One-Step Engineering Carbon Supported Magnetite Nanoparticles Composite in a Submicron Pomegranate Configuration for Superior Lithium-Ion Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Sample Characterization and Electrochemical Measurement
3. Results
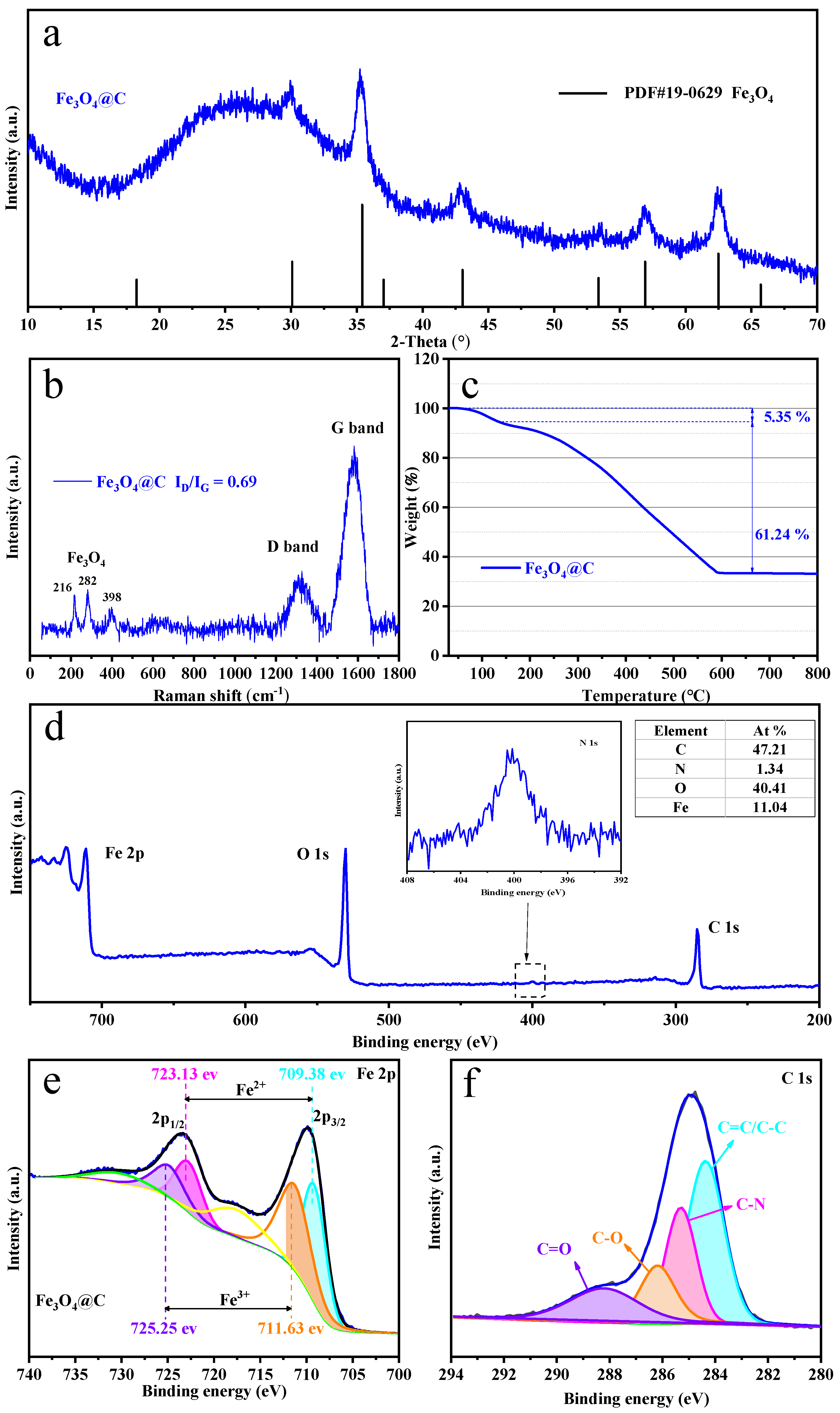
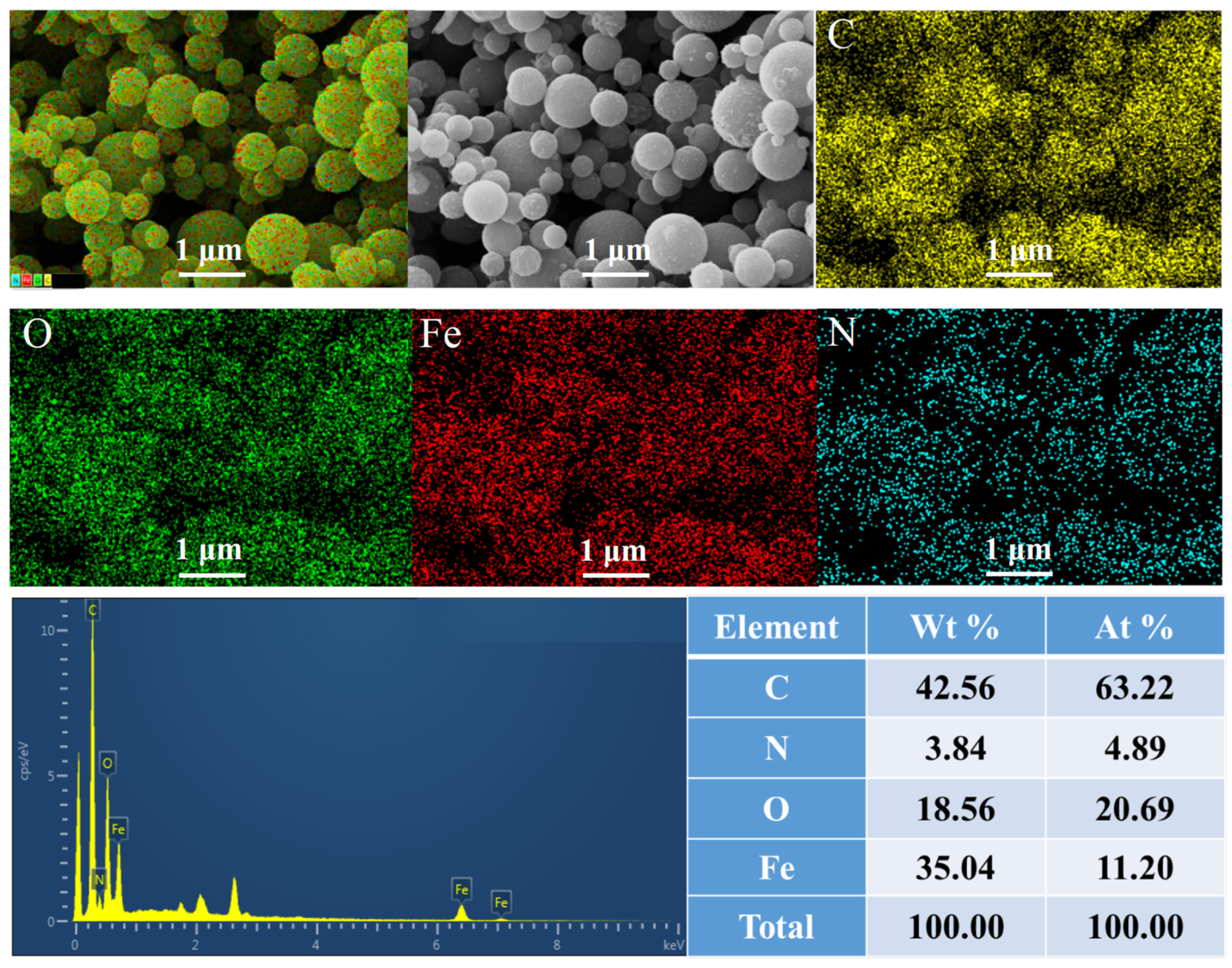
3.1. Structure and Morphology
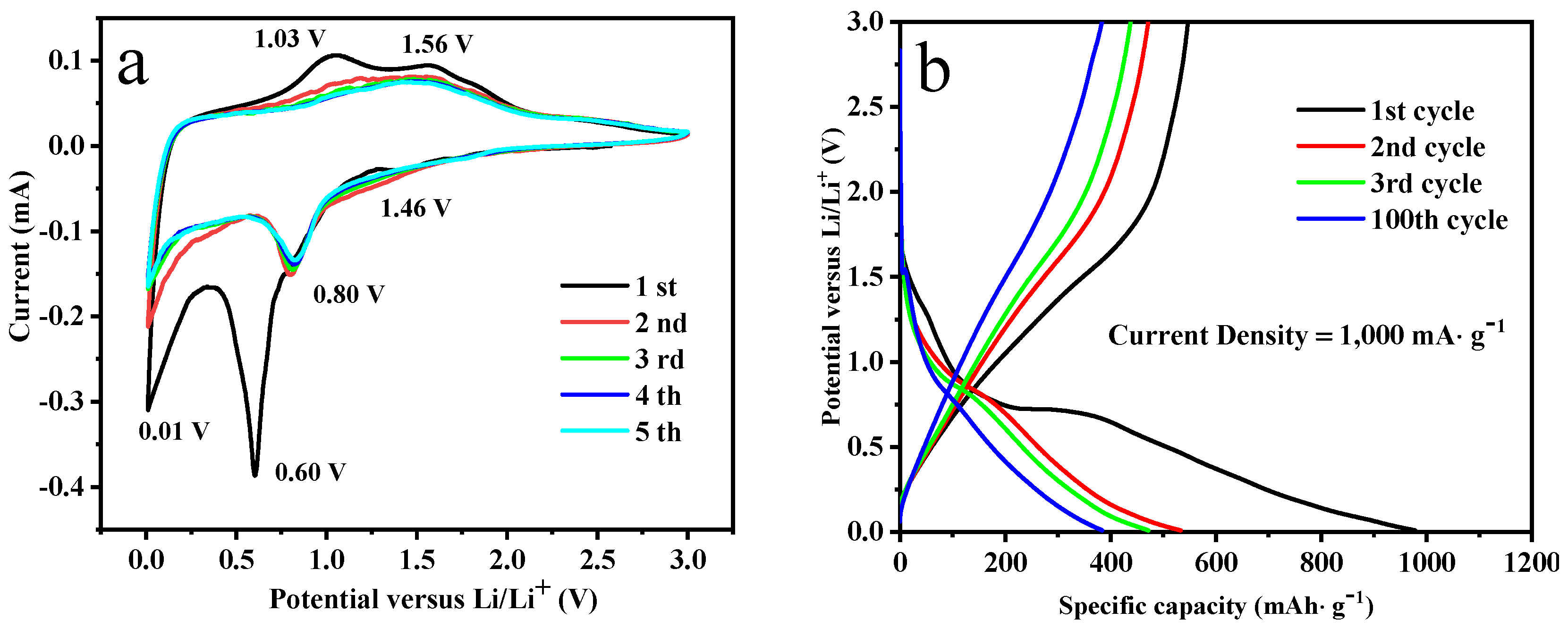
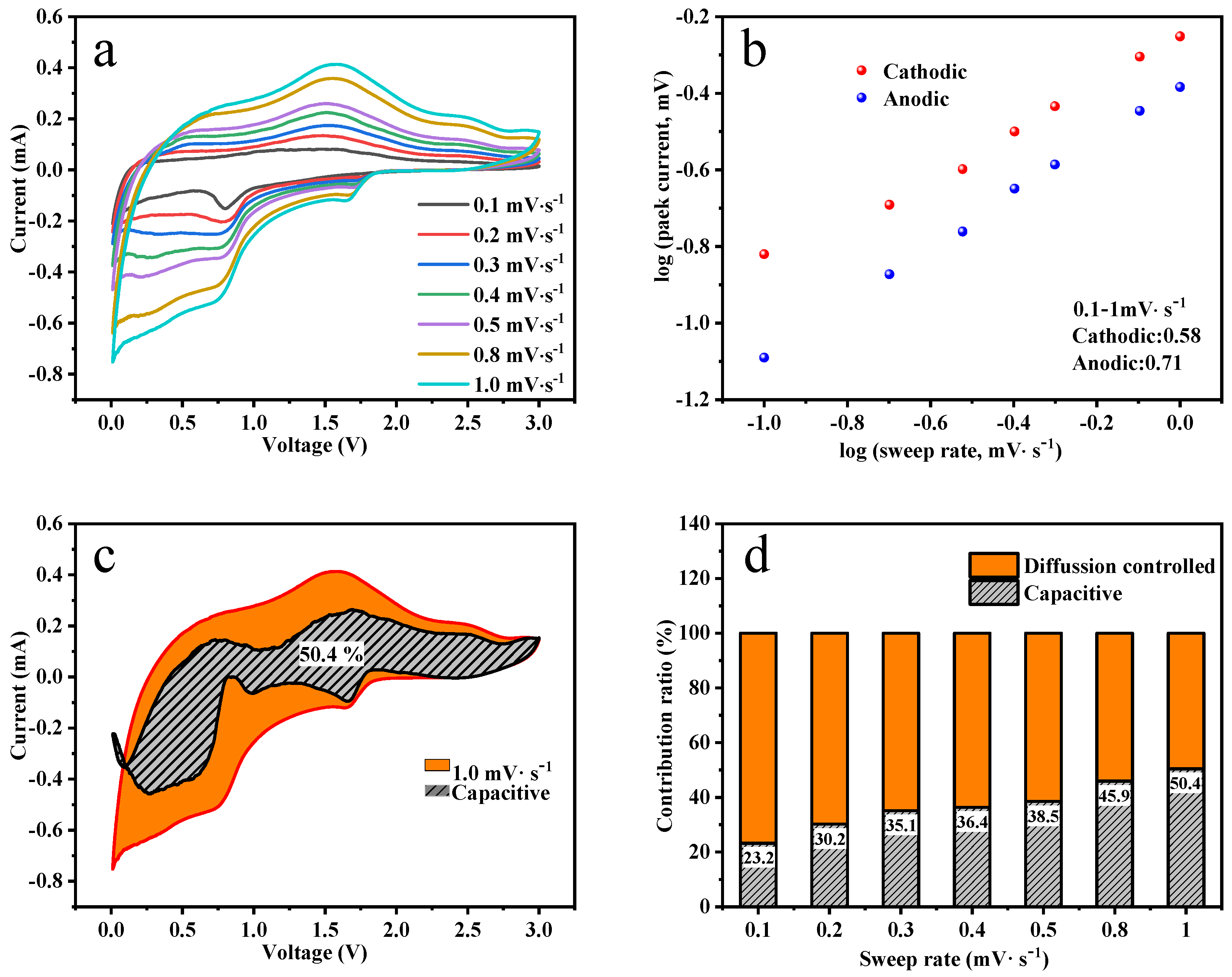
3.2. Lithium-Ion Storage Performances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metaloxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Gao, X.; Mao, L.; Hu, Y.; Lou, X.W. One-pot magnetic field induced formation of Fe3O4/C composite microrods with enhanced lithium storage capability. Small 2014, 10, 2815–2819. [Google Scholar] [CrossRef] [PubMed]
- Taberna, P.L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; He, M.; Du, H.; Wu, X.; Hao, J.; Li, B. Core-shells on nanosheets: Fe3O4@carbon-reduced graphene oxide composites for lithium-ion storage. J. Solid State Electrochem. 2018, 23, 237–244. [Google Scholar] [CrossRef]
- Liu, J.; Xu, X.; Hu, R.; Yang, L.; Zhu, M. Uniform Hierarchical Fe3O4@Polypyrrole Nanocages for Superior Lithium Ion Battery Anodes. Adv. Energy Mater. 2016, 6, 1600256. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Longoni, G.; Fiore, M.; Kim, J.-H.; Jung, Y.H.; Kim, D.K.; Mari, C.M.; Ruffo, R. Co3O4 negative electrode material for rechargeable sodium ion batteries: An investigation of conversion reaction mechanism and morphology-performances correlations. J. Power Sources 2016, 332, 42–50. [Google Scholar] [CrossRef]
- Luo, J.; Liu, J.; Zeng, Z.; Ng, C.F.; Ma, L.; Zhang, H.; Lin, J.; Shen, Z.; Fan, H.J. Three-dimensional graphene foam supported Fe3O4 lithium battery anodes with long cycle life and high rate capability. Nano Lett. 2013, 13, 6136–6143. [Google Scholar] [CrossRef]
- Tu, M.Y.; Wang, K.K.; Bao, S.C.; Zhang, R.; Tan, Q.K.; Kong, X.L.; Yu, L.B.; Wu, G.L.; Xu, B.H. Sodium carboxymethylcellulose induced engineering a porous carbon and graphene immobilized magnetite composite for lithium-ion storage. J. Colloid Interface Sci. 2022, 608, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bao, S.C.; Tan, Q.K.; Li, B.W.; Wang, C.; Shan, L.J.; Wang, C.; Xu, B.H. Facile synthesis of a rod-like porous carbon framework confined magnetite nanoparticle composite for superior lithium-ion storage. J. Colloid Interface Sci. 2021, 600, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.L.; Shan, L.J.; Zhang, R.; Bao, S.C.; Tu, M.Y.; Jia, R.X.; Yu, L.B.; Li, H.L.; Xu, B.H. Controllable engineering magnetite nanoparticles dispersed in a hierarchical amylose derived carbon and reduced graphene oxide framework for lithium-ion storage. J. Colloid. Interface Sci. 2022, 628, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.X.; Zhang, R.; Yu, L.B.; Kong, X.L.; Bao, S.C.; Tu, M.Y.; Liu, X.H.; Xu, B.H. Engineering a hierarchical carbon supported magnetite nanoparticles composite from metal organic framework and graphene oxide for lithium-ion storage. J Colloid Interface Sci 2022, 630, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohyd. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, B.T.; Zhu, L.; Shao, Z.P. Molten salt synthesis of nitrogen-doped carbon with hierarchical pore structures for use as high-performance electrodes in supercapacitors. Carbon 2015, 93, 48–58. [Google Scholar] [CrossRef]
- Han, C.P.; Xu, L.; Li, H.F.; Shi, R.Y.; Zhang, T.F.; Li, J.Q.; Wong, C.P.; Kang, F.Y.; Lin, Z.Q.; Li, B.H. Biopolymer-assisted synthesis of 3D interconnected Fe3O4@carbon core@shell as anode for asymmetric lithium ion capacitors. Carbon 2018, 140, 296–305. [Google Scholar] [CrossRef]
- Xu, Y.H.; Liu, Q.; Zhu, Y.J.; Liu, Y.H.; Langrock, A.; Zachariah, M.R.; Wang, C.S. Uniform nano-Sn/C composite anodes for lithium ion batteries. Nano Lett. 2013, 13, 470–474. [Google Scholar] [CrossRef]
- Xu, B.H.; Guan, X.G.; Zhang, L.Y.; Liu, X.W.; Jiao, Z.B.; Liu, X.H.; Hu, X.Q.; Zhao, X.S. A simple route to preparing gamma-Fe2O3/RGO composite electrode materials for lithium ion batteries. J. Mater. Chem. A 2018, 6, 4048–4054. [Google Scholar] [CrossRef]
- Liu, Z.; Guan, D.; Yu, Q.; Xu, L.; Zhuang, Z.; Zhu, T.; Zhao, D.; Zhou, L.; Mai, L. Monodisperse and homogeneous SiOX/C microspheres: A promising high-capacity and durable anode material for lithium-ion batteries. Energy Storage Mater. 2018, 13, 112–118. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Z.; Zuo, Z.; Pan, W.; Zhang, J. The ensemble effect of nitrogen doping and ultrasmall SnO2 nanocrystals on graphene sheets for efficient electroreduction of carbon dioxide. Appl. Catal. B-Environ. 2018, 239, 441–449. [Google Scholar] [CrossRef]
- Li, H.-H.; Saini, A.; Xu, R.-Y.; Wang, N.; Lv, X.-X.; Wang, Y.-P.; Yang, T.; Chen, L.; Jiang, H.-B. Hierarchical Fe3O4@C nanofoams derived from metal–organic frameworks for high-performance lithium storage. Rare Metals 2020, 39, 1072–1081. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, Y.; Zeng, J.; Wang, J.; Zhao, J. The facile preparation of hollow Fe3O4/C/CNT microspheres assisted by the spray drying method as an anode material for lithium-ion batteries. J. Mater. Sci. 2018, 53, 16447–16457. [Google Scholar] [CrossRef]
- Xu, B.; Wu, H.; Lin, C.X.; Wang, B.; Zhang, Z.; Zhao, X.S. Stabilization of silicon nanoparticles in graphene aerogel framework for lithium ion storage. RSC Adv. 2015, 5, 30624–30630. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Z.; Yang, L.; Hirano, S.-I. Synthesis of SnO2/Sn@carbon nanospheres dispersed in the interspaces of a three-dimensional SnO2/Sn@carbon nanowires network, and their application as an anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 12881–12887. [Google Scholar] [CrossRef]
- Meng, X.; Zhong, Y.; Sun, Y.; Banis, M.N.; Li, R.; Sun, X. Nitrogen-doped carbon nanotubes coated by atomic layer deposited SnO2 with controlled morphology and phase. Carbon 2011, 49, 1133–1144. [Google Scholar] [CrossRef]
- Liu, J.; Ni, J.; Zhao, Y.; Wang, H.; Gao, L. Grapecluster-like Fe3O4@C/CNT nanostructures with stable Li-storage capability. J. Mater. Chem. A 2013, 1, 12879–12884. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wang, G.; Xia, Y.; Wang, H. One-dimensional hybrid nanocomposite of high-density monodispersed Fe3O4 nanoparticles and carbon nanotubes for high-capacity storage of lithium and sodium. J. Mater. Chem. A 2016, 4, 18532–18542. [Google Scholar] [CrossRef]
- Cui, Y.; Feng, W.; Liu, W.; Li, J.; Zhang, Y.; Du, Y.; Li, M.; Huang, W.; Wang, H.; Liu, S. Template-assisted loading of Fe3O4 nanoparticles inside hollow carbon "rooms" to achieve high volumetric lithium storage. Nanoscale 2020, 12, 10816–10826. [Google Scholar] [CrossRef]
- Hao, S.; Zhang, B.; Ball, S.; Wu, J.; Srinivasan, M.; Huang, Y. Phase transition of hollow-porous α-Fe2O3 microsphere based anodes for lithium ion batteries during high rate cycling. J. Mater. Chem. A 2016, 4, 16569–16575. [Google Scholar] [CrossRef]
- Han, C.-G.; Zhu, C.; Sheng, N.; Aoki, Y.; Habazaki, H.; Akiyama, T. A facile one-pot synthesis of FeOx/carbon/graphene composites as superior anode materials for lithium-ion batteries. Electrochim. Acta 2017, 235, 88–97. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, X.; Engelbrekt, C.; Zhang, M.; Li, S.; Ulstrup, J.; Ci, L.; Feng, J.; Si, P.; Chi, Q. Graphene encapsulated Fe3O4 nanorods assembled into a mesoporous hybrid composite used as a high-performance lithium-ion battery anode material. Mater. Chem. Front. 2017, 1, 1185–1193. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energ. Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Wang, X.; Lin, C.; Zhao, X. Hollow Rutile Cuboid Arrays Grown on Carbon Fiber Cloth as a Flexible Electrode for Sodium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 2002629. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, M.; Yang, C.; Zhang, R.; Kong, X.; Jia, R.; Yu, L.; Xu, B. One-Step Engineering Carbon Supported Magnetite Nanoparticles Composite in a Submicron Pomegranate Configuration for Superior Lithium-Ion Storage. Materials 2023, 16, 313. https://doi.org/10.3390/ma16010313
Tu M, Yang C, Zhang R, Kong X, Jia R, Yu L, Xu B. One-Step Engineering Carbon Supported Magnetite Nanoparticles Composite in a Submicron Pomegranate Configuration for Superior Lithium-Ion Storage. Materials. 2023; 16(1):313. https://doi.org/10.3390/ma16010313
Chicago/Turabian StyleTu, Mengyao, Chun Yang, Rui Zhang, Xiangli Kong, Ruixin Jia, Longbiao Yu, and Binghui Xu. 2023. "One-Step Engineering Carbon Supported Magnetite Nanoparticles Composite in a Submicron Pomegranate Configuration for Superior Lithium-Ion Storage" Materials 16, no. 1: 313. https://doi.org/10.3390/ma16010313
APA StyleTu, M., Yang, C., Zhang, R., Kong, X., Jia, R., Yu, L., & Xu, B. (2023). One-Step Engineering Carbon Supported Magnetite Nanoparticles Composite in a Submicron Pomegranate Configuration for Superior Lithium-Ion Storage. Materials, 16(1), 313. https://doi.org/10.3390/ma16010313






