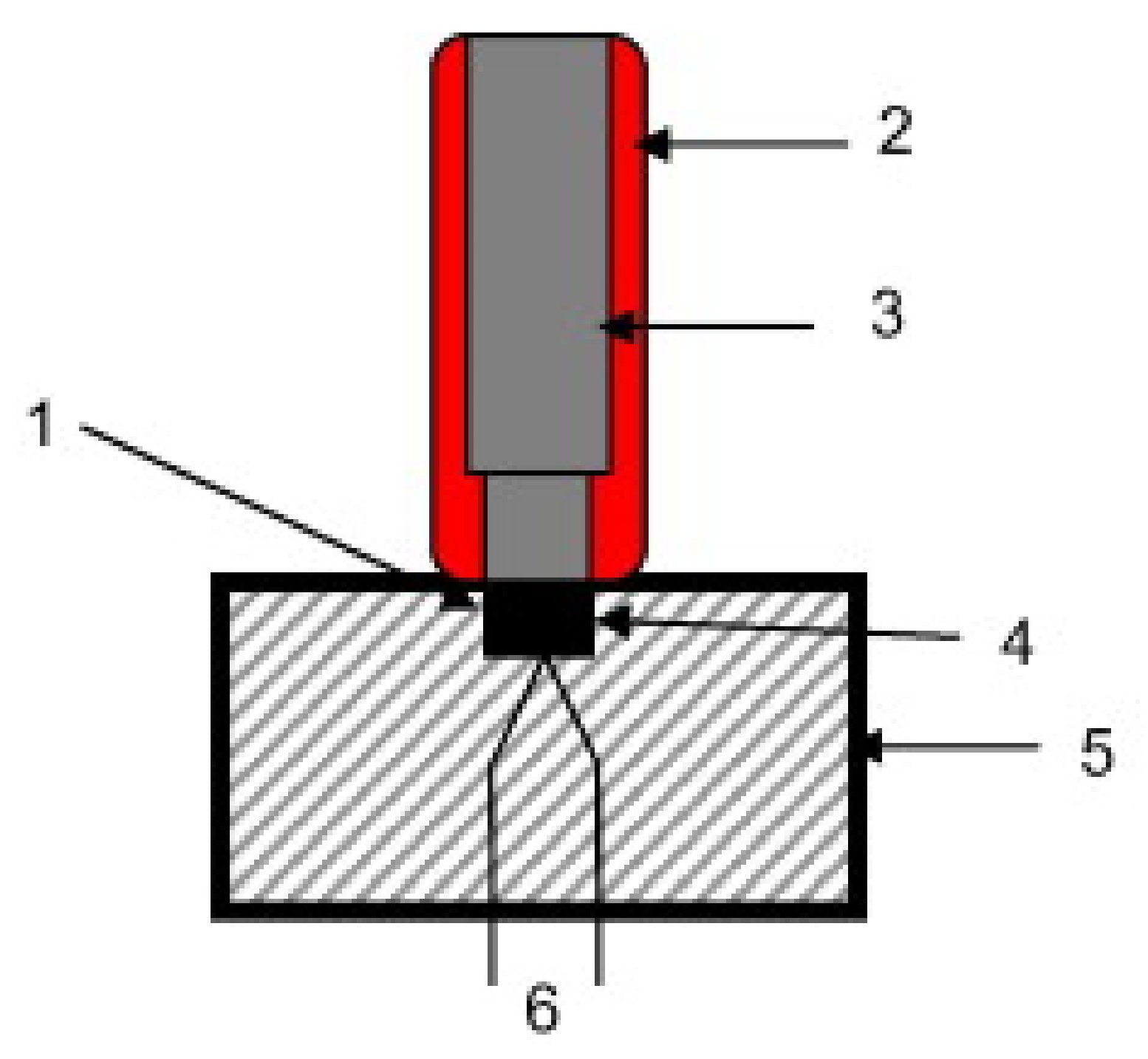
3.1.3. Sensitivity to Electric Discharge
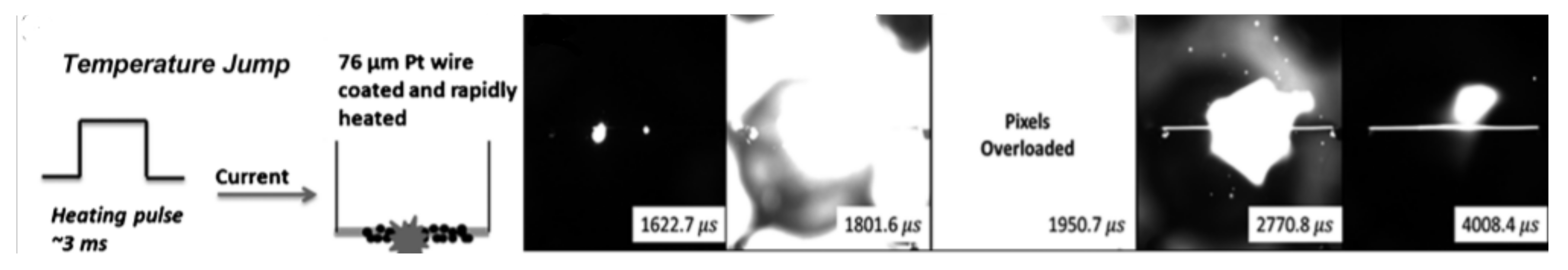
3.1.4. Sensitivity to Thermal Stimuli
3.2. Chemistry and Morphology
3.2.1. Heat of Explosion
3.2.2. Characterisation of Morphology and Composition
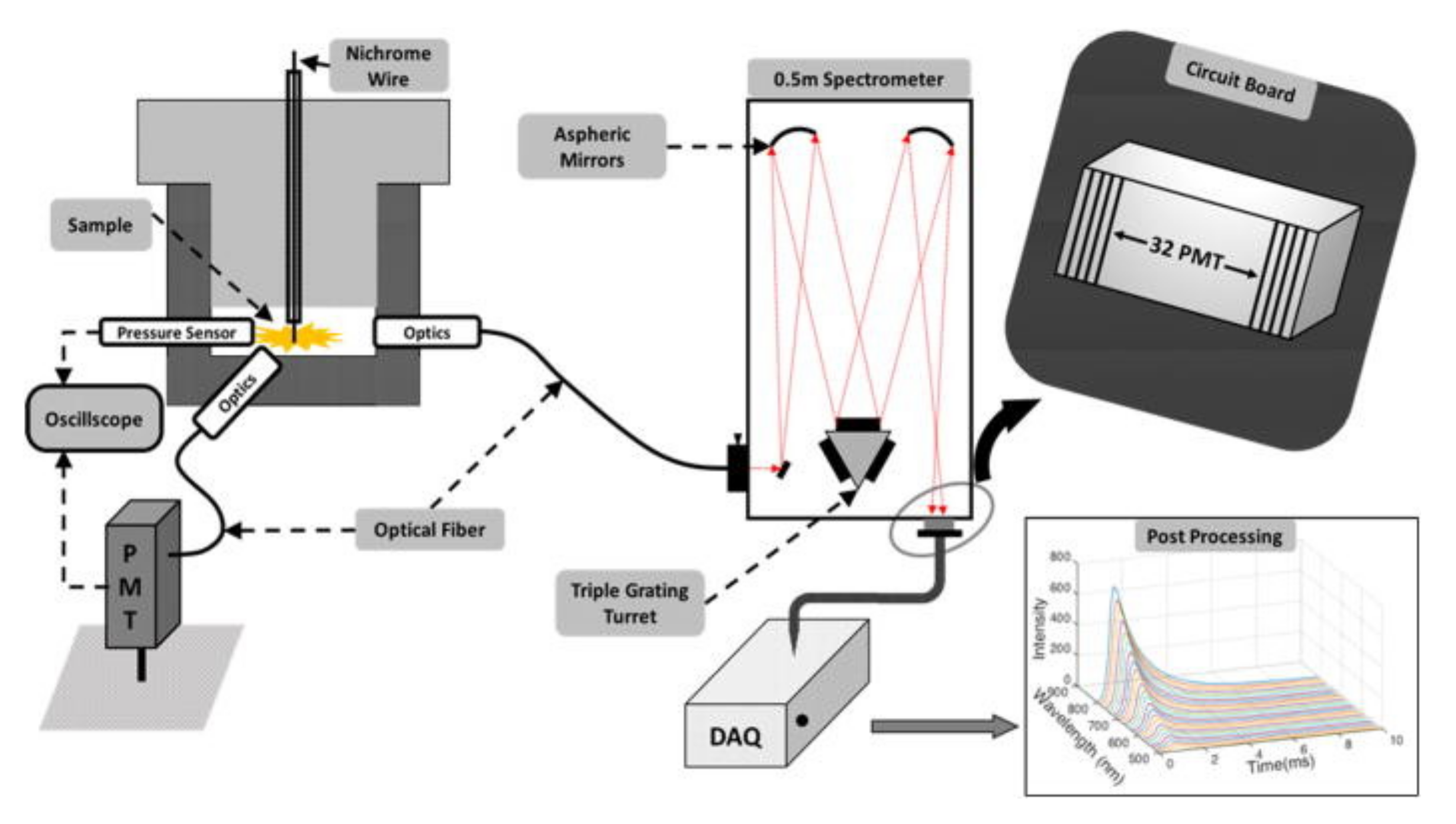
3.2.3. Temperature of Reaction
3.3. Velocity of Combustion
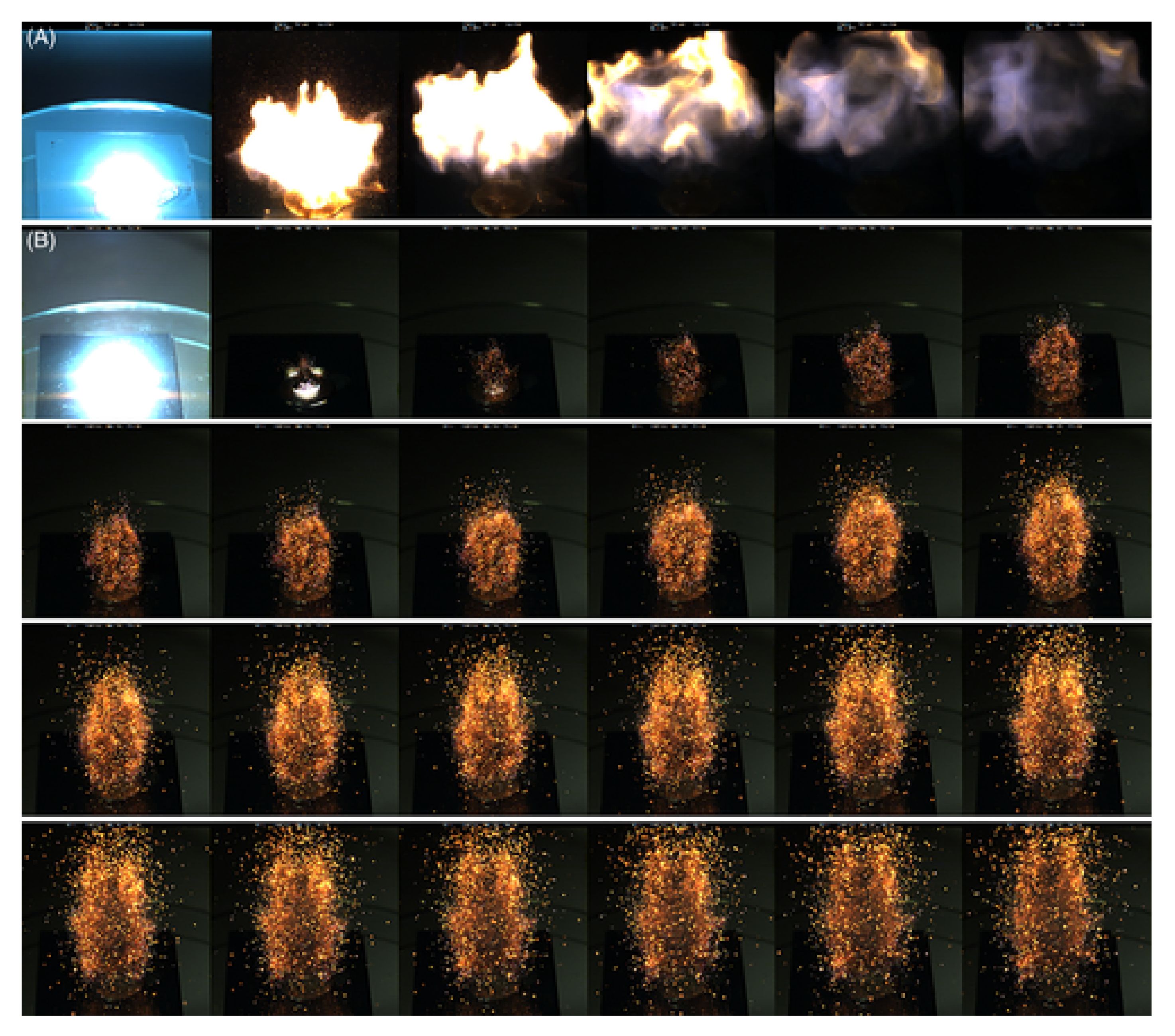
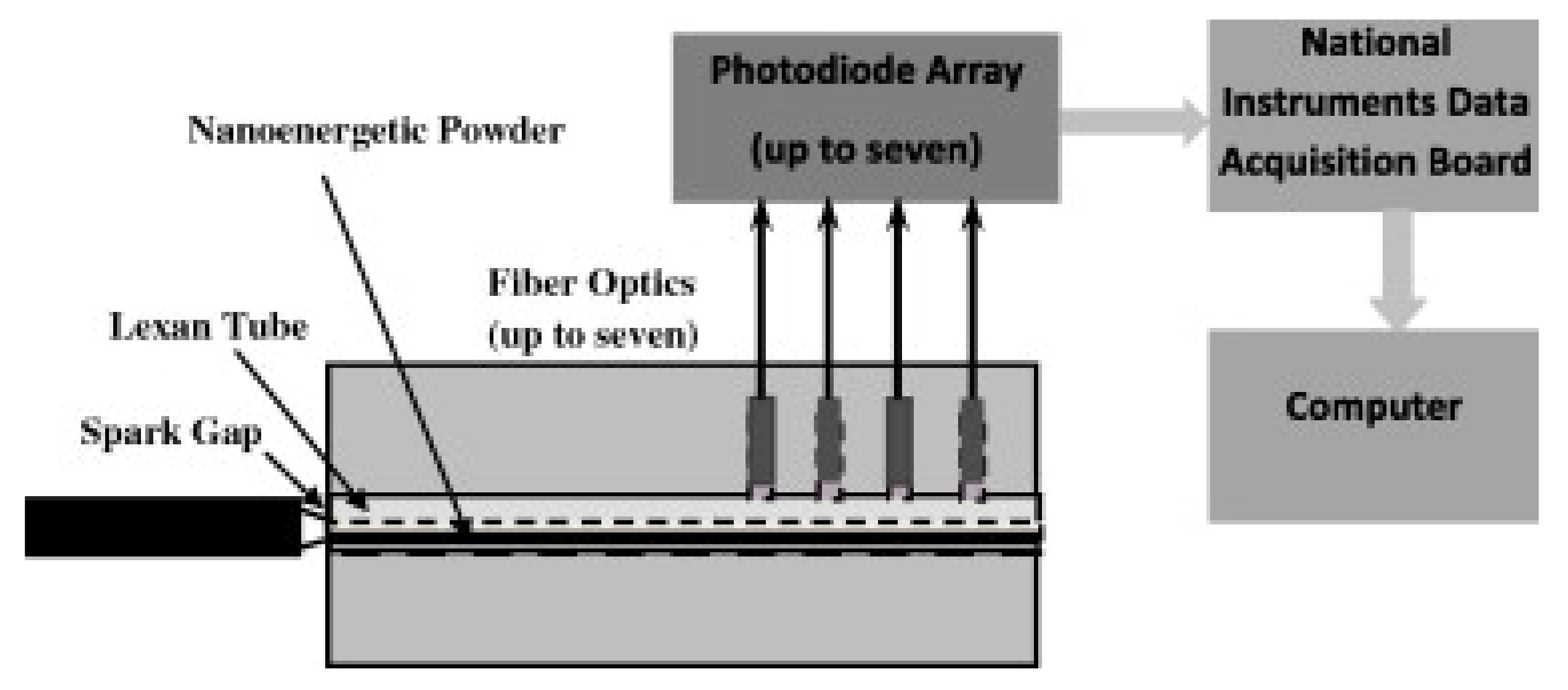
3.4. Open Air Combustions
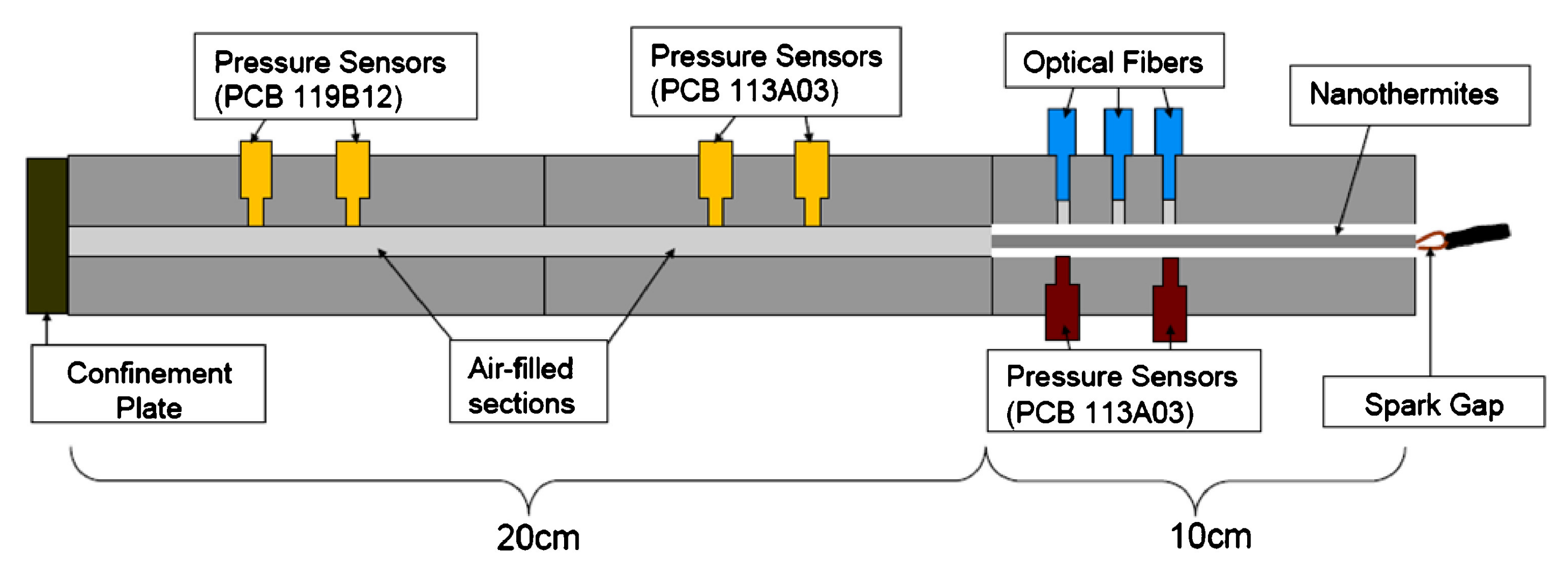
3.5. Pressure Parameters
3.6. DDT-Tube
3.7. Thrust Measurement
3.8. Ballistic Mortar
3.9. Brisance
4. Applications of Nanothermites and NSTEX
4.1. Devices Using Nanothermites and NSTEX
4.2. Materials Based Nanothermites and NSTEX
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CL-20 | 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.03, 11.05, 9]dodecane |
| NSTEX | NanoStructured Thermites and Explosives |
| PETN | 2,2-Bis[(nitrooxy)methyl]propane-1,3-diyl dinitrate |
| RDX | 1,3,5-Trinitro-1,3,5-triazinane |
| TMD | Theoretical maximum density |
| TNT | 2,4,6-Trinitromethylbenzene |
| TNRO | Lead(II) 2,4,6-trinitrobenzene-1,3-bis(olate) |
References
- Dixon, G.P.; Martin, J.A.; Thompson, D. Lead-Free Precussion Primer Mixes Based on Metastable Interstitial Composite (MIC) Technology. U.S. Patent 5,717,159, 1 January 1998. [Google Scholar]
- Focke, W.W.; Tichapondwa, S.M.; Montgomery, Y.C.; Grobler, J.M.; Kalombo, M.L. Review of gasless pyrotechnic time delays. Propellants Explos. Pyrotech. 2019, 44, 55–93. [Google Scholar] [CrossRef] [Green Version]
- Comet, M.; Martin, C.; Schnell, F.; Spitzer, D. Nanothermites: A short review. Factsheet for experimenters, present and future challenges. Propellants Explos. Pyrotech. 2019, 44, 18–36. [Google Scholar] [CrossRef] [Green Version]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Yu, C.; Ren, W.; Wu, G.; Zhang, W.; Hu, B.; Ni, D.; Zheng, Z.; Ma, K.; Ye, J.; Zhu, C. A Facile Preparation and Energetic Characteristics of the Core/Shell CoFe2O4/Al Nanowires Thermite Film. Micromachines 2020, 11, 516. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, L.; Ke, X.; Zhang, T.; Hao, G.; Hu, Y.; Zhang, G.; Guo, H.; Jiang, W. Energetic metastable Al/CuO/PVDF/RDX microspheres with enhanced combustion performance. Chem. Eng. Sci. 2021, 231, 116302. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, J.; Feng, H. Surface modification and functionalization of powder materials by atomic layer deposition: A review. RSC Adv. 2021, 11, 11918–11942. [Google Scholar] [CrossRef]
- Qiao, Z.; Shen, J.; Wang, J.; Huang, B.; Yang, Z.; Yang, G.; Zhang, K. Fast deflagration to detonation transition of energetic material based on a quasi-core/shell structured nanothermite composite. Compos. Sci. Technol. 2015, 107, 113–119. [Google Scholar] [CrossRef]
- Comet, M.; Martin, C.; Klaumünzer, M.; Schnell, F.; Spitzer, D. Energetic nanocomposites for detonation initiation in high explosives without primary explosives. Appl. Phys. Lett. 2015, 107, 243108. [Google Scholar] [CrossRef]
- Levitas, V.I.; Asay, B.W.; Son, S.F.; Pantoya, M. Melt dispersion mechanism for fast reaction of nanothermites. Appl. Phys. Lett. 2006, 89, 071909. [Google Scholar] [CrossRef]
- Nie, H.q.; Chan, H.Y.; Pisharath, S.; Hng, H.H. Combustion characteristic and aging behavior of bimetal thermite powders. Def. Technol. 2021, 17, 755–762. [Google Scholar] [CrossRef]
- Ji, F.; Yin, H.; Zhang, H.; Zhang, Y.; Lai, B. Treatment of military primary explosives wastewater containing lead styphnate (LS) and lead azide (LA) by mFe0-PS-O3 process. J. Clean. Prod. 2018, 188, 860–870. [Google Scholar] [CrossRef]
- Fronabarger, J.; Williams, M.; Bichay, M. Environmentally acceptable alternatives to lead azide and lead styphnate. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007; p. 5132. [Google Scholar]
- Bentley, R.; Sleight, B., III; Macek, K. Preliminary Evaluation of the Acute Toxicity of Desensitized Primer Compounds and Primer Waste Effluents to Representative Aquatic Organisims; Technical report; EG and G Bionomics: Wareham, MA, USA; FAO: Rome, Italy, 1975. [Google Scholar]
- Burrows, D.; Dacre, J.C. Toxicity to Aquatic Organisms and Chemistry of Nine Selected Waterborne Pollutants from Munitions Manufacture—A Literature Evaluation; Technical report; Army Medical Bioengineering Research and Development Laboratory: Fort Detrick, MD, USA; NTIS: Alexandria, VI, USA, 1975. [Google Scholar]
- Apperson, S.; Shende, R.; Subramanian, S.; Tappmeyer, D.; Gangopadhyay, S.; Chen, Z.; Gangopadhyay, K.; Redner, P.; Nicholich, S.; Kapoor, D. Generation of fast propagating combustion and shock waves with copper oxide/aluminum nanothermite composites. Appl. Phys. Lett. 2007, 91, 243109. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Ang, Q.; Li, N.; Shan, C.; Li, Y.; Zhang, L.; Zhu, S. Sulfate-based nanothermite: A green substitute of primary explosive containing lead. ACS Sustain. Chem. Eng. 2018, 6, 8584–8590. [Google Scholar] [CrossRef]
- Kabra, S.; Gharde, S.; Gore, P.M.; Jain, S.; Khire, V.H.; Kandasubramanian, B. Recent trends in nanothermites: Fabrication, characteristics and applications. Nano Express 2020, 1, 032001. [Google Scholar] [CrossRef]
- Guoli, F.; Weipeng, C.; Feng, L. Nanometer Core Shell Structure Thermite and Preparation Method Thereof. CN Patent 104551005A, 4 January 2017. [Google Scholar]
- Guizong, W.; Rong, P. Activated Boron Powder and Preparation Method Thereof. CN Patent 111689821A, 22 September 2020. [Google Scholar]
- Yifan, J.; Fengqi, Z.; Yanjing, Y.; Ting, A.; Hui, L.; Ming, Z.; Jiankan, Z.; Zhoufeng, J.; Na, L. Double-Layer Core-Shell Structure Thermite and Preparation Method Thereof. CN Patent 112250530A, 8 October 2021. [Google Scholar]
- Dai, J.; Wang, C.; Wang, Y.; Xu, W.; Xu, J.; Shen, Y.; Zhang, W.; Ye, Y.; Shen, R. From nanoparticles to on-chip 3D nanothermite: Electrospray deposition of reactive Al/CuO@ NC onto semiconductor bridge and its application for rapid ignition. Nanotechnology 2020, 31, 195712. [Google Scholar] [CrossRef]
- Wang, H.; Jian, G.; Egan, G.C.; Zachariah, M.R. Assembly and reactive properties of Al/CuO based nanothermite microparticles. Combust. Flame 2014, 161, 2203–2208. [Google Scholar] [CrossRef]
- Chen, L.; Ru, C.; Zhang, H.; Zhang, Y.; Chi, Z.; Wang, H.; Li, G. Assembling Hybrid Energetic Materials with Controllable Interfacial Microstructures by Electrospray. ACS Omega 2021, 6, 16816–16825. [Google Scholar] [CrossRef]
- Weeks, N.J.; Gazmin, E.; Iacono, S.T. Optimizing the Interfaces of Energetic Textiles with Perfluorinated Oligomer-Coated Aluminum Nanoparticles: Implications for Metastable Intermolecular Composites. ACS Appl. Nano Mater. 2021, 4, 6002–6011. [Google Scholar] [CrossRef]
- Sui, H.; Atashin, S.; Wen, J.Z. Thermo-chemical and energetic properties of layered nano-thermite composites. Thermochim. Acta 2016, 642, 17–24. [Google Scholar] [CrossRef]
- Guo, X.; Liang, T.; Bao, H.; Yuan, C.; Lai, C.; Tang, C.; Giwa, A. Novel electrophoretic assembly design of nano-aluminum@ tungsten trioxide (nano-Al@WO3) energetic coating with controllable exothermic performance. J. Mater. Sci. Mater. Electron. 2021, 32, 15242–15250. [Google Scholar] [CrossRef]
- Yang, H.; Yang, G.; Li, X.; Bao, H.; Yang, Y.; Guo, X.; Qiao, Z.; Li, X. Facile synthesis of high tightly ordered Al/CuO core-shell nanowire arrays and the effect of surface density on combustion. J. Alloy. Compd. 2021, 877, 160025. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, W.; Cheng, J.; Yu, C.; Shen, R.; Ye, J.; Qin, Z.; Chao, Y. A high energy output and low onset temperature nanothermite based on three-dimensional ordered macroporous nano-NiFe2O4. RSC Adv. 2016, 6, 93330–93334. [Google Scholar] [CrossRef] [Green Version]
- Salvagnac, L.; Assié-Souleille, S.; Rossi, C. Layered Al/CuO thin films for tunable ignition and actuations. Nanomaterials 2020, 10, 2009. [Google Scholar] [CrossRef]
- Zhao, N.; He, C.; Liu, J.; Gong, H.; An, T.; Xu, H.; Zhao, F.; Hu, R.; Ma, H.; Zhang, J. Dependence of catalytic properties of Al/Fe2O3 thermites on morphology of Fe2O3 particles in combustion reactions. J. Solid State Chem. 2014, 219, 67–73. [Google Scholar] [CrossRef]
- D’arche, S.P.; Swanson, T.; Molof, B. Thermite Torch Formulation Including Molybdenum Trioxide. U.S. Patent 7,998,291, 16 August 2011. [Google Scholar]
- Cudziło, S.; Maranda, A.; Nowaczewski, J.; Trębiński, R.; Trzciński, W. Wojskowe Materiały Wybuchowe; Wydawnictwo Politechniki: Częstochowskiej, Poland, 2000. [Google Scholar]
- EN 13631-3:2005; Explosives for Civil Uses—High Explosives—Part 3: Determination of Sensitiveness to Friction of Explosives. European Standard: Pilsen, Czech Republic, 2005.
- Agreement, S. 4487 (STANAG 4487); Explosives, Friction Sensitivity Tests; NATO: Brussels, Belgium, 2002. [Google Scholar]
- Walker, G.R.; Whitbread, E.G.; Hornig, D.C. Manual of Sensitiveness Tests; Canadian Armament Research and Development Establishment: Valcartier, QC, Canada, 1966. [Google Scholar]
- Fair, H.D.; Walker, R.F. Energetic Materials, Volume 2: Technology of the Inorganic Azides, 1st ed.; Plenum Press: New York, NY, USA, 1977. [Google Scholar]
- EN 13631-4:2002; Explosives for Civil Uses. High Explosives Determination of Sensitiveness to Impact of Explosives. European Standard: Pilsen, Czech Republic, 2002.
- NATO. 4489 (STANAG 4489), Explosives, Impact Sensitivity Tests; NATO: Brussels, Belgium, 1999. [Google Scholar]
- Gruhne, M.S.; Lommel, M.; Wurzenberger, M.H.; Szimhardt, N.; Klapötke, T.M.; Stierstorfer, J. OZM Ball Drop Impact Tester (BIT-132) vs. BAM Standard Method—A Comparative Investigation. Propellants Explos. Pyrotech. 2020, 45, 147–153. [Google Scholar] [CrossRef]
- MIL-STD-1751A; Department of Defense Test Method Standard: Safety and Performance Tests for the Qualification of Explosives (High Explosives, Propellants, and Pyrotechnics) Issued on 11 December 2001. United States Department of Defense: Washington, VA, USA, 2001.
- UN. Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria, 6th revised ed.; United Nations: New York, NY, USA; Geneva, Switzerland, 2015. [Google Scholar]
- Maranda, A. Metody badań wrażliwości materiałów wybuchowych na bodźce zewnętrzne w aspekcie przepisów ADR oraz norm polskich i europejskich. Górnictwo I Geoinżynieria 2004, 28, 349–360. [Google Scholar]
- Weston, A.M.; Eukel, W.W.G.L. Data Analysis of the Reaction Behaviour of Explosive Materials Subjected to Susan Test Impacts; U.S. Department of Energy, Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1970.
- Ma, D.; Chen, P.; Dai, K.; Zhou, Q. Specimen size effect of explosive sensitivity under low velocity impact. J. Phys. Conf. Ser. 2014, 500, 052026. [Google Scholar] [CrossRef]
- Ma, D.; Chen, P.; Zhou, Q.; Dai, K. Ignition criterion and safety prediction of explosives under low velocity impact. J. Appl. Phys. 2013, 114, 113505. [Google Scholar] [CrossRef]
- Chidester, S.; Tarver, C.; DePiero, A.; Garza, R. Single and multiple impact ignition of new and aged high explosives in the Steven impact test. AIP Conf. Proc. Am. Inst. Phys. 2000, 505, 663–666. [Google Scholar]
- Gibot, P.; Goetz, V. Aluminium/tin (IV) oxide thermite composite: Sensitivities and reaction propagation. J. Energ. Mater. 2020, 38, 295–308. [Google Scholar] [CrossRef]
- Gibot, P.; Goetz, V. SnO2–polyaniline composites for the desensitization of Al/SnO2 thermite composites. J. Appl. Polym. Sci. 2020, 137, 48947. [Google Scholar] [CrossRef]
- Gibot, P.; Puel, E. Study on Indium (III) Oxide/Aluminum Thermite Energetic Composites. J. Compos. Sci. 2021, 5, 166. [Google Scholar] [CrossRef]
- Kelly, D.G.; Beland, P.; Brousseau, P.; Petre, C.F. Formation of additive-containing nanothermites and modifications to their friction sensitivity. J. Energ. Mater. 2017, 35, 331–345. [Google Scholar] [CrossRef]
- Siegert, B.; Comet, M.; Muller, O.; Pourroy, G.; Spitzer, D. Reduced-sensitivity nanothermites containing manganese oxide filled carbon nanofibers. J. Phys. Chem. C 2010, 114, 19562–19568. [Google Scholar] [CrossRef]
- Phuoc, T.X. Laser-induced spark ignition fundamental and applications. Opt. Lasers Eng. 2006, 44, 351–397. [Google Scholar] [CrossRef]
- Chernai, A. On the mechanism of ignition of condensed secondary explosives by a laser pulse. Combust. Explos. Shock Waves 1996, 32, 8–15. [Google Scholar] [CrossRef]
- Menichelli, V.J. Technical Report 32-1474 Sensitivity of Explosives to Laser Energy; U.S. National Aeronautics and Space Administration, Jet Propulsion Laboratory, California Institute of Technology, Pasadena: La Cañada Flintridge, CA, USA, 1970.
- Belzowski, J. Koordynacyjne zwiąZki Metali Przejściowych Jako Materiały Wybuchowe Specjalnego Przeznaczenia; Silesian Universiy of Technology: Gliwice, Poland, 2011. [Google Scholar]
- He, S.; Chen, J.; Yang, G.; Qiao, Z.; Li, J. Controlled synthesis and application of nano-energetic materials based on the copper oxide/Al system. Cent. Eur. J. Energ. Mater. 2015, 12, 129–144. [Google Scholar]
- Dolgoborodov, A.Y.; Kirilenko, V.G.; Brazhnikov, M.A.; Grishin, L.I.; Kuskov, M.L.; Valyano, G.E. Ignition of nanothermites by a laser diode pulse. Def. Technol. 2021, 18, 194–204. [Google Scholar] [CrossRef]
- Granier, J.J.; Pantoya, M.L. Laser ignition of nanocomposite thermites. Combust. Flame 2004, 138, 373–383. [Google Scholar] [CrossRef]
- Saceleanu, F.; Idir, M.; Chaumeix, N.; Wen, J.Z. Combustion characteristics of physically mixed 40 nm aluminum/copper oxide nanothermites using laser ignition. Front. Chem. 2018, 6, 465. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, J.; Wang, S.; Li, H.; Yan, Q.; Xu, K. High-energy Al/graphene oxide/CuFe2O4 nanocomposite fabricated by self-assembly: Evaluation of heat release, ignition behavior, and catalytic performance. Energ. Mater. Front. 2021, 2, 22–31. [Google Scholar] [CrossRef]
- Fahd, A.; Baranovsky, A.; Dubois, C.; Chaouki, J.; Wen, J.Z. Superior performance of quaternary NC/GO/Al/KClO4 nanothermite for high speed impulse small-scale propulsion applications. Combust. Flame 2021, 232, 111527. [Google Scholar] [CrossRef]
- Petre, C.F.; Chamberland, D.; Ringuette, T.; Ringuette, S.; Paradis, S.; Stowe, R. Low-power laser ignition of aluminum/metal oxide nanothermites. Int. J. Energ. Mater. Chem. Propuls. 2014, 13, 479–494. [Google Scholar] [CrossRef]
- Kirilenko, V.; Grishin, L.; Dolgoborodov, A.Y.; Brazhnikov, M. Laser initiation of nanothermites Al/CuO and Al/Bi2O3. Goren Vzryv (Mosk.)-Combust. Explos. 2020, 13, 145e55. [Google Scholar]
- Guo, W.; Chang, S.; Cao, J.; Wu, L.; Shen, R.; Ye, Y. Precisely Controlled Reactive Multilayer Films with Excellent Energy Release Property for Laser-Induced Ignition. Nanoscale Res. Lett. 2019, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, W.; Shen, R.; Xu, X.; Cheng, J.; Ye, J.; Qin, Z.; Chao, Y. 3D ordered macroporous NiO/Al nanothermite film with significantly improved higher heat output, lower ignition temperature and less gas production. Mater. Des. 2016, 110, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; Zhang, W.; Shen, R.; Ye, J.; Qin, Z.; Chao, Y. Three-dimensionally ordered macroporous structure enabled nanothermite membrane of Mn2O3/Al. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Yu, H.; Zhang, W.; Shen, R.; Guo, W.; DeLuca, L.T.; Wang, H.; Ye, Y. Sponge-like Al/PVDF films with laser sensitivity and high combustion performance prepared by rapid phase inversion. Chem. Eng. J. 2020, 396, 124962. [Google Scholar] [CrossRef]
- Doorenbos, Z.; Walters, I.; Redner, P.; Kapoor, D.; Balas-Hummers, W.; Swiatkiewicz, J.; Puszynski, J. The effect of gaseous additives on dynamic pressure output and ignition sensitivity of nanothermites. AIP Conf. Proc. Am. Inst. Phys. 2012, 1426, 547–550. [Google Scholar]
- Qin, L.; Yan, N.; Li, J.; Hao, H.; Zhao, F.; Feng, H. Enhanced energy performance from core–shell structured Al@Fe2O3 nanothermite fabricated by atomic layer deposition. RSC Adv. 2017, 7, 7188–7197. [Google Scholar] [CrossRef] [Green Version]
- Puszynski, J.A.; Bulian, C.J.; Swiatkiewicz, J.J. Ignition characteristics of nanothermite systems. Int. J. Energ. Mater. Chem. Propuls. 2008, 7, 73–86. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Yang, Y.; Zhao, F.; Li, H.; Xu, K. Enhanced Thermal Decomposition and Laser Ignition Properties of Nanostructured NC/Al/RDX Composite Fibers Fabricated by Electrospinning. Cellulose 2021. [Google Scholar] [CrossRef]
- Chamberland, D. Fullerene Derivatives and Aluminum-Based Nanothermites as Potential New Ammunition Primers; Technical report; Numerica Technologies Inc.: Quebec, QC, Canada, 2013. [Google Scholar]
- Pantoya, M.L.; Granier, J.J. Combustion behavior of highly energetic thermites: Nano versus micron composites. Propellants, Explos. Pyrotech. Int. J. Deal. Sci. Technol. Asp. Energ. Mater. 2005, 30, 53–62. [Google Scholar] [CrossRef]
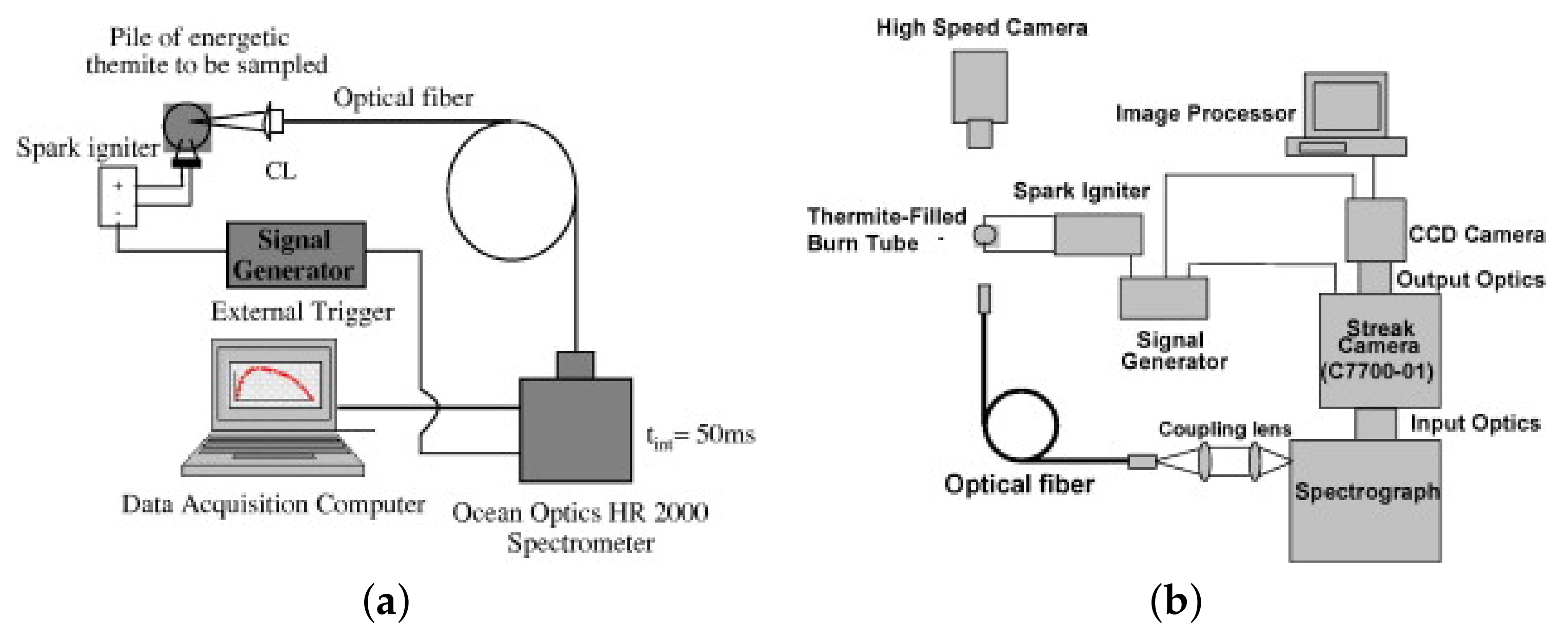
- Gottfried, J.L.; Wainwright, E.R.; Huang, S.; Jiang, Y.; Zheng, X. Probing boron thermite energy release at rapid heating rates. Combust. Flame 2021, 231, 111491. [Google Scholar] [CrossRef]
- Gottfried, J.L. Laboratory-Scale Method for Estimating Explosive Performance from Laser-Induced Shock Waves. Propellants Explos. Pyrotech. 2015, 40, 674–681. [Google Scholar] [CrossRef]
- Singh, R.K. Transient plasma shielding effects during pulsed laser ablation of materials. J. Electron. Mater. 1996, 25, 125–129. [Google Scholar] [CrossRef]
- Gottfried, J.L. Influence of exothermic chemical reactions on laser-induced shock waves. Phys. Chem. Chem. Phys. 2014, 16, 21452–21466. [Google Scholar] [CrossRef]
- Sommersel, O.; Bjerketvedt, D.; Christensen, S.; Krest, O.; Vaagsaether, K. Application of background oriented schlieren for quantitative measurements of shock waves from explosions. Shock Waves 2008, 18, 291–297. [Google Scholar] [CrossRef]
- Meir, Y.; Jerby, E. Thermite powder ignition by localized microwaves. Combust. Flame 2012, 159, 2474–2479. [Google Scholar] [CrossRef]
- Barkley, S.J.; Lawrence, A.R.; Zohair, M.; Smithhisler, O.L.; Pint, C.L.; Michael, J.B.; Sippel, T.R. Smart Electromagnetic Thermites: GO/rGO Nanoscale Thermite Composites with Thermally Switchable Microwave Ignitability. ACS Appl. Mater. Interfaces 2021, 13, 39678–39688. [Google Scholar] [CrossRef]
- Crane, C.; Pantoya, M.; Weeks, B. Investigating the trade-offs of microwave susceptors in energetic composites: Microwave heating versus combustion performance. J. Appl. Phys. 2014, 115, 104106. [Google Scholar] [CrossRef]
- Barkley, S.J.; Zhu, K.; Lynch, J.E.; Michael, J.B.; Sippel, T.R. Microwave plasma enhancement of multiphase flames: On-demand control of solid propellant burning rate. Combust. Flame 2019, 199, 14–23. [Google Scholar] [CrossRef]
- Hwang, J.; Bae, C.; Park, J.; Choe, W.; Cha, J.; Woo, S. Microwave-assisted plasma ignition in a constant volume combustion chamber. Combust. Flame 2016, 167, 86–96. [Google Scholar] [CrossRef]
- Crane, C.; Pantoya, M.; Weeks, B. Spatial observation and quantification of microwave heating in materials. Rev. Sci. Instrum. 2013, 84, 084705. [Google Scholar] [CrossRef] [PubMed]
- EN 13938-2:2005; Explosives for Civil Uses—Propellants and Rocket Propellants—Part 2: Determination of Resistance to Electrostatic Energy. European Standard: Pilsen, Czech Republic, 2005.
- Dai, J.; Xu, J.; Wang, F.; Tai, Y.; Shen, Y.; Shen, R.; Ye, Y. Facile formation of nitrocellulose-coated Al/Bi2O3 nanothermites with excellent energy output and improved electrostatic discharge safety. Mater. Des. 2018, 143, 93–103. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Shen, Y.; Wang, C.-a.; Zhang, Z.; Xu, J.; Ye, Y.; Shen, R. Fabrication of high electrostatic safety metastable Al/CuO nanocomposites doped with nitro-functionalized graphene with fast initiation ability and tunable reaction performance. Combust. Flame 2021, 233, 111580. [Google Scholar] [CrossRef]
- NATO. STANAG 4490 Explosives, Electrostatic Discharge Sensitivity Test(S); NATO: Brussels, Belgium, 2001. [Google Scholar]
- Goetz, V.; Gibot, P.; Spitzer, D. Spark sensitivity and light signature mitigation of an Al/SnO2 nanothermite by the controlled addition of a conductive polymer. Chem. Eng. J. 2022, 427, 131611. [Google Scholar] [CrossRef]
- Gibot, P.; Goetz, V. Polypyrrole material for the electrostatic discharge sensitivity mitigation of Al/SnO2 energetic composites. J. Appl. Polym. Sci. 2021, 138, 50752. [Google Scholar] [CrossRef]
- Gordeev, V.; Kazutin, M.; Kozyrev, N. Effect of additives on CuO/Al nanothermite properties. J. Phys. Conf. Ser. 2017, 894, 012116. [Google Scholar] [CrossRef] [Green Version]
- Foley, T.; Pacheco, A.; Malchi, J.; Yetter, R.; Higa, K. Development of nanothermite composites with variable electrostatic discharge ignition thresholds. Propellants, Explos. Pyrotech. Int. J. Deal. Sci. Technol. Asp. Energ. Mater. 2007, 32, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.; Beland, P.; Brousseau, P.; Petre, C.F. Electrostatic discharge sensitivity and resistivity measurements of Al nanothermites and their fuel and oxidant precursors. Cent. Eur. J. Energ. Mater. 2017, 14, 105–119. [Google Scholar] [CrossRef]
- Weir, C.; Pantoya, M.L.; Ramachandran, G.; Dallas, T.; Prentice, D.; Daniels, M. Electrostatic discharge sensitivity and electrical conductivity of composite energetic materials. J. Electrost. 2013, 71, 77–83. [Google Scholar] [CrossRef]
- Fuh, C.; Lee, J.; Liaw, C. The design aspect of the bruceton test for pyrotechnics sensitivity analysis. J. Data Sci. 2003, 1, 83–101. [Google Scholar] [CrossRef]
- Weir, C.; Pantoya, M.L.; Daniels, M.A. The role of aluminum particle size in electrostatic ignition sensitivity of composite energetic materials. Combust. Flame 2013, 160, 2279–2281. [Google Scholar] [CrossRef]
- Gibot, P.; Oudot, F.; Lallemand, B.; Schnell, F.; Spitzer, D. Nanosized niobium (V) and tantalum (V) oxide ceramics as competitive oxidizers within aluminium-based nanothermites. Energ. Mater. Front. 2021, 2, 167–173. [Google Scholar] [CrossRef]
- Bach, A.; Gibot, P.; Vidal, L.; Gadiou, R.; Spitzer, D. Modulation of the reactivity of a WO3/Al energetic material with graphitized carbon black as additive. J. Energ. Mater. 2015, 33, 260–276. [Google Scholar] [CrossRef]
- Thiruvengadathan, R.; Belarde, G.M.; Bezmelnitsyn, A.; Shub, M.; Balas-Hummers, W.; Gangopadhyay, K.; Gangopadhyay, S. Combustion Characteristics of Silicon-Based Nanoenergetic Formulations with Reduced Electrostatic Discharge Sensitivity. Propellants Explos. Pyrotech. 2012, 37, 359–372. [Google Scholar] [CrossRef]
- Comet, M.; Vidick, G.; Schnell, F.; Suma, Y.; Baps, B.; Spitzer, D. Sulfates-based nanothermites: An expanding horizon for metastable interstitial composites. Angew. Chem. Int. Ed. 2015, 54, 4458–4462. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Damiri, S.; Ghaemi, E.F. Non-isothermal studies on the thermal decomposition of C4 explosive using the TG/DTA technique. Cent. Eur. J. Energ. Mater. 2014, 11, 405–416. [Google Scholar]
- Lafontaine, E.; Comet, M. Nanothermites; Wiley-ISTE: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cheng, J.; Hng, H.; Lee, Y.; Du, S.; Thadhani, N. Kinetic study of thermal-and impact-initiated reactions in Al–Fe2O3 nanothermite. Combust. Flame 2010, 157, 2241–2249. [Google Scholar] [CrossRef]
- Jian, G.; Feng, J.; Jacob, R.J.; Egan, G.C.; Zachariah, M.R. Super-reactive nanoenergetic gas generators based on periodate salts. Angew. Chem. 2013, 125, 9925–9928. [Google Scholar] [CrossRef]
- Zhou, W.; DeLisio, J.B.; Li, X.; Liu, L.; Zachariah, M.R. Persulfate salt as an oxidizer for biocidal energetic nano-thermites. J. Mater. Chem. A 2015, 3, 11838–11846. [Google Scholar] [CrossRef]
- Wang, C.-a.; Xu, J.-b.; Shen, Y.; Wang, Y.-t.; Yang, T.-l.; Zhang, Z.-h.; Li, F.-w.; Shen, R.-q.; Ye, Y.-h. Thermodynamics and performance of Al/CuO nanothermite with different storage time. Def. Technol. 2021, 17, 741–747. [Google Scholar] [CrossRef]
- Jian, G.; Chowdhury, S.; Sullivan, K.; Zachariah, M.R. Nanothermite reactions: Is gas phase oxygen generation from the oxygen carrier an essential prerequisite to ignition? Combust. Flame 2013, 160, 432–437. [Google Scholar] [CrossRef]
- Sarkar, S. Platinum RTD sensor based multi-channel high-precision temperature measurement system for temperature range −100 °C to +100 °C using single quartic function. Cogent Eng. 2018, 5, 1558687. [Google Scholar] [CrossRef]
- Jian, G.; Zhou, L.; Piekiel, N.W.; Zachariah, M.R. Low Effective Activation Energies for Oxygen Release from Metal Oxides: Evidence for Mass-Transfer Limits at High Heating Rates. ChemPhysChem 2014, 15, 1666–1672. [Google Scholar] [CrossRef]
- Li, S.; Guo, T.; Yao, M.; Ding, W.; Chen, J.; Song, J.; Zhang, S.; Zhu, R. Thermal behavior and combustion performance of Al/Bi2O3 nano thermites with the effect of potassium perchlorate. Mater. Res. Express 2021, 8, 035507. [Google Scholar] [CrossRef]
- Chen, J.; Guo, T.; Ding, W.; Song, J.; Yao, M.; Bei, F.; Li, S. Effect of CuO on the thermal kinetics and combustion properties of Al/MoO3 thermite prepared by ball milling. Ceram. Int. 2021, 47, 16500–16510. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Kline, D.J.; Eckman, N.; Agrawal, N.R.; Wu, T.; Wang, P.; Zachariah, M.R. Direct writing of a 90 wt% particle loading nanothermite. Adv. Mater. 2019, 31, 1806575. [Google Scholar] [CrossRef]
- Wang, H.; DeLisio, J.B.; Jian, G.; Zhou, W.; Zachariah, M.R. Electrospray formation and combustion characteristics of iodine-containing Al/CuO nanothermite microparticles. Combust. Flame 2015, 162, 2823–2829. [Google Scholar] [CrossRef]
- Sullivan, K.; Piekiel, N.; Chowdhury, S.; Wu, C.; Zachariah, M.; Johnson, C. Ignition and combustion characteristics of nanoscale Al/AgIO3: A potential energetic biocidal system. Combust. Sci. Technol. 2010, 183, 285–302. [Google Scholar] [CrossRef]
- Rehwoldt, M.C.; Yang, Y.; Wang, H.; Holdren, S.; Zachariah, M.R. Ignition of nanoscale titanium/potassium perchlorate pyrotechnic powder: Reaction mechanism study. J. Phys. Chem. C 2018, 122, 10792–10800. [Google Scholar] [CrossRef]
- Xu, F.; Biswas, P.; Nava, G.; Schwan, J.; Kline, D.J.; Rehwoldt, M.C.; Mangolini, L.; Zachariah, M.R. Tuning the reactivity and energy release rate of I2O5 based ternary thermite systems. Combust. Flame 2021, 228, 210–217. [Google Scholar] [CrossRef]
- Wu, T.; Wang, X.; Zavalij, P.Y.; DeLisio, J.B.; Wang, H.; Zachariah, M.R. Performance of iodine oxides/iodic acids as oxidizers in thermite systems. Combust. Flame 2018, 191, 335–342. [Google Scholar] [CrossRef]
- Gibot, P.; Miesch, Q.; Bach, A.; Schnell, F.; Gadiou, R.; Spitzer, D. Mechanical desensitization of an Al/WO3 nanothermite by means of carbonaceous coatings derived from carbohydrates. C 2019, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Yao, B.; Chan, Y.; Zhang, X.Y. Enhanced photothermal effect in Si nanowires. Nano Lett. 2003, 3, 475–477. [Google Scholar] [CrossRef]
- Ohkura, Y.; Rao, P.M.; Zheng, X. Flash ignition of Al nanoparticles: Mechanism and applications. Combust. Flame 2011, 158, 2544–2548. [Google Scholar] [CrossRef]
- Ohkura, Y.; Rao, P.M.; Sun Cho, I.; Zheng, X. Reducing minimum flash ignition energy of Al microparticles by addition of WO3 nanoparticles. Appl. Phys. Lett. 2013, 102, 043108. [Google Scholar] [CrossRef]
- Zheng, X. Quantification of Ignition Properties of Porous Si Based Energetics; Technical report; Stanford University Stanford United States: Stanford, CA, USA, 2018. [Google Scholar]
- Son, S.F.; Asay, B.; Foley, T.; Yetter, R.; Wu, M.; Risha, G. Combustion of nanoscale Al/MoO3 thermite in microchannels. J. Propuls. Power 2007, 23, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Mason, B.A.; Groven, L.J.; Son, S.F.; Yetter, R.A. Combustion performance of several nanosilicon-based nanoenergetics. J. Propuls. Power 2013, 29, 1435–1444. [Google Scholar] [CrossRef]
- Epps, J.M.; Hickey, J.P.; Wen, J.Z. Modelling reaction propagation for Al/CuO nanothermite pellet combustion. Combust. Flame 2021, 229, 111374. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, J.; Xu, J.; Shen, Y.; Wang, C.a.; Ye, Y.; Shen, R. Experimental and numerical investigations of the effect of charge density and scale on the heat transfer behavior of Al/CuO nano-thermite. Vacuum 2021, 184, 109878. [Google Scholar] [CrossRef]
- Fried, L.; Souers, P. Cheetah: A Next Generation Thermochemical Code; Technical report; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1994. [Google Scholar]
- Sućeska, M. Evaluation of detonation energy from EXPLO5 computer code results. Propellants Explos. Pyrotech. 1999, 24, 280–285. [Google Scholar] [CrossRef]
- Farley, C.W.; Pantoya, M.L.; Losada, M.; Chaudhuri, S. Linking molecular level chemistry to macroscopic combustion behavior for nano-energetic materials with halogen containing oxides. J. Chem. Phys. 2013, 139, 074701. [Google Scholar] [CrossRef]
- Němec, O.; Musil, T.; Künzel, M. The Effect of Detonator Shell Materials on Detonation Calorimetry Results. Cent. Eur. J. Energ. Mater. 2020, 17, 552–565. [Google Scholar] [CrossRef]
- Shen, L.; Li, G.; Luo, Y.; Gao, K.; Ge, Z. Preparation and characterization of Al/B/Fe2O3 nanothermites. Sci. China Chem. 2014, 57, 797–802. [Google Scholar] [CrossRef]
- Korchagina, E.; Ermakova, E.; Belyakov, V. A comparative analysis of the technical and metrological characteristics of bomb calorimeters used in Russia. Meas. Tech. 2011, 54, 186–193. [Google Scholar] [CrossRef]
- Nagano, Y.; Sugimoto, T. Micro-combustion calorimetry aiming at 1 mg samples. J. Therm. Anal. Calorim. 1999, 57, 867–874. [Google Scholar] [CrossRef]
- McEwan, W.S.; Anderson, C.M. Miniature bomb calorimeter for the determination of heats of combustion of samples of the order of 50 mg mass. Rev. Sci. Instrum. 1955, 26, 280–284. [Google Scholar] [CrossRef]
- Rojas-Aguilar, A. An isoperibol micro-bomb combustion calorimeter for measurement of the enthalpy of combustion. Application to the study of fullerene C60. J. Chem. Thermodyn. 2002, 34, 1729–1743. [Google Scholar] [CrossRef]
- Overdeep, K.R.; Weihs, T.P. Design and functionality of a high-sensitivity bomb calorimeter specialized for reactive metallic foils. J. Therm. Anal. Calorim. 2015, 122, 787–794. [Google Scholar] [CrossRef]
- Fahd, A.; Dubois, C.; Chaouki, J.; Wen, J.; Youssef, E. Synthesis and Characterization of Tertiary Nanothermite CNMs/Al/KClO4 with Enhanced Combustion Characteristics. Propellants Explos. Pyrotech. 2021, 46, 995–1005. [Google Scholar] [CrossRef]
- Cervantes, O.G.; Kuntz, J.D.; Gash, A.E.; Munir, Z.A. Heat of combustion of tantalum–tungsten oxide thermite composites. Combust. Flame 2010, 157, 2326–2332. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Jiang, Y.; Huang, S.; Shi, X.; Zhao, J.; Zheng, X. Tuning the morphological, ignition and combustion properties of micron-Al/CuO thermites through different synthesis approaches. Combust. Flame 2018, 195, 303–310. [Google Scholar] [CrossRef]
- Becker, C.; Currano, L. Thermal Analysis and Novel On-Chip Velocity Characterization of Energetic Porous Silicon with Sodium Perchlorate Oxidizer; ARL Summer Student Research Symposium, Volume I: Select Papers; U.S. Army Research Laboratory: Adelphi, MD, USA, 2010; p. 59. [Google Scholar]
- Pivkina, A.; Ulyanova, P.; Frolov, Y.; Zavyalov, S.; Schoonman, J. Nanomaterials for heterogeneous combustion. Propellants, Explos. Pyrotech. Int. J. Deal. Sci. Technol. Asp. Energ. Mater. 2004, 29, 39–48. [Google Scholar] [CrossRef]
- Thakur, P.; Sharma, V.; Thakur, N. Exothermic behaviour of aluminium and graphene as a fuel in Fe2O3 based nanothermite. Zeitschrift für Naturforschung A 2021, 76, 703–710. [Google Scholar] [CrossRef]
- Zhao, W.; Jiao, Q.; Ou, Y.; Yang, R.; Zhu, Y.; Wang, F. Perfluoroalkyl Acid-Functionalized Aluminum Nanoparticles for Fluorine Fixation and Energy Generation. ACS Appl. Nano Mater. 2021, 4, 6337–6344. [Google Scholar] [CrossRef]
- Berthe, J.E.; Comet, M.; Schnell, F.; Suma, Y.; Spitzer, D. Propellants reactivity enhancement with nanothermites. Propellants Explos. Pyrotech. 2016, 41, 994–998. [Google Scholar] [CrossRef]
- Patel, V.K.; Saurav, J.R.; Gangopadhyay, K.; Gangopadhyay, S.; Bhattacharya, S. Combustion characterization and modeling of novel nanoenergetic composites of Co3O4/nAl. RSC Adv. 2015, 5, 21471–21479. [Google Scholar] [CrossRef]
- Valluri, S.K.; Schoenitz, M.; Dreizin, E. Preparation and characterization of silicon-metal fluoride reactive composites. Nanomaterials 2020, 10, 2367. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Li, F. Nanometer ammonium perchlorate and ammonium nitrate prepared with 2D network structure via rapid freezing technology. Nanomaterials 2019, 9, 1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, T.; Wang, Y.; Huang, H.; Shang, F.; Song, X. An electrospun preparation of the NC/GAP/nano-LLM-105 nanofiber and its properties. Nanomaterials 2019, 9, 854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhammada, A.; Trache, D.; Kesraoui, M.; Chelouche, S. Hydrothermal synthesis of hematite nanoparticles decorated on carbon mesospheres and their synergetic action on the thermal decomposition of nitrocellulose. Nanomaterials 2020, 10, 968. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Xu, J.; Ma, X.; Ye, Y.; Yang, G.; Zhang, K. In situ preparation of explosive embedded CuO/Al/CL20 nanoenergetic composite with enhanced reactivity. Chem. Eng. J. 2018, 354, 885–895. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.; Lu, J.; Zhang, K. CuO/Mg/fluorocarbon sandwich-structure superhydrophobic nanoenergetic composite with anti-humidity property. Chem. Eng. J. 2015, 266, 163–170. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Z.; Luo, Q.; Duan, X.; Pei, C. Preparation and thermal decomposition properties of nitrated graphene oxide (NGO)/RDX nano-energetic composites. J. Therm. Anal. Calorim. 2020, 139, 1671–1679. [Google Scholar] [CrossRef]
- Elbasuney, S. Novel colloidal nanothermite particles (MnO2/Al) for advanced highly energetic systems. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1793–1800. [Google Scholar] [CrossRef]
- Luo, Q.; Long, X.; Nie, F.; Liu, G.; Zhu, M. The safety properties of a potential kind of novel green primary explosive: Al/Fe2O3/RDX nanocomposite. Materials 2018, 11, 1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Singh, G.; Kulkarni, N.; Mathe, V.; Bhoraskar, S. Synthesis, characterization and catalytic activity of Al/Fe2O3 nanothermite. J. Therm. Anal. Calorim. 2015, 119, 309–317. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, L.; Jian, X.; Zhou, W. Integration of nano-Al with one-step synthesis of MoO3 nanobelts to realize high exothermic nanothermite. Sci. Eng. Compos. Mater. 2018, 25, 579–585. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, J.Y.; Park, H.S.; Kim, S.H. Optical ignition of nanoenergetic materials: The role of single-walled carbon nanotubes as potential optical igniters. Combust. Flame 2013, 160, 830–834. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, S.; Ren, W.; Zheng, Z.; Chen, J.; Ma, K.; Yu, C.; Zhou, X.; Zhang, W. A Favorable Improvement in Reactivity between n-Al and Sheet-like Porous CuO as a Nanoenergetic Composite by Graphene Oxide Additives. Ind. Eng. Chem. Res. 2020, 59, 12934–12942. [Google Scholar] [CrossRef]
- Mehendale, B.; Shende, R.; Subramanian, S.; Gangopadhyay, S.; Redner, P.; Kapoor, D.; Nicolich, S. Nanoenergetic composite of mesoporous iron oxide and aluminum nanoparticles. J. Energ. Mater. 2006, 24, 341–360. [Google Scholar] [CrossRef]
- Vorozhtsov, A.; Lerner, M.; Rodkevich, N.; Sokolov, S.; Perchatkina, E.; Paravan, C. Preparation and characterization of Al/HTPB composite for high energetic materials. Nanomaterials 2020, 10, 2222. [Google Scholar] [CrossRef]
- Ke, X.; Zhou, X.; Gao, H.; Hao, G.; Xiao, L.; Chen, T.; Liu, J.; Jiang, W. Surface functionalized core/shell structured CuO/Al nanothermite with long-term storage stability and steady combustion performance. Mater. Des. 2018, 140, 179–187. [Google Scholar] [CrossRef]
- Guo, X.; Sun, Q.; Liang, T.; Giwa, A. Controllable electrically guided nano-Al/MoO3 energetic-film formation on a semiconductor bridge with high reactivity and combustion performance. Nanomaterials 2020, 10, 955. [Google Scholar] [CrossRef]
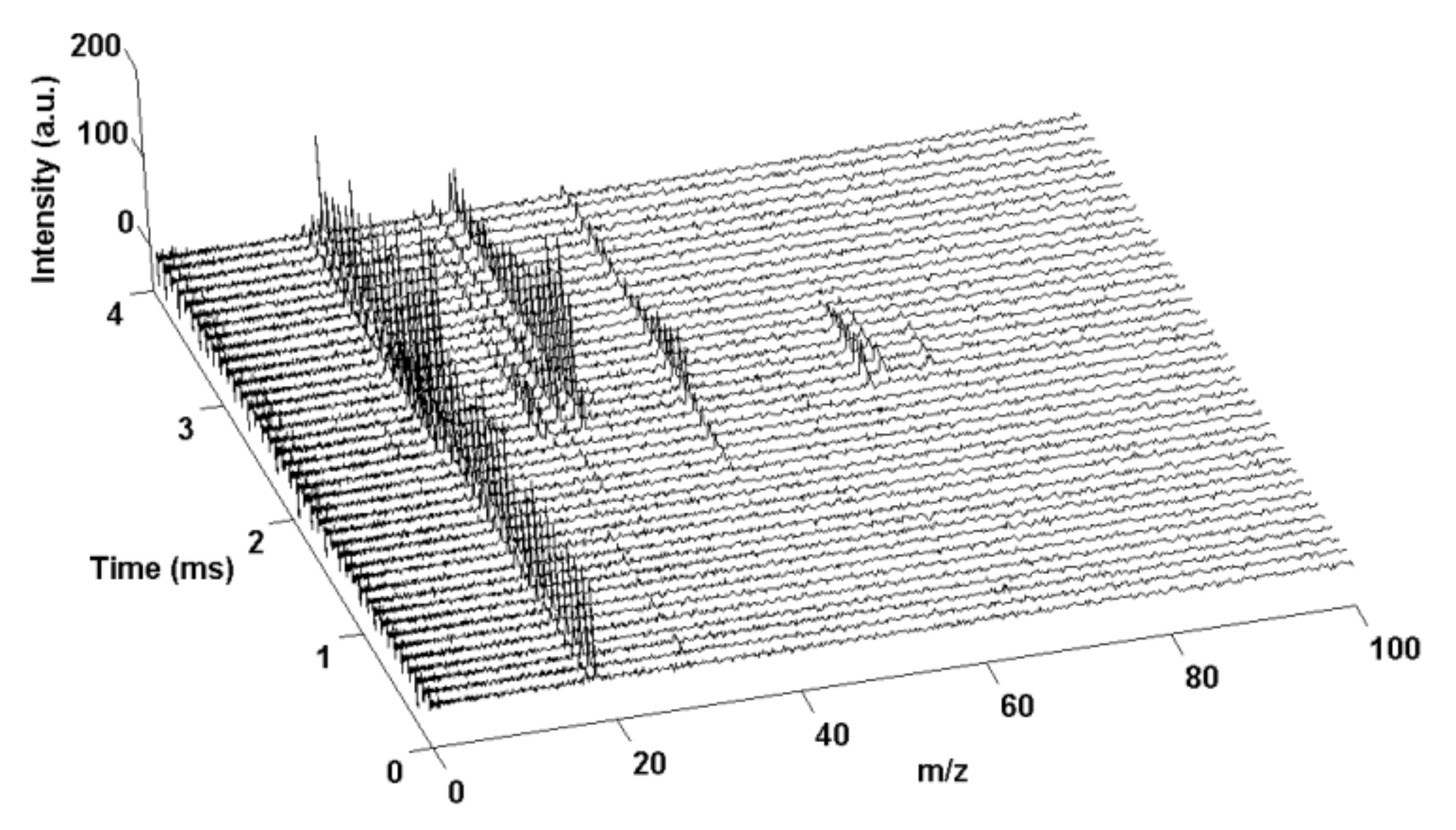
- Zhou, L.; Piekiel, N.; Chowdhury, S.; Zachariah, M. Time-resolved mass spectrometry of the exothermic reaction between nanoaluminum and metal oxides: The role of oxygen release. J. Phys. Chem. C 2010, 114, 14269–14275. [Google Scholar] [CrossRef]
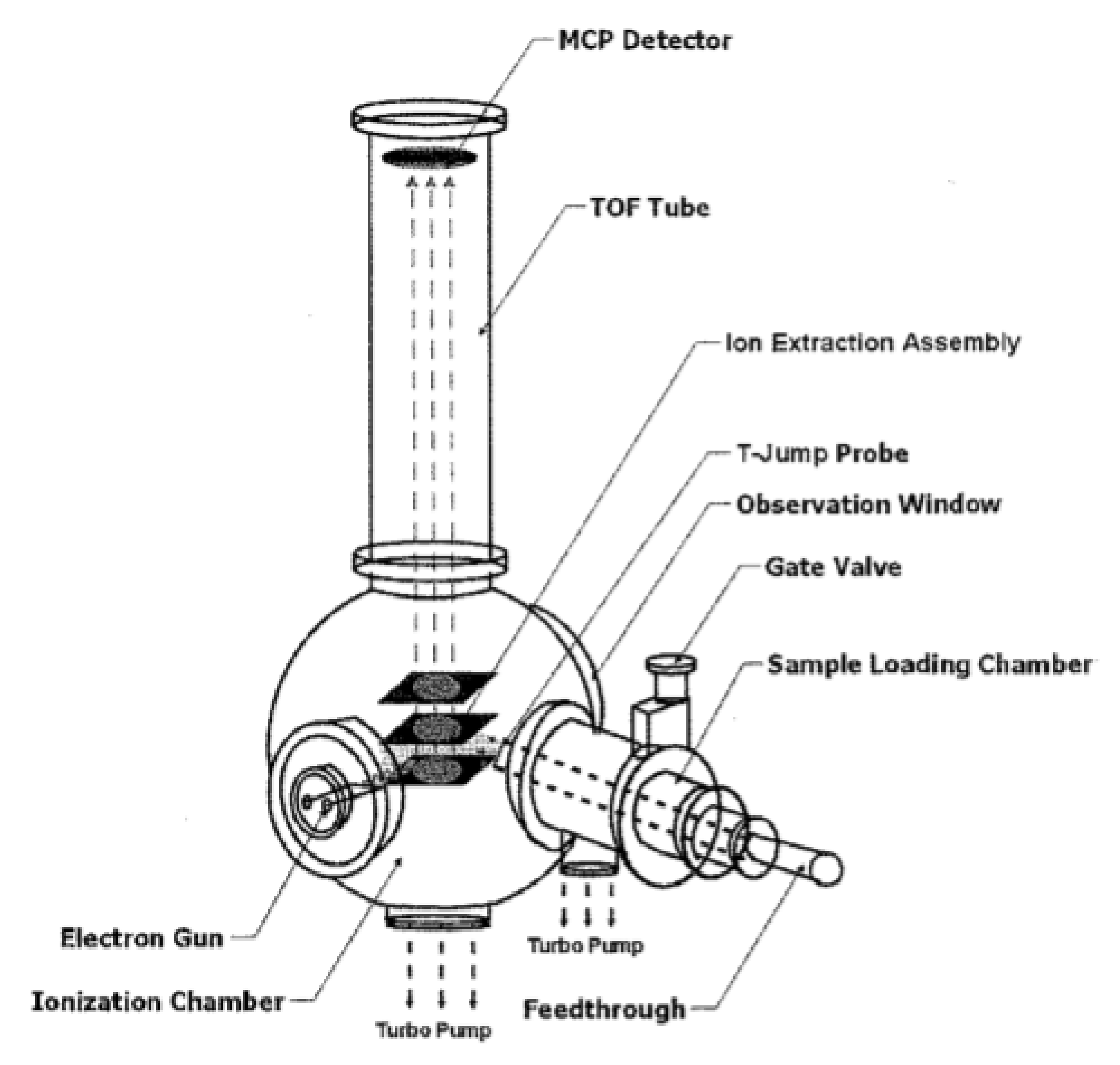
- Zhou, L.; Piekiel, N.; Chowdhury, S.; Zachariah, M.R. T-Jump/time-of-flight mass spectrometry for time-resolved analysis of energetic materials. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up-Res. Mass Spectrom. 2009, 23, 194–202. [Google Scholar] [CrossRef]
- Egan, G.C.; Sullivan, K.T.; Olson, T.Y.; Han, T.Y.J.; Worsley, M.A.; Zachariah, M.R. Ignition and combustion characteristics of nanoaluminum with copper oxide nanoparticles of differing oxidation state. J. Phys. Chem. C 2016, 120, 29023–29029. [Google Scholar] [CrossRef]
- Wu, T.; Zachariah, M.R. Silver ferrite: A superior oxidizer for thermite-driven biocidal nanoenergetic materials. RSC Adv. 2019, 9, 1831–1840. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, K.T.; Wu, C.; Piekiel, N.W.; Gaskell, K.; Zachariah, M.R. Synthesis and reactivity of nano-Ag2O as an oxidizer for energetic systems yielding antimicrobial products. Combust. Flame 2013, 160, 438–446. [Google Scholar] [CrossRef]
- Jian, G.; Chowdhury, S.; Feng, J.; Zachariah, M.R. The ignition and combustion study of nano-al and iodine pentoxide thermite. In Proceedings of the 8th US National Combustion Meeting, Park City, UT, USA, 19–22 May 2013; pp. 1287–1299. [Google Scholar]
- Piekiel, N.; Sullivan, K.; Chowdhury, S.; Zachariah, M. The Role of Metal Oxides in Nanothermite Reactions: Evidence of Condensed Phase Initiation; Technical report; Maryland Univ.: College Park, MD, USA, 2010. [Google Scholar]
- Sui, H.; Huda, N.; Shen, Z.; Wen, J.Z. Al–NiO energetic composites as heat source for joining silicon wafer. J. Mater. Process. Technol. 2020, 279, 116572. [Google Scholar] [CrossRef]
- Jacob, R.J.; Ortiz-Montalvo, D.L.; Overdeep, K.R.; Weihs, T.P.; Zachariah, M.R. Incomplete reactions in nanothermite composites. J. Appl. Phys. 2017, 121, 054307. [Google Scholar] [CrossRef] [Green Version]
- Rafiei, M.; Enayati, M.; Karimzadeh, F. Kinetic analysis of thermite reaction in Al-Ti-Fe2O3 system to produce (Fe, Ti) 3Al-Al2O3 nanocomposite. Powder Technol. 2014, 253, 553–560. [Google Scholar] [CrossRef]
- Miller, H.A.; Kusel, B.S.; Danielson, S.T.; Neat, J.W.; Avjian, E.K.; Pierson, S.N.; Budy, S.M.; Ball, D.W.; Iacono, S.T.; Kettwich, S.C. Metastable nanostructured metallized fluoropolymer composites for energetics. J. Mater. Chem. A 2013, 1, 7050–7058. [Google Scholar] [CrossRef] [Green Version]
- Shende, R.; Subramanian, S.; Hasan, S.; Apperson, S.; Thiruvengadathan, R.; Gangopadhyay, K.; Gangopadhyay, S.; Redner, P.; Kapoor, D.; Nicolich, S.; et al. Nanoenergetic composites of CuO nanorods, nanowires, and Al-nanoparticles. Propellants Explos. Pyrotech. Int. J. Deal. Sci. Technol. Asp. Energ. Mater. 2008, 33, 122–130. [Google Scholar] [CrossRef]
- Yao, E.; Zhao, N.; Qin, Z.; Ma, H.; Li, H.; Xu, S.; An, T.; Yi, J.; Zhao, F. Thermal decomposition behavior and thermal safety of nitrocellulose with different shape CuO and Al/CuO nanothermites. Nanomaterials 2020, 10, 725. [Google Scholar] [CrossRef] [Green Version]
- Martirosyan, K.S. Nanoenergetic gas-generators: Principles and applications. J. Mater. Chem. 2011, 21, 9400–9405. [Google Scholar] [CrossRef]
- Thakur, P.; Sharma, V.; Thakur, N. Study of energy release in Fe2O3/Al nano-thermite with graphene as an additional fuel. Phys. B Condens. Matter 2021, 610, 412803. [Google Scholar] [CrossRef]
- Perry, W.L.; Smith, B.L.; Bulian, C.J.; Busse, J.R.; Macomber, C.S.; Dye, R.C.; Son, S.F. Nano-scale tungsten oxides for metastable intermolecular composites. Propellants Explos. Pyrotech. Int. J. Deal. Sci. Technol. Asp. Energ. Mater. 2004, 29, 99–105. [Google Scholar] [CrossRef]
- Bezmelnitsyn, A.; Thiruvengadathan, R.; Barizuddin, S.; Tappmeyer, D.; Apperson, S.; Gangopadhyay, K.; Gangopadhyay, S.; Redner, P.; Donadio, M.; Kapoor, D.; et al. Modified nanoenergetic composites with tunable combustion characteristics for propellant applications. Propellants Explos. Pyrotech. 2010, 35, 384–394. [Google Scholar] [CrossRef]
- Sahoo, S.; Danali, S.; Arya, P. Ignition behavior of Al/Fe2O3 metastable intermolecular composites. Int. J. Eng. Res. Sci 2017, 3, 61–71. [Google Scholar] [CrossRef]
- Yermekova, Z.; Mansurov, Z.; Mukasyan, A. Combustion synthesis of silicon nanopowders. Int. J. Self-Propagating High-Temp. Synth. 2010, 19, 94–101. [Google Scholar] [CrossRef]
- Thiruvengadathan, R.; Bezmelnitsyn, A.; Apperson, S.; Staley, C.; Redner, P.; Balas, W.; Nicolich, S.; Kapoor, D.; Gangopadhyay, K.; Gangopadhyay, S. Combustion characteristics of novel hybrid nanoenergetic formulations. Combust. Flame 2011, 158, 964–978. [Google Scholar] [CrossRef]
- Elbasuney, S.; Yehia, M.; El-Sayyad, G.S. Bio-inspired metastable intermolecular nanothermite composite based on Manganese dioxide/Polydopamine/Aluminium. J. Mater. Sci. Mater. Electron. 2021, 32, 9158–9170. [Google Scholar] [CrossRef]
- Patel, V.K.; Bhattacharya, S. High-performance nanothermite composites based on aloe-vera-directed CuO nanorods. ACS Appl. Mater. Interfaces 2013, 5, 13364–13374. [Google Scholar] [CrossRef]
- Deng, P.; Liu, Y.; Luo, P.; Wang, J.; Liu, Y.; Wang, D.; He, Y. Two-steps synthesis of sandwich-like graphene oxide/LLM-105 nanoenergetic composites using functionalized graphene. Mater. Lett. 2017, 194, 156–159. [Google Scholar] [CrossRef]
- Lin, C.; Huang, B.; Gong, F.; Yang, Z.; Liu, J.; Zhang, J.; Zeng, C.; Li, Y.; Li, J.; Guo, S. Core@ double-shell structured energetic composites with reduced sensitivity and enhanced mechanical properties. ACS Appl. Mater. Interfaces 2019, 11, 30341–30351. [Google Scholar] [CrossRef]
- Jacob, R.J.; Hill, K.J.; Yang, Y.; Pantoya, M.L.; Zachariah, M.R. Pre-stressing aluminum nanoparticles as a strategy to enhance reactivity of nanothermite composites. Combust. Flame 2019, 205, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, K.T.; Chiou, W.A.; Fiore, R.; Zachariah, M.R. In situ microscopy of rapidly heated nano-Al and nano-Al/WO3 thermites. Appl. Phys. Lett. 2010, 97, 133104. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Piekiel, N.; Chowdhury, S.; Zachariah, M. T-Jump/Time-of-Flight Mass Spectrometery for Time Resolved Analysis of Fast Condensed Stated Reactions. In Proceedings of the 47th AIAA Aerospace Sciences Meeting Including The New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 5–8 January 2009; p. 639. [Google Scholar]
- DeLisio, J.B.; Yi, F.; LaVan, D.A.; Zachariah, M.R. High heating rate reaction dynamics of Al/CuO nanolaminates by nanocalorimetry-coupled time-of-flight mass spectrometry. J. Phys. Chem. C 2017, 121, 2771–2777. [Google Scholar] [CrossRef]
- Weismiller, M.; Lee, J.; Yetter, R. Temperature measurements of Al containing nano-thermite reactions using multi-wavelength pyrometry. Proc. Combust. Inst. 2011, 33, 1933–1940. [Google Scholar] [CrossRef]
- Ng, D.; Fralick, G. Use of a multiwavelength pyrometer in several elevated temperature aerospace applications. Rev. Sci. Instrum. 2001, 72, 1522–1530. [Google Scholar] [CrossRef] [Green Version]
- Bazyn, T.; Krier, H.; Glumac, N. Evidence for the transition from the diffusion-limit in aluminum particle combustion. Proc. Combust. Inst. 2007, 31, 2021–2028. [Google Scholar] [CrossRef]
- Goroshin, S.; Mamen, J.; Higgins, A.; Bazyn, T.; Glumac, N.; Krier, H. Emission spectroscopy of flame fronts in aluminum suspensions. Proc. Combust. Inst. 2007, 31, 2011–2019. [Google Scholar] [CrossRef]
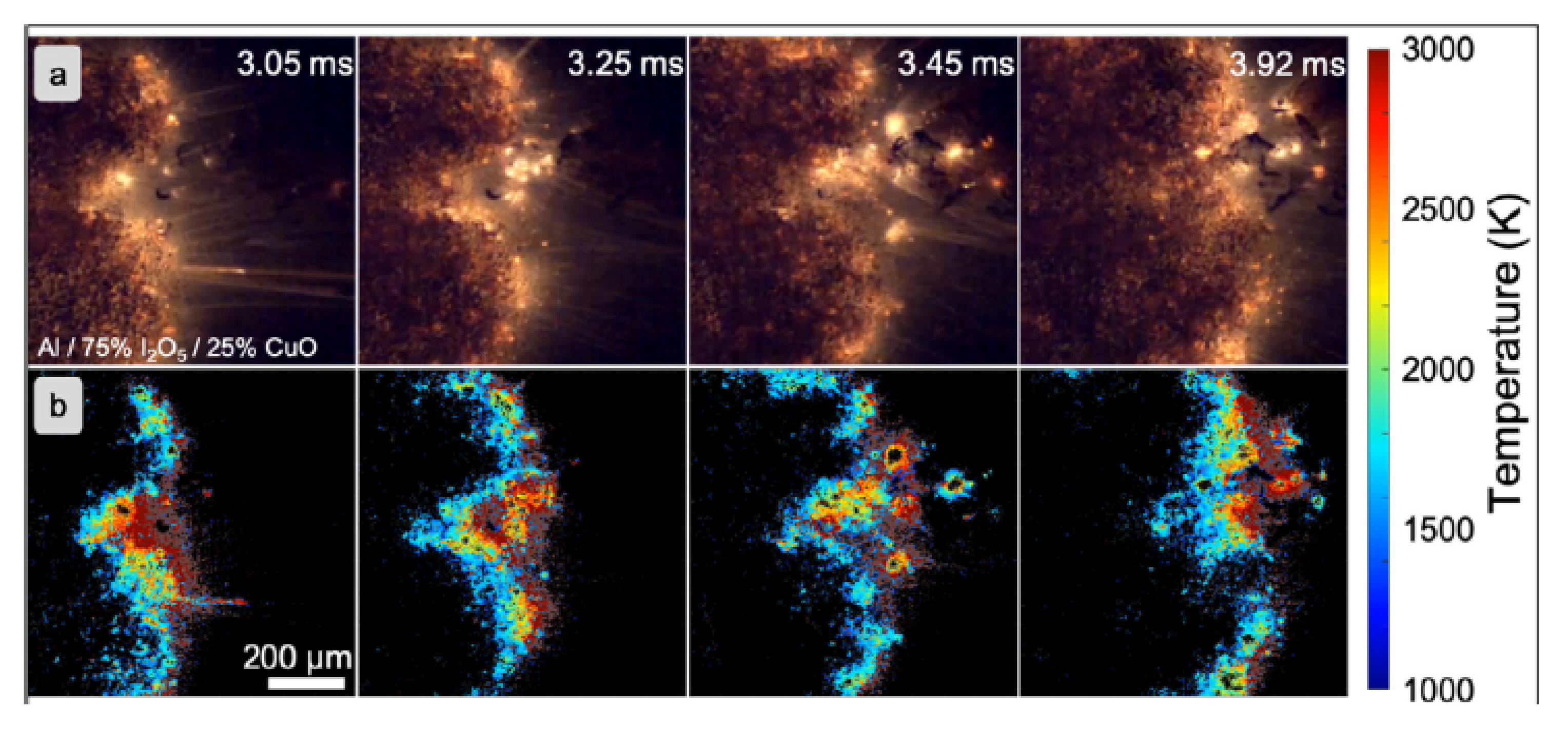
- Kline, D.J.; Alibay, Z.; Rehwoldt, M.C.; Idrogo-Lam, A.; Hamilton, S.G.; Biswas, P.; Xu, F.; Zachariah, M.R. Experimental observation of the heat transfer mechanisms that drive propagation in additively manufactured energetic materials. Combust. Flame 2020, 215, 417–424. [Google Scholar] [CrossRef]
- Jacob, R.J.; Kline, D.J.; Zachariah, M.R. High speed 2-dimensional temperature measurements of nanothermite composites: Probing thermal vs. Gas generation effects. J. Appl. Phys. 2018, 123, 115902. [Google Scholar] [CrossRef]
- Wang, H.; Kline, D.J.; Biswas, P.; Zachariah, M.R. Connecting agglomeration and burn rate in a thermite reaction: Role of oxidizer morphology. Combust. Flame 2021, 231, 111492. [Google Scholar] [CrossRef]
- Weismiller, M.; Malchi, J.; Lee, J.; Yetter, R.; Foley, T. Effects of fuel and oxidizer particle dimensions on the propagation of aluminum containing thermites. Proc. Combust. Inst. 2011, 33, 1989–1996. [Google Scholar] [CrossRef]
- Wang, H.; Kline, D.J.; Rehwoldt, M.C.; Zachariah, M.R. Carbon Fibers Enhance the Propagation of High Loading Nanothermites: In Situ Observation of Microscopic Combustion. ACS Appl. Mater. Interfaces 2021, 13, 30504–30511. [Google Scholar] [CrossRef]
- Moore, D.S.; Son, S.F.; Asay, B.W. Time-Resolved Spectral Emission of Deflagrating Nano-Al and Nano-MoO3 Metastable Interstitial Composites. Propellants Explos. Pyrotech. Int. J. Deal. Sci. Technol. Asp. Energ. Mater. 2004, 29, 106–111. [Google Scholar] [CrossRef]
- Khasainov, B.; Comet, M.; Veyssiere, B.; Spitzer, D. Comparison of Performance of Fast-Reacting Nanothermites and Primary Explosives. Propellants Explos. Pyrotech. 2017, 42, 754–772. [Google Scholar] [CrossRef]
- Tappan, A.; Long, G.; Renlund, A.; Kravitz, S. Microenergetic materials-microscale energetic material processing and testing. In Proceedings of the 41st Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 6–9 January 2003; p. 242. [Google Scholar]
- Tappan, A.S.; Brundage, A.L.; Long, G.T.; Renlund, A.M.; Kravitz, S.H.; Nogan, J.J.; Wroblewski, B.; Palmer, J.A.; Baer, M.R.; Baer, M.R. Microenergetic Research Involving a Coupled Experimental and Computational Approach to Evaluate Microstructural Effects on Detonation and Combustion at Sub-Millimeter Geometries; Technical report; Sandia National Lab. (SNL-NM): Albuquerque, NM, USA, 2006. [Google Scholar]
- Tappan, A.S. Microenergetics: Combustion and detonation at sub-millimeter scales. AIP Conf. Proc. Am. Inst. Phys. 2007, 955, 997–1002. [Google Scholar]
- Wu, T.; Lahiner, G.; Tenailleau, C.; Reig, B.; Hungría, T.; Estève, A.; Rossi, C. Unexpected enhanced reactivity of aluminized nanothermites by accelerated aging. Chem. Eng. J. 2021, 418, 129432. [Google Scholar] [CrossRef]
- Mao, Y.; He, Q.; Wang, J.; Li, Z.; Yang, Z.; Nie, F.; Wang, D. Rational design of gradient structured fluorocarbon/Al composites towards tunable combustion performance. Combust. Flame 2021, 230, 111436. [Google Scholar] [CrossRef]
- Zhong, L.; Zhou, X.; Huang, X.; Zheng, D.; Mao, Y.; Wang, R.; Wang, D. Combustion/decomposition characteristics of 3D-printed Al/CuO, Al/Fe2O3, Al/Bi2O3 and Al/PTFE hollow filaments. Mater. Chem. Phys. 2021, 271, 124874. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Zhang, W.; Zheng, Z.; Yu, C.; Wang, J.; Song, C.; Chen, J.; Lei, X.; Ma, K. Direct ink writing of nAl/pCuO/HPMC with outstanding combustion performance and ignition performance. Combust. Flame 2022, 236, 111747. [Google Scholar] [CrossRef]
- Sanders, V.; Asay, B.; Foley, T.; Tappan, B.; Pacheco, A.; Son, S. Combustion and reaction propagation of four nanoscale energetic composites. In Proceedings of the Conference: International Pyrotechnics Symposium, Fort Collins, CO, USA, 16–21 July 2006; pp. 113–121. [Google Scholar]
- Valliappan, S.; Puszynski, J.A. Combustion characteristics of metal-based nanoenergetic systems. Proc. South Dak. Acad. Sci. 2003, 82, 97. [Google Scholar]
- Moore, K.; Pantoya, M.L. Combustion of environmentally altered molybdenum trioxide nanocomposites. Propellants Explos. Pyrotech. Int. J. Deal. Sci. Technol. Asp. Energ. Mater. 2006, 31, 182–187. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Yan, H.; Wang, X.; Miao, Y. New approaches for evaluating detonation properties of commercial explosives using a novel continuous velocity probe. Meas. Sci. Technol. 2018, 29, 115901. [Google Scholar] [CrossRef]
- Mertuszka, P.; Pytlik, M. Analysis and comparison of the continuous detonation velocity measurement method with the standard method. Mater. Wysokoenergetyczne 2019, 11, 63–72. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gao, Y.; Apperson, S.; Subramaniam, S.; Shende, R.; Gangopadhyay, S.; Talantsev, E. A novel on-chip diagnostic method to measure burn rates of energetic materials. J. Energ. Mater. 2006, 24, 1–15. [Google Scholar] [CrossRef]
- He, Q.; Wang, J.; Mao, Y.; Yin, H.; Cao, W.; Nie, F. Fabrication of gradient structured HMX/Al and its combustion performance. Combust. Flame 2021, 226, 222–228. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Kim, K.J.; Kim, S.H. Tuning the ignition and combustion properties of nanoenergetic materials by incorporating with carbon black nanoparticles. Combust. Flame 2018, 194, 264–270. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, Y.; Cheng, S.; Zheng, H.; Liu, Y.; Qiao, Z.; Yang, G.; Zhang, K. Energetic composites based on nano-Al and energetic coordination polymers (ECPs): The “father-son” effect of ECPs. Chem. Eng. J. 2020, 392, 123719. [Google Scholar] [CrossRef]
- Wang, H.; Jian, G.; Zhou, W.; DeLisio, J.; Lee, V.T.; Zachariah, M.R. Metal iodate-based energetic composites and their combustion and biocidal performance. ACS Appl. Mater. Interfaces 2015, 7, 17363–17370. [Google Scholar] [CrossRef]
- Sullivan, K.; Zachariah, M. Simultaneous pressure and optical measurements of nanoaluminum thermites: Investigating the reaction mechanism. J. Propuls. Power 2010, 26, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Guo, X.; Chen, L.; Huang, S.; Zhao, L.; Ji, J.; Zhou, X. Alcohol-thermal synthesis of approximately core-shell structured Al@ CuO nanothermite with improved heat-release and combustion characteristics. Combust. Flame 2021, 228, 331–339. [Google Scholar] [CrossRef]
- Prakash, A.; McCormick, A.V.; Zachariah, M.R. Synthesis and Reactivity of a Super-Reactive Metastable Intermolecular Composite Formulation of Al/KMnO4. Adv. Mater. 2005, 17, 900–903. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Mao, Y.; Gong, F. An effective way to enhance energy output and combustion characteristics of Al/PTFE. Combust. Flame 2020, 214, 419–425. [Google Scholar] [CrossRef]
- Williams, A.; Shancita, I.; Altman, I.; Tamura, N.; Pantoya, M.L. On the Pressure Generated by Thermite Reactions Using Stress-Altered Aluminum Particles. Propellants Explos. Pyrotech. 2021, 46, 99–106. [Google Scholar] [CrossRef]
- Hill, K.J.; Tamura, N.; Levitas, V.I.; Pantoya, M.L. Impact ignition and combustion of micron-scale aluminum particles pre-stressed with different quenching rates. J. Appl. Phys. 2018, 124, 115903. [Google Scholar] [CrossRef] [Green Version]
- Yarrington, C.D.; Son, S.F.; Foley, T.J. Combustion of silicon/teflon/viton and aluminum/teflon/viton energetic composites. J. Propuls. Power 2010, 26, 734–743. [Google Scholar] [CrossRef]
- Bockmon, B.; Pantoya, M.; Son, S.; Asay, B.; Mang, J. Combustion velocities and propagation mechanisms of metastable interstitial composites. J. Appl. Phys. 2005, 98, 064903. [Google Scholar] [CrossRef]
- Maček, A. Transition from deflagration to detonation in cast explosives. J. Chem. Phys. 1959, 31, 162–167. [Google Scholar] [CrossRef]
- Miner, T.; Dalton, D.; Romero, D.; Heine, M.; Gorby, A.; Todd, S.; Asay, B. Deflagration-to-detonation transition in Bullseye powder. Shock Waves 2019, 29, 905–911. [Google Scholar] [CrossRef]
- Asay, B. Shock Wave Science and Technology Reference Library, Vol. 5: Non-Shock Initiation of Explosives; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Comet, M.; Schwartz, C.; Schnell, F.; Oudot, F.; Lallemand, B.; Spitzer, D. New Detonating Compositions from Ammonium Dinitramide. Propellants Explos. Pyrotech. 2021, 46, 742–750. [Google Scholar] [CrossRef]
- Comet, M.; Martin, C.; Schnell, F.; Schwartz, C.; Lallemand, B.; Galland, G.; Spitzer, D. Submicron Potassium Perchlorate: A Key Component to Reach Detonation in Binary Mixtures with Titanium Hydride. Propellants Explos. Pyrotech. 2021, 46, 1345–1351. [Google Scholar] [CrossRef]
- Trzciński, W.A. Numerical analysis of the deflagration to detonation transition in primary explosives. Cent. Eur. J. Energ. Mater. 2012, 9, 17–38. [Google Scholar]
- Apperson, S.J.; Bezmelnitsyn, A.V.; Thiruvengadathan, R.; Gangopadhyay, K.; Gangopadhyay, S.; Balas, W.A.; Anderson, P.E.; Nicolich, S.M. Characterization of nanothermite material for solid-fuel microthruster applications. J. Propuls. Power 2009, 25, 1086–1091. [Google Scholar] [CrossRef]
- Apperson, S.J. Characterization and MEMS Applications of Nanothermite Materials; University of Missouri-Columbia: Columbia, MO, USA, 2010. [Google Scholar]
- Staley, C.S.; Raymond, K.E.; Thiruvengadathan, R.; Herbst, J.J.; Swaszek, S.M.; Taylor, R.J.; Gangopadhyay, K.; Gangopadhyay, S. Effect of nitrocellulose gasifying binder on thrust performance and high-g launch tolerance of miniaturized nanothermite thrusters. Propellants Explos. Pyrotech. 2014, 39, 374–382. [Google Scholar] [CrossRef]
- Staley, C.S.; Raymond, K.E.; Thiruvengadathan, R.; Apperson, S.J.; Gangopadhyay, K.; Swaszek, S.M.; Taylor, R.J.; Gangopadhyay, S. Fast-impulse nanothermite solid-propellant miniaturized thrusters. J. Propuls. Power 2013, 29, 1400–1409. [Google Scholar] [CrossRef]
- Hobosyan, M.; Martirosyan, K.S.; Lyshevski, S.E. Design and evaluations of 3D-printed microthrusters with nanothermite propellants. In Proceedings of the 2018 IEEE 38th International Conference on Electronics and Nanotechnology (ELNANO), Kyiv, Ukraine, 24–26 April 2018; pp. 478–482. [Google Scholar]
- Suceska, M. Test Methods for Explosives; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Szastok, M. Comparative study of anfo’s and dynamite’s capability to perform work determined with lead block and ballistic pendulum. In Zeszyty Naukowe Instytutu Gospodarki Surowcami Mineralnymi i Energiią; Polska Akademia Nauk: Warsaw, Poland, 2018. [Google Scholar]
- Zaky, M.G.; Abdalla, A.M.; Sahu, R.P.; Puri, I.K.; Radwan, M.; Elbasuney, S. Nanothermite colloids: A new prospective for enhanced performance. Def. Technol. 2019, 15, 319–325. [Google Scholar] [CrossRef]
- Li, K.; Dong, X.; Li, X.; Wang, Y.; Wang, W. Determination of JWL parameters from underwater explosion test of spherical explosives by continuous velocity probe. J. Energ. Mater. 2021, 39, 479–493. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Wang, X.; Yan, H.; Yang, C.; Chen, X. A simple electrometric method for parametric determination of Jones-Wilkins-Lee equation of state from underwater explosion test. J. Appl. Phys. 2018, 124, 215906. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Ismael, S.; Yehia, M. Colloid thermite nanostructure: A novel high energy density material for enhanced explosive performance. J. Inorg. Organomet. Polym. Mater. 2021, 31, 559–565. [Google Scholar] [CrossRef]
- Trzciński, W.; Cudziło, S.; Szymańczyk, L. Determination of the detonation pressure from a water test. Eng. Trans. 2001, 49, 443–458. [Google Scholar]
- Duan, Z.; Liu, Y.; Pi, A.; Huang, F. Foil-like manganin gauges for dynamic high pressure measurements. Meas. Sci. Technol. 2011, 22, 075206. [Google Scholar] [CrossRef]
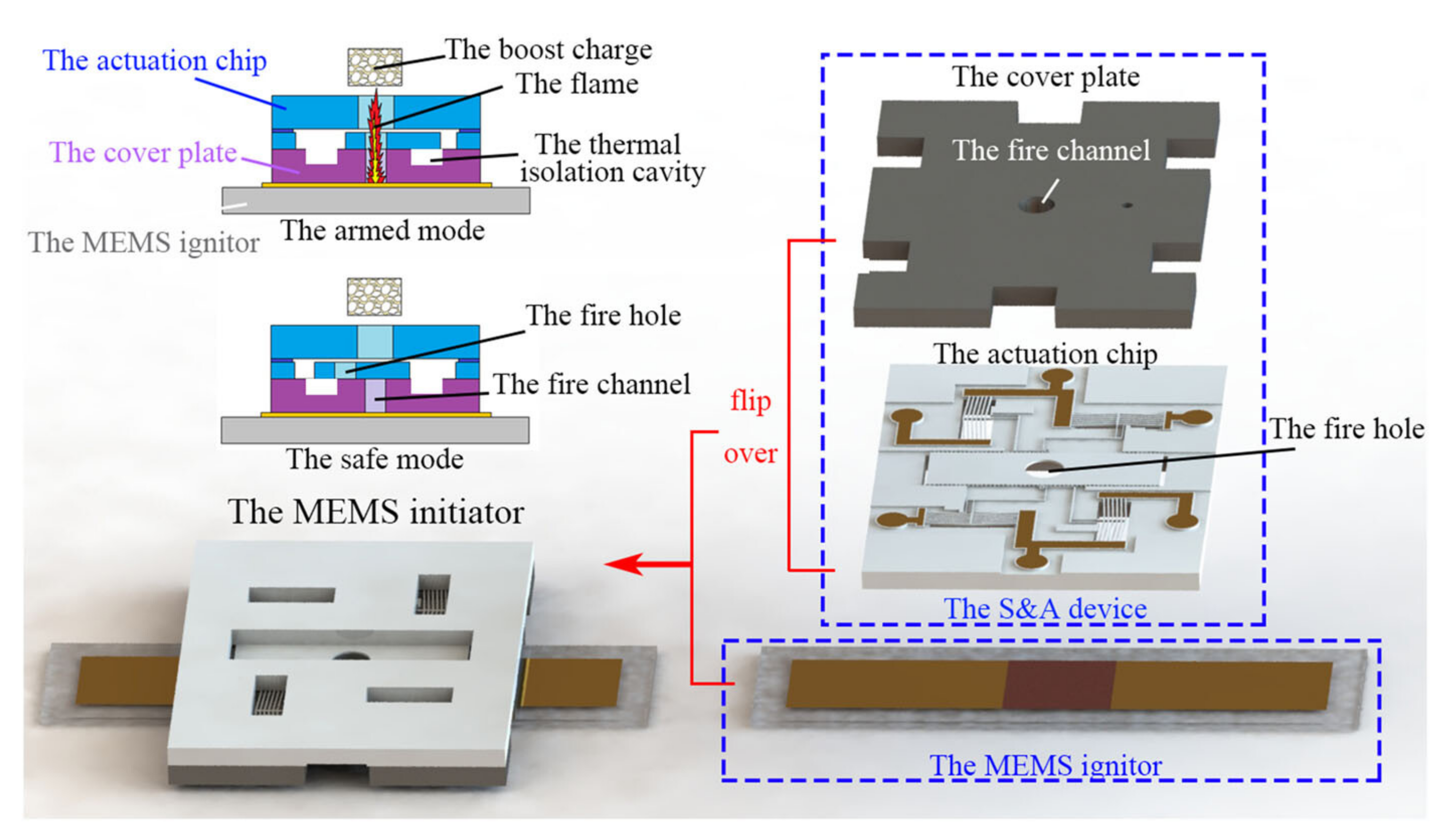
- Hu, T.; Zhao, Y.; Wang, K.; Fang, K.; Ren, W. The development of an on-chip microinitiator with a built-in safety-and-arming device. Rev. Sci. Instrum. 2021, 92, 025007. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Kim, S.B.; Kim, J.H.; Jang, N.S.; Kim, D.H.; Lee, H.W.; Kim, J.M.; Kim, S.H. A micro-chip initiator with controlled combustion reactivity realized by integrating Al/CuO nanothermite composites on a microhotplate platform. J. Micromech. Microeng. 2015, 26, 015002. [Google Scholar] [CrossRef]
- Staley, C.; Morris, C.; Thiruvengadathan, R.; Apperson, S.; Gangopadhyay, K.; Gangopadhyay, S. Silicon-based bridge wire micro-chip initiators for bismuth oxide–aluminum nanothermite. J. Micromech. Microeng. 2011, 21, 115015. [Google Scholar] [CrossRef]
- Oqab, H.B.; Dietrich, G.B. Thermal Power Plant. WIPO Patent WO2021028823A1, 18 February 2021. [Google Scholar]
- Nicollet, A.; Charlot, S.; Baijot, V.; Estève, A.; Rossi, C. Ultra-rapid and fully integrated active pyrotechnic safety switches integrating nanothermites. In Proceedings of the 2017 Materials Research Society Fall Meeting & Exhibit, Boston, MA, USA, 18–21 September 2017. [Google Scholar]
- Lopez, A.; Drukier, A.; Freese, K.; Kurdak, C.; Tarle, G. New dark matter detector using nanoscale explosives. arXiv 2014, arXiv:1403.8115. [Google Scholar]
- Li, Z.; Yaqing, C.; Zhenxin, Y.; Shunguan, Z.; Yan, L.; Jingyan, M. Method for Preparing Bridgeless Electric Explosive Device through Functionalization Carbon Fibers. CN Patent 110686567A, 1 January 2020. [Google Scholar]
- Ni, D.; Dang, P.; Yu, G.; Chu, E. An initiator integrated the AI/MoO3 multilayer nanothermite and bridge-wire electrode plug. J. Phys. Conf. Ser. 2020, 1507, 042011. [Google Scholar] [CrossRef]
- Wilson, D.E.; Schroder, K.A. Ordnance Neutralization Method and Device Using Energetic Compounds. U.S. Patent 8,505,427, 13 August 2013. [Google Scholar]
- Koleza, I.; Elmar, M. Detonator Initiator. WO Patent 2011106803A1, 2 June 2011. [Google Scholar]
- Ying, Z.; Xiaomia, M.; Jian, L.; Kaili, Z. Micro-Miniature Igniter Based on Micro-Heater and Structural Energetic Material and Preparation of Micro-Miniature Igniter. CN Patent 111947522A, 17 November 2020. [Google Scholar]
- Tappan, A.S.; Cesarano, J., III; Stuecker, J.N. Nanocomposite Thermite Ink. U.S. Patent 8,048,242, 1 November 2011. [Google Scholar]
- Kangzhen, X.; Jinghua, W.; Weimin, W.; Yanjing, Y.; Fengqi, Z. Preparation Method and Application of High-Energy Composite Copper Ferrite/GO/Al. CN Patent 110937965A, 27 October 2020. [Google Scholar]
- Wenjun, X.; Tao, L.; Wei, W. Thermite Reaction Synthesized Namometer Aluminum Oxide Particle Reinforced Composite Material and Preparation Method Thereof. CN Patent 103170598A, 26 June 2013. [Google Scholar]
- Qin, Y.; Zhao, T.; Zhang, Y.; Xu, Y. High-Strength Dissolvable Aluminum Alloy and Preparation Method Therefor. U.S. Patent 11,047,025, 29 June 2021. [Google Scholar]
- Li, K.; Yao, H.; Shi, J.; Chen, M.; Li, Q.; Zhang, R.; Xu, K. Micro-Nano Particle Reinforced Aluminum-Based Composite Material and Preparation Method Thereof. CN Patent 102102158A, 4 July 2012. [Google Scholar]
- Hearn, D.D.; Hirschmann, C.; Shafer, R.S. Nano-Thermite Well Plug. U.S. Patent 10,871,050B2, 22 December 2020. [Google Scholar]





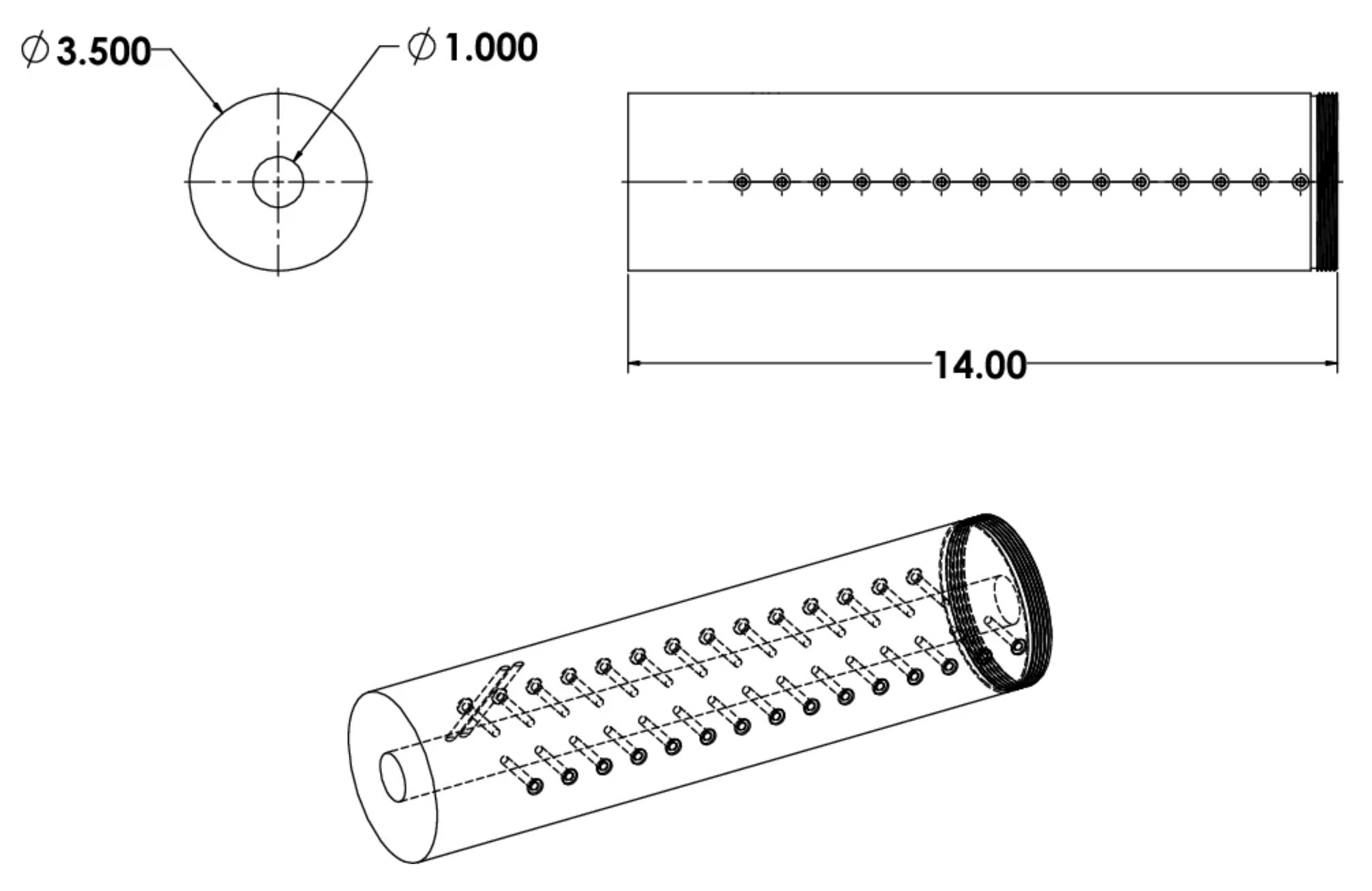
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polis, M.; Stolarczyk, A.; Glosz, K.; Jarosz, T. Quo Vadis, Nanothermite? A Review of Recent Progress. Materials 2022, 15, 3215. https://doi.org/10.3390/ma15093215
Polis M, Stolarczyk A, Glosz K, Jarosz T. Quo Vadis, Nanothermite? A Review of Recent Progress. Materials. 2022; 15(9):3215. https://doi.org/10.3390/ma15093215
Chicago/Turabian StylePolis, Mateusz, Agnieszka Stolarczyk, Karolina Glosz, and Tomasz Jarosz. 2022. "Quo Vadis, Nanothermite? A Review of Recent Progress" Materials 15, no. 9: 3215. https://doi.org/10.3390/ma15093215
APA StylePolis, M., Stolarczyk, A., Glosz, K., & Jarosz, T. (2022). Quo Vadis, Nanothermite? A Review of Recent Progress. Materials, 15(9), 3215. https://doi.org/10.3390/ma15093215





