Effect of Mechanically Activated Nepheline-Syenite Additive on the Physical–Mechanical Properties and Frost Resistance of Ceramic Materials Composed of Illite Clay and Mineral Wool Waste
Abstract
1. Introduction
2. Materials and Methods
3. Results
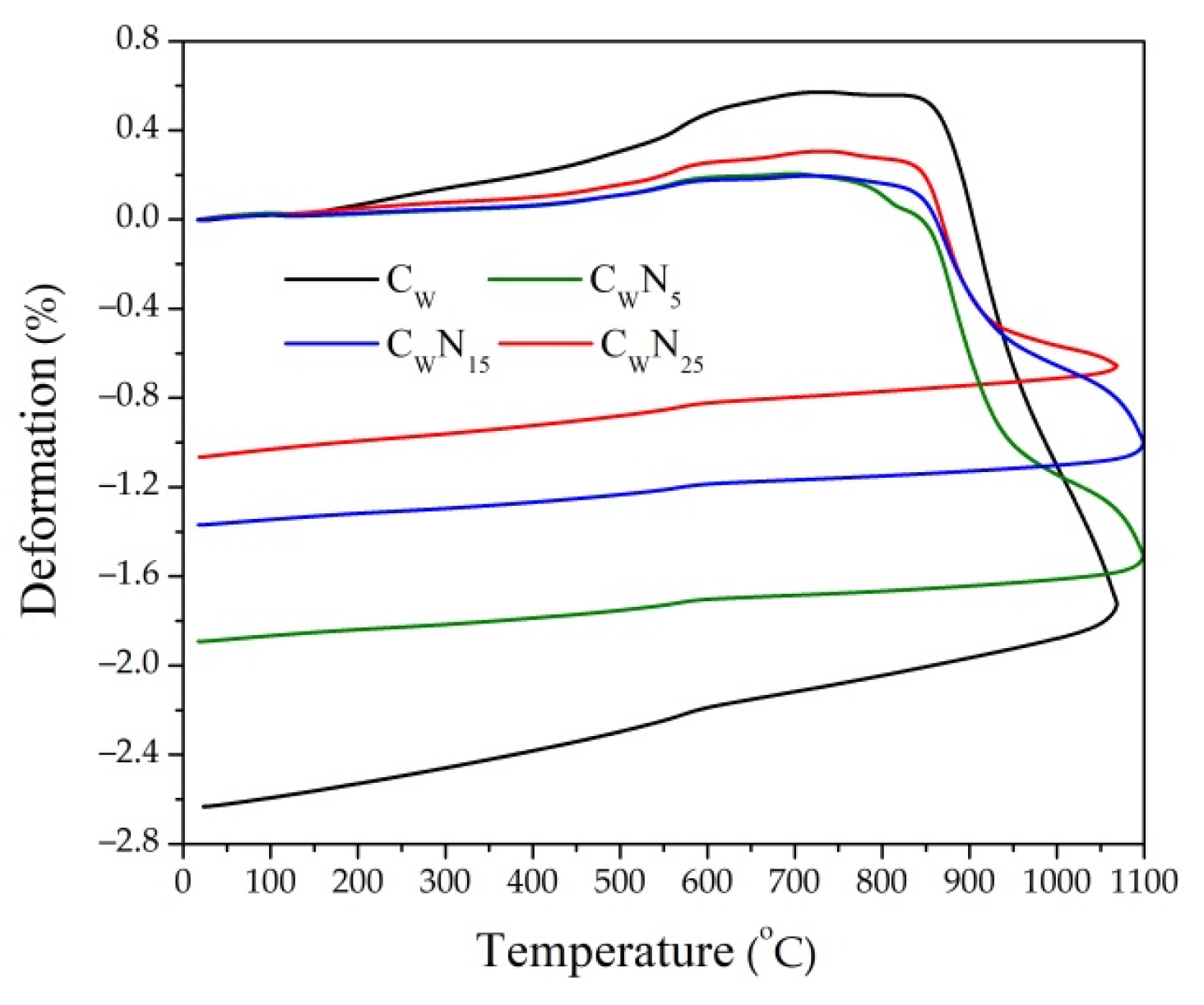
3.1. Dilatometric Analysis
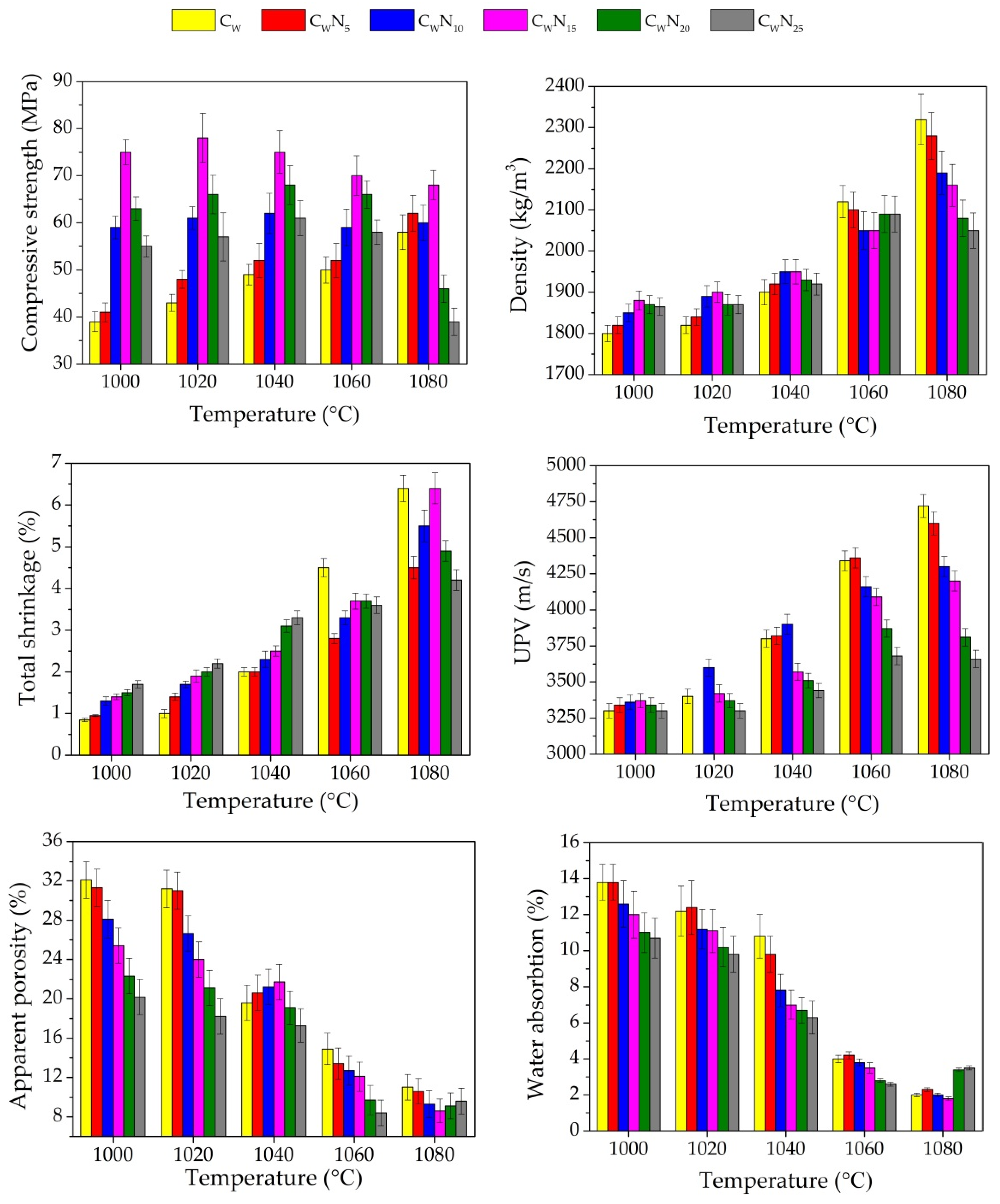
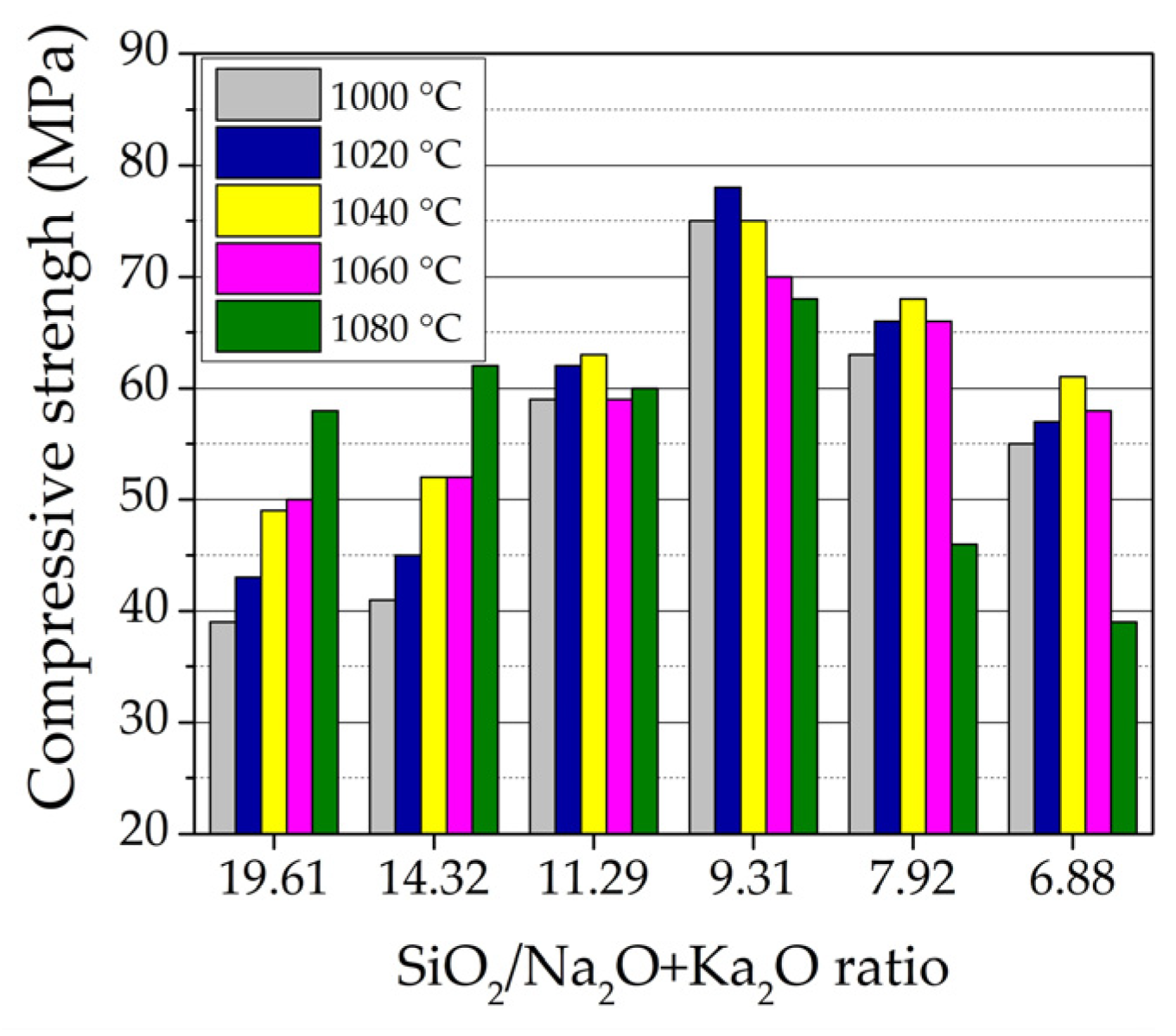
3.2. Physical and Mechanical Properties
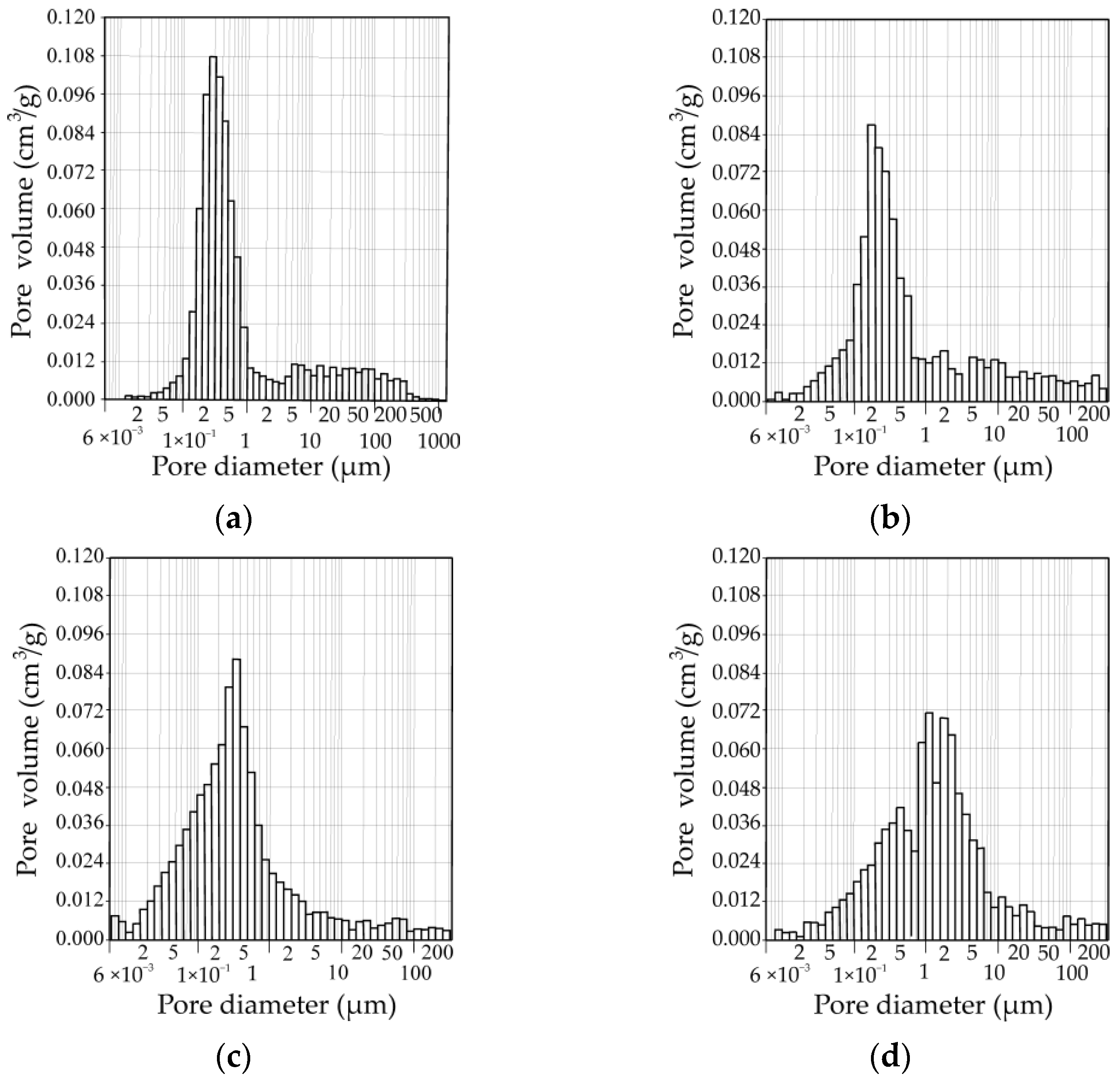
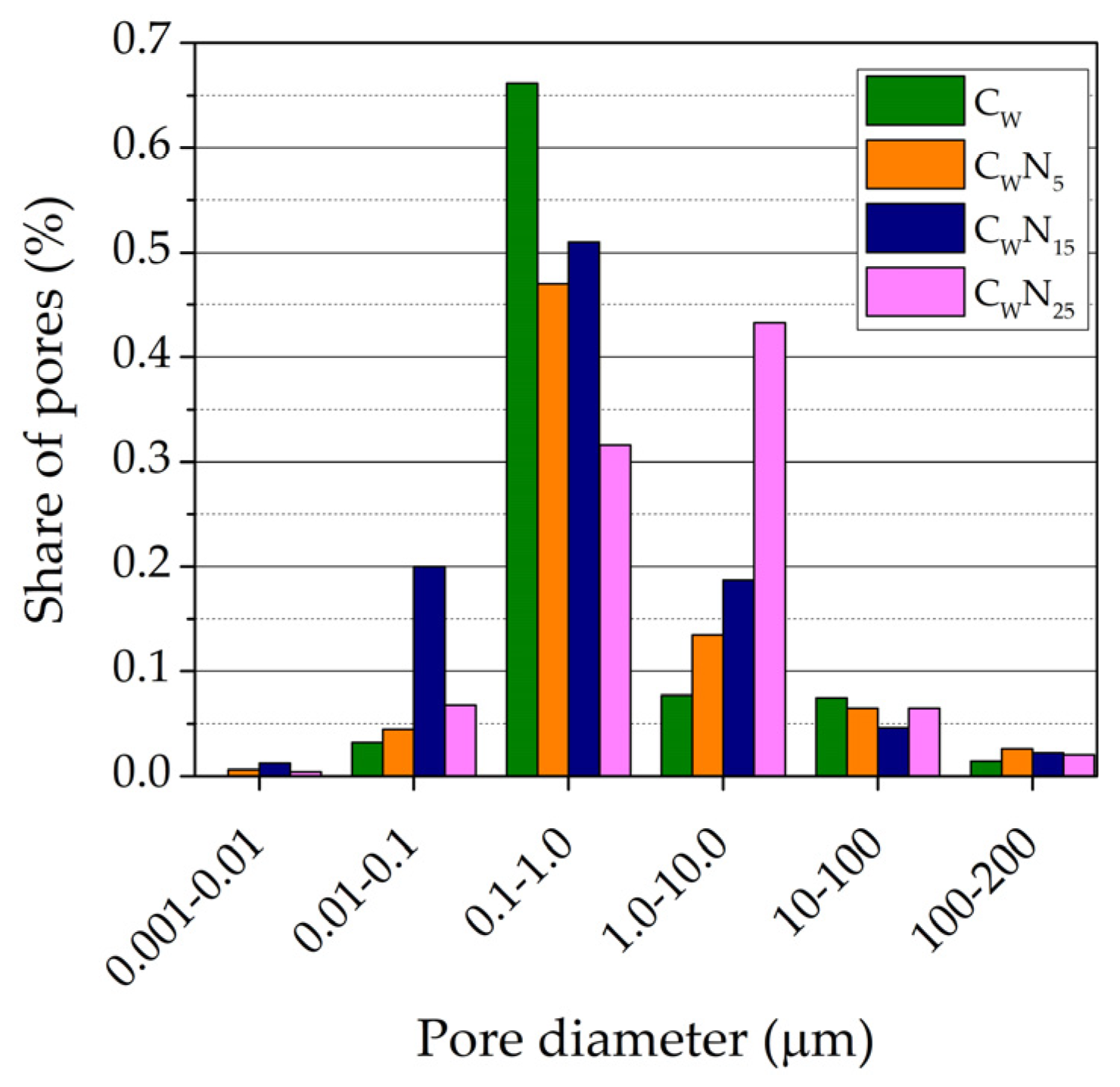
3.3. Porosity
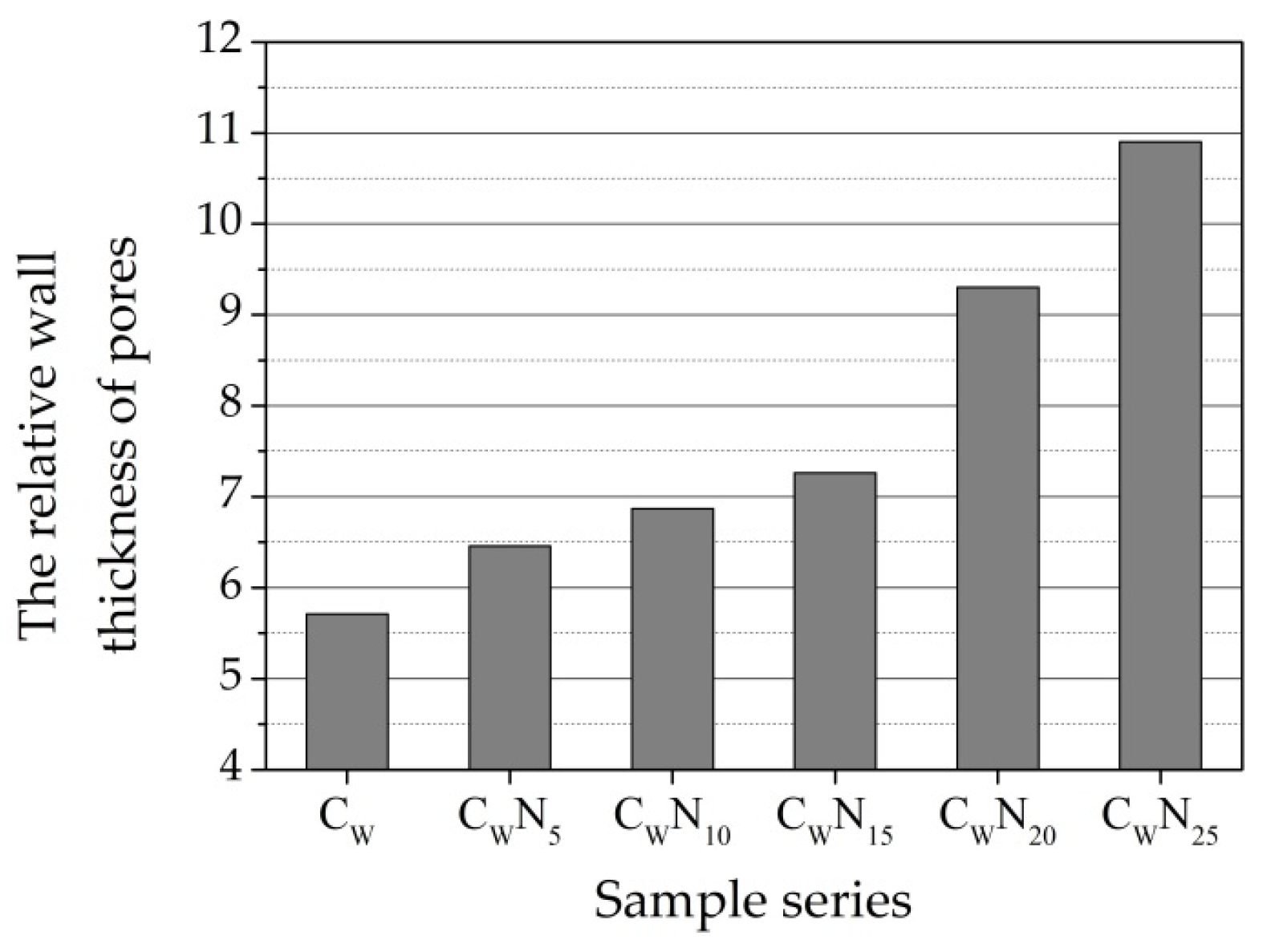
3.4. Structural Parameters
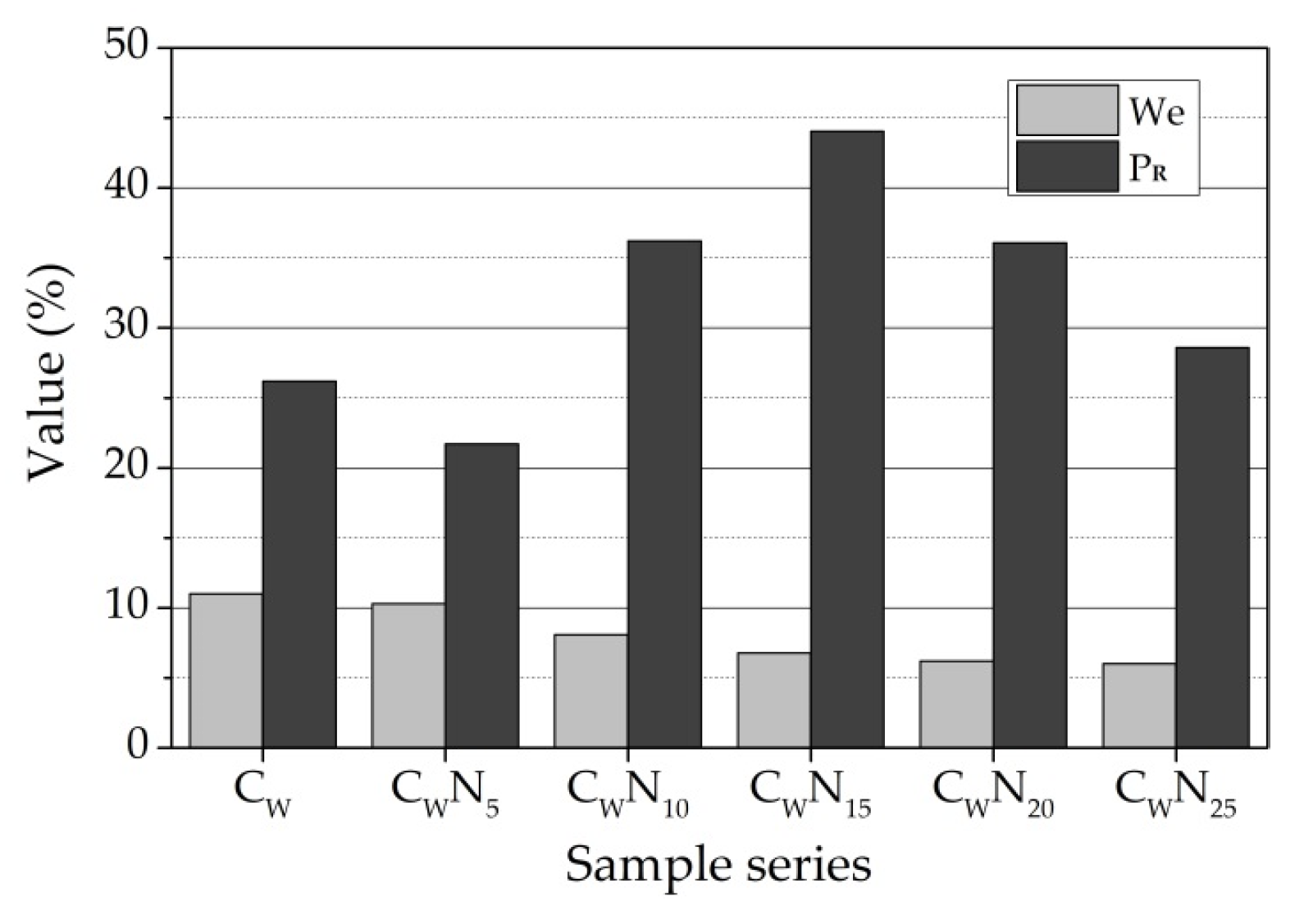
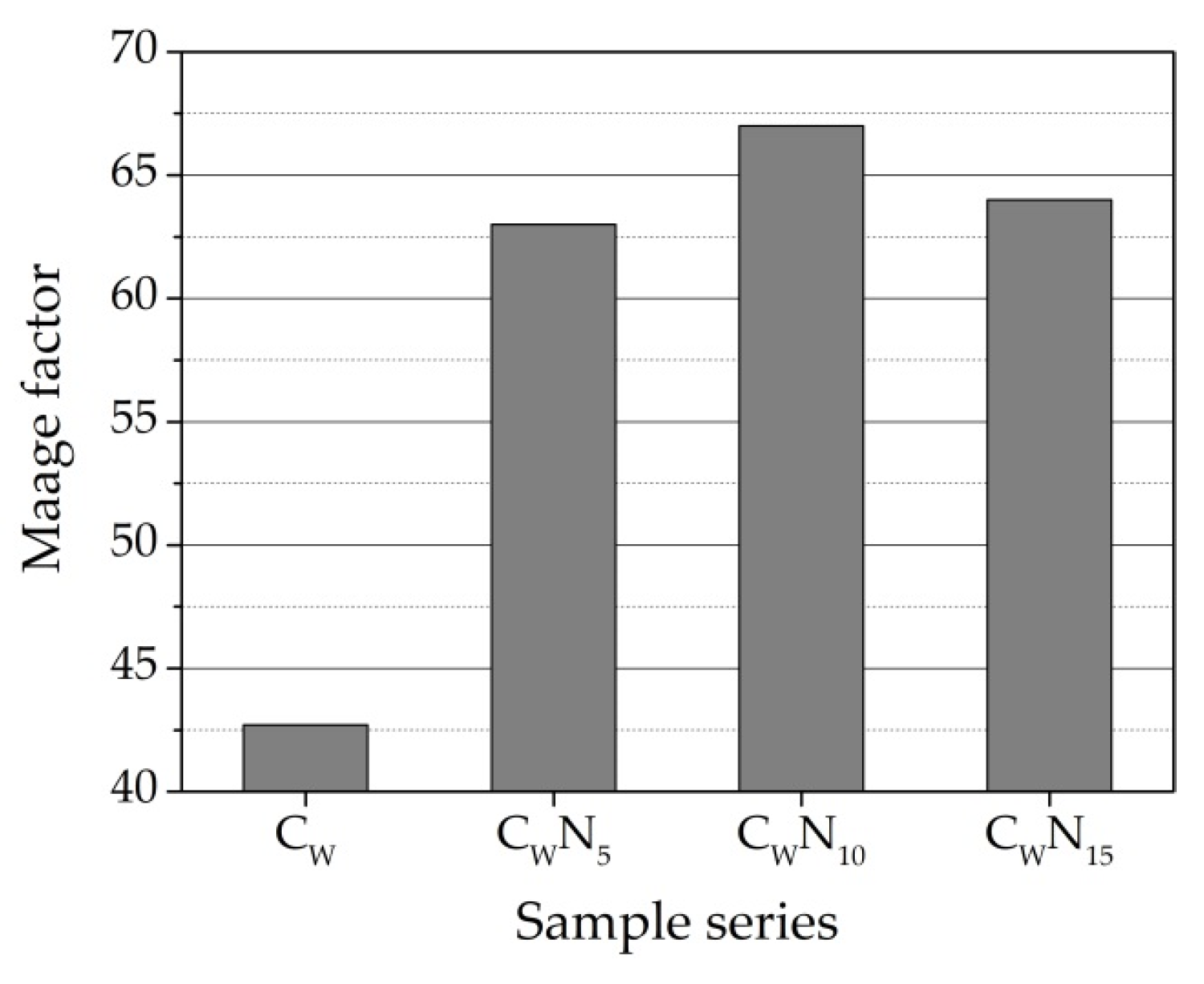
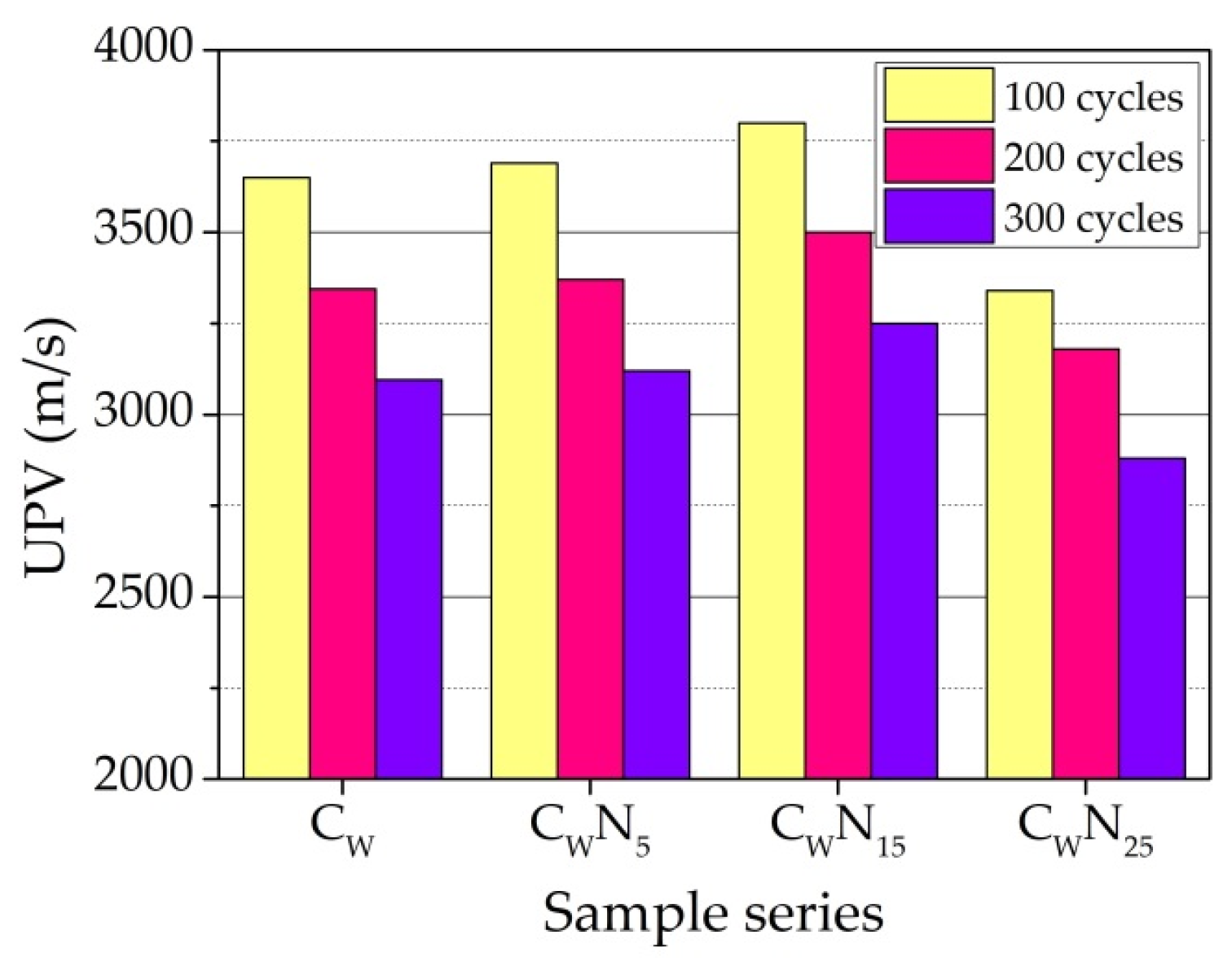
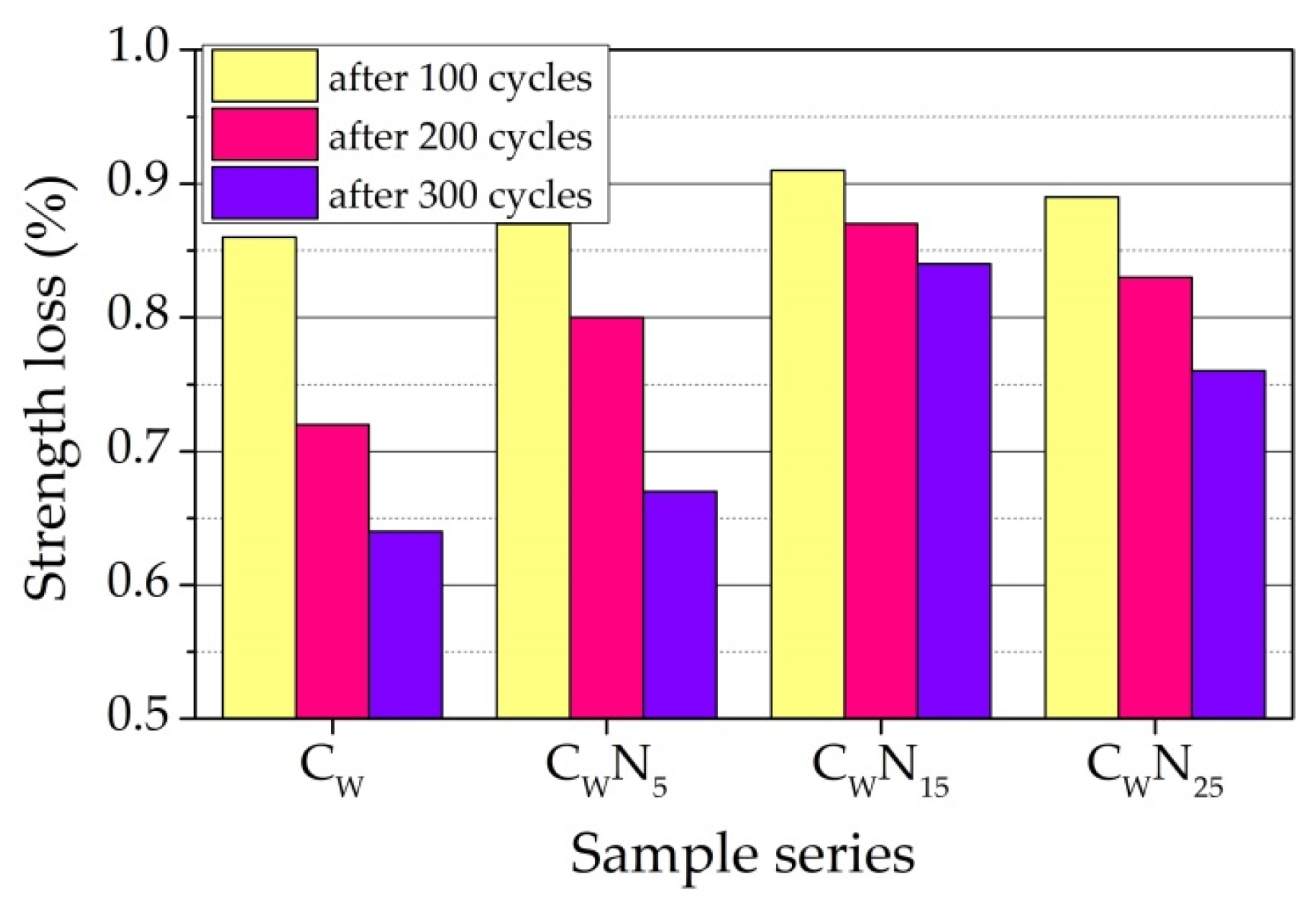
3.5. Frost Resistance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernardo, E.; Bonomo, E.; Dattoli, A. Optimisation of Sintered Glass-Ceramics from an Industrial Waste Glass. Ceram. Int. 2010, 36, 1675–1680. [Google Scholar] [CrossRef]
- Boltakova, N.V.; Faseeva, G.R.; Kabirov, R.R.; Nafikov, R.M.; Zakharov, Y.A. Utilization of Inorganic Industrial Wastes in Producing Construction Ceramics. Review of Russian Experience for the Years 2000–2015. Waste Manag. 2017, 60, 230–246. [Google Scholar] [CrossRef] [PubMed]
- El-Shimy, Y.N.; Amin, S.K.; El-Sherbiny, S.A.; Abadir, M.F. The Use of Cullet in the Manufacture of Vitrified Clay Pipes. Constr. Build. Mater. 2014, 73, 452–457. [Google Scholar] [CrossRef]
- Mymrin, V.; Klitzke, W.; Alekseev, K.; Catai, R.E.; Nagalli, A.; Izzo, R.L.d.S.; Romano, C.A. Red Clay Application in the Utilization of Paper Production Sludge and Scrap Glass to Fabricate Ceramic Materials. Appl. Clay Sci. 2015, 107, 28–35. [Google Scholar] [CrossRef]
- Sena da Fonseca, B.; Galhano, C.; Seixas, D. Technical Feasibility of Reusing Coal Combustion By-Products from a Thermoelectric Power Plant in the Manufacture of Fired Clay Bricks. Appl. Clay Sci. 2015, 104, 189–195. [Google Scholar] [CrossRef]
- Naganathan, S.; Mohamed, A.Y.O.; Mustapha, K.N. Performance of Bricks Made Using Fly Ash and Bottom Ash. Constr. Build. Mater. 2015, 96, 576–580. [Google Scholar] [CrossRef]
- Bourtsalas, A.; Vandeperre, L.J.; Grimes, S.M.; Themelis, N.; Cheeseman, C.R. Production of Pyroxene Ceramics from the Fine Fraction of Incinerator Bottom Ash. Waste Manag. 2014, 45, 217–225. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, W.; Li, J.; Qiao, L.; Zheng, J.; Sheng, J. Utilization of Coal Fly Ash in the Glass-Ceramic Production. J. Hazard. Mater. 2007, 149, 523–526. [Google Scholar] [CrossRef]
- Pranckeviciene, I.; Pundiene, I. Effect of Co-Use of Mineral Wool Production Waste and Catalytic Cracking Catalyst Waste on Ceramic Structure and Properties. Glas. Ceram. 2021, 77, 34–40. [Google Scholar] [CrossRef]
- Kizinievič, O.; Balkevičius, V.; Pranckevičienė, J.; Kizinievič, V. Analysis of the Effect of Syenite Alkali Aluminium Concentrate (SAAC) on the Properties of Ceramic Products Containing the Centrifugation Waste of a Mineral Wool Melt. Ceram. Int. 2015, 41, 11234–11241. [Google Scholar] [CrossRef]
- Kizinievič, O.; Balkevičius, V.; Pranckevičiene, J.; Kizinievič, V. Investigation of the Usage of Centrifuging Waste of Mineral Wool Melt (CMWW), Contaminated with Phenol and Formaldehyde, in Manufacturing of Ceramic Products. Waste Manag. 2014, 34, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Kizinevich, O.; Machyulaitis, R.; Kizinevich, V.; Yakovlev, G.I. Utilization of Technogenic Material from an Oil-Processing Company in the Production of Building Ceramics. Glas. Ceram. 2006, 63, 64–67. [Google Scholar] [CrossRef]
- Feng, C.; Roels, S.; Janssen, H. Towards a More Representative Assessment of Frost Damage to Porous Building Materials. Build. Environ. 2019, 164, 106343. [Google Scholar] [CrossRef]
- Raut, S.P.; Ralegaonkar, R.V.; Mandavgane, S.A. Development of Sustainable Construction Material Using Industrial and Agricultural Solid Waste: A Review of Waste-Create Bricks. Constr. Build. Mater. 2011, 25, 4037–4042. [Google Scholar] [CrossRef]
- Zhang, L. Production of Bricks from Waste Materials—A Review. Constr. Build. Mater. 2013, 47, 643–655. [Google Scholar] [CrossRef]
- Muñoz Velasco, P.; Morales Ortíz, M.P.; Mendívil Giró, M.A.; Muñoz Velasco, L. Fired Clay Bricks Manufactured by Adding Wastes as Sustainable Construction Material—A Review. Constr. Build. Mater. 2014, 63, 97–107. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Vieira, C.M.F. On the Production of Fired Clay Bricks from Waste Materials: A Critical Update. Constr. Build. Mater. 2014, 68, 599–610. [Google Scholar] [CrossRef]
- Shigetaka, F. Freeze-Thaw Stable Stoneware Tile and Process for Production Thereof. U.S. Patent 4,542,058, 17 September 1985. [Google Scholar]
- Sánchez De Rojas, M.I.; Marín, F.P.; Frías, M.; Valenzuela, E.; Rodríguez, O. Influence of Freezing Test Methods, Composition and Microstructure on Frost Durability Assessment of Clay Roofing Tiles. Constr. Build. Mater. 2011, 25, 2888–2897. [Google Scholar] [CrossRef]
- Koroth, S.R.; Fazio, P.; Feldman, D. Evaluation of Clay Brick Durability Using Ultrasonic Pulse Velocity. J. Archit. Eng. 1998, 4, 142–147. [Google Scholar] [CrossRef]
- Stryszewska, T.; Kańka, S. Forms of Damage of Bricks Subjected to Cyclic Freezing and Thawing in Actual Conditions. Materials 2019, 12, 1165. [Google Scholar] [CrossRef]
- Ingham, J.P. Predicting the Frost Resistance of Building Stone. Q. J. Eng. Geol. Hydrogeol. 2005, 38, 387–399. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, P.C. Pore Size Distribution in Cement Pastes in Relation to Freeze-Thaw Distress. J. Mater. Civ. Eng. 2015, 27, 04014123. [Google Scholar] [CrossRef]
- Bracka, A.; Rusin, Z. Comparison of Pore Characteristics and Water Absorption in Ceramic Materials with Frost Resistance Factor, Fc. Struct. Environ. 2012, 4, 15–19. [Google Scholar]
- Smits, A.; Gregoire, Y.; Tirlocq, J.; Lefort, V.; Andre, S. Frost Resistance of Clay Masonry Units: In-Depth Experimental Study of the European Method. In Proceedings of the 8th International Masonry Conference 2010 Dresden, Dresden, Alemanha, 4–7 July 2010; pp. 1–10. [Google Scholar]
- Rusin, Z.; Świercz, P. Frost Resistance of Rock Materials. Constr. Build. Mater. 2017, 148, 704–714. [Google Scholar] [CrossRef]
- Elert, K.; Cultrone, G.; Rodriguez Navarro, C.; Sebastián Pardo, E. Durability of Bricks Used in the Conservation of Historic Buildings—Influence of Composition and Microstructure. J. Cult. Herit. 2003, 4, 91–99. [Google Scholar] [CrossRef]
- Koroth, S.R. Evaluation and Improvement of Frost Durability of Clay Bricks. Ph.D. Thesis, Concordia University, Montreal, QC, Canada, 1997. [Google Scholar]
- Kung, J.H.; Officer, R. Frost Durability of Canadian Clay Bricks. In Proceedings of the 7th International Brick Masonry Conference, Melbourne, Australia, 17–20 February 1985; Volume 1, pp. 245–251. [Google Scholar]
- Mensinga, P. Determining the Critical Degree of Saturation of Brick Using Frost Dilatometry. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2009. [Google Scholar]
- Smits, A.; Dirkx, I.; Gregorie, Y. Research Concerning the Durability Assessment of Lime Masonry Mortars. In Proceedings of the Historical Mortars Conference Characterization, Diagnosis, Conservation, Repair and Compatibility, Lissabon, Portugal, 24–26 September 2008. [Google Scholar]
- Cultrone, G.; Sebastián, E.; Elert, K.; de la Torre, M.J.; Cazalla, O.; Rodriguez-Navarro, C. Influence of Mineralogy and Firing Temperature on the Porosity of Bricks. J. Eur. Ceram. Soc. 2004, 24, 547–564. [Google Scholar] [CrossRef]
- Esposito, L.; Salem, A.; Tucci, A.; Gualtieri, A.; Jazayeri, S.H. The Use of Nepheline-Syenite in a Body Mix for Porcelain Stoneware Tiles. Ceram. Int. 2005, 31, 233–240. [Google Scholar] [CrossRef]
- Salem, A.; Jazayeri, S.H.; Rastelli, E.; Timellini, G. Dilatometeric Study of Shrinkage during Sintering Process for Porcelain Stoneware Body in Presence of Nepheline Syenite. J. Mater. Process. Technol. 2009, 209, 1240–1246. [Google Scholar] [CrossRef]
- Burat, F.; Kangal, O.; Onal, G. An Alternative Mineral in the Glass and Ceramic Industry: Nepheline Syenite. Miner. Eng. 2006, 19, 370–371. [Google Scholar] [CrossRef]
- Rostami, N.; Salem, A.; Paknjhad, A. Influence of Nepheline Syenite on Mechanical Reliability of Ceramic Raschig Rings. Int. J. Appl. Ceram. Technol. 2011, 8, 446–454. [Google Scholar] [CrossRef]
- Kamseu, E.; Bakop, T.; Djangang, C.; Melo, U.C.; Hanuskova, M.; Leonelli, C. Porcelain Stoneware with Pegmatite and Nepheline Syenite Solid Solutions: Pore Size Distribution and Descriptive Microstructure. J. Eur. Ceram. Soc. 2013, 33, 2775–2784. [Google Scholar] [CrossRef]
- Pranckevičienė, J. Impact of Mineral Wool Production Waste on Properties of Sintered Ceramics. Ph.D. Thesis, Vilnius Gediminas Technical University, Vilnius, Lithuania, 2011. [Google Scholar]
- Mena Silva, C.A.; Ellefmo, S.L.; Sandøy, R.; Sørensen, B.E.; Aasly, K. A Neural Network Approach for Spatial Variation Assessment—A Nepheline Syenite Case Study. Miner. Eng. 2020, 149, 106178. [Google Scholar] [CrossRef]
- Dondi, M. Feldspathic Fluxes for Ceramics: Sources, Production Trends and Technological Value. Resour. Conserv. Recycl. 2018, 133, 191–205. [Google Scholar] [CrossRef]
- Chiwona, A.G.; Cortés, J.A.; Gaulton, R.G.; Manning, D.A.C. Petrology and Geochemistry of Selected Nepheline Syenites from Malawi and Their Potential as Alternative Potash Sources. J. Afr. Earth Sci. 2020, 164, 103769. [Google Scholar] [CrossRef]
- Zanelli, C.; Ardit, M.; Conte, S.; Soldati, R.; Cruciani, G.; Dondi, M. Viscous Flow Sintering of Porcelain Stoneware Revisited. In Proceedings of the 15th World Congress on Ceramic Tile Quality, QUALICER 2018, Castellón, Spain, 12–13 February 2018; pp. 1–9. [Google Scholar]
- Dias, F.G.; Segadães, A.M.; Perottoni, C.A.; Cruz, R.C.D. Assessment of the Fluxing Potential of Igneous Rocks in the Traditional Ceramics Industry. Ceram. Int. 2017, 43, 16149–16158. [Google Scholar] [CrossRef]
- El-Maghraby, H.F.; El-Omla, M.M.; Bondioli, F.; Naga, S.M. Granite as Flux in Stoneware Tile Manufacturing. J. Eur. Ceram. Soc. 2011, 31, 2057–2063. [Google Scholar] [CrossRef]
- Echeverrigaray, S.G.; Emiliano, J.V.; Segadães, A.M.; Cruz, R.C.D. Low-Valued Raw Materials Challenge the Common Eligibility Criteria for Triaxial Ceramics. Ceram. Int. 2016, 42, 10671–10681. [Google Scholar] [CrossRef]
- Tamsu, N.; Bayrak, A.V.; Ozdemir, H. Eects of Na2O/K2O Ratio on the Deformation Behaviour of the Floor Tile Bodies. Acta Phys. Pol. A 2013, 123, 283–284. [Google Scholar] [CrossRef]
- Suvorova, O.; Kumarova, V.; Nekipelov, D.; Selivanova, E.; Makarov, D.; Masloboev, V. Construction Ceramics from Ore Dressing Waste in Murmansk Region, Russia. Constr. Build. Mater. 2017, 153, 783–789. [Google Scholar] [CrossRef]
- Salem, A.; Jazayeri, S.H.; Rastelli, E.; Timellini, G. Kinetic Model for Isothermal Sintering of Porcelain Stoneware Body in Presence of Nepheline Syenite. Thermochim. Acta 2010, 503–504, 1–7. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Panyachai, T.; Kaewsimork, K.; Siritai, C. Effects of Recycled Glass Substitution on the Physical and Mechanical Properties of Clay Bricks. Waste Manag. 2009, 29, 2717–2721. [Google Scholar] [CrossRef] [PubMed]
- Andreola, F.; Barbieri, L.; Karamanova, E.; Lancellotti, I.; Pelino, M. Recycling of CRT Panel Glass as Fluxing Agent in the Porcelain Stoneware Tile Production. Ceram. Int. 2008, 34, 1289–1295. [Google Scholar] [CrossRef]
- Storozhenko, G.; Stolboushkin, A.U. Ceramic Bricks from Industrial Waste. Ceram. Sakhteman Seas. Mag. Ceram. Build. Winter 2010, 2, 2–6. [Google Scholar]
- Zanelli, C.; Raimondo, M.; Guarini, G.; Dondi, M. The Vitreous Phase of Porcelain Stoneware: Composition, Evolution during Sintering and Physical Properties. J. Non. Cryst. Solids 2011, 357, 3251–3260. [Google Scholar] [CrossRef]
- Sedmale, G.P.; Ya Sedmalis, U. Sintered Ceramic Materials Produced from Hydromica Clays. Glas. Ceram. 2000, 57, 26–28. [Google Scholar] [CrossRef]
- EN 772-13:2003; Methods of Test for Masonry Units—Part 13: Determination of Net and Gross Dry Density of Masonry Units (except for Natural Stone). European Committee for Standardization: Brussels, Belgium, 2003.
- EN 772-21:2011; Methods of Test for Masonry Units—Part 21: Determination of Water Absorption of Clay and Calcium Silicate Masonry Units by Cold Water Absorption. European Committee for Standardization: Brussels, Belgium, 2011.
- EN 772-1:2011+A1:2015; Methods of Test for Masonry Units—Part 1: Determination of Compressive Strength. European Committee for Standardization: Brussels, Belgium, 2015.
- Netinger Grubeša, I.; Vračević, M.; Ranogajec, J.; Vučetić, S. Influence of Pore-Size Distribution on the Resistance of Clay Brick to Freeze–Thaw Cycles. Materials 2020, 13, 2364. [Google Scholar] [CrossRef] [PubMed]
- Løland, K.E. Continuous Damage Model for Load-Response Estimation of Concrete. Cem. Concr. Res. 1980, 10, 395–402. [Google Scholar] [CrossRef]
- Mačiulaitis, R.; Malaiškiene, J. Possibilities to Control Ceramics Properties by Changing Firing Cycles. Constr. Build. Mater. 2009, 23, 226–232. [Google Scholar] [CrossRef]
- Kizinievič, O.; Žurauskiene, R.; Kizinievič, V.; Žurauskas, R. Utilisation of Sludge Waste from Water Treatment for Ceramic Products. Constr. Build. Mater. 2013, 41, 464–473. [Google Scholar] [CrossRef]
- Mačiulaitis, R. Frost Resistance and Durability of Ceramic Facade Products; Technika: Vilnius, Lithuania, 1996. [Google Scholar]
- Maage, M. Frost Resistance and Pore Size Distribution in Bricks. Matériaux Constr. 1984, 17, 345–350. [Google Scholar] [CrossRef]
- ASTM C666/C666M-15; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. ASTM International: West Conshohocken, PA, USA, 2015.
- Janssen, H.; Feng, C.; Roels, S. Impact of Frost Temperature and Moisture Content on Frost Damage to Ceramic Bricks. MATEC Web Conf. 2019, 282, 02013. [Google Scholar] [CrossRef]
- EN 12371:2010; Natural Stone Test Methods—Determination of Frost Resistance. European Committee for Standardization: Brussels, Belgium, 2010.
- Sánchez Muñoz, L. Transformaciones Con La Temperatura. In Materias Primas y Aditivos Cerámicos: Enciclopedia Cerámica; Faenza Editrice Ibérica: Castellón de la Plana, Spain, 2003; pp. 88–120. [Google Scholar]
- Wang, S.; Gainey, L.; Mackinnon, I.D.R.; Allen, C.; Gu, Y.; Xi, Y. Thermal Behaviors of Clay Minerals as Key Components and Additives for Fired Brick Properties: A Review. J. Build. Eng. 2023, 66, 105802. [Google Scholar] [CrossRef]
- Bakop, T.T.; Fongang, R.T.T.; Melo, U.C.; Kamseu, E.; Miselli, P.; Leonelli, C. Sintering Behaviors of Two Porcelainized Stoneware Compositions Using Pegmatite and Nepheline Syenite Minerals. J. Therm. Anal. Calorim. 2013, 114, 113–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, M.; Chen, D.; Wu, J. Leucite Crystallization Kinetics with Kalsilite as a Transition Phase. Mater. Lett. 2007, 61, 2978–2981. [Google Scholar] [CrossRef]
- Kudabienë, G. Synthesis and Properties of Feldspar Ceramics Designated for Metal—Ceramics Composites. Chemija 2004, 15, 42–48. [Google Scholar]
- Wang, S.; Gainey, L.; Mackinnon, I.D.R.; Xi, Y. High- and Low-Defect Kaolinite for Brick Making: Comparisons of Technological Properties, Phase Evolution and Microstructure. Constr. Build. Mater. 2023, 366, 130250. [Google Scholar] [CrossRef]
- Il’Ina, V.P.; Lebedeva, G.A. Use of Wastes from Concentration of Alkali Syenites from the Elet’ozerskoe Deposit for Manufacture of Ceramic Tiles. Glas. Ceram. 2010, 67, 199–202. [Google Scholar] [CrossRef]
- Kizinievič, O.; Kizinievič, V. Influence of Structural Parameters on the Frost Resistance of Clay Masonry Units. Mater. Sci. 2009, 15, 59–63. [Google Scholar]
- Pranckeviciene, J.; Pundiene, I.; Balkevicius, V.R.; Balciunas, G. Investigation of the Influence of Paraffin Production Wastes on the Properties of Ceramic Articles. Glas. Ceram. 2019, 76, 155–159. [Google Scholar] [CrossRef]
- Mačiulaitis, R.; Malaiškiene, J. New Quality Regulation System for Manufacture of Ceramic Products. Constr. Build. Mater. 2007, 21, 258–268. [Google Scholar] [CrossRef]
- Kizinievič, O.; Kizinievič, V.; Malaiškienė, J. Analysis of the Effect of Paper Sludge on the Properties, Microstructure and Frost Resistance of Clay Bricks. Constr. Build. Mater. 2018, 169, 689–696. [Google Scholar] [CrossRef]

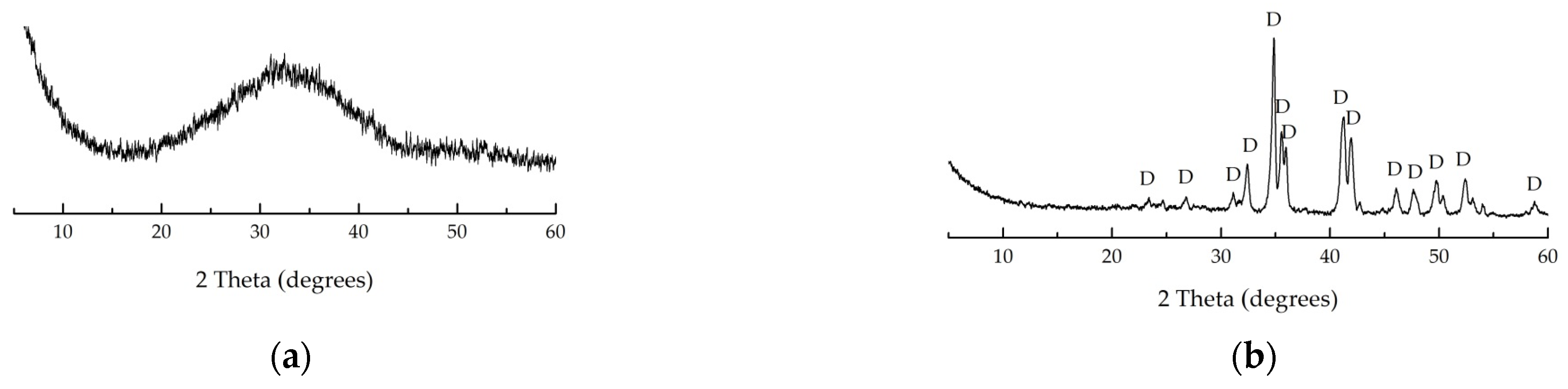

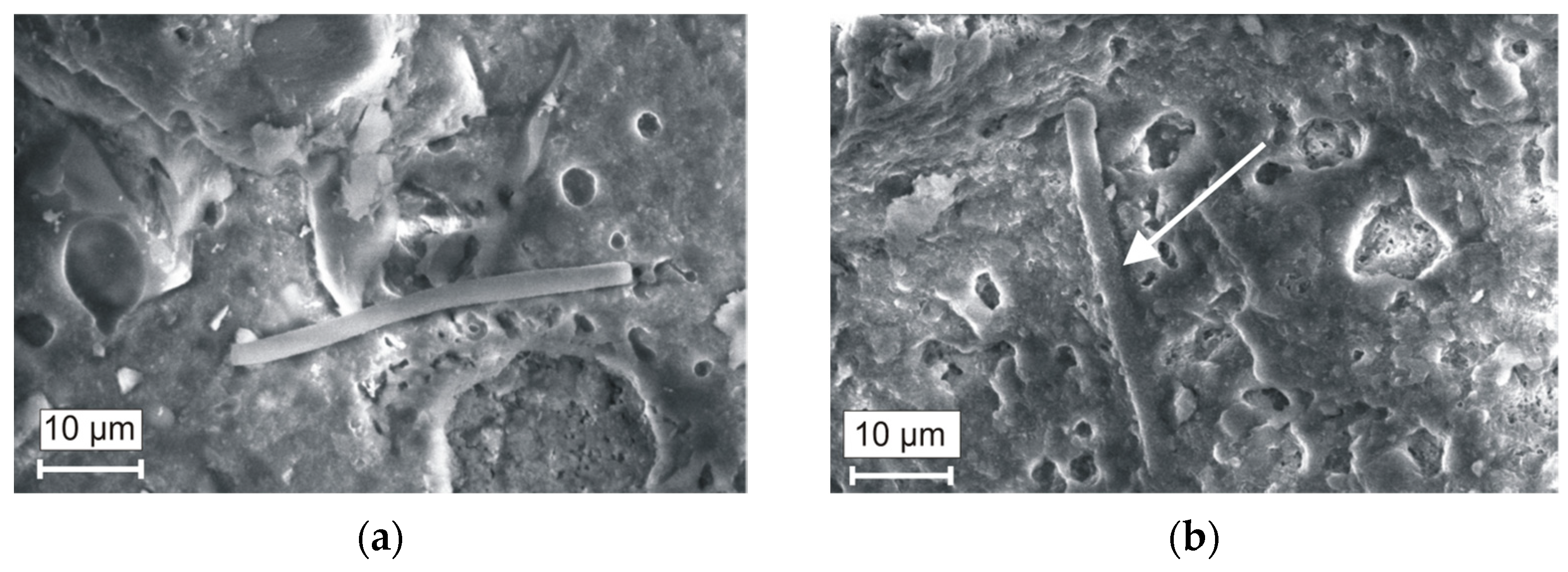



| Components | Residue on Sieve (mm)% | |||||||
|---|---|---|---|---|---|---|---|---|
| 2.0 | 1.0 | 0.5 | 0.25 | 0.125 | 0.08 | 0.045 | <0.045 | |
| Clay | 0.083 | 0.004 | 0.006 | 0.006 | 0.07 | 0.80 | 88.24 | 9.92 |
| MWMW | - | - | 0.29 | 3.92 | 19.62 | 39.55 | 26.84 | 9.79 |
| NS | - | - | - | - | 0.80 | 99.2 | 37.95 | 61.25 |
| Components | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O + K2O | Loss on Ignition |
|---|---|---|---|---|---|---|---|
| MWMW | 42.13 | 18.30 | 5.81 | 16.15 | 13.94 | 1.73 | 1.40 |
| Clay | 46.88 | 17.93 | 5.31 | 10.36 | 4.37 | 2.67 | 14.50 |
| NS | 44.80 | 28.60 | 2.60 | 1.20 | 0.60 | 19.5 | 0.90 |
| Batch | Composition of Forming Masses, % | ||
|---|---|---|---|
| Clay | MWMW | NS | |
| CW | 80.0 | 20.0 | - |
| CWN5 | 75.0 | 20.0 | 5.0 |
| CWN10 | 70.0 | 20.0 | 10.0 |
| CWN15 | 65.0 | 20.0 | 15.0 |
| CWN20 | 60.0 | 20.0 | 20.0 |
| CWN25 | 55.0 | 20.0 | 25.0 |
| Batch | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O + K2O | SiO2/Na2O + K2O | Al2O3/Na2O + K2O |
|---|---|---|---|---|---|---|---|---|
| CW | 51.38 | 20.06 | 5.94 | 12.96 | 7.03 | 2.62 | 19.61 | 7.66 |
| CWN5 | 50.98 | 20.54 | 5.76 | 12.38 | 6.78 | 3.56 | 14.32 | 5.77 |
| CWN10 | 50.59 | 21.02 | 5.58 | 11.81 | 6.5 | 4.48 | 11.29 | 4.69 |
| CWN15 | 50.20 | 21.49 | 5.40 | 11.24 | 6.29 | 5.39 | 9.31 | 3.99 |
| CWN20 | 49.81 | 21.96 | 5.22 | 10.68 | 6.05 | 6.29 | 7.92 | 3.49 |
| CWN25 | 49.43 | 22.42 | 5.04 | 10.12 | 5.81 | 7.18 | 6.88 | 3.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pranckevičienė, J.; Pundienė, I. Effect of Mechanically Activated Nepheline-Syenite Additive on the Physical–Mechanical Properties and Frost Resistance of Ceramic Materials Composed of Illite Clay and Mineral Wool Waste. Materials 2023, 16, 4943. https://doi.org/10.3390/ma16144943
Pranckevičienė J, Pundienė I. Effect of Mechanically Activated Nepheline-Syenite Additive on the Physical–Mechanical Properties and Frost Resistance of Ceramic Materials Composed of Illite Clay and Mineral Wool Waste. Materials. 2023; 16(14):4943. https://doi.org/10.3390/ma16144943
Chicago/Turabian StylePranckevičienė, Jolanta, and Ina Pundienė. 2023. "Effect of Mechanically Activated Nepheline-Syenite Additive on the Physical–Mechanical Properties and Frost Resistance of Ceramic Materials Composed of Illite Clay and Mineral Wool Waste" Materials 16, no. 14: 4943. https://doi.org/10.3390/ma16144943
APA StylePranckevičienė, J., & Pundienė, I. (2023). Effect of Mechanically Activated Nepheline-Syenite Additive on the Physical–Mechanical Properties and Frost Resistance of Ceramic Materials Composed of Illite Clay and Mineral Wool Waste. Materials, 16(14), 4943. https://doi.org/10.3390/ma16144943






