Ternary Copper Tungsten Sulfide (Cu2WS4) Nanoparticles Obtained through a Solvothermal Approach: A Bi-Functional Electrocatalyst for the Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER)
Abstract
:1. Introduction
2. Results and Discussion
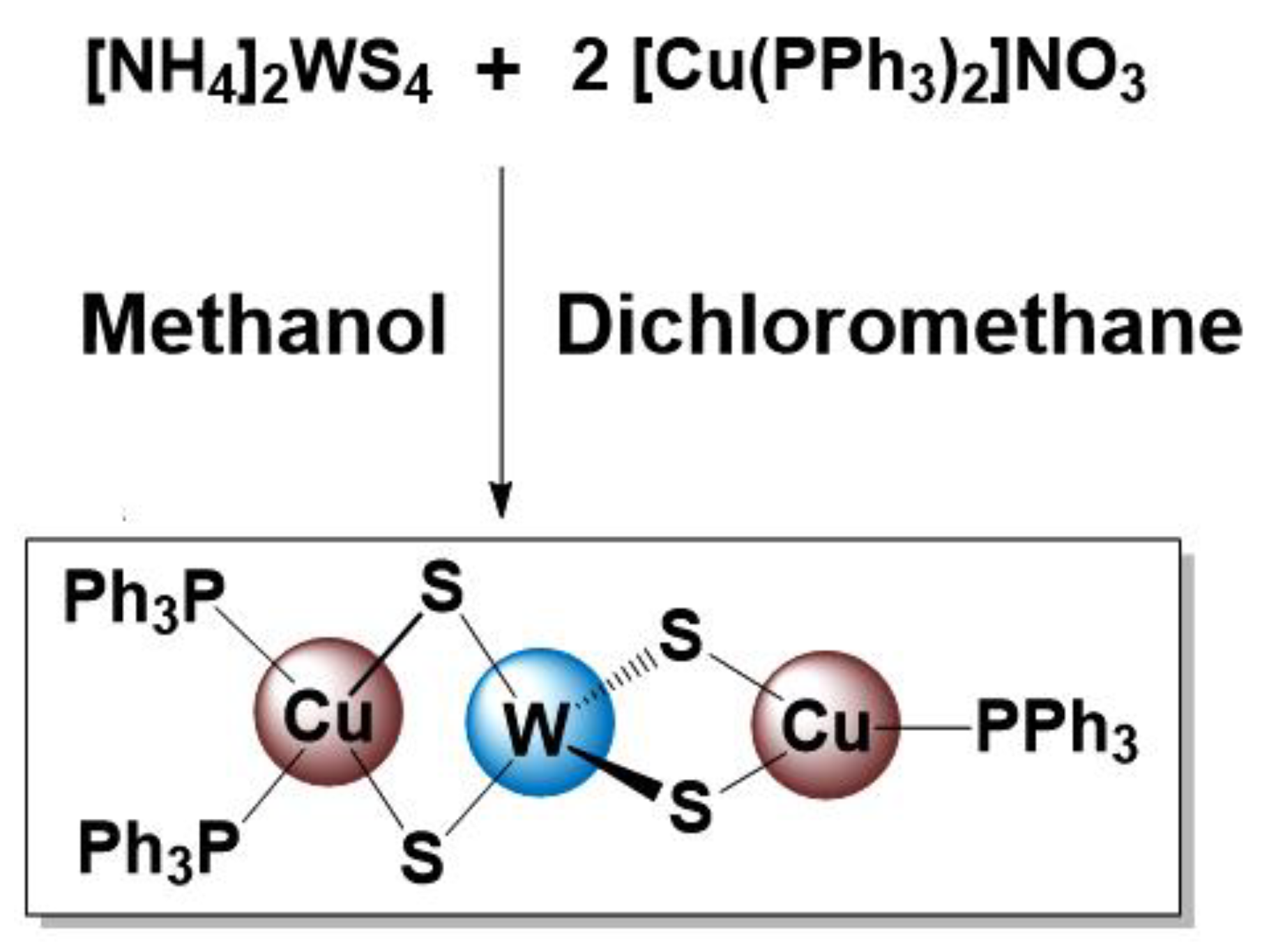
2.1. Synthesis of WCu2S4(PPh3)3 (SSP)
2.2. Spectroscopy
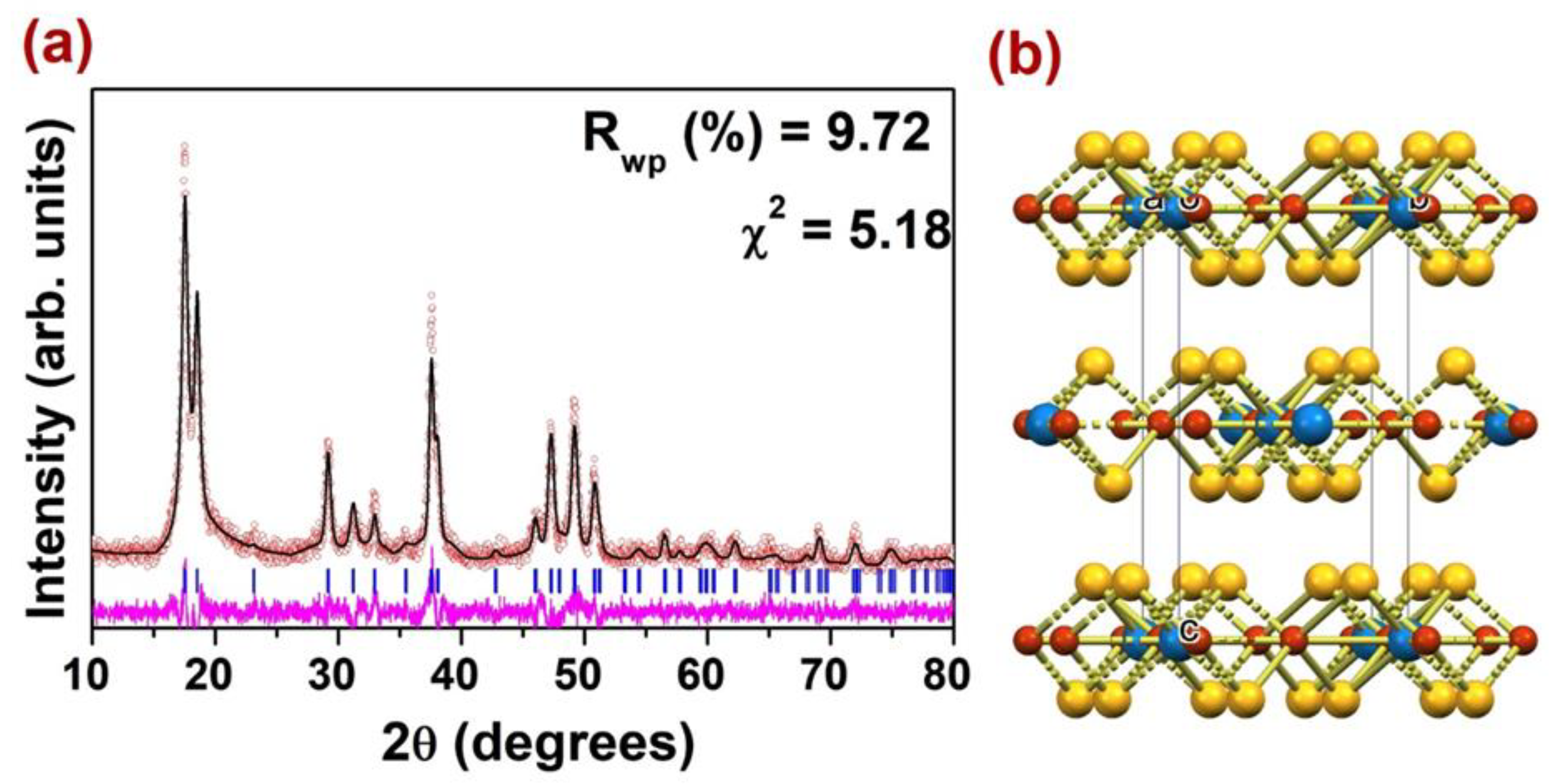
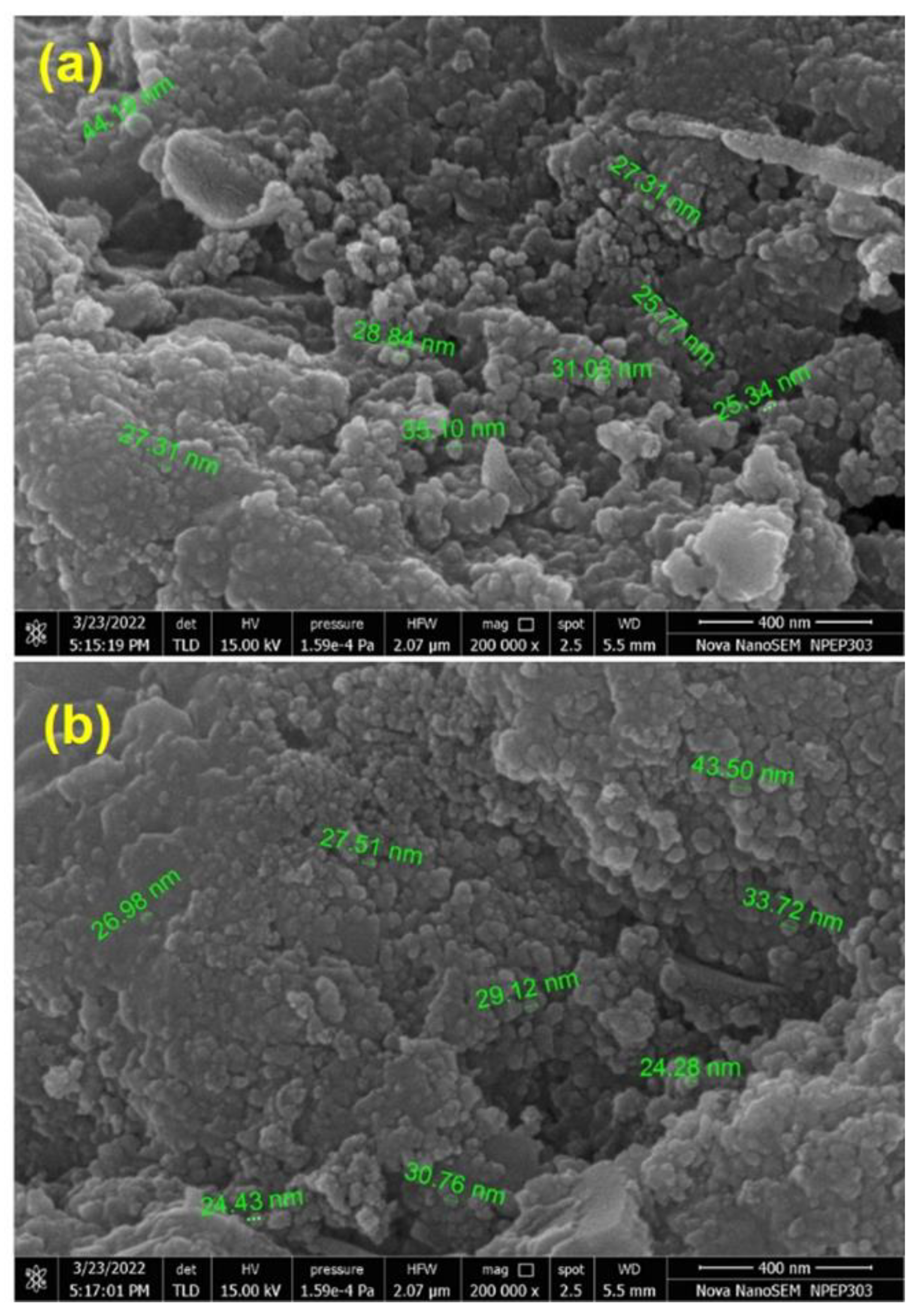
2.3. PXRD and SEM
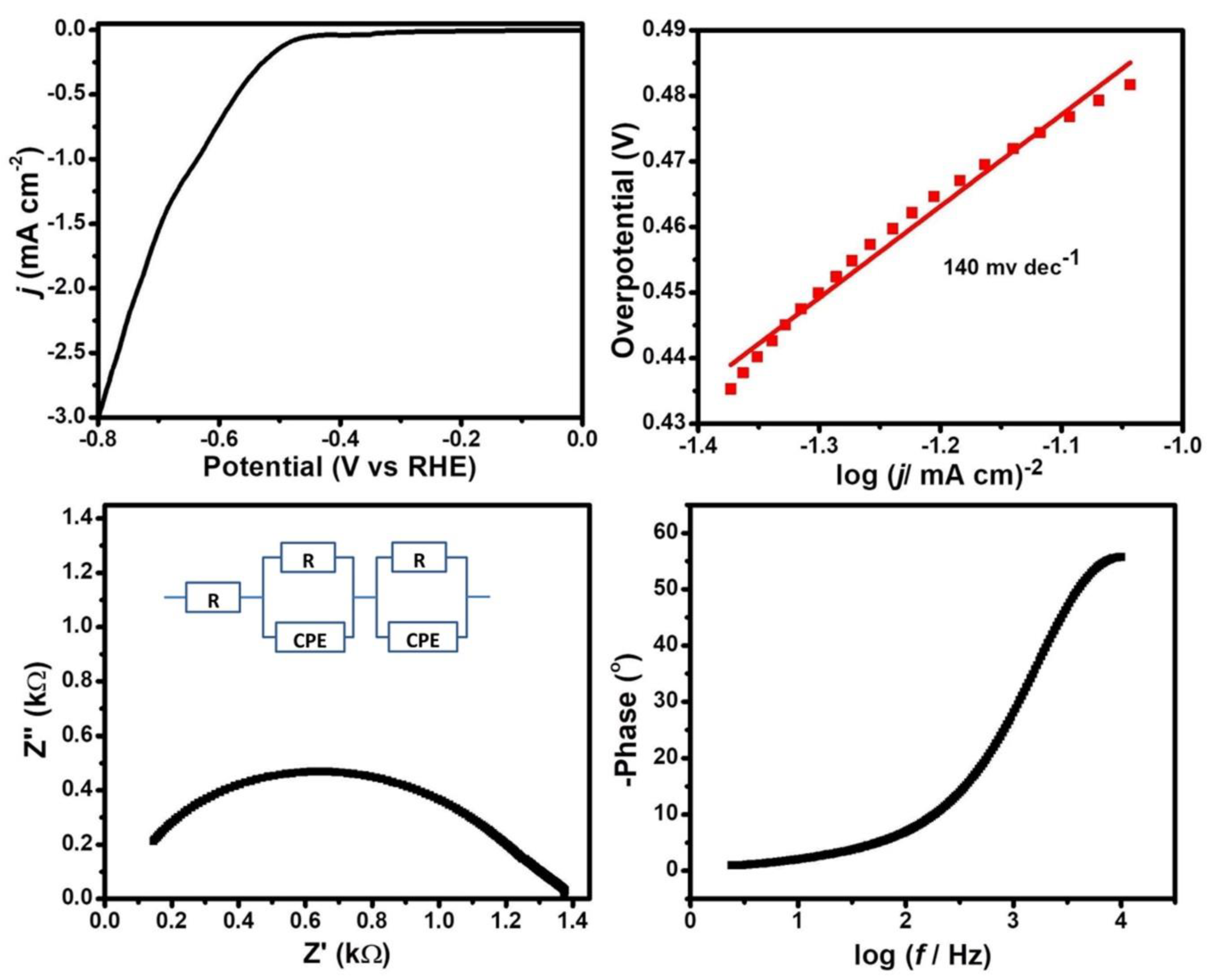
2.4. Electrochemical Study
3. Conclusions
4. Experimental
4.1. Materials and Methods
4.2. Syntheses
4.2.1. Synthesis of SSP (WCu2S4(PPh3)3)
4.2.2. Synthesis of Cu2WS4 Nanoparticles
4.3. Electrochemical Studies of Cu2WS4 Nanoparticles
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, H.X.; Wang, Z.L.; Meng, F.L.; Zhang, X.B. Integrated three-dimensional carbon paper/carbon tubes/cobalt-sulfide sheets as a bifunctional electrode for overall water splitting. ACS Nano 2016, 10, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.M.; Meng, X.Y.; Li, S.N.; Zeng, J.H.; Chen, Y. Ultrathin Co3O4 nanomeshes for the oxygen evolution reaction. ACS Catal. 2018, 8, 1913–1920. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Su, J.; Jiang, P.; Xia, G.; Chen, Q. Enhanced activity for hydrogen evolution reaction over CoFe catalysts by alloying with small amount of Pt. ACS Appl. Mater. Interfaces 2017, 9, 3596–3601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lai, W.; Cao, R. Energy-related small molecule activation reactions: Oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin-and corrole-based systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Aralekallu, S.; Sajjan, V.A.; Palanna, M.; Prabhu, C.P.K.; Hojamberdiev, M.; Sannegowda, L.K. Ni foam-supported azo linkage cobalt phthalocyanine as an efficient electrocatalyst for oxygen evolution reaction. J. Power Sources 2020, 449, 227516. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, R.; Cui, L.; Liu, D.; Hao, S.; Ma, Y.; Du, G.; Asiri, A.M.; Sun, X. High-performance electrolytic oxygen evolution in neutral media catalyzed by a cobalt phosphate nanoarray. Angew. Chem. Int. Ed. 2017, 56, 1064–1068. [Google Scholar] [CrossRef]
- Dong, B.; Zhao, X.; Han, G.Q.; Li, X.; Shang, X.; Liu, Y.R.; Hu, W.-H.; Chai, Y.-M.; Zhao, H.; Liu, C.-G. Two-step synthesis of binary Ni–Fe sulfides supported on nickel foam as highly efficient electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 13499–13508. [Google Scholar] [CrossRef]
- Shang, X.; Yan, K.L.; Rao, Y.; Dong, B.; Chi, J.Q.; Liu, Y.R.; Li, X.; Chai, Y.-M.; Liu, C.-G. In situ cathodic activation of V-incorporated Ni x S y nanowires for enhanced hydrogen evolution. Nanoscale 2017, 9, 12353–12363. [Google Scholar] [CrossRef]
- Shang, X.; Yan, K.L.; Lu, S.S.; Dong, B.; Gao, W.K.; Chi, J.Q.; Liu, Z.-Z.; Chai, Y.-M.; Liu, C.-G. Controlling electrodeposited ultrathin amorphous Fe hydroxides film on V-doped nickel sulfide nanowires as efficient electrocatalyst for water oxidation. J. Power Sources 2017, 363, 44–53. [Google Scholar] [CrossRef]
- Tian, G.L.; Zhao, M.Q.; Yu, D.; Kong, X.Y.; Huang, J.Q.; Zhang, Q.; Wei, F. Nitrogen-doped graphene/carbon nanotube hybrids: In situ formation on bifunctional catalysts and their superior electrocatalytic activity for oxygen evolution/reduction reaction. Small 2014, 10, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs. alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Koper, M.T.M. A basic solution. Nat. Chem. 2013, 5, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; et al. A heterostructure coupling of exfoliated Ni–Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef]
- Wang, F.; Shifa, T.A.; Zhan, X.; Huang, Y.; Liu, K.; Cheng, Z.; Jiang, C.; He, J. Recent advances in transition-metal dichalcogenide based nanomaterials for water splitting. Nanoscale 2015, 7, 19764–19788. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. Recent advances in transition-metal dichalcogenide based nanomaterials for water splitting. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Q.; Asiri, A.M.; Sun, X.; Luo, Y. Self-supported FeP nanorod arrays: A cost-effective 3D hydrogen evolution cathode with high catalytic activity. ACS Catal. 2014, 4, 4065–4069. [Google Scholar] [CrossRef]
- Tian, L.; Yan, X.; Chen, X.; Liu, L.; Chen, X. One-pot, large-scale, simple synthesis of Co x P nanocatalysts for electrochemical hydrogen evolution. J. Mater. Chem. A 2016, 4, 13011–13016. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Jia, G.; Chen, S.; Rawat, R.S.; Fan, H.J. Rapid synthesis of cobalt nitride nanowires: Highly efficient and low-cost catalysts for oxygen evolution. Angew. Chem. Int. Ed. 2016, 55, 8670–8674. [Google Scholar] [CrossRef]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.-Y.; Lou, X.W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512–6519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, L.A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Read, C.G.; Callejas, J.F.; Holder, C.F.; Schaak, R.E. General strategy for the synthesis of transition metal phosphide films for electrocatalytic hydrogen and oxygen evolution. ACS Appl. Mater. Interfaces 2016, 8, 12798–12803. [Google Scholar] [CrossRef]
- Liu, M.; Li, J. Cobalt phosphide hollow polyhedron as efficient bifunctional electrocatalysts for the evolution reaction of hydrogen and oxygen. ACS Appl. Mater. Interfaces 2016, 8, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, X.-J.; Cao, X.; Huang, X.; Tan, C.; Tian, J.; Liu, H.; Wang, J.; Zhang, H. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energy Environ. Sci. 2013, 6, 2921–2924. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, A.; Li, L.; Ai, L. Nickel–cobalt layered double hydroxide nanosheets as high-performance electrocatalyst for oxygen evolution reaction. J. Power Sources 2015, 278, 445–451. [Google Scholar] [CrossRef]
- Yu, L.; Yang, J.F.; Guan, B.Y.; Lu, Y.; Lou, X.W.D. Hierarchical Hollow Nanoprisms Based on Ultrathin Ni-Fe Layered Double Hydroxide Nanosheets with Enhanced Electrocatalytic Activity towards Oxygen Evolution. Angew. Chem. 2018, 130, 178–182. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, Y.; Edstr€om, K.; Niklasson, G.A.; Edvinsson, T. Controlled crystal growth orientation and surface charge effects in self-assembled nickel oxide nanoflakes and their activity for the oxygen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 28397–28407. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Monteverde Videla, A.H.A.; Ciura, K.; Specchia, S. Oxygen evolution catalysis in alkaline conditions over hard templated nickel-cobalt based spinel oxides. Int. J. Hydrogen Energy 2017, 42, 27910–27918. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Cui, W.; Jiang, P.; Cheng, N.; Asiri, A.M.; Sun, X. Carbon Nanotubes Decorated with CoP Nanocrystals: A Highly Active Non-Noble-Metal Nanohybrid Electrocatalyst for Hydrogen Evolution. Angew. Chem. Int. Ed. 2014, 126, 6828–6832. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Arellano-Jimenez, M.J.; Ye, G.; Dong Kim, N.; Samuel, E.L.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668–8675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Zhao, J.W.; Zhou, L.; Li, Z.H.; Shao, M.F.; Wei, M. Layer-by-layer assembly of exfoliated layered double hydroxide nanosheets for enhanced electrochemical oxidation of water. J. Mater. Chem. A 2016, 4, 11516–11523. [Google Scholar] [CrossRef]
- Siracusano, S.; Hodnik, N.; Jovanovic, P.; Ruiz-Zepeda, F.; Sala, M.; Baglio, V.; Arico, A.S. New insights into the stability of a high performance nanostructured catalyst for sustainable water electrolysis. Nano Energy 2017, 40, 618–632. [Google Scholar] [CrossRef]
- Liu, W.; Bao, J.; Guan, M.; Zhao, Y.; Lian, J.; Qiu, J.; Xu, L.; Huang, Y.; Qian, J.; Li, H. Nickel–cobalt-layered double hydroxide nanosheet arrays on Ni foam as a bifunctional electrocatalyst for overall water splitting. Dalton Trans. 2017, 46, 8372–8376. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, K.; Sims, H.; Butler, W.H.; Gupta, A. Mono-, few-, and multiple layers of copper antimony sulfide (CuSbS2): A ternary layered sulfide. J. Am. Chem. Soc. 2014, 136, 1587–1598. [Google Scholar] [CrossRef]
- Pruss, E.A.; Snyder, B.S.; Stacy, A.M. A new layered ternary sulfide: Formation of Cu2WS4 by reaction of WS and Cu+ ions. Angew. Chem. Int. Ed. 1993, 32, 256–257. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, Y.; Peng, R.; Huang, B.; Dai, Y. Single-layer Cu2WS4 with promising electrocatalytic activity toward hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2019, 11, 45818–45824. [Google Scholar] [CrossRef]
- Jing, D.; Liu, M.; Chen, Q.; Guo, L. Efficient photocatalytic hydrogen production under visible light over a novel W-based ternary chalcogenide photocatalyst prepared by a hydrothermal process. Int. J. Hydrogen Energy 2010, 35, 8521–8527. [Google Scholar] [CrossRef]
- Ozel, F.; Aslan, E.; Sarilmaz, A.; Patir, I.H. Hydrogen evolution catalyzed by Cu2WS4 at liquid–liquid interfaces. ACS Appl. Mater. Interfaces 2016, 8, 25881–25887. [Google Scholar] [CrossRef]
- Aslan, E.; Gonce, M.K.; Yigit, M.Z.; Sarilmaz, A.; Stathatos, E.; Ozel, F.; Can, M.; Patir, I.H. Photocatalytic H2 evolution with a Cu2WS4 catalyst on a metal free D-π-A organic dye-sensitized TiO2. Appl. Catal. B Environ. 2017, 210, 320–327. [Google Scholar] [CrossRef]
- Patir, I.H.; Aslan, E.G.; Yanalak, M.; Karaman, A.; Sarilmaz, M.; Can, M.; Can, M.; Ozel, F. Donor-π-acceptor dye-sensitized photoelectrochemical and photocatalytic hydrogen evolution by using Cu2WS4 co-catalyst. Int J. Hydrogen Energy 2019, 44, 1441–1450. [Google Scholar] [CrossRef]
- Novak, T.G.; Prakash, O.; Tiwari, A.P.; Jeon, S. Solution-phase phosphorus substitution for enhanced oxygen evolution reaction in Cu2WS4. RSC Adv. 2019, 9, 234–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.; Kim, S.J.; Kim, N.H.; Lee, J.H. All-solid-state asymmetric supercapacitor with MWCNT-based hollow NiCo2O4 positive electrode and porous Cu2WS4 negative electrode. Chem. Eng. J. 2021, 415, 128188. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Sahoo, S.; Mariappan, V.K.; Kim, S.J. Copper tungsten sulfide anchored on Ni-foam as a high-performance binder free negative electrode for asymmetric supercapacitor. Chem. Eng. J. 2019, 359, 409–418. [Google Scholar] [CrossRef]
- Gulen, M.; Sarilmaz, A.; Patir, I.H.; Ozel, F.; Sonmezoglu, S. Ternary copper-tungsten-disulfide nanocube inks as catalyst for highly efficient dye-sensitized solar cells. Electrochim. Acta 2018, 269, 119–127. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, Y.C.; Li, J.; Chen, Y.; Xu, B. Hydrothermal synthesis of Cu2WS4 as a visible-light-activated photocatalyst in the reduction of aqueous Cr (VI). Mater. Lett. 2014, 117, 24–27. [Google Scholar] [CrossRef]
- Kannan, S.; Vinitha, P.; Mohanraj, K.; Sivakumar, G. Antibacterial studies of novel Cu2WS4 ternary chalcogenide synthesized by hydrothermal process. J. Solid State Chem. 2018, 258, 376–382. [Google Scholar] [CrossRef]
- Shan, J.; Li, X.; Yang, K.; Xiu, W.; Wen, Q.; Zhang, Y.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. Efficient Bacteria Killing by Cu2WS4 Nanocrystals with Enzyme-like Properties and Bacteria-Binding Ability. ACS Nano 2019, 13, 13797–13808. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Kociok-Köhn, G.; Bhimireddi, R.; Singh, A.; Singh, A.K.; Kumar, A.; Muddassir, M. Ternary copper molybdenum sulfide (Cu2MoS4) nanoparticles anchored on PANI/rGO as electrocatalysts for oxygen evolution reaction (OER). Appl. Organomet. Chem. 2022, 36, e6683. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Kociok-Köhn, G.; Molloy, K.C.; Singh, A.K.; Kumar, A.; Muddassir, M. Ni (ii) dithiolate anion composites with two-dimensional materials for electrochemical oxygen evolution reactions (OERs). New J. Chem. 2021, 45, 16264–16270. [Google Scholar] [CrossRef]
- Müller, A.; Bögge, H.; Schimanski, U. The preparation of different types of polynuclear transition metal sulfur compounds by thiometallates, including cubane-like ones. Crystal structures of {Cu3WS3Cl}(PPh3)3S, {Cu3WS3Cl}(PPh3)3O, {Cu3MoS3Cl}(PPh3)3S, {Cu3MoS3Cl}(PPh33O, (PPh3)3Cu2WS4·0.8CH2Cl2 and (PPh3)3Ag2MoS4·0.8CH2Cl2. Inorg. Chim. Acta 1983, 69, 5–16. [Google Scholar]
- Doyle, R.L.; Lyons, M.E.G. Photoelectrochem Solar Fuel Production; Springer: Cham, The Netherland, 2016; pp. 41–104. [Google Scholar]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Liu, T.; Li, L.; Song, S.; Ding, R. Nickel-based electrodes as catalysts for hydrogen evolution reaction in alkaline media. Ionics 2018, 24, 1121. [Google Scholar] [CrossRef]
- Alobaid, A.; Wang, C.; Adomaitis, R.A. Mechanism and kinetics of HER and OER on NiFe LDH films in an alkaline electrolyte. J. Electrochem. Soc. 2018, 165, J3395. [Google Scholar] [CrossRef]
- Ding, Y.; Liang, J.; Liu, G.; Ni, W.; Shen, L. Preparation and anticorrosive property of soluble aniline tetramer. Coatings 2019, 9, 399. [Google Scholar] [CrossRef] [Green Version]
- Mcdonald, J.W.; Friesen, G.D.; Rosenhein, L.D.; Newton, W.E. Syntheses and characterization of ammonium and tetraalkylammonium thiomolybdates and thiotungstates. Inorg. Chim. Acta 1983, 72, 205–210. [Google Scholar] [CrossRef]
- Gysling, H.J.; Kubas, G.J. Coordination Complexes of Copper(I) Nitrate. In Inorganic Syntheses; Wiley: Hoboken, NJ, USA, 2007; Volume 19, pp. 93–94. [Google Scholar]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter. 2017, 29, 465901. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009, 21, 395502. Available online: http://www.quantum-espresso.org (accessed on 19 December 2022). [CrossRef]

| Solution | Rs (Ω) | Qc | Rc (Ω) | Qct | Rct (Ω) | ||
|---|---|---|---|---|---|---|---|
| Yo (10−6) | n | Y (10−7) | n | ||||
| 0.5 M H2SO4 | 46.1 | 4711 | 0.8 | 327.7 | 2.826 | 0.8 | 1024 |
| 0.1 M H2SO4 | 967.5 | 0.3271 | 0.8436 | 3369 | 65.29 | 0.6714 | 3844 |
| 0.1 M KOH | 138.1 | 2.455 | 0.7097 | 1215 × 103 | 29.85 | 0.7679 | 1622 × 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muddassir, M.; Alarifi, A.; Abduh, N.A.Y.; Saeed, W.S.; Karami, A.M.; Afzal, M. Ternary Copper Tungsten Sulfide (Cu2WS4) Nanoparticles Obtained through a Solvothermal Approach: A Bi-Functional Electrocatalyst for the Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER). Materials 2023, 16, 299. https://doi.org/10.3390/ma16010299
Muddassir M, Alarifi A, Abduh NAY, Saeed WS, Karami AM, Afzal M. Ternary Copper Tungsten Sulfide (Cu2WS4) Nanoparticles Obtained through a Solvothermal Approach: A Bi-Functional Electrocatalyst for the Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER). Materials. 2023; 16(1):299. https://doi.org/10.3390/ma16010299
Chicago/Turabian StyleMuddassir, Mohd., Abdullah Alarifi, Naaser A. Y. Abduh, Waseem Sharaf Saeed, Abdulnasser Mahmoud Karami, and Mohd Afzal. 2023. "Ternary Copper Tungsten Sulfide (Cu2WS4) Nanoparticles Obtained through a Solvothermal Approach: A Bi-Functional Electrocatalyst for the Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER)" Materials 16, no. 1: 299. https://doi.org/10.3390/ma16010299






