Construction of Durable Self-Cleaning PDMS Film on Polyester Fabric Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of Polyester Fabric
2.3. Preparation and Characterization of PDMS Emulsion
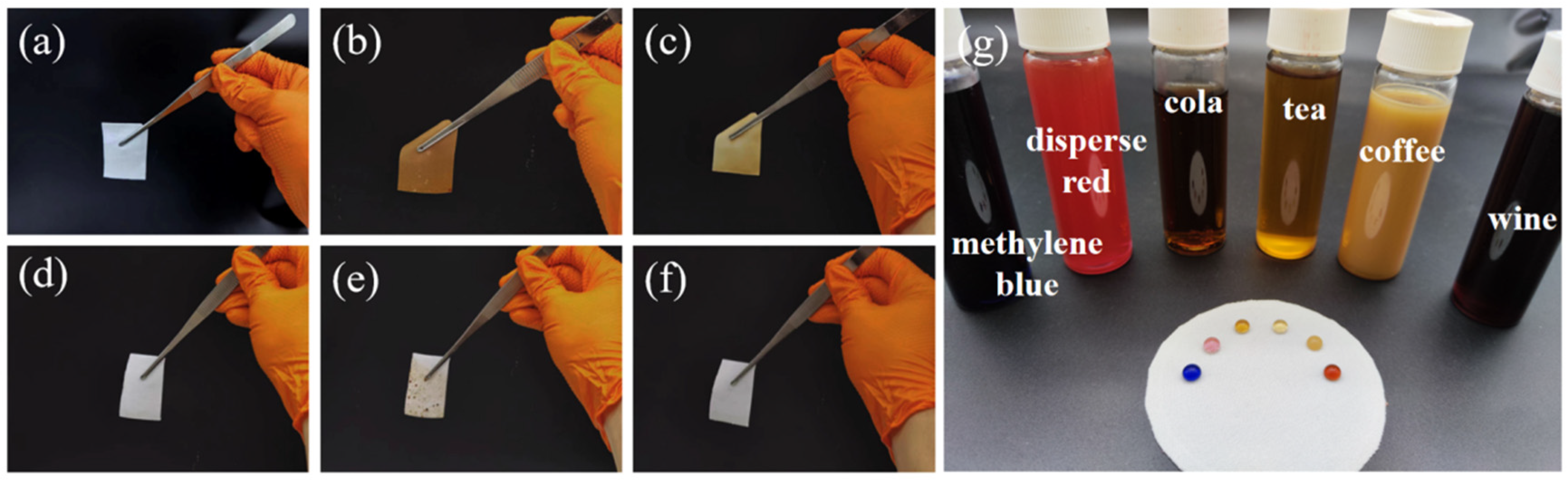
2.4. Preparation and Characterization of Self-Cleaning Polyester Fabric
2.5. Water Repellency Test
2.6. Washing Fastness Test
2.7. Test of Rubbing Fastness
2.8. Spray Wetting Grade Test
2.9. Oil–Water Separation Efficiency Test
3. Results and Discussion
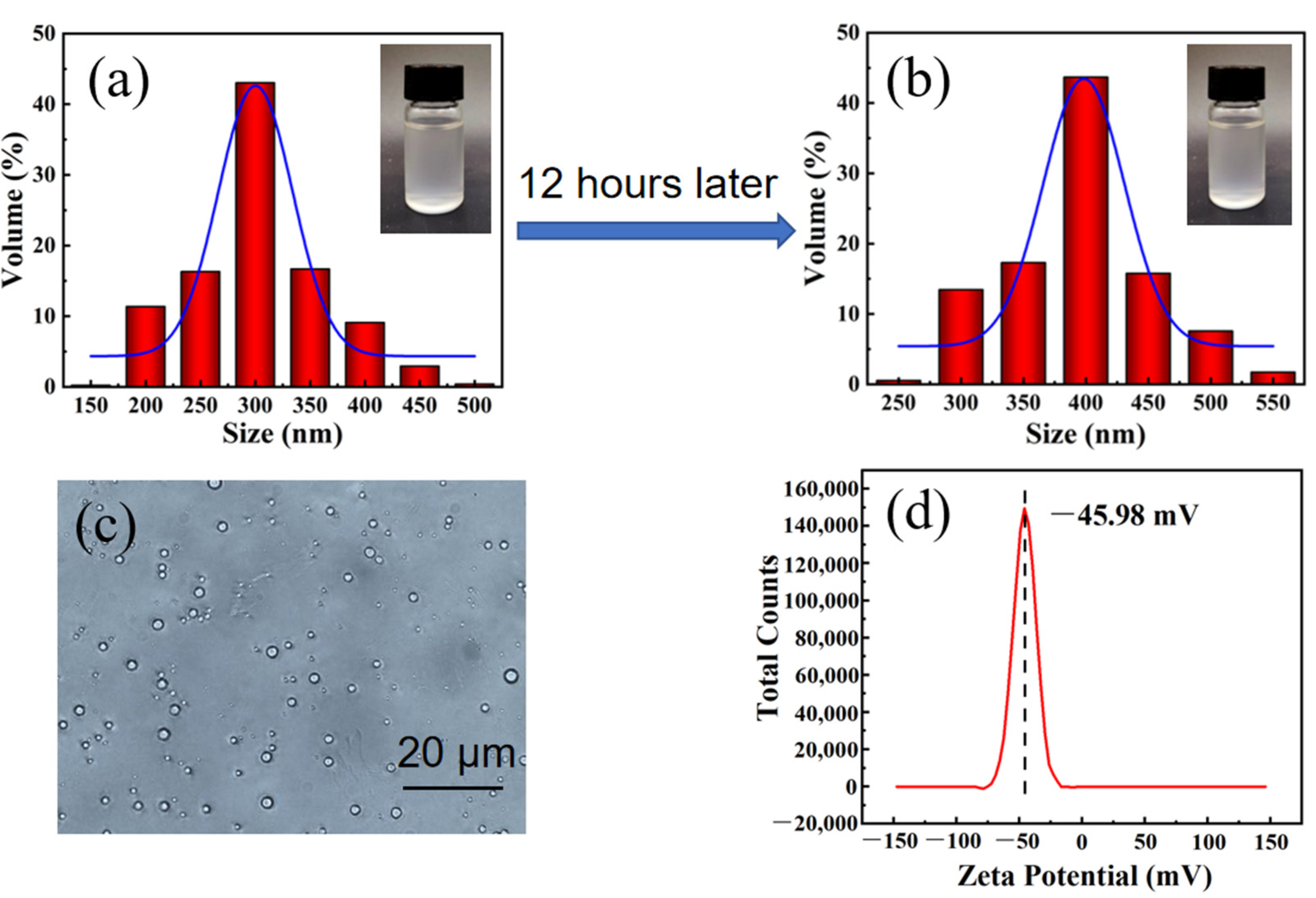
3.1. Preparation and Characterization of PDMS Emulsion
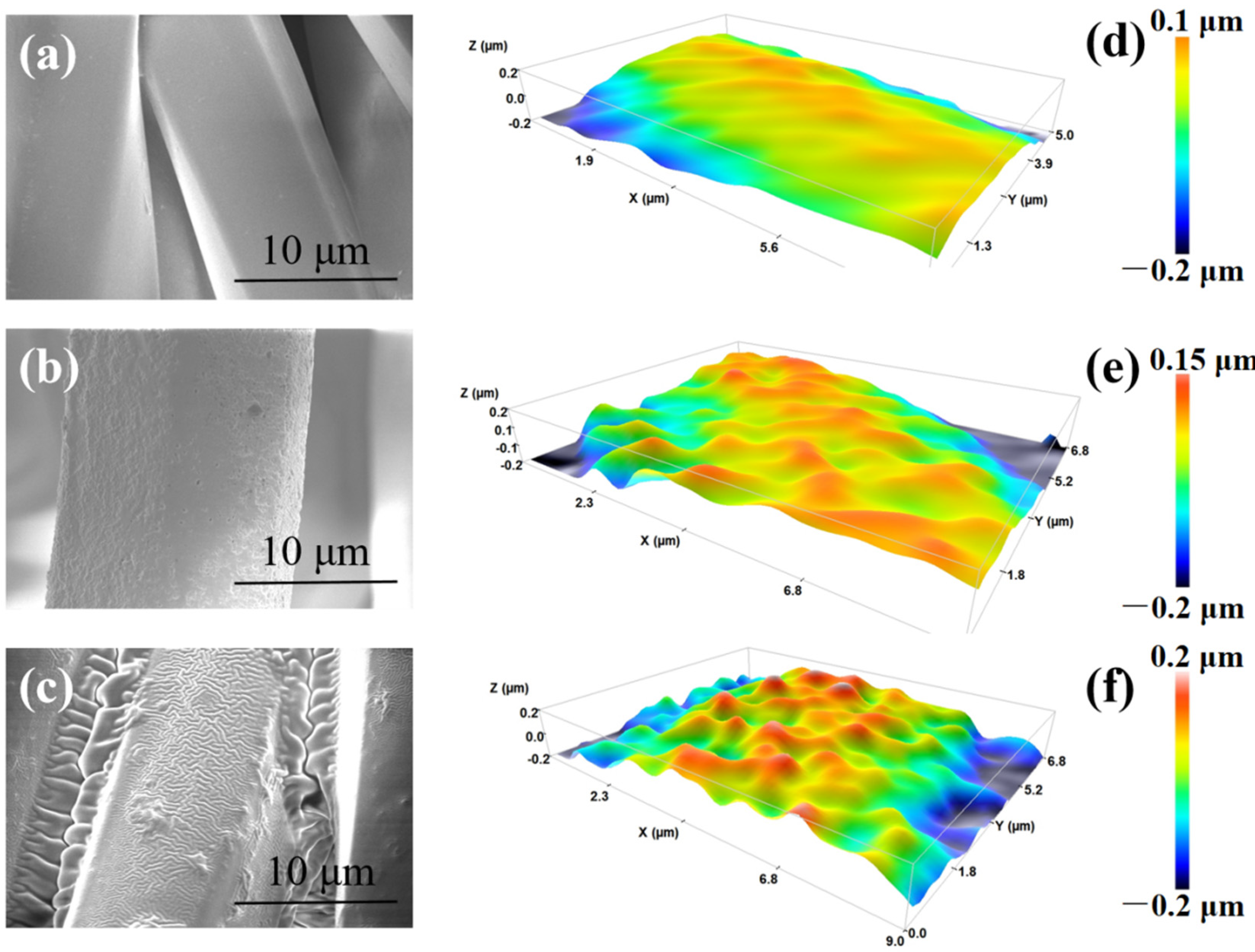
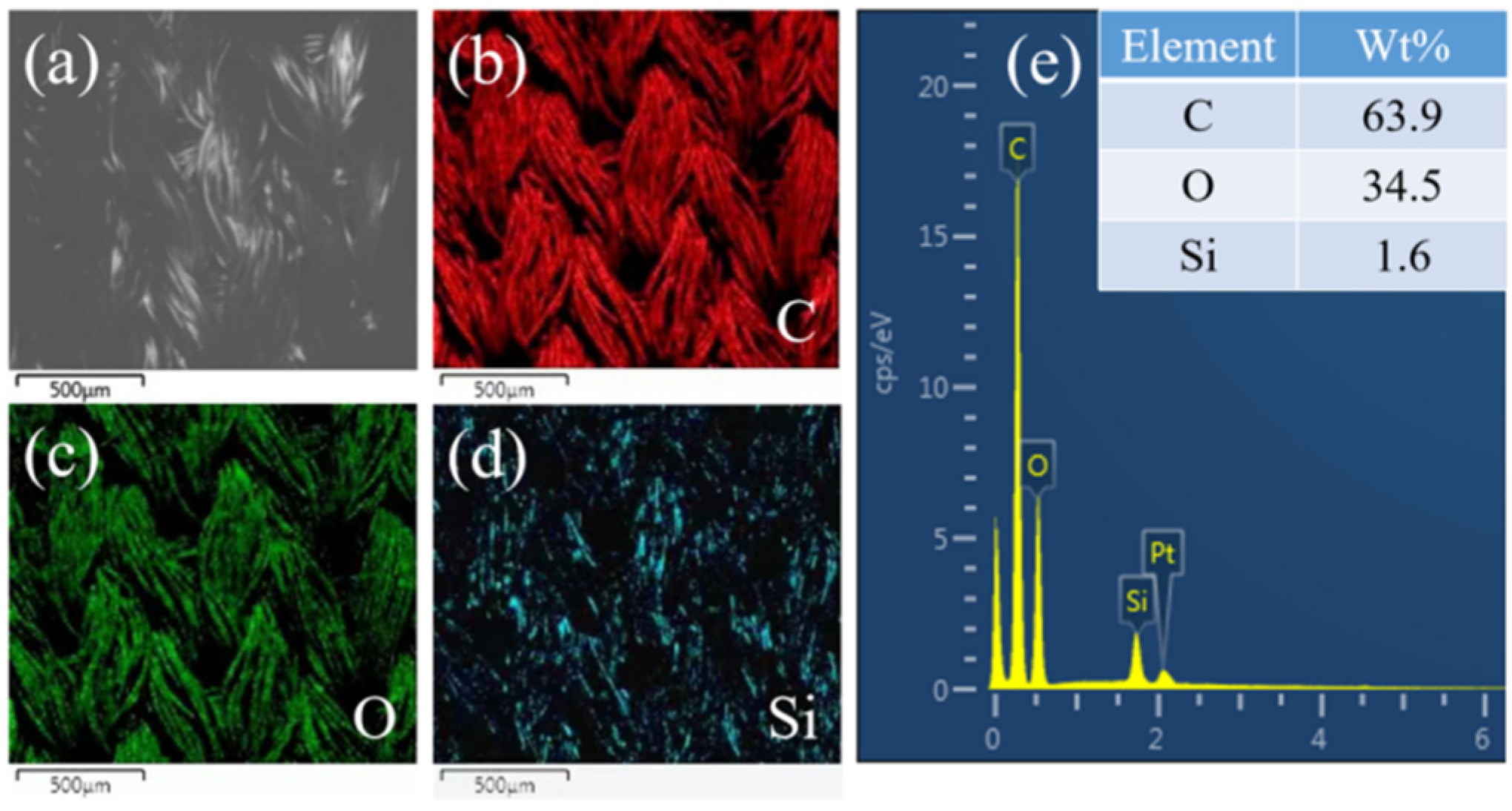
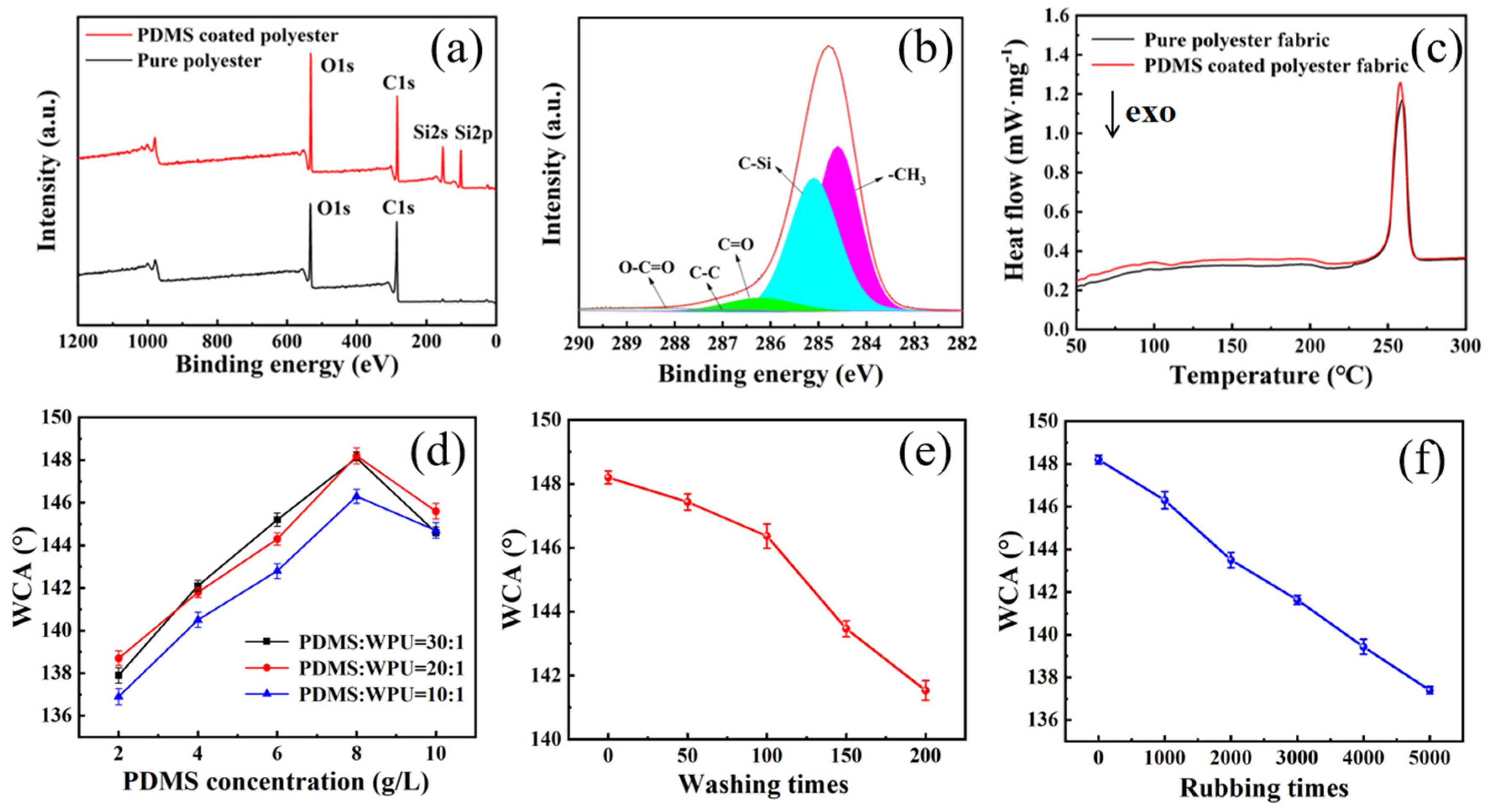
3.2. Preparation and Characterization of Self-Cleaning Polyester Fabric
4. Conclusions
- (a)
- The WCA of the self-cleaning polyester fabric is 148.2°, and the wetting grade is 5.
- (b)
- After 200 times of washing, the WCA drops to 141.5°, the wetting grade drops to 4, and the WCA drops to 138.6° after 5000 times of rubbing.
- (c)
- Compared with before and after PDMS coating, the tensile strength of polyester fabric increased from 489.4N to 536.4N, and the water vapor permeability decreased from 13,535.7 g/(m2·d) to 12,224.3 g/(m2·d).
- (d)
- In addition, we also tested the oil–water separation. The oil–water separation efficiency of the self-cleaning polyester fabric obtained by this method reached 98.37%, and decreased to 94.67% after 50 times of washing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Shen, C.; Li, J.; Zhong, L.; Xu, X.; Xiao, X.; Xu, G.; Shi, J. Superhydrophobic polytetrafluoroethylene film deposited on solar selective absorber by electron beam evaporation. Mater. Chem. Phys. 2021, 257, 123828. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Wu, Y.; Chen, M.; Li, Z. One-step spontaneous grafting via diazonium chemistry for the fabrication of robust bionic multifunctional superhydrophobic fabric. Surf. Coat. Technol. 2021, 407, 126802. [Google Scholar] [CrossRef]
- Roy, S.; Zhai, L.; Kim, J.W.; Kim, H.C.; Kim, J. A novel approach of developing sustainable cellulose coating for selfcleaning-healing fabric. Prog. Org. Coat. 2020, 140, 105500. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, Y.; Zhang, D.; Zhang, Z.; Fu, F.; Zhang, X.; Yang, Y.; Lin, H.; Chen, Y. Fabrication of robust and self-healing superhydrophobic PET fabrics based on profiled fiber structure. Colloids Surf. A 2021, 609, 125686. [Google Scholar] [CrossRef]
- Xu, L.; Xie, K.; Liu, Y.; Zhang, C. Stable super-hydrophobic and comfort PDMS-coated polyester fabric. e-Polymers 2021, 21, 654–661. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Zhan, Y.; Lan, J.; Shang, J.; Zhou, M.; Lin, S. A robust and antibacterial superhydrophobic cotton fabric with sunlight-driven self-cleaning performance for oil/water separation. Cellulose 2021, 28, 1715–1729. [Google Scholar] [CrossRef]
- Celik, N.; Torun, I.; Ruzi, M.; Onses, M.S. Robust superhydrophobic fabrics by infusing structured polydimethylsiloxane films. J. Appl. Polym. Sci. 2021, 138, e51358. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y.; Mi, T.; Wang, X.; Zhang, X. Dip-coating approach to fabricate durable PDMS/STA/SiO2 superhydrophobic polyester fabrics. Coatings 2021, 11, 326. [Google Scholar] [CrossRef]
- Dong, L.; Shi, M.; Xu, S.; Sun, Q.; Pan, G.; Zhu, C. Surface construction of fluorinated TiO2 nanotube networks to develop uvioresistant superhydrophobic aramid fabric. RSC Adv. 2020, 10, 22578. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J.; Khaled, M. Effect of silylating agents on the superhydrophobic and self-cleaning properties of siloxane/polydimethylsiloxane nanocomposite coatings on cellulosic fabric fifilters for oil–water separation. RSC Adv. 2021, 11, 9586. [Google Scholar] [CrossRef]
- Kang, Z.; He, Y.; Sang, J.; Hirahara, H.; Chen, D. Superhydrophobic and conductive cotton fabric composite with excellent corrosion resistance for wearable electronics. Adv. Mater. Interfaces 2021, 8, 2100651. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, L.; Shi, C.; Wu, C.; Long, Z.; Qiao, H.; Wang, K.; Fan, Q. Nature-inspired hierarchical protrusion structure construction for washable and wear-resistant superhydrophobic textiles with self-cleaning ability. ACS Appl. Mater. Interfaces 2021, 12, 18142–18151. [Google Scholar] [CrossRef]
- Pakdel, E.; Zhao, H.; Wang, J.; Tang, B.; Varley, R.J. Superhydrophobic and photocatalytic self-cleaning cotton fabric using flower-like N-doped TiO2/PDMS coating. Cellulose 2021, 28, 8807–8820. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Lin, T. Recent progress in durable and self-healing super-nonwettable fabrics. Adv. Mater. Interfaces 2018, 5, 1800461. [Google Scholar] [CrossRef]
- Xia, Y.; He, L.; Feng, J.; Xu, S.; Yao, L.; Pan, G. Waterproof and moisture-permeable polyurethane nanofiber membrane with high strength, launderability, and durable antimicrobial properties. Nanomaterials 2022, 12, 1813. [Google Scholar] [CrossRef]
- Shi, T.; Liang, J.; Li, W.; Zhang, C.; Yang, H. Improving the Corrosion Resistance of Aluminum Alloy by Creating a Superhydrophobic Surface Structure through a Two-Step Process of Etching Followed by Polymer Modification. Polymers 2022, 14, 4509. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Y.; Zhang, X.; Luo, B. Effects of superhydrophobic surface on tribological properties: Mechanism, status and prospects. Prog. Chem. 2020, 32, 320–330. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Zhao, Y.; Lu, Q. Advance of wetting behavior research on the superhydrophobic surface with micro- and nano-structures. Prog. Chem. 2006, 18, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Zhang, L.; Zhang, W.; Yu, X.; Zhang, Y. Thermal stability of typical superhydrophobic surfaces. J. Bionic Eng. 2018, 15, 1025–1034. [Google Scholar] [CrossRef]
- Luo, G.; Jin, Z.; Dong, Y.; Huang, J.; Zhang, R.; Wang, J.; Li, M.; Shen, Q.; Zhang, L. Preparation and performance enhancements of wear-resistant, transparent PU/SiO2 superhydrophobic coating. Surf. Eng. 2018, 34, 139–145. [Google Scholar] [CrossRef]
- Gong, A.; Zheng, Y.; Yang, Z.; Guo, X.; Guo, Y.; Li, X. Spray fabrication of superhydrophobic coating on aluminum alloy for corrosion mitigation. Mater. Today Commun. 2021, 26, 101828. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Altiparmak, F.; Nguyen, N.; Tuduri, L.; Ouellet-Plamondon, C.M.; Prud’homme, R.E. Robust superhydrophobic cotton fibers prepared by simple dip- coating approach using chemical and plasma-etching pretreatments. ACS Omega 2019, 4, 7829–7837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Zeng, X.; Li, H.; Lai, X.; Wu, T. One-pot fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via sol-gel reaction. J. Colloid Interface Sci. 2018, 533, 198–206. [Google Scholar] [CrossRef]
- Yan, Y.; Gao, N.; Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Colloid Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Jia, S.; Zhang, J.; Ma, J. Large-area fabrication of superhydrophobic surfaces for practical applications: An overview. Sci. Technol. Adv. Mater. 2010, 11, 033002. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yang, J.; Yue, Y.; Zhang, H. A review of recent advances in superhydrophobic surfaces and their applications in drag reduction and heat transfer. Nanomaterials 2022, 12, 44. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Liang, F.; Liu, R.; Zhang, Y.; Zhang, W.; Zhu, T.; Yi, B.; Tang, Y.; Lai, Y. A “PDMS-in-water” emulsion enables mechanochemically robust superhydrophobic surfaces with self-healing nature. Nanoscale Horiz. 2020, 5, 65–73. [Google Scholar] [CrossRef]
- Wen, H.; Jia, Y.; Xiang, B.; Zhang, W.; Luo, S.; Liu, T. A facile preparation of the superhydrophobic polydimethylsiloxane materials and its performances based on the supercritical fluid foaming. J. Appl. Polym. Sci. 2021, 138, e50858. [Google Scholar] [CrossRef]
- Tropmann, A.; Tanguy, L.; Koltay, P.; Zengerle, R.; Riegger, L. Completely superhydrophobic PDMS surfaces for microfluidics. Langmuir 2012, 28, 8292–8295. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Li, H.; Lai, X.; Xie, H. Effects of calcination temperature on the microstructure and wetting behavior of superhydrophobic polydimethylsiloxane/silica coating. Colloids Surf. A 2014, 445, 111–118. [Google Scholar] [CrossRef]
- Shao, H.; Yu, Y.; Li, Y.; Shuai, M.; He, Z.; Tang, C.; Li, X.; Mei, J.; Fu, Q. Building a mechanically stable polydimethylsiloxane/silica superhydrophobic coating on poly(chloro-p-xylylene) film by introducing a polydimethylsiloxane adhesive layer. Surf. Coat. Technol. 2018, 350, 201–210. [Google Scholar] [CrossRef]
- Shan, Y.; Liang, S.; Mao, X.; Lu, J.; Liu, L.; Huang, Y.; Yang, J. Stretchable dual cross-linked silicon elastomer with a superhydrophobic surface and fast triple self-healing ability at room temperature. Soft Matter 2021, 17, 4643–4652. [Google Scholar] [CrossRef]
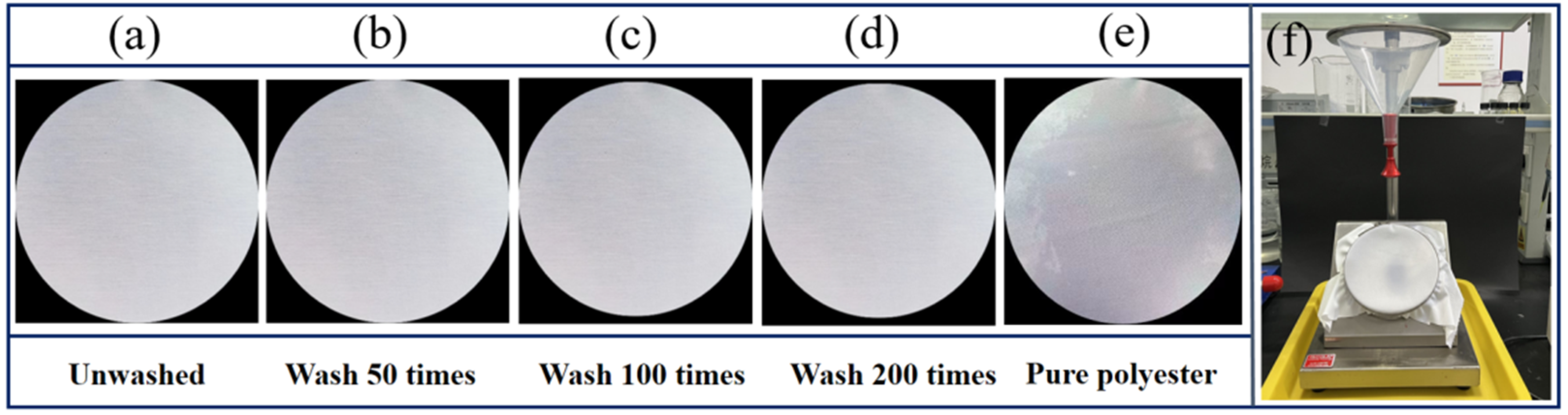

| Samples | Wet Grade | |
|---|---|---|
| PDMS coated polyester fabric | Unwashed | 5 |
| Wash 50 times | 5 | |
| Wash 100 times | 4 | |
| Wash 200 times | 4 | |
| Pure polyester | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Zhu, N.; Zhao, Y.; Zhu, J.; Chen, H.; Xu, L.; Yao, L. Construction of Durable Self-Cleaning PDMS Film on Polyester Fabric Surface. Materials 2023, 16, 52. https://doi.org/10.3390/ma16010052
Xia Y, Zhu N, Zhao Y, Zhu J, Chen H, Xu L, Yao L. Construction of Durable Self-Cleaning PDMS Film on Polyester Fabric Surface. Materials. 2023; 16(1):52. https://doi.org/10.3390/ma16010052
Chicago/Turabian StyleXia, Yong, Nan Zhu, Ying Zhao, Jiehui Zhu, Huajie Chen, Liyun Xu, and Lirong Yao. 2023. "Construction of Durable Self-Cleaning PDMS Film on Polyester Fabric Surface" Materials 16, no. 1: 52. https://doi.org/10.3390/ma16010052




