Bacterial Adhesion of TESPSA and Citric Acid on Different Titanium Surfaces Substrate Roughness: An In Vitro Study with a Multispecies Oral Biofilm Model
Abstract
1. Introduction
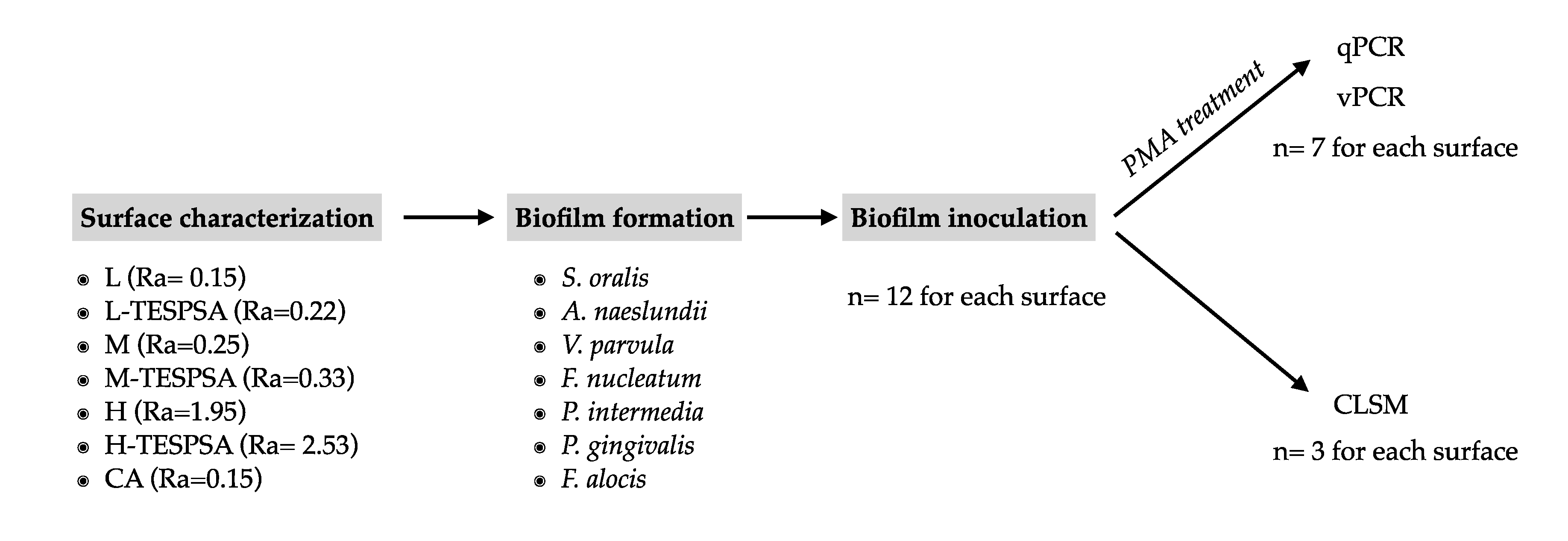
2. Materials and Methods
2.1. Study Groups
2.2. Bacterial Strains and Growth Conditions
2.3. Biofilm Formation
2.4. Propidium Monoazide (PMA) Treatment
2.5. DNA Extraction and qPCR
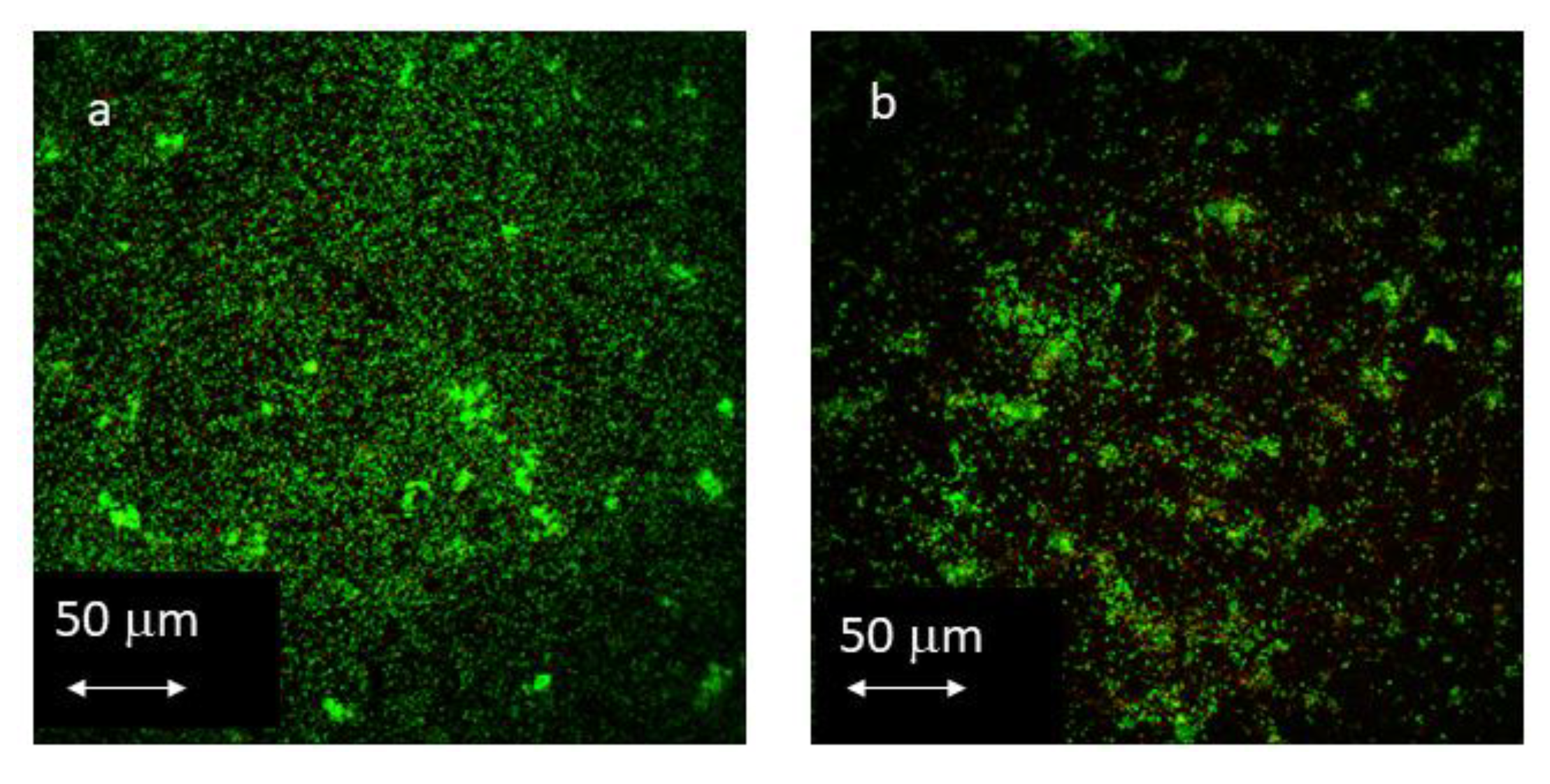
2.6. Confocal Laser Scanning Microscope (CLSM)
2.7. Statistical Analysis
3. Results
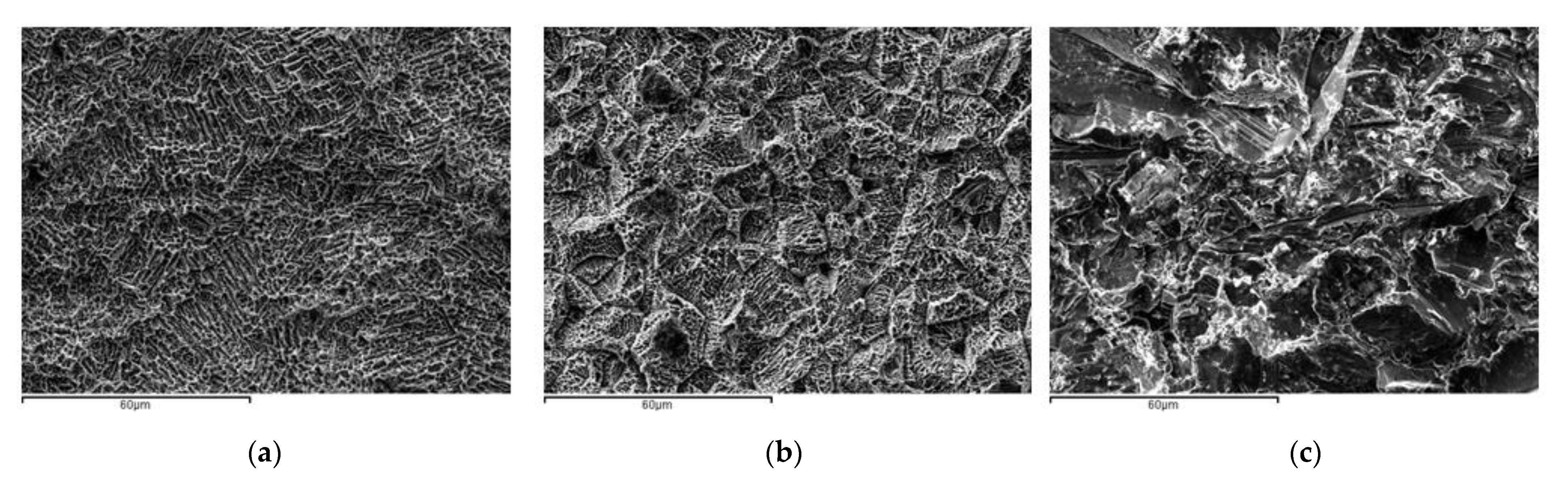
3.1. Characterization of the Titanium Surfaces
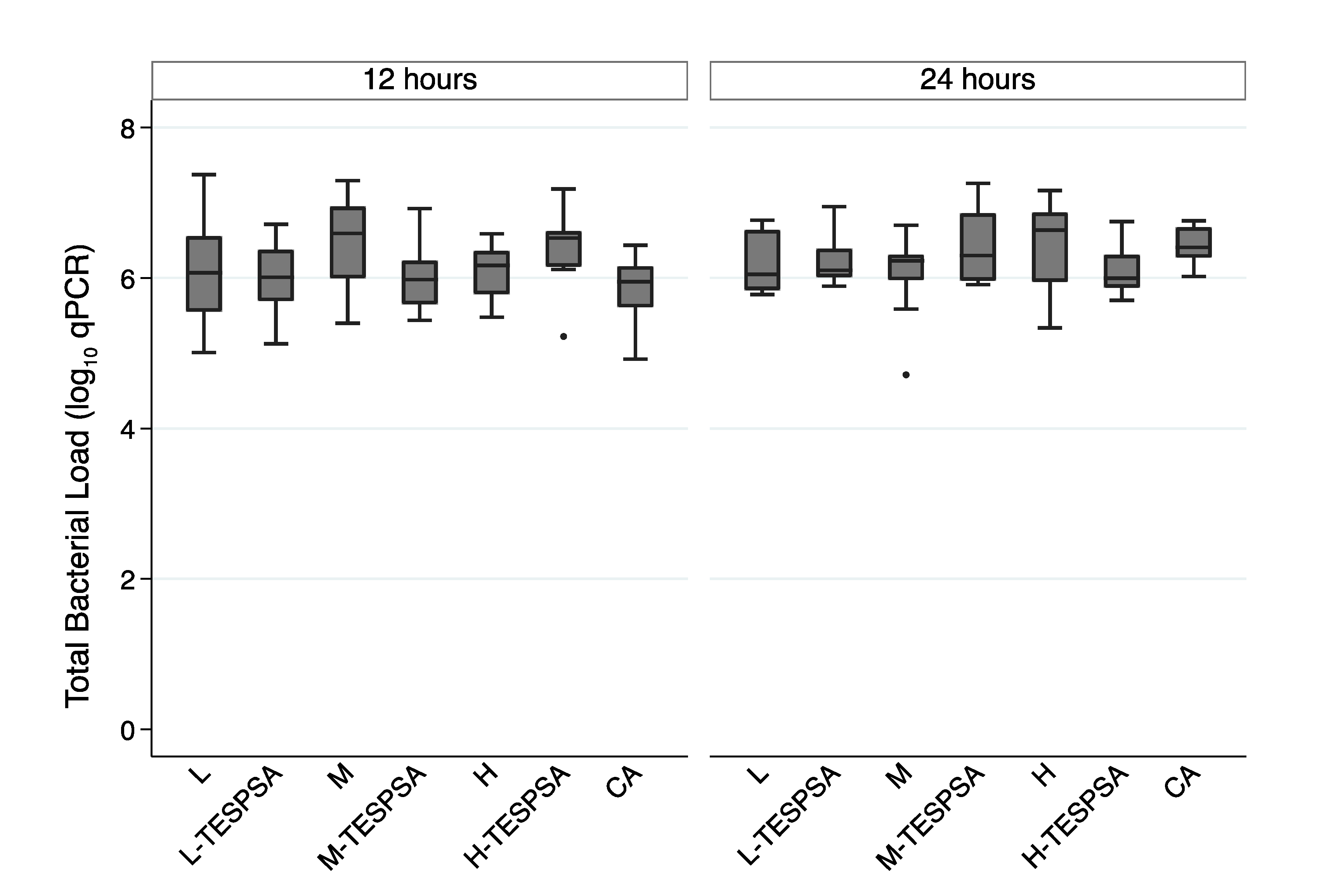
3.2. qPCR
3.2.1. Influence of Surface Topography on Bacterial Adhesion
3.2.2. Influence of Substrate Roughness of Coated Surfaces on Bacterial Adhesion
3.2.3. Influence of Antibacterial Coating on Bacterial Proliferation
3.2.4. Influence of Antibacterial Coating on Biofilm Mortality
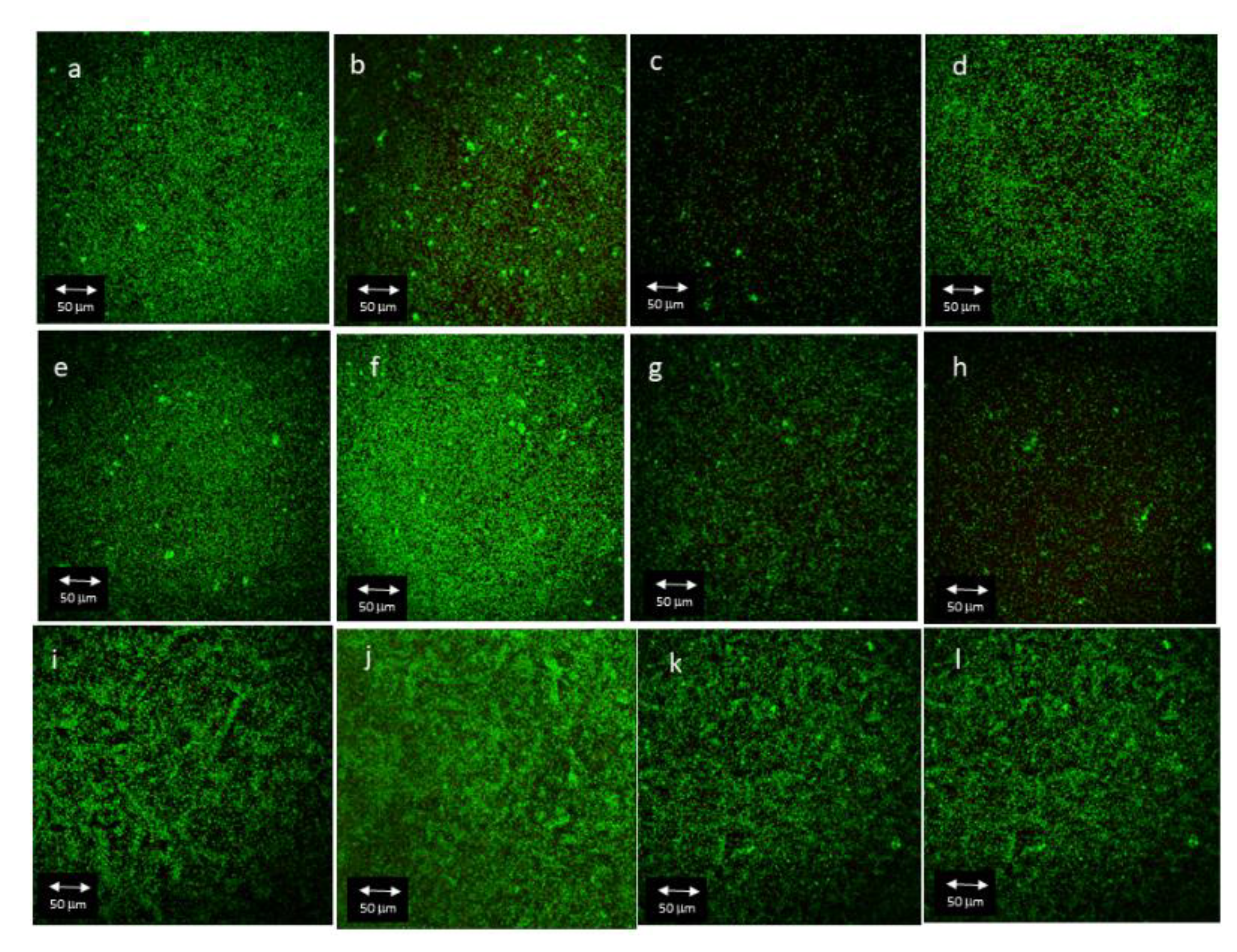
3.3. CLSM
3.3.1. Influence of Antibacterial Coating on Total Biofilm Area
3.3.2. Influence of Antibacterial Coating on Total Biofilm Volume
3.3.3. Influence of Antibacterial Coating on Live/Dead Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.L.; Cochran, D.; Sculean, A.; Canullo, L. How Frequent Does Peri-Implantitis Occur? A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, D.; Sanz-Sánchez, I.; Figuero, E.; Llodrá, J.C.; Bravo, M.; Caffesse, R.G.; Vallcorba, N.; Guerrero, A.; Herrera, D. Prevalence and Risk Indicators of Peri-Implant Diseases in Spain. J. Clin. Periodontol. 2018, 45, 1510–1520. [Google Scholar] [CrossRef]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef]
- Monje, A.; Insua, A.; Wang, H.-L. Understanding Peri-Implantitis as a Plaque-Associated and Site-Specific Entity: On the Local Predisposing Factors. J. Clin. Med. 2019, 8, 279. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-Implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef]
- Sanz-Martin, I.; Doolittle-Hall, J.; Teles, R.P.; Patel, M.; Belibasakis, G.N.; Hämmerle, C.H.F.; Jung, R.E.; Teles, F.R.F. Exploring the Microbiome of Healthy and Diseased Peri-Implant Sites Using Illumina Sequencing. J. Clin. Periodontol. 2017, 44, 1274–1284. [Google Scholar] [CrossRef]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.F.; Cardaropoli, G.; van Thompson, P.; Lemons, J.E. Basic Research Methods and Current Trends of Dental Implant Surfaces. J. Biomed. Mater. Res.—Part B Appl. Biomater. Febr. 2009, 88B, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Berglundh, T.; Linder, E.; Lang, N.P.; Lindhe, J. Early Bone Formation Adjacent to Rough and Turned Endosseous Implant Surfaces. An Experimental Study in the Dog. Clin. Oral Implant. Res. 2004, 15, 381–392. [Google Scholar] [CrossRef]
- de Bruyn, H.; Christiaens, V.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant Surface Roughness and Patient Factors on Long-Term Peri-Implant Bone Loss. Periodontology 2000 2017, 73, 218–227. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial Colonization Immediately after Installation on Oral Titanium Implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Drake, D.R.; Paul, J.; Keller, J.C. Primary Bacterial Colonization of Implant Surfaces. Int. J. Oral Maxillofac. Implant. 1999, 14, 226–232. [Google Scholar]
- Quirynen, M.; van der Mei, H.; Bollen, C.; Schotte, A.; Marechal, M.; Doornbusch, G.; Busscher, H.; van Steenberghe, D. An In Vivo Study of the Influence of the Surface Roughness of Implants on the Microbiology of Supra-and Subgingival Plaque. J. Dent. Res. 1993, 72, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The Interaction of Cells and Bacteria with Surfaces Structured at the Nanometre Scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Teughels, W.; van Assche, N.; Sliepen, I.; Quirynen, M. Effect of Material Characteristics and/or Surface Topography on Biofilm Development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Adatrow, P.; Haggard, W.O.; Norowski, A. Emerging Antibacterial Biomaterial Strategies for the Prevention of Peri-Implant Inflammatory Diseases. Int. J. Oral Maxillofac. Implant. 2011, 26, 553–560. [Google Scholar]
- Pera, F.; Menini, M.; Alovisi, M.; Crupi, A.; Ambrogio, G.; Asero, S.; Marchetti, C.; Canepa, C.; Merlini, L.; Pesce, P.; et al. Can Abutment with Novel Superlattice CrN/NbN Coatings Influence Peri-Implant Tissue Health and Implant Survival Rate Compared to Machined Abutment? 6-Month Results from a Multi-Center Split-Mouth Randomized Control Trial. Materials 2023, 16, 246. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Buxadera-Palomero, J.; Godoy-Gallardo, M.; Molmeneu, M.; Punset, M.; Gil, F.J. Antibacterial Properties of Triethoxysilylpropyl Succinic Anhydride Silane (TESPSA) on Titanium Dental Implants. Polymers 2020, 12, 773. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-Functional Silane Immobilized onto Titanium Surfaces Induces Osteoblast Cell Differentiation and Reduces Bacterial Adhesion and Biofilm Formation. Mater. Sci. Eng. C 2016, 59, 524–532. [Google Scholar] [CrossRef]
- Vilarrasa, J.; Delgado, L.M.; Galofré, M.; Àlvarez, G.; Violant, D.; Manero, J.M.; Blanc, V.; Gil, F.J.; Nart, J. In Vitro Evaluation of a Multispecies Oral Biofilm over Antibacterial Coated Titanium Surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 164. [Google Scholar] [CrossRef] [PubMed]
- Punset, M.; Villarrasa, J.; Nart, J.; Manero, J.M.; Bosch, B.; Padrós, R.; Perez, R.A.; Gil, F.J. Citric Acid Passivation of Titanium Dental Implants for Minimizing Bacterial Colonization Impact. Coatings 2021, 11, 214. [Google Scholar] [CrossRef]
- Verdeguer, P.; Gil, J.; Punset, M.; Manero, J.M.; Nart, J.; Vilarrasa, J.; Ruperez, E. Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior. Materials 2022, 15, 545. [Google Scholar] [CrossRef] [PubMed]
- Atefyekta, S.; Blomstrand, E.; Rajasekharan, A.K.; Svensson, S.; Trobos, M.; Hong, J.; Webster, T.J.; Thomsen, P.; Andersson, M. Antimicrobial Peptide-Functionalized Mesoporous Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014, 10, 258–266. [Google Scholar] [CrossRef]
- Mishra, B.; Lushnikova, T.; Golla, R.M.; Wang, X.; Wang, G. Design and surface immobilization of short anti-biofilm peptides. Acta Biomater. 2017, 49, 316–328. [Google Scholar] [CrossRef]
- Abu-Ghazaleh, B. Effects of ascorbic acid, citric acid, lactic acid, NaCl, potassium sorbate and Thymus vulgarisextract on Staphylococcus aureus and Escherichia coli. Afr. J. Microbiol. Res. 2013, 7, 7–12. [Google Scholar] [CrossRef]
- Àlvarez, G.; González, M.; Isabal, S.; Blanc, V.; León, R. Method to Quantify Live and Dead Cells in Multi-Species Oral Biofilm by Real-Time PCR with Propidium Monoazide. AMB Express 2013, 3, 1. [Google Scholar] [CrossRef]
- Nadkarni, M.A.; Martin, F.E.; Jacques, N.A.; Hunter, N. Determination of Bacterial Load by Real-Time PCR Using a Broad-Range (Universal) Probe and Primers Set. Microbiology 2002, 148, 257–266. [Google Scholar] [CrossRef]
- Polymeri, A.; van der Horst, J.; Buijs, M.J.; Zaura, E.; Wismeijer, D.; Crielaard, W.; Loos, B.G.; Laine, M.L.; Brandt, B.W. Submucosal Microbiome of Peri-Implant Sites: A Cross-Sectional Study. J. Clin. Periodontol. 2021, 48, 1228–1239. [Google Scholar] [CrossRef]
- Zaugg, L.K.; Astasov-Frauenhoffer, M.; Braissant, O.; Hauser-Gerspach, I.; Waltimo, T.; Zitzmann, N.U. Determinants of Biofilm Formation and Cleanability of Titanium Surfaces. Clin. Oral Implant. Res. 2017, 28, 469–475. [Google Scholar] [CrossRef]
- Bermejo, P.; Sánchez, M.C.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz, M. Topographic Characterization of Multispecies Biofilms Growing on Dental Implant Surfaces: An in Vitro Model. Clin. Oral Implant. Res. 2019, 30, 229–241. [Google Scholar] [CrossRef]
- Bevilacqua, L.; Milan, A.; del Lupo, V.; Maglione, M.; Dolzani, L. Biofilms Developed on Dental Implant Titanium Surfaces with Different Roughness: Comparison between In Vitro and In Vivo Studies. Curr. Microbiol. 2018, 75, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.G.S.; Cordeiro, J.M.; Lima, C.V.; Barão, V.A.R. Citric Acid Reduces Oral Biofilm and Influences the Electrochemical Behavior of Titanium: An in Situ and In Vitro Study. J. Periodontol. 2019, 90, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of Bone Loss in Antibacterial Coated Dental Implants: An Experimental Study in Dogs. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef]
- Badihi Hauslich, L.; Sela, M.N.; Steinberg, D.; Rosen, G.; Kohavi, D. The Adhesion of Oral Bacteria to Modified Titanium Surfaces: Role of Plasma Proteins and Electrostatic Forces. Clin. Oral Implant. Res. 2013, 24, 49–56. [Google Scholar] [CrossRef]
- Alovisi, M.; Carossa, M.; Mandras, N.; Roana, J.; Costalonga, M.; Cavallo, L.; Pira, E.; Putzu, M.G.; Bosio, D.; Roato, I.; et al. Disinfection and Biocompatibility of Titanium Surfaces Treated with Glycine Powder Airflow and Triple Antibiotic Mixture: An In Vitro Study. Materials 2022, 15, 4850. [Google Scholar] [CrossRef]
- Efstratiou, M.; Papaioannou, W.; Nakou, M.; Ktenas, E.; Vrotsos, I.A.; Panis, V. Contamination of a Toothbrush with Antibacterial Properties by Oral Microorganisms. J. Dent. 2007, 35, 331–337. [Google Scholar] [CrossRef]
- Quirynen, M.; De Soete, M.; Pauwels, M.; Gizani, S.; Van Meerbeek, B.; van Steenberghe, D. Can Toothpaste or a Toothbrush with Antibacterial Tufts Prevent Toothbrush Contamination? J. Periodontol. 2003, 74, 312–322. [Google Scholar] [CrossRef]
- Hickok, N.J.; Shapiro, I.M.; Chen, A.F. The Impact of Incorporating Antimicrobials into Implant Surfaces. J. Dent. Res. 2018, 97, 14–22. [Google Scholar] [CrossRef] [PubMed]
| Surface Name | Roughness(Ra) (μm) | Contact Angle (◦) | Surface Energy (mJ/m2) | |
|---|---|---|---|---|
| H2O | Formamide | |||
| L | 0.15 ± 0.06 | 55.4 ± 6 | 33.6 ± 4 | 49.6 ± 3 |
| L-TESPSA | 0.22 ± 0.02 | 51.5 ± 2 | 26.5 ± 3 | 53.4 ± 2 |
| M | 0.25 ± 0.07 | 58.4 ± 2 | 34.6 ± 6 | 48.5 ± 3 |
| M-TESPSA | 0.33 ± 0.07 | 40.4 ± 2 | 13.0 ± 11 | 59.2 ± 3 |
| H | 1.95 ± 0.19 | 89.9 ± 10 | 63.2 ± 10 | 38.8 ± 14 |
| H-TESPSA | 2.53 ± 0.15 | 20.3 ± 4 | 0 ± 0 | 68.3 ± 2 |
| CA | 0.15 ± 0.04 | 51.8 ± 15 | 37.6 ± 10 | 49.9 ± 9 |
| Group | L-TESPSA | M-TESPSA | H-TESPSA |
|---|---|---|---|
| M-TESPSA | 0.403 | ||
| H-TESPSA | 0.062 | 0.072 | |
| CA | 0.308 | 0.279 | 0.021 * |
| 12 h | 24 h | |||
|---|---|---|---|---|
| Group | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) |
| L | 0.32 ± 0.25 | 0.26 (0.38) | 0.26 ± 0.22 | 0.14 (0.29) |
| L-TESPSA | 0.49 ± 0.20 | 0.42 (0.28) | 0.25 ± 0.15 | 0.22 (0.22) |
| M | 0.43 ± 0.07 | 0.41 (0.13) | 0.36 ± 0.16 | 0.29 (0.15) |
| M-TESPSA | 0.58 ± 0.16 | 0.53 (0.25) | 0.38 ± 0.24 | 0.29 (0.08) |
| H | 0.54 ± 0.24 | 0.52 (0.42) | 0.38 ± 0.15 | 0.29 (0.28) |
| H-TESPSA | 0.44 ± 0.08 | 0.43 (0.07) | 0.28 ± 0.12 | 0.27 (0.19) |
| CA | 0.61 ± 0.18 | 0.49 (0.61) | 0.39 ± 0.18 | 0.36 (0.20) |
| 12 h | 24 h | |||
|---|---|---|---|---|
| Group | Live | Dead | Live | Dead |
| L | 3.40 (0.12) | 1.44 (0.49) | 3.53 (0.18) | 1.8 (0.36) |
| L-TESPSA | 3.43 (0.48) | 1.92 (0.54) | 3.48 (0.22) | 1.58 (0.93) |
| M | 3.44 (0.35) | 1.58 (1.25) | 3.36 (0.60) | 1.52 (0.60) |
| M-TESPSA | 3.30 (0.37) | 1.13 (0.49) | 3.3 (0.47) | 1.55 (0.58) |
| H | 3.13 (0.56) | 1.23 (0.31) | 3.25 (0.36) | 2.36 (0.46) |
| H-TESPSA | 3.09 (0.34) | 1.11 (0.09) | 3.65 (0.36) | 2.12 (0.05) |
| CA | 3.04 (0.37) | 1.52 (0.60) | 3.51 (0.32) | 2.12 (0.76) |
| 12 h | 24 h | |||
|---|---|---|---|---|
| Group | Live | Dead | Live | Dead |
| L | 4.83 (0.27) | 2.79 (0.28) | 5.06 (0.21) | 3.36 (0.05) |
| L-TESPSA | 4.71 (0.46) | 3.19 (0.42) | 4.97 (0.47) | 3.45 (0.89) |
| M | 4.81 (0.30) | 3.01 (1.11) | 4.94 (0.87) | 3.15 (0.56) |
| M-TESPSA | 4.58 (0.42) | 2.42 (0.54) | 4.81 (0.97) | 3.23 (0.63) |
| H | 4.58 (0.52) | 2.6 (0.28) | 4.7 (0.30) | 3.68 (0.59) |
| H-TESPSA | 4.48 (0.35) | 2.51 (0.02) | 5.21 (0.38) | 3.74 (0.14) |
| CA | 4.48 (0.53) | 2.96 (0.68) | 5.14 (0.48) | 3.68 (0.76) |
| 12 h | 24 h | |||
|---|---|---|---|---|
| Group | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) |
| L | 2.45 ± 0.78 | 2.36 (0.81) | 2.02 ± 0.23 | 1.98 (0.36) |
| L-TESPSA | 1.88 ± 0.38 | 1.73 (0.67) | 2.33 ± 0.71 | 2.15 (0.81) |
| M | 2.98 ± 3.21 | 3.12 (2.62) | 2.28 ± 0.78 | 2.31 (1.35) |
| M-TESPSA | 4.72 ± 0.78 | 3.09 (1.61) | 1.99 ± 0.47 | 2.01 (0.77) |
| H | 2.56 ± 0.40 | 2.56 (0.29) | 1.48 ± 0.27 | 1.40 (0.46) |
| H-TESPSA | 2.96 ± 0.30 | 3.06 (0.46) | 1.70 ± 0.22 | 1.66 (0.26) |
| CA | 1.86 ± 0.20 | 1.84 (0.36) | 1.79 ± 0.46 | 1.73 (0.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilarrasa, J.; Àlvarez, G.; Soler-Ollé, A.; Gil, J.; Nart, J.; Blanc, V. Bacterial Adhesion of TESPSA and Citric Acid on Different Titanium Surfaces Substrate Roughness: An In Vitro Study with a Multispecies Oral Biofilm Model. Materials 2023, 16, 4592. https://doi.org/10.3390/ma16134592
Vilarrasa J, Àlvarez G, Soler-Ollé A, Gil J, Nart J, Blanc V. Bacterial Adhesion of TESPSA and Citric Acid on Different Titanium Surfaces Substrate Roughness: An In Vitro Study with a Multispecies Oral Biofilm Model. Materials. 2023; 16(13):4592. https://doi.org/10.3390/ma16134592
Chicago/Turabian StyleVilarrasa, Javi, Gerard Àlvarez, Agnès Soler-Ollé, Javier Gil, José Nart, and Vanessa Blanc. 2023. "Bacterial Adhesion of TESPSA and Citric Acid on Different Titanium Surfaces Substrate Roughness: An In Vitro Study with a Multispecies Oral Biofilm Model" Materials 16, no. 13: 4592. https://doi.org/10.3390/ma16134592
APA StyleVilarrasa, J., Àlvarez, G., Soler-Ollé, A., Gil, J., Nart, J., & Blanc, V. (2023). Bacterial Adhesion of TESPSA and Citric Acid on Different Titanium Surfaces Substrate Roughness: An In Vitro Study with a Multispecies Oral Biofilm Model. Materials, 16(13), 4592. https://doi.org/10.3390/ma16134592







