Fabrication and Characterization of Eco-Friendly Thin Films as Potential Optical Absorbers for Efficient Multi-Functional Opto-(Electronic) and Solar Cell Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
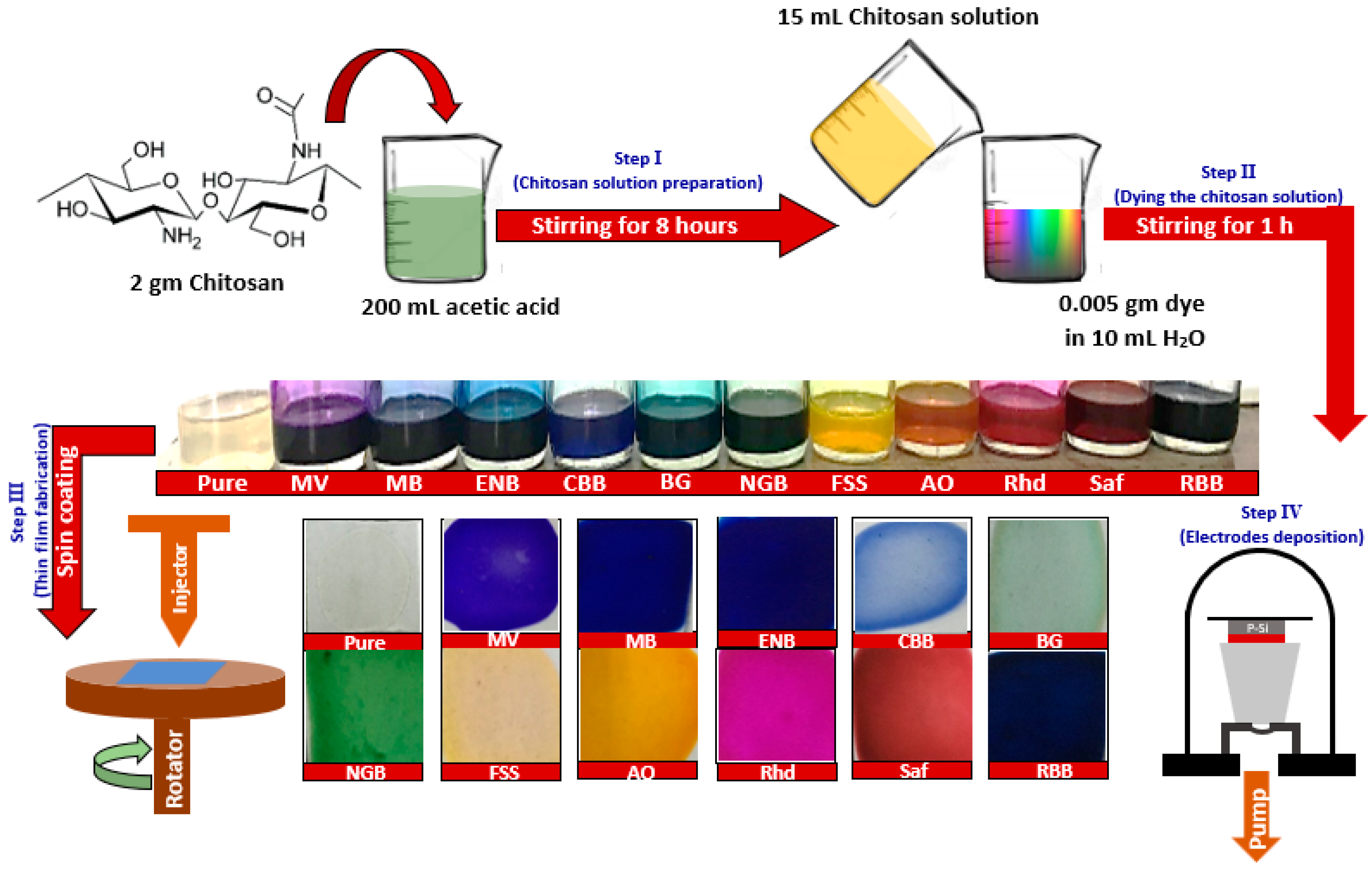
2.2. Preparation of Thin Films
2.3. Characterization Techniques
3. Results
3.1. FTIR Molecular Structure Characterization
3.2. Spectrophotometric Optical Characterizations
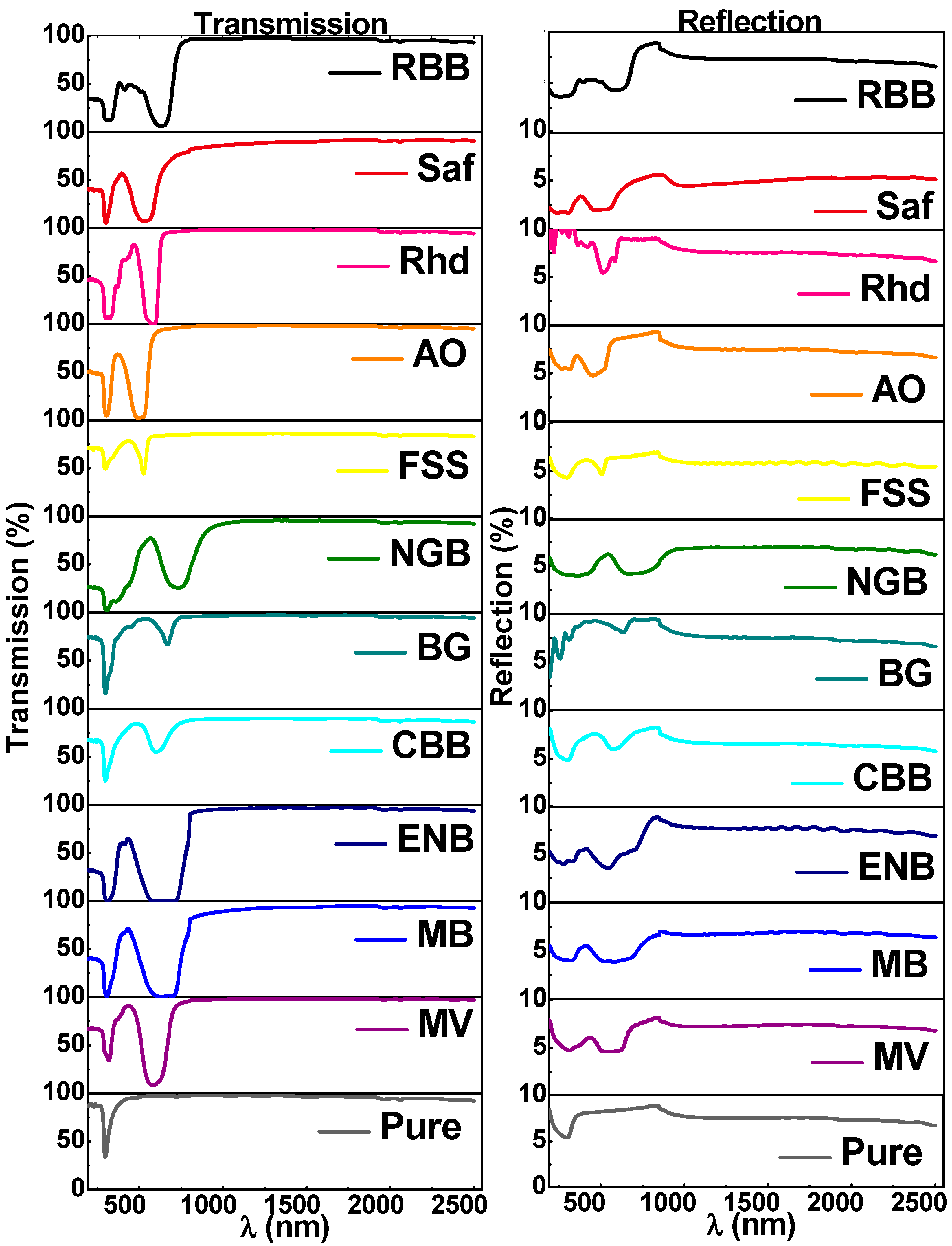
3.2.1. Transmission and Reflection Spectra
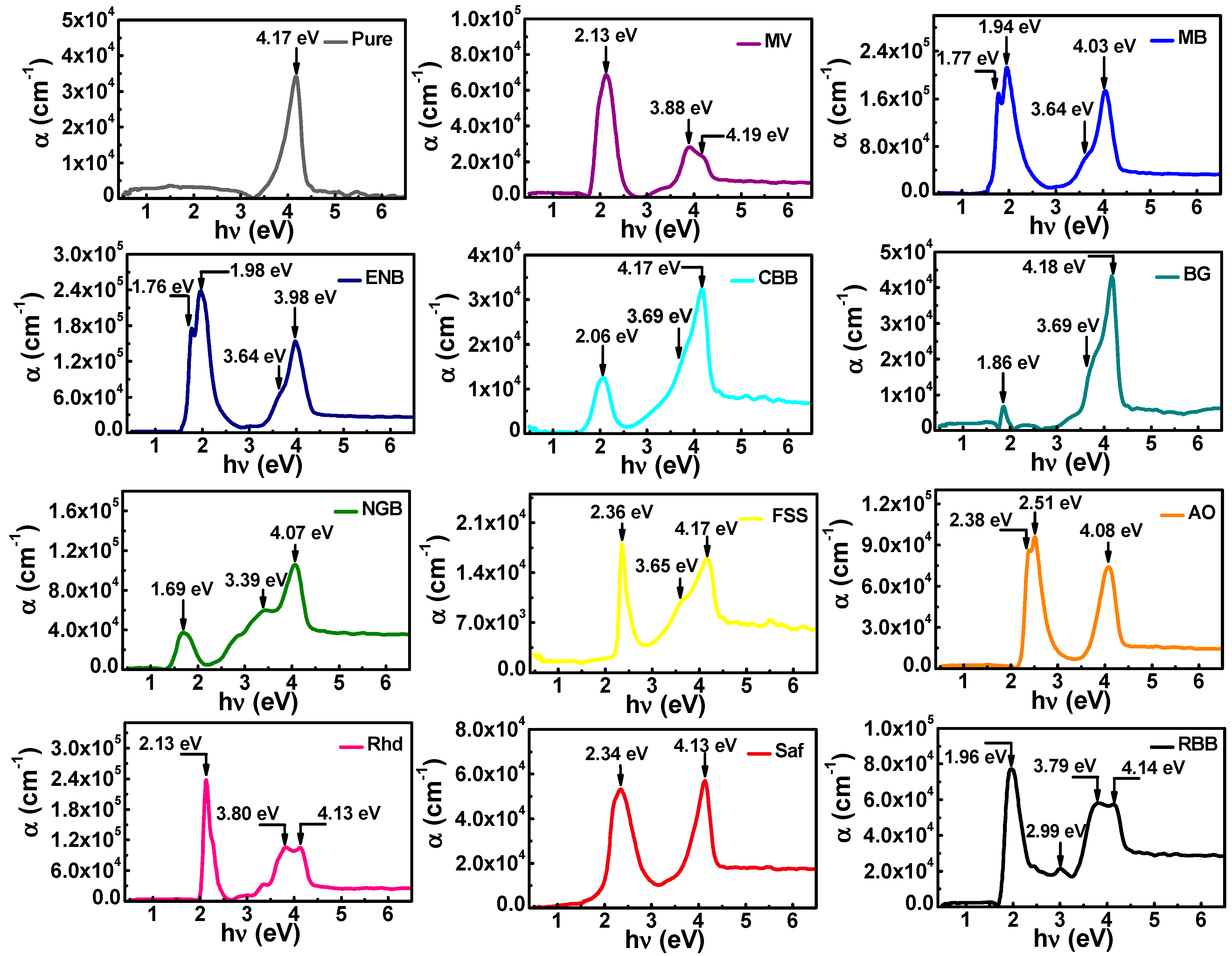
3.2.2. Absorption Coefficient, Energy Gap, and Urbach Energy
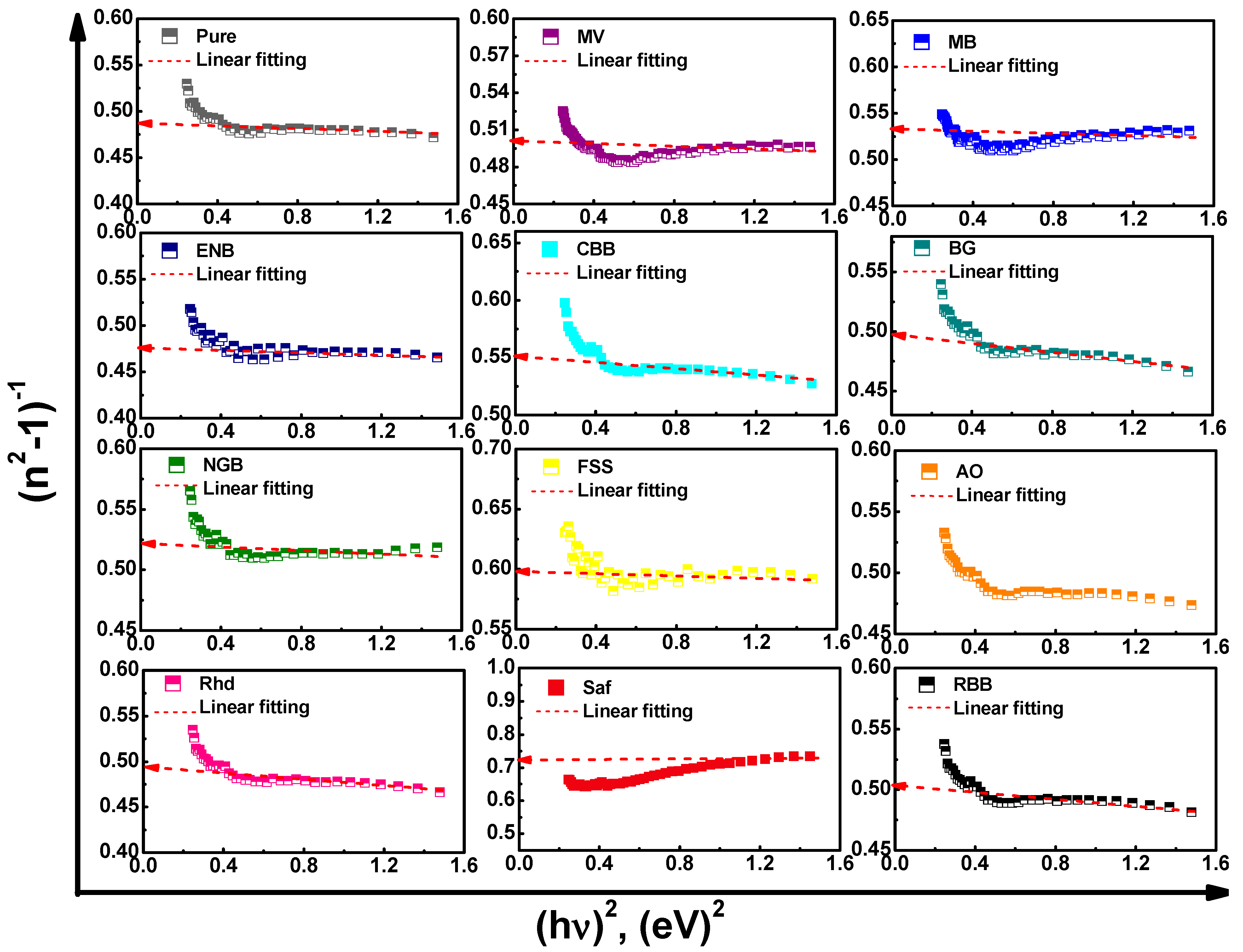
3.2.3. Dispersion Behavior and Dispersive Parameters
3.2.4. Nonlinear Optical Parameters
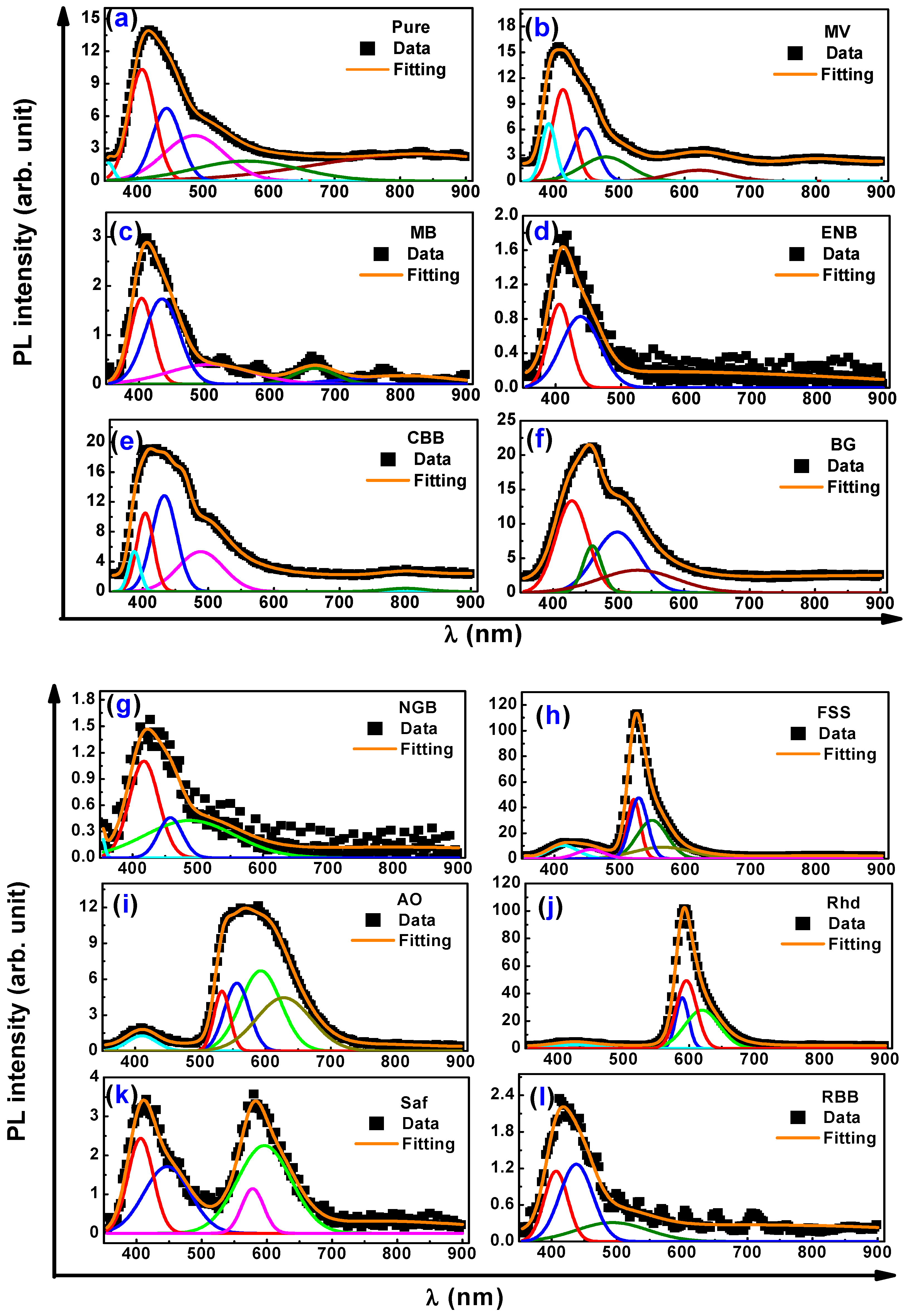
3.3. Photoluminescence and Color Chromaticity Characterizations
3.4. Laser Cut-Off Filter Performances
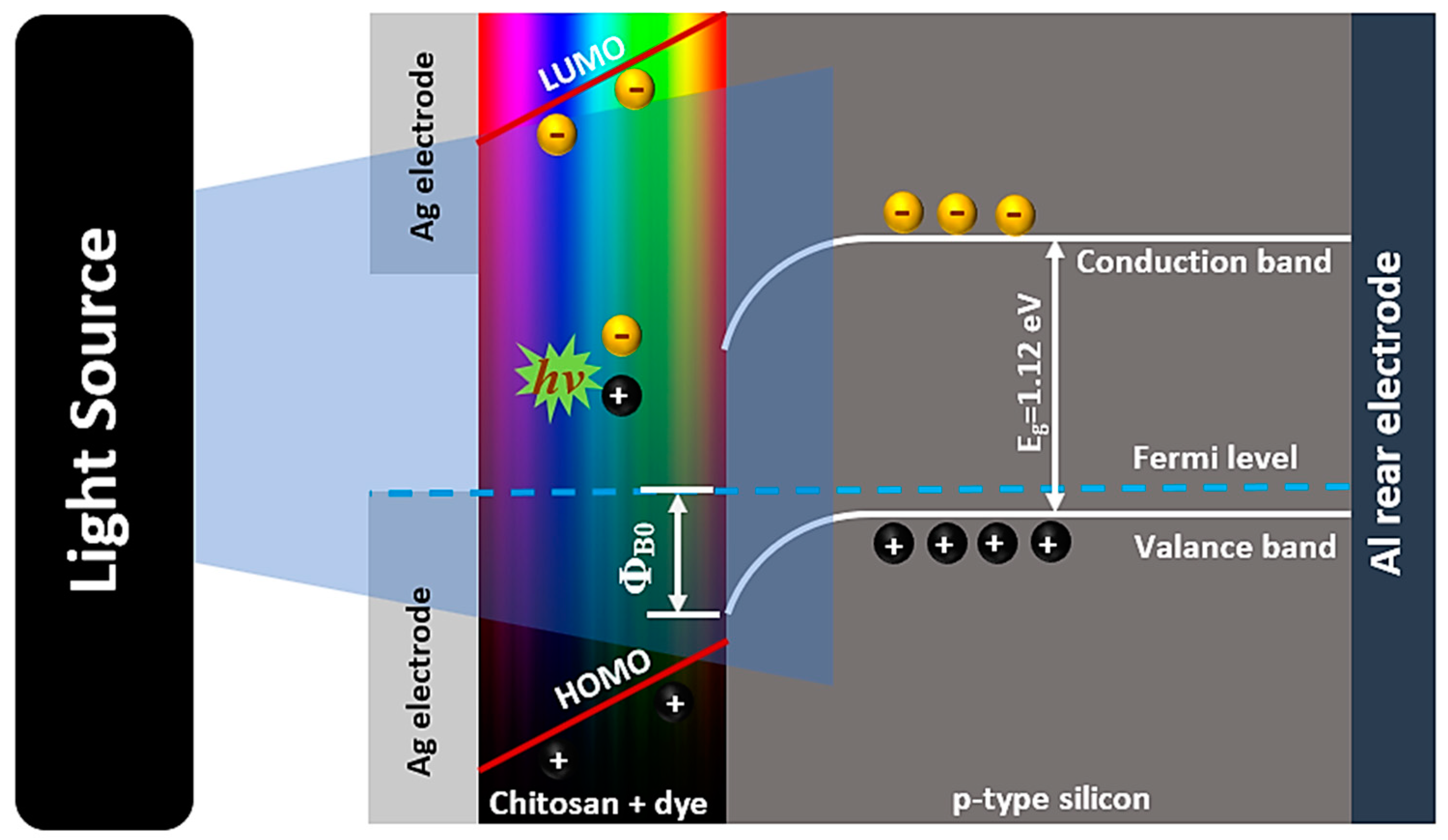
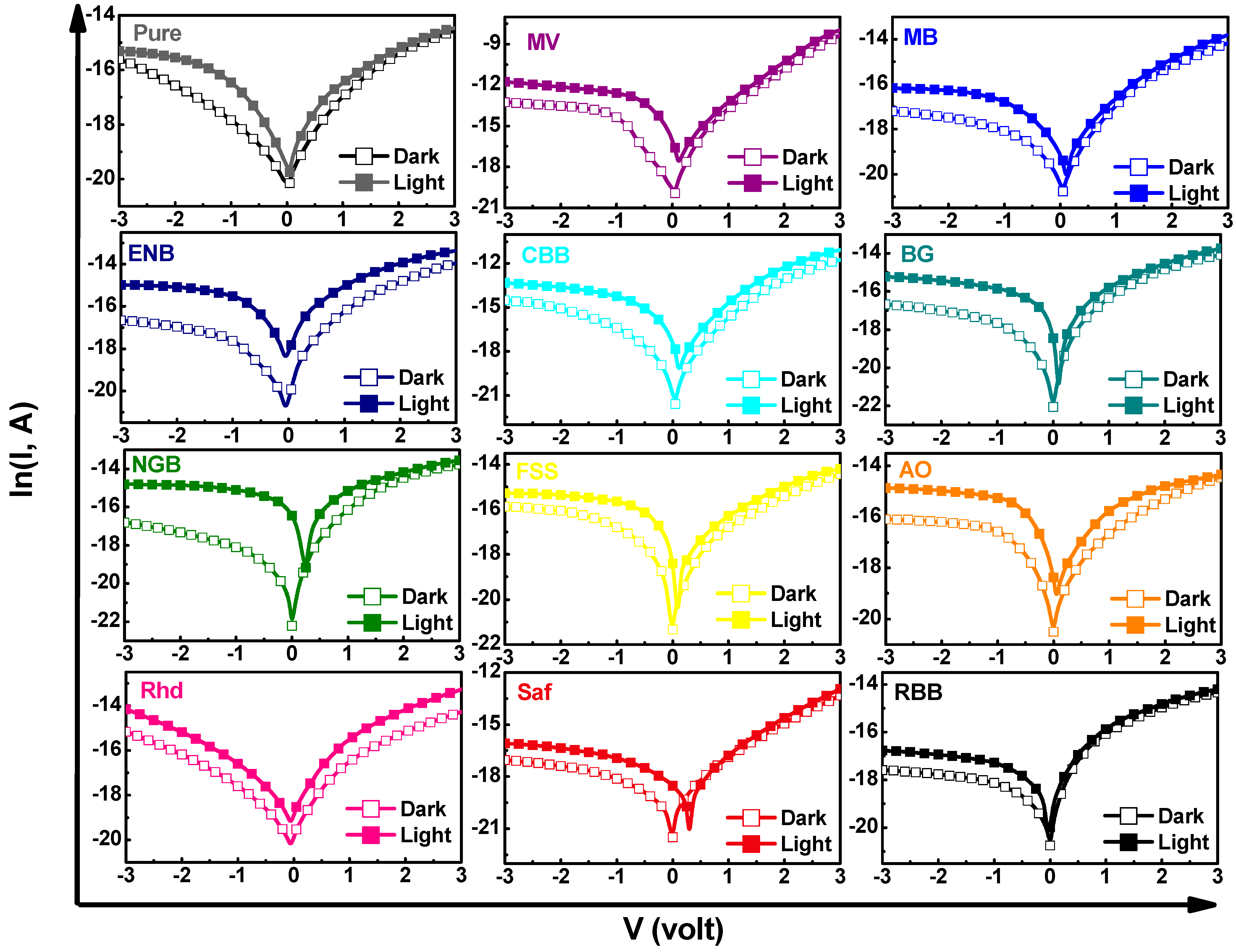
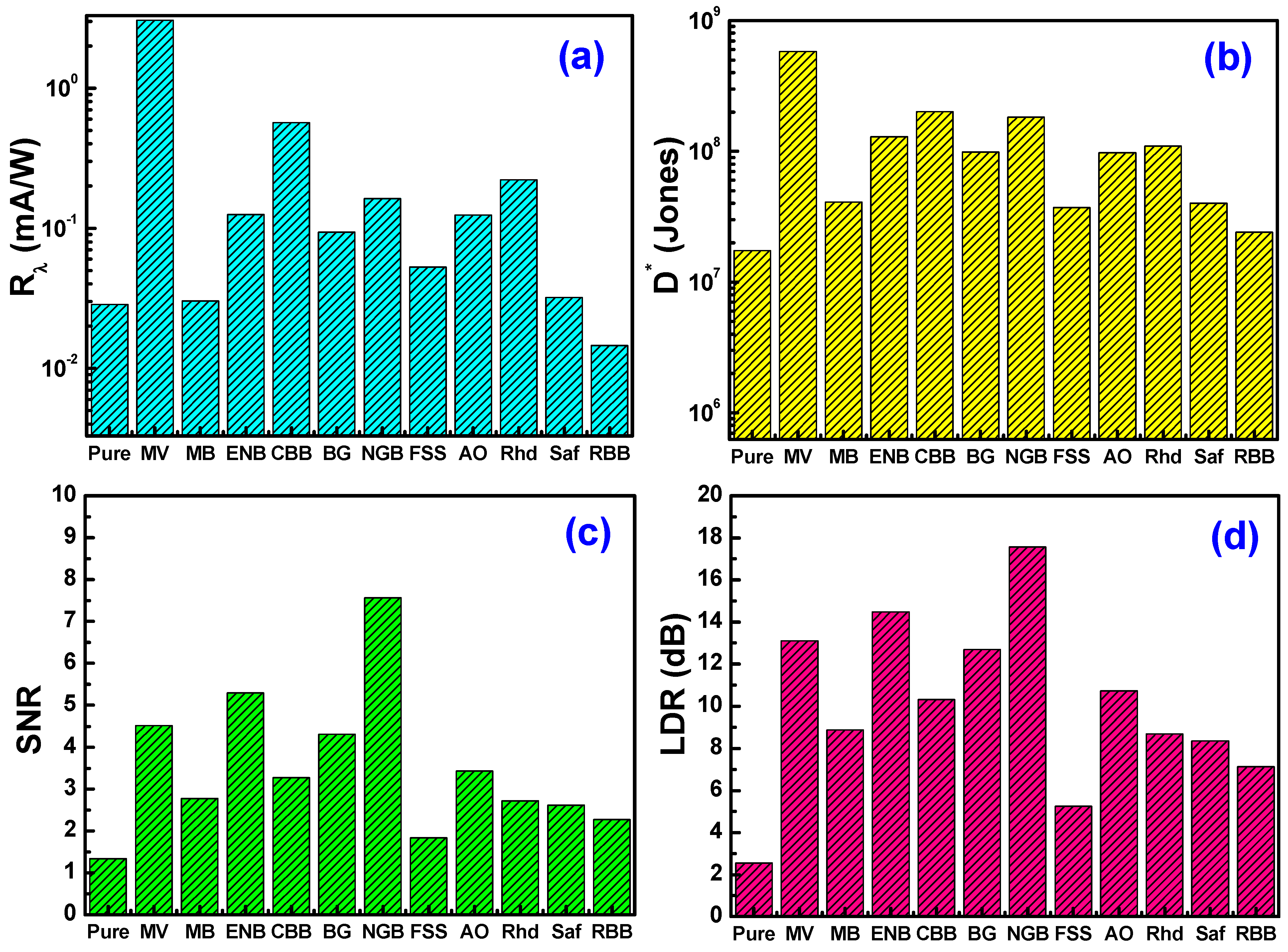
3.5. Photosensing Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X. Eco-Friendly Holocellulose Materials for Mechanical Performance and Optical Transmittance 2019; KTH Royal Institute of Technology: Stockholm, Sweden, 2019. [Google Scholar]
- Ullal, N.; Muthamma, K.; Sunil, D. Carbon Dots from Eco-Friendly Precursors for Optical Sensing Application: An up-to-Date Review. Chem. Pap. 2022, 76, 6097–6127. [Google Scholar] [CrossRef]
- Mariotti, N.; Bonomo, M.; Fagiolari, L.; Barbero, N.; Gerbaldi, C.; Bella, F.; Barolo, C. Recent Advances in Eco-Friendly and Cost-Effective Materials towards Sustainable Dye-Sensitized Solar Cells. Green Chem. 2020, 22, 7168–7218. [Google Scholar] [CrossRef]
- Giraldo, J.D.; Rivas, B.L. Direct Ionization and Solubility of Chitosan in Aqueous Solutions with Acetic Acid. Polym. Bull. 2021, 78, 1465–1488. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of Chitosan in Environmental Remediation: A Review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M. Chitosan-Based Nanoparticles against Bacterial Infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Luyt, A.S. Development of Multifunctional Nano/Ultrafiltration Membrane Based on a Chitosan Thin Film on Alginate Electrospun Nanofibres. J. Clean. Prod. 2017, 156, 470–479. [Google Scholar] [CrossRef]
- Pirillo, S.; Pedroni, V.; Rueda, E.; Luján Ferreira, M. Elimination of Dyes from Aqueous Solutions Using Iron Oxides and Chitosan as Adsorbents: A Comparative Study. Quim. Nova 2009, 32, 1239–1244. [Google Scholar] [CrossRef]
- Fereidooni, L.; Abbaspourrad, A.; Enayati, M. Electrolytic Transesterification of Waste Frying Oil Using Na+/Zeolite–Chitosan Biocomposite for Biodiesel Production. Waste Manag. 2021, 127, 48–62. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan Biopolymer for Fuel Cell Applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef]
- Akkılıç, K.; Yakuphanoglu, F. Electrical and Photovoltaic Properties of a N-Si/Chitosan/Ag Photodiode. Microelectron. Eng. 2008, 85, 1826–1830. [Google Scholar] [CrossRef]
- Kacus, H.; Aydogan, S.; Incekara, U.; Yilmaz, M.; Biber, M. On Thermal and Optical Sensor Applications of Chitosan Molecule in the Co/Chitosan/p-Si Hybrid Heterojunction Design. J. Mater. Sci. Mater. Electron. 2021, 32, 6586–6597. [Google Scholar] [CrossRef]
- Kocyigit, A.; Yilmaz, M.; Aydogan, S.; Incekara, U.; Sahin, Y. The Performance of Chitosan Layer in Au/n-Si Sandwich Structures as a Barrier Modifier. Polym. Test. 2020, 89, 106546. [Google Scholar] [CrossRef]
- Tran, K.M.; Do, D.P.; Thi, K.H.T.; Pham, N.K. Influence of Top Electrode on Resistive Switching Effect of Chitosan Thin Films. J. Mater. Res. 2019, 34, 3899–3906. [Google Scholar] [CrossRef]
- Do, P.D.; Doan, T.U.T.; Tran, T.D.; Van Hoang, D.; Pham, K.N. The Effect of Content and Thickness of Chitosan Thin Films on Resistive Switching Characteristics. VNUHCM J. Sci. Technol. Dev. 2020, 23, 637–644. [Google Scholar] [CrossRef]
- Ni, J.; Huang, X.; Bai, Y.; Zhao, B.; Han, Y.; Han, S.; Xu, T.; Si, C.; Zhang, C. Resistance to Aggregation-Caused Quenching: Chitosan-Based Solid Carbon Dots for White Light-Emitting Diode and 3D Printing. Adv. Compos. Hybrid Mater. 2022, 5, 1865–1875. [Google Scholar] [CrossRef]
- Jang, J.; Kang, K.; Raeis-Hosseini, N.; Ismukhanova, A.; Jeong, H.; Jung, C.; Kim, B.; Lee, J.; Park, I.; Rho, J. Self-powered Humidity Sensor Using Chitosan-based Plasmonic Metal–Hydrogel–Metal Filters. Adv. Opt. Mater. 2020, 8, 1901932. [Google Scholar] [CrossRef]
- Sisman, O.; Kaur, N.; Sberveglieri, G.; Núñez-Carmona, E.; Sberveglieri, V.; Comini, E. UV-Enhanced Humidity Sensing of Chitosan–SnO2 Hybrid Nanowires. Nanomaterials 2020, 10, 329. [Google Scholar] [CrossRef]
- Ahmad, H.; Ooi, S.I.; Yusoff, N.; Lim, H.S.; MatJafri, M.Z.; Thambiratnam, K.; Tiu, Z.C. Tungsten Disulfide-Chitosan Film as Optical Pulse and Amplitude Modulator in C-Band Region. Laser Phys. 2019, 29, 105102. [Google Scholar] [CrossRef]
- Sergeev, A.A.; Voznesenskiy, S.S.; Bratskaya, S.Y.; Mironenko, A.Y.; Lagutkin, R.V. Investigation of Humidity Influence upon Waveguide Features of Chitosan Thin Films. Phys. Procedia 2012, 23, 115–118. [Google Scholar] [CrossRef][Green Version]
- Lee, C.W.; Lee, S.H.; Yang, Y.; Ryu, G.; Kim, H. Fabrication of Photochromic Hydrogels Using an Interpenetrating Chitosan Network. J. Appl. Polym. Sci. 2017, 134, 45120. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Guo, L.; Nardeli, J.V.; Oukhrib, R. The Application of Chitosan-Based Compounds against Metallic Corrosion. Chitin Chitosan-Physicochem. Prop. Ind. Appl. 2021, 11, 231. [Google Scholar]
- de Marzo, G.; Mastronardi, V.M.; Algieri, L.; Vergari, F.; Pisano, F.; Fachechi, L.; Marras, S.; Natta, L.; Spagnolo, B.; Brunetti, V. Sustainable, Flexible, and Biocompatible Enhanced Piezoelectric Chitosan Thin Film for Compliant Piezosensors for Human Health. Adv. Electron. Mater. 2022, 2200069. [Google Scholar] [CrossRef]
- Rahman, N.A.; Hanifah, S.A.; Mobarak, N.N.; Ahmad, A.; Ludin, N.A.; Bella, F.; Su’ait, M.S. Chitosan as a Paradigm for Biopolymer Electrolytes in Solid-State Dye-Sensitised Solar Cells. Polymer 2021, 230, 124092. [Google Scholar] [CrossRef]
- Zaki, A.A.; Abdel-Basset, T.A.; Haggar, M.; Bashal, A.H. Dielectric and Optical Properties of Chitosan-Pb and Chitosan-Bi Nanocomposites. J. Mater. Sci. Mater. Electron. 2021, 32, 3603–3611. [Google Scholar] [CrossRef]
- Houser, K.W.; Esposito, T.; Royer, M.P.; Christoffersen, J. A Method and Tool to Determine the Colorimetric and Photobiological Properties of Light Transmitted through Glass and Other Optical Materials. Build. Environ. 2022, 215, 108957. [Google Scholar] [CrossRef]
- Yue, W.; Wang, Z.; Wang, Z.; Xu, Q.; Zheng, C.; Zha, X.; Gui, H.; Zhang, H. Synthesis of CdS with Chitosan for Photodegradation to Rhodamine B. J. Nanopart. Res. 2021, 23, 2. [Google Scholar] [CrossRef]
- Fatima, B. Quantitative Analysis by IR: Determination of Chitin/Chitosan DD. Mod. Spectrosc. Tech. Appl. 2020, 7, 107. [Google Scholar]
- Queiroz, M.F.; Teodosio Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Lustriane, C.; Dwivany, F.M.; Suendo, V.; Reza, M. Effect of Chitosan and Chitosan-Nanoparticles on Post Harvest Quality of Banana Fruits. J. Plant Biotechnol. 2018, 45, 36–44. [Google Scholar] [CrossRef]
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical Properties of Chitin and Chitosan Biopolymers with Application to Structural Color Analysis. Opt. Mater. 2012, 35, 175–183. [Google Scholar] [CrossRef]
- Koralewski, M.; Bodek, K.H.; Marczewska, K. Optical Properties of Chitosan in Aqueous Solution. Polish Chitin Soc. 2006, 4, 29–39. [Google Scholar]
- Salem, M.S.; Wassel, A.R.; Fedawy, M.; Shaker, A.; Al-Bagawia, A.H.; Alanazi, A.; El-Mahalawy, A.M. Elucidation the Effectiveness of Acridine Orange as Light-Harvesting Layer for Photosensing Applications: Structural, Spectroscopic and Electrical Investigations. Opt. Mater. 2022, 133, 112928. [Google Scholar] [CrossRef]
- Amin, F.M.; Abdel-Khalek, H.; El-Sagheer, A.M.; Abd-El Salam, M.; El-Mahalawy, A.M. Detailed Investigations on Structural, Spectroscopic, and Optoelectronic Properties of Ethyl Nile Blue Thin Films. Phys. B Condens. Matter 2022, 642, 414141. [Google Scholar] [CrossRef]
- Negrea, P.; Caunii, A.; Sarac, I.; Butnariu, M. The Study of Infrared Spectrum of Chitin and Chitosan Extract as Potential Sources of Biomass. Dig. J. Nanomater. Biostruct. 2015, 10, 1129–1138. [Google Scholar]
- Fahmy, T.; Sarhan, A. Characterization and Molecular Dynamic Studies of Chitosan–Iron Complexes. Bull. Mater. Sci. 2021, 44, 142. [Google Scholar] [CrossRef]
- Salem, M.S.; Wassel, A.R.; Fedawy, M.; Shaker, A.; Al-Bagawia, A.H.; Aleid, G.M.; El-Mahalawy, A.M. Integration of Biocompatible Coomassie Brilliant Blue Dye on Silicon in Organic/Inorganic Heterojunction for Photodetection Applications. J. Phys. Chem. Solids 2022, 169, 110890. [Google Scholar] [CrossRef]
- Almotiri, R.A.; Alkhamisi, M.M.; Wassel, A.R.; El-Mahalawy, A.M. Optical Dispersion and Photovoltaic Performance of Safranin Thin Films Solar Cells in Hybrid Organic-Inorganic Isotype Heterojunction Configuration. Mater. Res. Bull. 2022, 151, 111824. [Google Scholar] [CrossRef]
- Kubba, R.; Singh, M.K.; Yadav, O.; Kumar, A. Förster Resonance Energy Transfer (FRET) between CdSe Quantum Dots and ABA Phosphorus (V) Corroles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 291, 122345. [Google Scholar] [CrossRef]
- El-Mahalawy, A.M.; Wassel, A.R. Enhancement of Organic/Inorganic Hybrid Photodetector Based on Pentacene/n-Si by Surface Plasmonic Effect of Gold and Silver Nanoparticles: A Comparative Study. Opt. Laser Technol. 2020, 131, 106395. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Mohamad, A.D.M. Synthesis, Structural, Thermal, Optical and Dielectric Properties of Chitosan Biopolymer; Influence of PVP and α-Fe2O3 Nanorods. J. Polym. Res. 2018, 25, 175. [Google Scholar] [CrossRef]
- Wemple, S.H.; DiDomenico Jr, M. Behavior of the Electronic Dielectric Constant in Covalent and Ionic Materials. Phys. Rev. B 1971, 3, 1338. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Detection of Mercury and Copper Ions Using Surface Plasmon Resonance Optical Sensor. Sens. Mater. 2011, 23, 325–334. [Google Scholar]
- Penn, D.R. Wave-Number-Dependent Dielectric Function of Semiconductors. Phys. Rev. 1962, 128, 2093. [Google Scholar] [CrossRef]
- El-Mahalawy, A.M.; Wassel, A.R. Structural, Optical and Photoelectrical Characteristics of 4-Methoxy-2-Nitroaniline for Optoelectronic Applications. Mater. Sci. Semicond. Process. 2020, 116, 105124. [Google Scholar] [CrossRef]
- Abdel-Khalek, H.; Amin, F.M.; Wassel, A.R.; El-Mahalawy, A.M. Enhancement of Structure and Optical Dispersion Properties of N, N′-Bis (3-Methylphenyl)-N, N′-Diphenylbenzidine Thin Films: Impact of UV Irradiation. Opt. Mater. 2021, 113, 110867. [Google Scholar] [CrossRef]
- Sang, L.; Liao, M.; Sumiya, M. A Comprehensive Review of Semiconductor Ultraviolet Photodetectors: From Thin Film to One-Dimensional Nanostructures. Sensors 2013, 13, 10482–10518. [Google Scholar] [CrossRef] [PubMed]
- Tung, R.T. The Physics and Chemistry of the Schottky Barrier Height. Appl. Phys. Rev. 2014, 1, 11304. [Google Scholar]
- Akkılıç, K.; Uzun, İ.; Kılıçoğlu, T. The Calculation of Electronic Properties of an Ag/Chitosan/n-Si Schottky Barrier Diode. Synth. Met. 2007, 157, 297–302. [Google Scholar] [CrossRef]
- Bednorz, M.; Matt, G.J.; Głowacki, E.D.; Fromherz, T.; Brabec, C.J.; Scharber, M.C.; Sitter, H.; Sariciftci, N.S. Silicon/Organic Hybrid Heterojunction Infrared Photodetector Operating in the Telecom Regime. Org. Electron. 2013, 14, 1344–1350. [Google Scholar] [CrossRef]
- Tubtimsri, S.; Limmatvapirat, C.; Limsirichaikul, S.; Akkaramongkolporn, P.; Inoue, Y.; Limmatvapirat, S. Fabrication and Characterization of Spearmint Oil Loaded Nanoemulsions as Cytotoxic Agents against Oral Cancer Cell. Asian J. Pharm. Sci. 2018, 13, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.A.M.; Elkanzi, N.A.A.; Ammar, A.H.; Roushdy, N. Performance and Photoresponse Characterizations of Pyrimidine Quinolone Carboxylate Derivatives Films-Based Heterojunction Devices. Optik 2021, 231, 166426. [Google Scholar] [CrossRef]
- Yilmaz, M.; Kocyigit, A.; Aydogan, S.; Incekara, U.; Tursucu, A.; Kacus, H. Light-Sensing Behaviors of Organic/n-Si Bio-Hybrid Photodiodes Based on Malachite Green (MG) Organic Dye. J. Mater. Sci. Mater. Electron. 2020, 31, 21548–21556. [Google Scholar] [CrossRef]
- Farag, A.A.M.; Roushdy, N.; Halim, S.A. Towards Significant Enhancement of Structural and Optoelectronic Properties of Porphyrin Palladium (II) Complex: A Theoretical and Experimental Analysis. J. Mol. Struct. 2021, 1232, 129933. [Google Scholar] [CrossRef]
- Roushdy, N.; Farag, A.A.M.; Ibrahim, M.A.; Halim, S.A.; El-Gohary, N.M. Synthesis, Spectral Characterization, DFT and Photosensitivity Studies of 1-{[(4-Methoxy-5-Oxo-5H-Furo [3, 2-g] Chromen-6-Yl) Methylidene] Amino}-4, 6-Dimethyl-2-Oxo-1, 2-Dihydropyridine-3-Carbonitrile (MFCMP). Optik 2019, 178, 1163–1176. [Google Scholar] [CrossRef]


| Assignment | Wavenumber (cm−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure | MV | MB | ENB | CBB | BG | NGB | FSS | AO | Rhd | Saf | RBB | |
| 409 | 403 | - | 407 | - | 402 | 401 | - | 406 | - | 409 | 407 | |
| 418 | 418 | 414 | 421 | 426 | 416 | 421 | 410 | 426 | 419 | 429 | 417 | |
| 442 | 443 | 44 | 434 | - | 430 | 438 | 435 | - | 439 | 444 | 434 | |
| 454 | - | 456 | 453 | 455 | 451 | 449 | 459 | 457 | - | 459 | 445 | |
| 464 | 462 | 465 | 467 | - | 464 | 465 | - | - | 465 | 460 | 467 | |
| - | - | - | - | 480 | 479 | 472 | - | - | - | - | ||
| - | 492 | 492 | 485 | 495 | 495 | 494 | - | 506 | 490 | 488 | 501 | |
| 525 | 522 | 527 | 513 | 512 | 518 | 517 | 519 | 521 | 524 | 520 | 523 | |
| - | - | - | - | 537 | - | - | - | - | - | - | - | |
| δ (NH) + δ (C-O) | 559 | 559 | 556 | 553.69 | 560 | 558 | 554 | 566 | 558 | 558 | 554 | 559 |
| - | 588 | 567 | 577 | 588 | 597 | 598 | 578 | 581 | - | 592 | - | |
| 605 | 604 | 603 | 605 | 604 | 607 | 617 | 604 | 605 | 600 | 608 | 607 | |
| 659 | 659 | 659 | 662 | 659 | 658 | 659 | - | 660 | 659 | 658 | 659 | |
| δ (NH) | 707 | 704 | 706 | 710 | 690 | 701 | 607 | 710 | 698 | - | 709 | 705 |
| - | - | - | - | - | - | 740 | 746 | - | - | 747 | - | |
| - | 801 | - | 810 | 808 | 809 | 801 | 805 | - | - | - | 800 | |
| ν (C-N) | 895 | 895 | 894 | 890 | 895 | 895 | 895 | 895 | 894 | 895 | 892 | 894 |
| - | - | 992 | 988 | - | 993 | 991 | 987 | 989 | 990 | 986 | 993 | |
| ν (C-O) | 1027 | 1024 | 1027 | 1025 | 1028 | 1026 | 1027 | 1024 | 1025 | 1026 | 1024 | 1025 |
| δ (C-O) + ν (C-O) | 1063 | 1062 | 1064 | 1061 | 1065 | 1063 | 1066 | 1062 | 1063 | 1064 | 1061 | 1064 |
| νas (C-O-C) | 1151 | 1151 | 1151 | 1151 | 1152 | 1151 | 1150 | 1150 | 1151 | 1151 | 1151 | 1151 |
| - | - | - | - | - | 1198 | 1199 | - | 1200 | 1198 | 1201 | 1198 | |
| ν (NH2) + δ (OH) | 1261 | 1261 | 1262 | 1259 | 1258 | 1262 | 1261 | 1260 | 1259 | 1256 | - | 1261 |
| ν (C-N) amide III | 1326 | 1327 | 1327 | 1330 | 1327 | 1326 | 1322 | 1329 | 1325 | 1335 | 1322 | 1335 |
| δ(C=O) + τ (C-H) in CH3 group | 1376 | 1372 | 1378 | 1380 | 1375 | 1376 | 1376 | 1377 | 1376 | 1376 | 1376 | 1377 |
| δ(C-H) + ω(CH2) | 1419 | 1416 | 1418 | 1415 | 1420 | 1416 | 1409 | 1408 | 1415 | 1413 | 1415 | 1414 |
| ν (C=O) + ν (NH) amide II + δ (N-H) amine I | 1583 | 1584 | 1583 | 1596 | 1580 | 1582 | 1560 | 1580 | 1581 | 1587 | 1573 | 1580 |
| ν (C=O) + ν (C-O) + ν (NH) amide I | 1646 | 1645 | 1648 | 1651 | 1647 | 1646 | 1645 | 1645 | 1646 | 1635 | 1645 | 1649 |
| ν (C=O) | - | - | - | - | - | - | 1737 | - | 1754 | - | - | |
| νas(C-H) of methylene | 2867 | 2865 | 2865 | 2865 | 2858 | 2867 | 2862 | 2870 | 2864 | 2867 | 2850 | 2863 |
| νs(C-H) | 2917 | 2919 | 2914 | - | 2919 | 2917 | 2914 | 2907 | 2914 | 2913 | 2918 | 2907 |
| ν(OH) + ν(NH) | 3292 | 3287 | 3290 | - | 3272 | 3289 | 3271 | 3284 | 3275 | 3294 | 3282 | 3285 |
| 3356 | 3350 | 3355 | 3352 | 3347 | 3359 | 3359 | 3366 | 3357 | 3359 | 3341 | 3353 | |
| Optical Parameter | Pure | MV | MB | ENB | CBB | BG | NGB | FSS | AO | Rhd | Saf | RBB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eg (eV) | Eg1 | 3.82 | 3.64 | 3.71 | 3.44 | 3.43 | 3.53 | 2.88 | 3.52 | 3.42 | 3.78 | 3.78 | 3.81 |
| Eg2 | - | 1.86 | 1.69 | 1.80 | 1.79 | 1.80 | 1.48 | 2.29 | 1.80 | 2.27 | 2.04 | 2.06 | |
| Eu (meV) | 243 | 229 | 274 | 191 | 541 | 161 | 840 | 662 | 297 | 207 | 188 | 418 | |
| Ed (eV) | 19.3 | 18.2 | 11.6 | 7.60 | 11.5 | 10.2 | 19.1 | 31.7 | 14.5 | 14.1 | 5.57 | 16.3 | |
| Eo (eV) | 9.36 | 8.87 | 5.94 | 3.60 | 6.36 | 5.10 | 9.72 | 18.8 | 7.10 | 6.86 | 3.71 | 8.11 | |
| no | 1.75 | 1.75 | 1.72 | 1.76 | 1.68 | 1.73 | 1.72 | 1.64 | 1.74 | 1.75 | 1.58 | 1.73 | |
| ε∞ | 3.06 | 3.05 | 2.95 | 3.11 | 2.81 | 3.00 | 2.97 | 2.69 | 3.04 | 3.05 | 2.5 | 3.01 | |
| εL | 3.24 | 3.19 | 3.07 | 3.28 | 2.99 | 3.22 | 3.09 | 2.81 | 3.22 | 3.24 | 2.54 | 3.18 | |
| N/m* (×1046 g−1·cm−3) | 6.05 | 5.07 | 4.55 | 6.25 | 5.49 | 6.52 | 5.48 | 4.33 | 6.25 | 6.50 | 0.86 | 5.67 | |
| ωp (×1014 Hz) | 2.32 | 2.14 | 2.07 | 2.35 | 2.31 | 2.42 | 2.26 | 2.11 | 2.37 | 2.41 | 0.99 | 2.27 | |
| ψ (eV) | 0.152 | 0.141 | 0.136 | 0.154 | 0.152 | 0.159 | 0.148 | 0.139 | 0.156 | 0.158 | 0.065 | 0.149 | |
| EP (eV) | 0.094 | 0.098 | 0.097 | 0.106 | 0.113 | 0.112 | 0.105 | 0.107 | 0.109 | 0.100 | 0.053 | 0.105 | |
| EF (eV) | 0.024 | 0.022 | 0.021 | 0.024 | 0.024 | 0.025 | 0.023 | 0.021 | 0.025 | 0.025 | 0.008 | 0.023 | |
| χ(1) (e.s.u) | 0.164 | 0.163 | 0.163 | 0.168 | 0.144 | 0.159 | 0.156 | 0.134 | 0.163 | 0.164 | 0.119 | 0.160 | |
| χ(3) (×10−13 e.s.u) | 1.23 | 1.21 | 1.21 | 1.36 | 0.730 | 1.09 | 1.02 | 0.552 | 1.19 | 1.22 | 0.347 | 1.11 | |
| n2 (×10−12 e.s.u) | 2.66 | 2.61 | 2.65 | 2.90 | 1.64 | 2.38 | 2.23 | 1.27 | 2.57 | 2.63 | 0.827 | 2.43 | |
| Color Parameter | Pure | MV | MB | ENB | CBB | BG | NGB | FSS | AO | Rhd | Saf | RBB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | 0.231 | 0.245 | 0.297 | 0.311 | 0.213 | 0.211 | 0.306 | 0.261 | 0.431 | 0.562 | 0.355 | 0.300 |
| Y | 0.21 | 0.188 | 0.277 | 0.299 | 0.201 | 0.234 | 0.300 | 0.569 | 0.449 | 0.385 | 0.322 | 0.286 |
| L* | 47.29 | 29.91 | 26.15 | 26.70 | 34.78 | 38.97 | 33.62 | 39.35 | 34.74 | 32.95 | 31.98 | 25.44 |
| a* | −0.81 | 8.87 | 2.92 | 1.03 | −1.39 | −2.94 | −9.39 | −1.84 | 8.01 | 17.07 | 9.80 | −0.58 |
| b* | −1.34 | −12.17 | −9.54 | −4.47 | −6.45 | −1.25 | 6.43 | 8.32 | 16.97 | −7.85 | 3.30 | −6.07 |
| dE* | 7.65 | 18.18 | 16.90 | 13.84 | 8.09 | 2.78 | 12.61 | 8.55 | 0.25 | 20.38 | 13.36 | 15.50 |
| C* | 1.57 | 15.06 | 9.98 | 4.59 | 6.59 | 3.19 | 11.38 | 8.52 | 18.76 | 18.79 | 10.34 | 6.10 |
| h | 238.77 | 306.08 | 286.99 | 282.95 | 257.85 | 202.94 | 145.60 | 102.47 | 64.74 | 335.32 | 18.62 | 264.58 |
| Device | Pin (mW/cm2) | R (mA/W) | D* (Jones) | SNR | LDR (dB) | Ref. |
|---|---|---|---|---|---|---|
| Ag/Cs/p-Si | 100 | 0.029 | 1.74 × 107 | 1.340 | 2.545 | Present work |
| Ag/Cs-MV/p-Si | 3.046 | 5.79 × 108 | 4.517 | 13.09 | ||
| Ag/Cs-MB/p-Si | 0.030 | 4.10 × 107 | 2.778 | 8.875 | ||
| Ag/Cs-ENB/p-Si | 0.125 | 1.29 × 108 | 5.290 | 14.47 | ||
| Ag/Cs-CBB/p-Si | 0.569 | 2.01 × 108 | 3.27 | 10.30 | ||
| Ag/Cs-BG/p-Si | 0.094 | 9.85 × 107 | 4.308 | 12.69 | ||
| Ag/Cs-NGB/p-Si | 0.163 | 1.82 × 108 | 7.557 | 17.57 | ||
| Ag/Cs-FSS/p-Si | 0.053 | 3.71 × 107 | 1.830 | 5.251 | ||
| Ag/Cs-AO/p-Si | 0.125 | 9.74 × 107 | 3.434 | 10.72 | ||
| Ag/Cs-Rhd/p-Si | 0.223 | 1.09 × 108 | 2.718 | 8.686 | ||
| Ag/Cs-Saf/p-Si | 0.032 | 4.01 × 107 | 2.614 | 8.348 | ||
| Ag/Cs-RBB/p-Si | 0.015 | 2.40 × 107 | 2.269 | 7.118 | ||
| Al/PTCDI/p-Si | 200 | 0.2 | 7.0 × 107 | - | - | [51] |
| Au/α-6 T/n-Si | 2560 | 0.2 | 1.2 × 108 | 57 | 35 | [52] |
| Au/ABPQC/p-Si | 100 | 6.5 × 10−5 | 1.25 × 106 | - | 33.5 | [53] |
| Co/MG/n-Si | 400 | 0.08 | - | - | - | [54] |
| Au/PaOEP/p-Si | 80 | 8.5 × 10−4 | 1.2 × 103 | - | - | [55] |
| Au/MFCMP/p-Si | 100 | 4.1 × 10−5 | 1.8 × 106 | - | - | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Newehy, M.H.; El-Mahalawy, A.M.; Thamer, B.M.; Moydeen Abdul Hameed, M. Fabrication and Characterization of Eco-Friendly Thin Films as Potential Optical Absorbers for Efficient Multi-Functional Opto-(Electronic) and Solar Cell Applications. Materials 2023, 16, 3475. https://doi.org/10.3390/ma16093475
El-Newehy MH, El-Mahalawy AM, Thamer BM, Moydeen Abdul Hameed M. Fabrication and Characterization of Eco-Friendly Thin Films as Potential Optical Absorbers for Efficient Multi-Functional Opto-(Electronic) and Solar Cell Applications. Materials. 2023; 16(9):3475. https://doi.org/10.3390/ma16093475
Chicago/Turabian StyleEl-Newehy, Mohamed H., Ahmed M. El-Mahalawy, Badr M. Thamer, and Meera Moydeen Abdul Hameed. 2023. "Fabrication and Characterization of Eco-Friendly Thin Films as Potential Optical Absorbers for Efficient Multi-Functional Opto-(Electronic) and Solar Cell Applications" Materials 16, no. 9: 3475. https://doi.org/10.3390/ma16093475
APA StyleEl-Newehy, M. H., El-Mahalawy, A. M., Thamer, B. M., & Moydeen Abdul Hameed, M. (2023). Fabrication and Characterization of Eco-Friendly Thin Films as Potential Optical Absorbers for Efficient Multi-Functional Opto-(Electronic) and Solar Cell Applications. Materials, 16(9), 3475. https://doi.org/10.3390/ma16093475








