Synthesis of Nickel and Cobalt Ferrite-Doped Graphene as Efficient Catalysts for Improving the Hydrogen Storage Kinetics of Lithium Borohydride
Abstract
:1. Introduction
2. Materials and Methods
3. Results
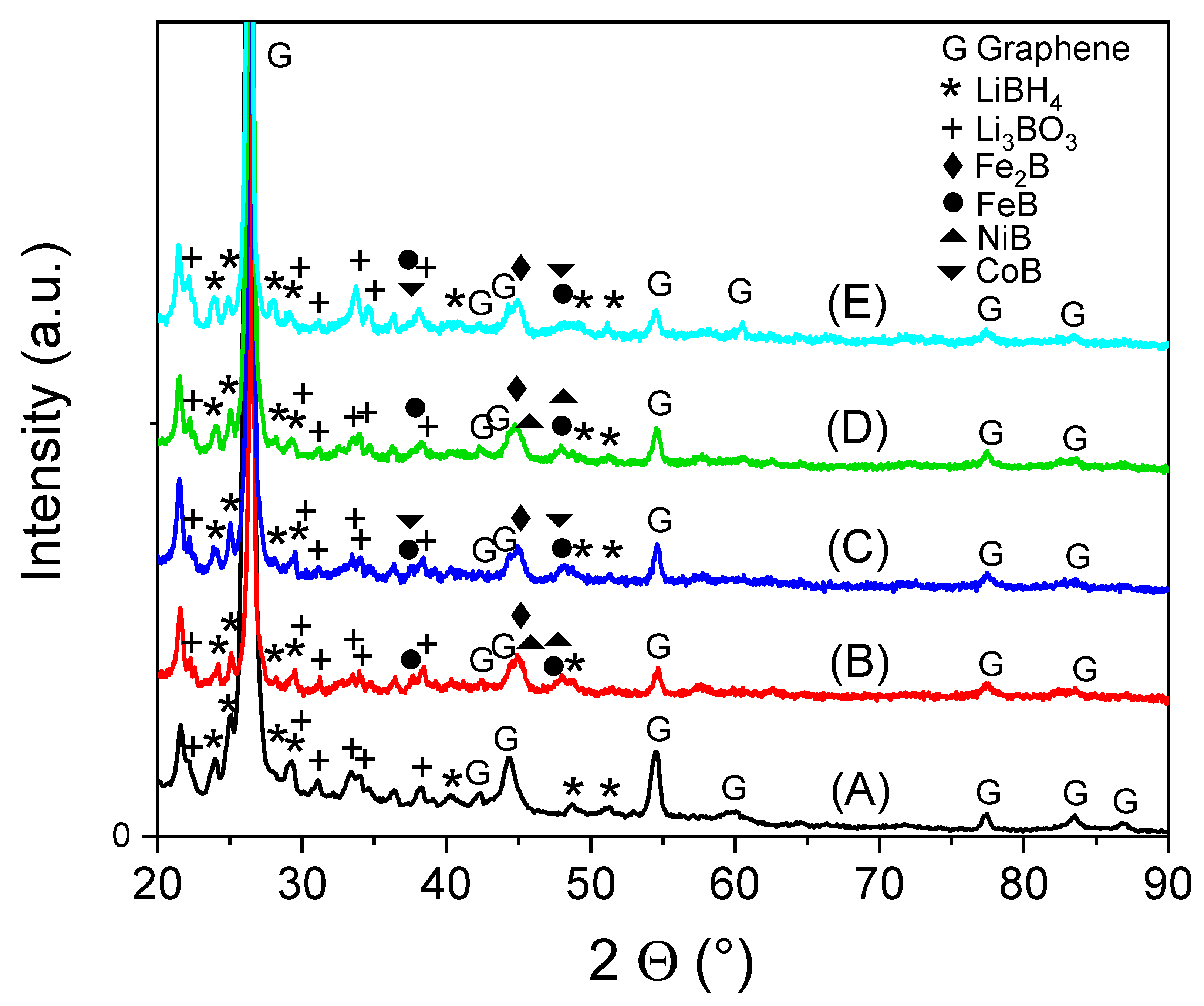
3.1. X-ray Diffraction Analysis
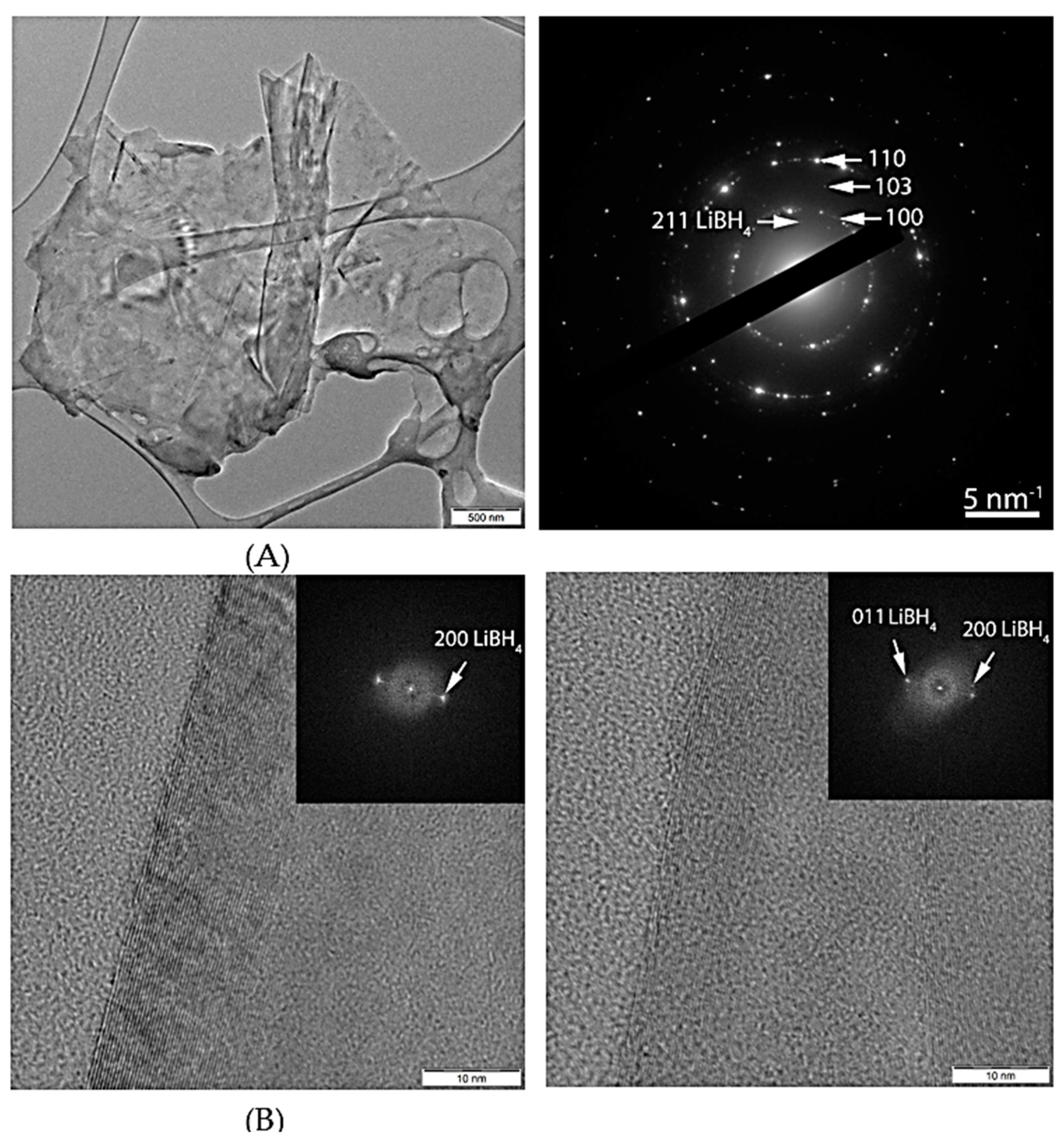
3.2. Morphological and Compositional Investigation by TEM and STEM-EDS
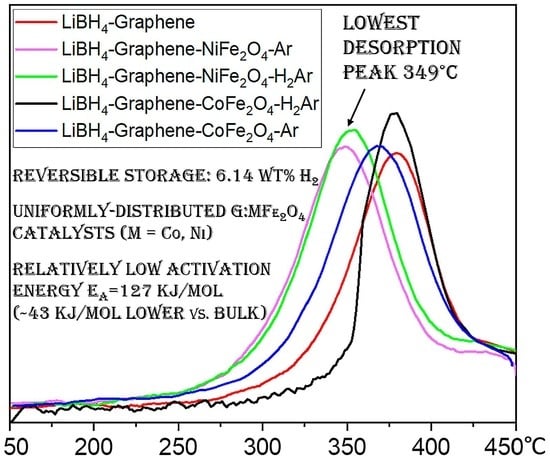
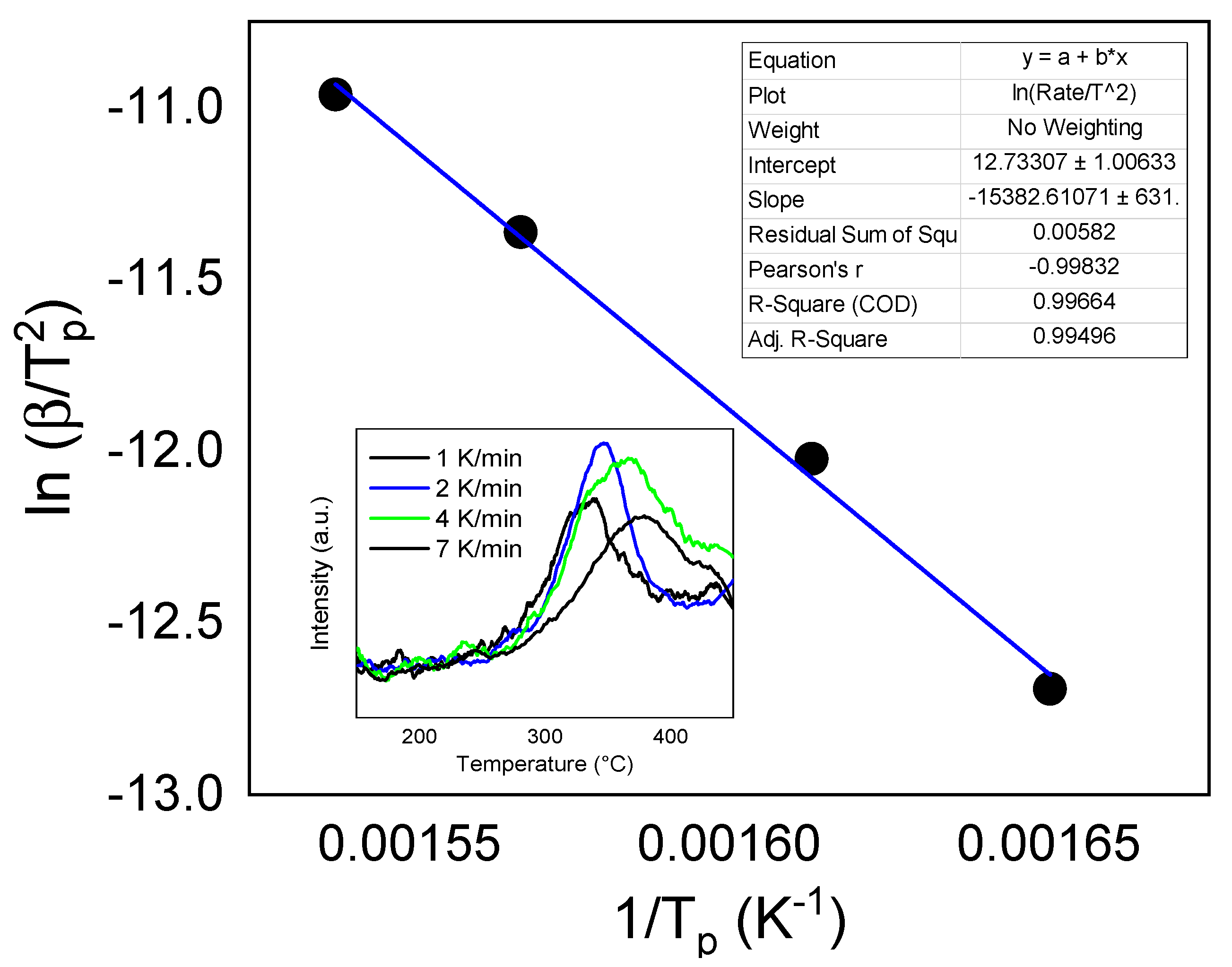
3.3. Hydrogen Storage Property Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comanescu, C. Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications. Materials 2022, 15, 2286. [Google Scholar] [CrossRef] [PubMed]
- Comanescu, C. Recent Development in Nanoconfined Hydrides for Energy Storage. Int. J. Mol. Sci. 2022, 23, 7111. [Google Scholar] [CrossRef]
- Morse, J.R.; Zugell, D.A.; Patterson, E.; Baldwin, J.W.; Willauer, H.D. Hydrogenated graphene: Important material properties regarding its application for hydrogen storage. J. Power Sources 2021, 494, 229734. [Google Scholar] [CrossRef]
- Wang, S.; Gao, M.; Yao, Z.; Liu, Y.; Wu, M.; Li, Z.; Liu, Y.; Sun, W.; Pan, H. A nanoconfined-LiBH4 system using a unique multifunctional porous scaffold of carbon wrapped ultrafine Fe3O4 skeleton for reversible hydrogen storage with high capacity. Chem. Eng. J. 2022, 428, 131056. [Google Scholar] [CrossRef]
- Gasnier, A.; Amica, G.; Juan, J.; Troiani, H.; Gennari, F.C. N-Doped Graphene-Rich Aerogels Decorated with Nickel and Cobalt Nanoparticles: Effect on Hydrogen Storage Properties of Nanoconfined LiBH4. J. Phys. Chem. C 2020, 124, 115–125. [Google Scholar] [CrossRef]
- de Kort, L.M.; Harmel, J.; de Jongh, P.E.; Ngene, P. The effect of nanoscaffold porosity and surface chemistry on the Li-ion conductivity of LiBH4-LiNH2/metal oxide nanocomposites. J. Mater. Chem. A 2020, 8, 20687–20697. [Google Scholar] [CrossRef]
- Wang, S.; Gao, M.; Xian, K.; Li, Z.; Shen, Y.; Yao, Z.; Liu, Y.; Pan, H. LiBH4 Nanoconfined in Porous Hollow Carbon Nanospheres with High Loading, Low Dehydrogenation Temperature, Superior Kinetics, and Favorable Reversibility. ACS Appl. Energy Mater. 2020, 3, 3928–3938. [Google Scholar] [CrossRef]
- Martínez, A.A.; Gasnier, A.; Gennari, F.C. Pore Filling of a Carbon Matrix by Melt-Impregnated LiBH4. J. Phys. Chem. C 2022, 126, 66–78. [Google Scholar] [CrossRef]
- Puszkiel, J.; Gasnier, A.; Amica, G.; Gennari, F. Tuning LiBH4 for Hydrogen Storage: Destabilization, Additive, and Nanoconfinement Approaches. Molecules 2020, 25, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.; Zhang, X.; Liu, Y.; Zhang, L.; Hu, J.; Gao, M.; Pan, H. A Unique Double-Layered Carbon Nanobowl-Confined Lithium Borohydride for Highly Reversible Hydrogen Storage. Small 2020, 16, 2001963. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, W.; Ren, Z.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Nano-synergy enables highly reversible storage of 9.2 wt% hydrogen at mild conditions with lithium borohydride. Nano Energy 2021, 83, 105839. [Google Scholar] [CrossRef]
- Ye, J.K.; Xia, G.L.; Yu, X.B. In-situ constructed destabilization reaction of LiBH4 wrapped with graphene toward stable hydrogen storage reversibility. Mater. Today Energy 2021, 22, 100885. [Google Scholar] [CrossRef]
- Comanescu, C.; Guran, C.; Palade, P. Improvements of kinetic properties of LiBH4 by supporting on MSU-H type mesoporous silica. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 705–708. [Google Scholar]
- Cahen, S.; Eymery, J.B.; Janot, R.; Tarascon, J.M. Improvement of the LiBH4 hydrogen desorption by inclusion into mesoporous carbons. J. Power Sources 2009, 189, 902–908. [Google Scholar] [CrossRef]
- Gross, A.F.; Vajo, J.J.; Van Atta, S.L.; Olson, G.L. Enhanced hydrogen storage kinetics of LiBH4 in nanoporous carbon scaffolds. J. Phys. Chem. C 2008, 112, 5651–5657. [Google Scholar] [CrossRef]
- Comanescu, C.; Capurso, G.; Maddalena, A. Nanoconfinement in activated mesoporous carbon of calcium borohydride for improved reversible hydrogen storage. Nanotechnology 2012, 23, 385401. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, S.; Huang, Q.; Li, J.; Cen, X.; Zhang, H.; Chu, H.; Sun, L.; Xu, F.; Huang, P. Facile synthesis of NiCo2O4-anchored reduced graphene oxide nanocomposites as efficient additives for improving the dehydrogenation behavior of lithium alanate. Inorg. Chem. Front. 2020, 7, 1257–1272. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Zhang, L.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Single-pot solvothermal strategy toward support-free nanostructured LiBH4 featuring 12 wt% reversible hydrogen storage at 400 °C. Chem. Eng. J. 2022, 428, 132566. [Google Scholar] [CrossRef]
- Friedrichs, O.; Borgschulte, A.; Kato, S.; Buchter, F.; Gremaud, R.; Remhof, A.; Zuttel, A. Low-temperature synthesis of LiBH4 by gas-solid reaction. Chem. Eur. J. 2009, 15, 5531–5534. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Remhof, A.; Hwang, S.-J.; Li, H.-W.; Mauron, P.; Orimo, S.-I.; Zuttel, A. Pressure and temperature dependence of the decomposition pathway of LiBH4. Phys. Chem. Chem. Phys. 2012, 14, 6514–6519. [Google Scholar] [CrossRef] [Green Version]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef]
- Mosegaard, L.; Møller, B.; Jørgensen, J.E.; Bosenberg, U.; Dornheim, M.; Hanson, J.C.; Cerenius, Y.; Walker, G.S.; Jakobsen, H.J.; Besenbacher, F.; et al. Intermediate phases observed during decomposition of LiBH4. J. Alloys Compd. 2007, 446, 301–305. [Google Scholar] [CrossRef]
- Kato, S.; Bielmann, M.; Borgschulte, A.; Zakaznova-Herzog, V.; Remhof, A.; Orimo, S.; Zuttel, A. Effect of the surface oxidation of LiBH4 on the hydrogen desorption mechanism. Phys. Chem. Chem. Phys. 2010, 12, 10950–10955. [Google Scholar] [CrossRef]
- Verdal, N.; Udovic, T.J.; Rush, J.J.; Liu, X.; Majzoub, E.H.; Vajo, J.J.; Gross, A.F. Dynamical perturbations of tetrahydroborate anions in LiBH4 due to nanoconfinement in controlled-pore carbon scaffolds. J. Phys. Chem. C 2013, 117, 17983–17995. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Liao, Q.; Zhang, W.; Huang, Z.; Chen, X.; Shao, Y.; Dong, H.; Liu, Q.; Li, H. Modulation the electronic structure of hollow structured CuO-NiCo2O4 nanosphere for enhanced catalytic activity towards methanolysis of ammonia borane. Fuel 2023, 332, 126045. [Google Scholar] [CrossRef]
- Liao, J.; Shao, Y.; Feng, Y.; Zhang, J.; Song, C.; Zeng, W.; Tang, J.; Dong, H.; Liu, Q.; Li, H. Interfacial charge transfer induced dual-active-sites of heterostructured Cu0.8Ni0.2WO4 nanoparticles in ammonia borane methanolysis for fast hydrogen production. Appl. Catal. B Environ. 2023, 320, 121973. [Google Scholar] [CrossRef]
- Comanescu, C. Paving the Way to the Fuel of the Future—Nanostructured Complex Hydrides. Int. J. Mol. Sci. 2023, 24, 143. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Wan, Q.; Zhai, F.; Volinsky, A.A.; Qu, X. Superior destabilization effects of LiBH4 with the addition of nano-sized nickel ferrite NiFe2O4. RSC Adv. 2015, 5, 81212–81219. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.; Zhai, F.; Wan, Q.; Li, X.; Qu, X.; Volinsky, A.A. NiFe2O4 Nanoparticles Catalytic Effects of Improving LiAlH4 Dehydrogenation Properties. J. Phys. Chem. C 2013, 117, 25917–25925. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhai, F.; Wan, Q.; Liu, Z.; Shan, J.; Li, P.; Volinskyc, A.A.; Qua, X. Enhanced hydrogen storage properties of LiAlH4 catalyzed by CoFe2O4 nanoparticles. RSC Adv. 2014, 4, 18989–18997. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, P.; Wan, Q.; Zhang, J.; Li, Y.; Li, R.; Dong, X.; Qu, X. Improved dehydrogenation performance of NaAlH4 using NiFe2O4 nanoparticles. J. Alloys Compd. 2017, 709, 850–856. [Google Scholar] [CrossRef]


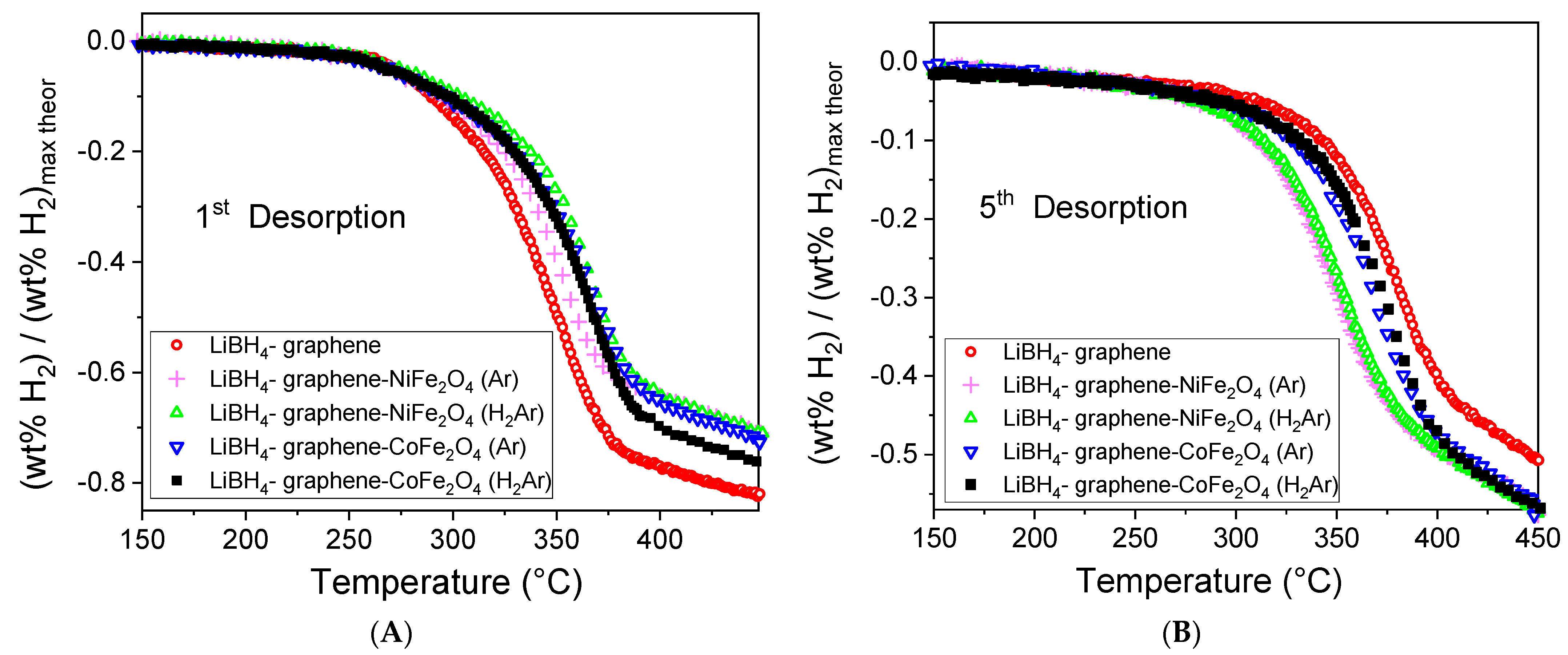
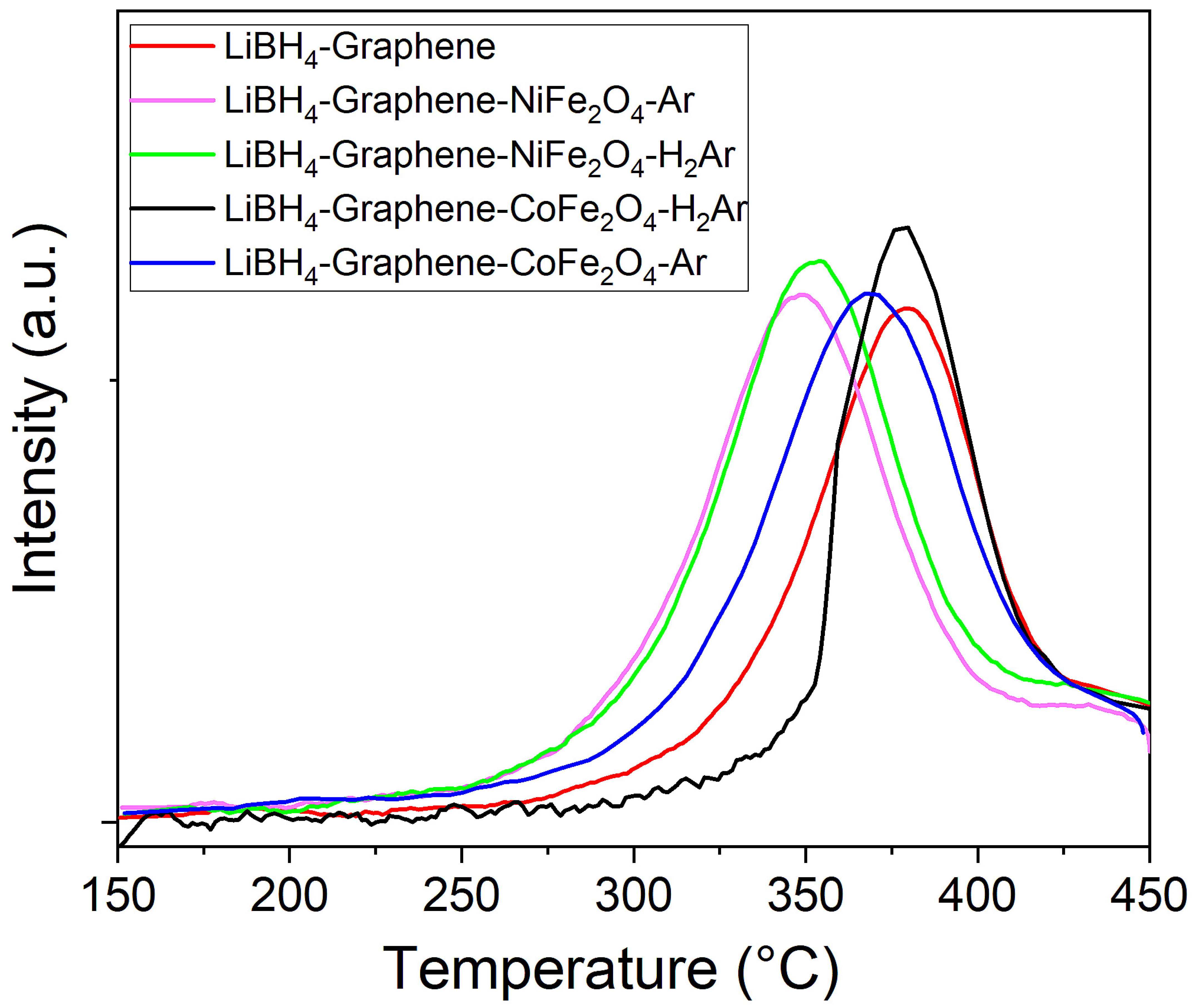
| Sample | Normalized 1st Des. <450 °C/Complete | Normalized 5th Des. <450 °C/Complete | T Des. Peak (°C) |
|---|---|---|---|
| LiBH4-G | 0.83/0.95 | 0.51/0.86 | 379.5 |
| LiBH4-G-NFO-Ar | 0.72/0.99 | 0.57/0.89 | 349 |
| LiBH4-G-NFO-H2Ar | 0.71/0.99 | 0.58/0.91 | 353 |
| LiBH4-G-CFO-Ar | 0.72/0.99 | 0.56/0.87 | 369 |
| LiBH4-G-CFO-H2Ar | 0.77/0.99 | 0.57/0.87 | 378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palade, P.; Comanescu, C.; Radu, C. Synthesis of Nickel and Cobalt Ferrite-Doped Graphene as Efficient Catalysts for Improving the Hydrogen Storage Kinetics of Lithium Borohydride. Materials 2023, 16, 427. https://doi.org/10.3390/ma16010427
Palade P, Comanescu C, Radu C. Synthesis of Nickel and Cobalt Ferrite-Doped Graphene as Efficient Catalysts for Improving the Hydrogen Storage Kinetics of Lithium Borohydride. Materials. 2023; 16(1):427. https://doi.org/10.3390/ma16010427
Chicago/Turabian StylePalade, Petru, Cezar Comanescu, and Cristian Radu. 2023. "Synthesis of Nickel and Cobalt Ferrite-Doped Graphene as Efficient Catalysts for Improving the Hydrogen Storage Kinetics of Lithium Borohydride" Materials 16, no. 1: 427. https://doi.org/10.3390/ma16010427
APA StylePalade, P., Comanescu, C., & Radu, C. (2023). Synthesis of Nickel and Cobalt Ferrite-Doped Graphene as Efficient Catalysts for Improving the Hydrogen Storage Kinetics of Lithium Borohydride. Materials, 16(1), 427. https://doi.org/10.3390/ma16010427