Antibacterial Structure Design of Porous Ti6Al4V by 3D Printing and Anodic Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Morphology and Contact Angle Characterization of the TiO2 Oxide Layer
2.4. Bacteriostatic Test
3. Results
3.1. Characteristics of 3D-Printed Porous Titanium Alloys
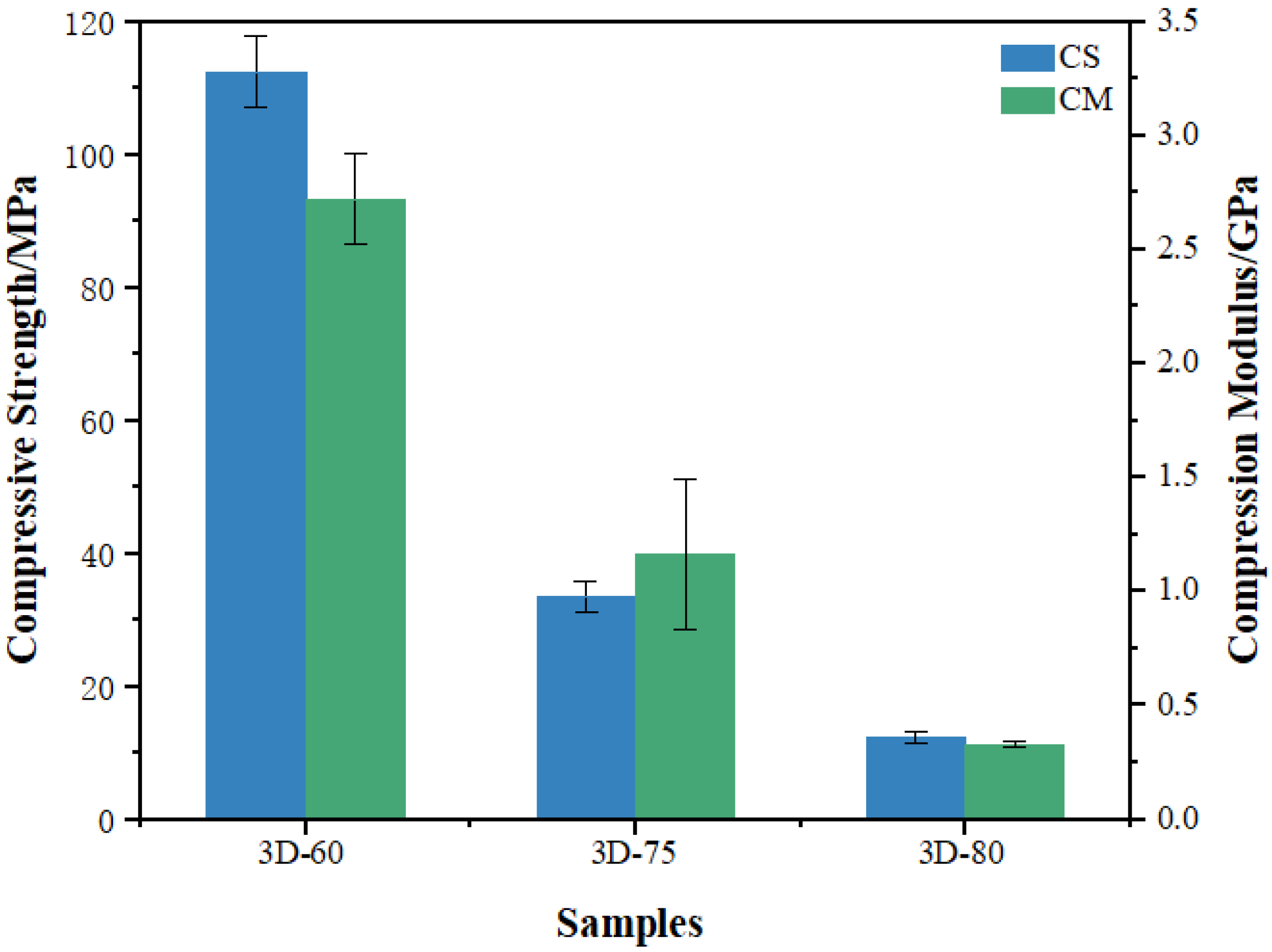
3.2. Mechanical Properties Evaluation
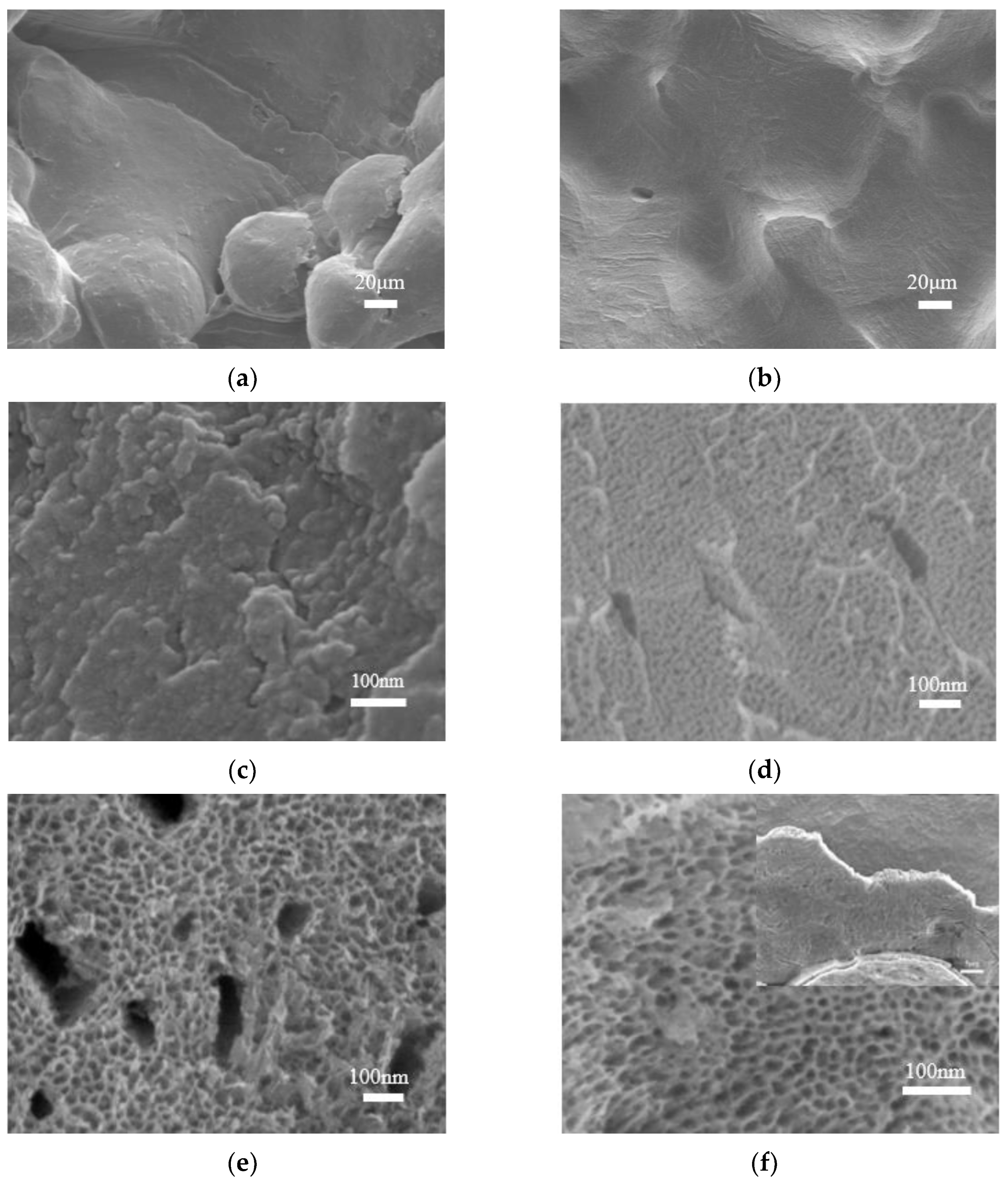
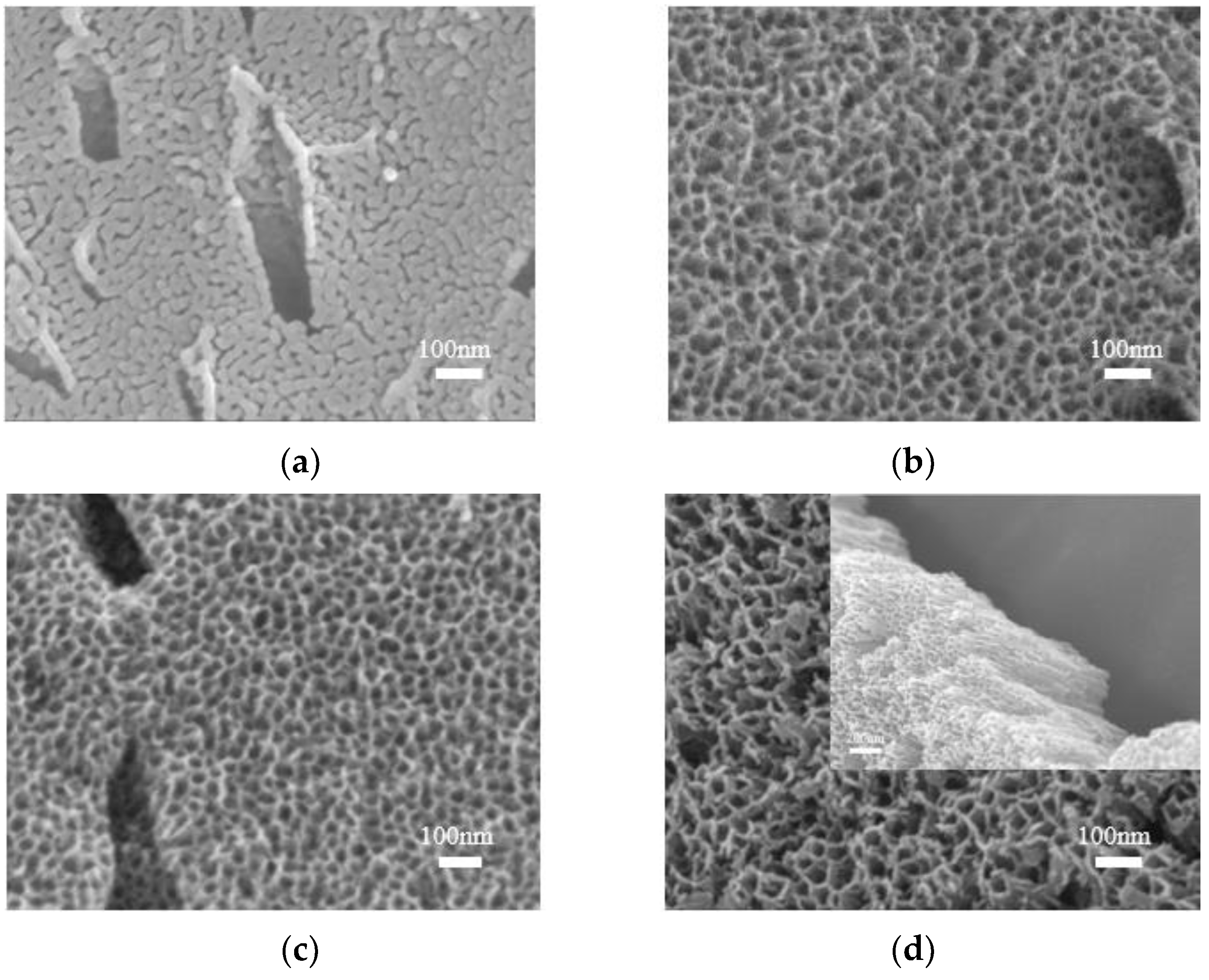
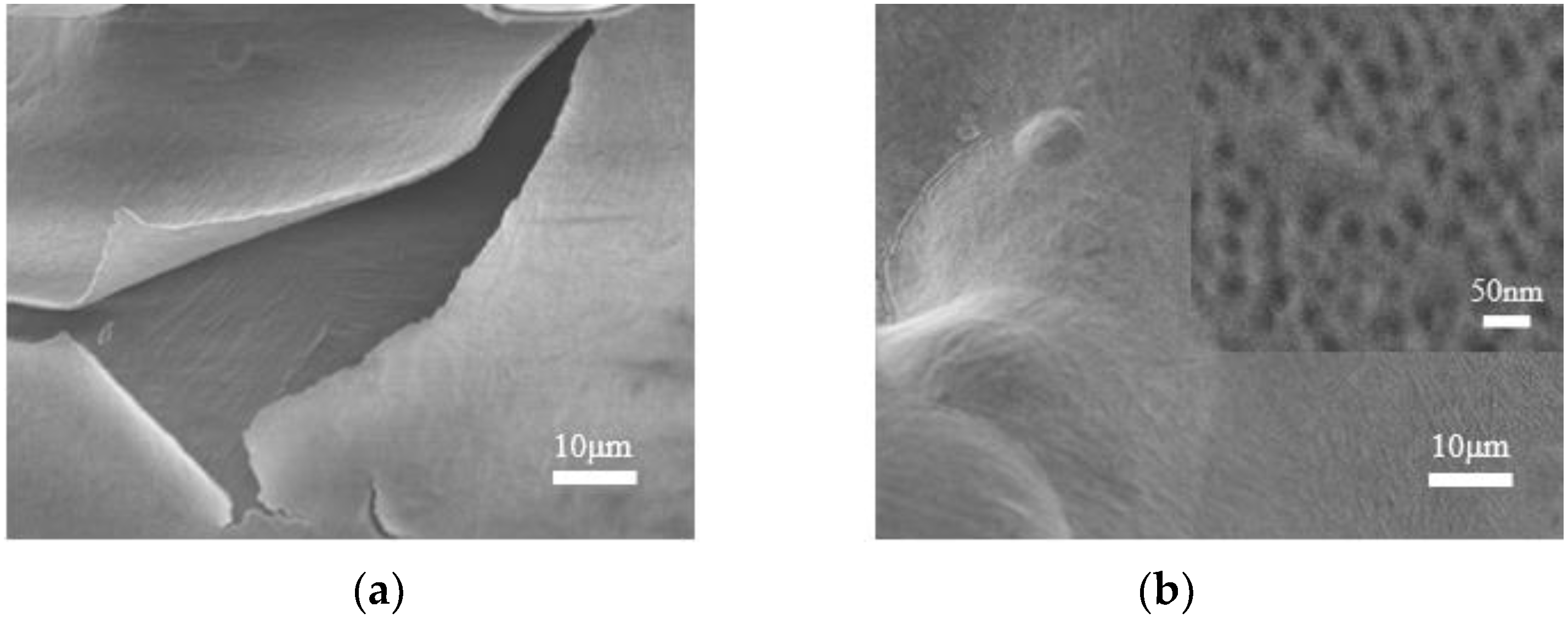
3.3. Morphological Characterization
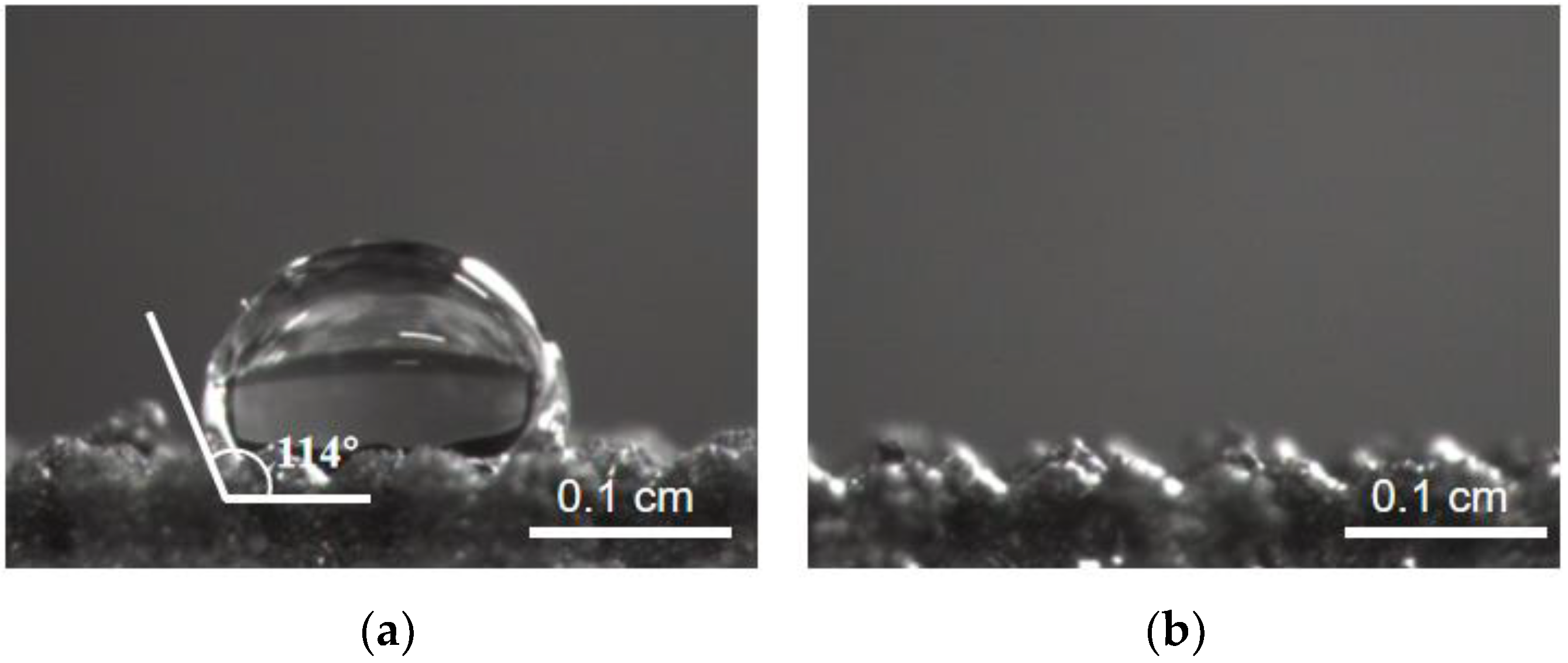
3.4. Wettability
3.5. Antibacterial Activity of 3D-Printed Porous Titanium Alloy with TiO2 Oxide Layer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Review Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Raffa, M.L.; Nguyen, V.-H.; Hernigou, P.; Flouzat, C.-H.; Lachaniette; Haiat, G. Stress shielding at the bone-implant interface: Influence of surface roughness and of the bone-implant contact ratio. J. Orthop. Res. 2021, 39, 1174–1183. [Google Scholar] [CrossRef]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater 2016, 33, 311–321. [Google Scholar] [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, M.; Liu, Z.; Wang, Y.; Dong, W.; Zhao, S.; Sun, D. Biomimetic design strategy of complex porous structure based on 3D printing Ti-6Al-4V scaffolds for enhanced osseointegration. Mater. Des. 2022, 218, 110721. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Wu, Y.; Zhang, Z.; Jiang, D.; Jia, R.; Wang, X.; Liu, Z. In Vitro and In Vivo Analysis of the Effects of 3D-Printed Porous Porous Titanium Alloy Scaffold Structure on Osteogenic Activity. Biomed Res. Int. 2022, 2022, 8494431. [Google Scholar] [CrossRef]
- Adamek, G.; Junka, A.; Wirstlein, P.; Jurczyk, M.U.; Siwak, P.; Koper, J.; Jakubowicz, J. Biomedical Ti-Nb-Zr Foams Prepared by Means of Thermal Dealloying Process and Electrochemical Modification. Materials 2022, 15, 2130. [Google Scholar] [CrossRef]
- Zhao, L.; Pei, X.; Jiang, L.; Hu, C.; Sun, J.; Xing, F.; Zhou, C.; Fan, Y.; Zhang, X. Bionic design and 3D printing of porous titanium alloy scaffolds for bone tissue repair. Compos. Part B Eng. 2019, 162, 154–161. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials 2013, 34, 8018–8029. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ni, S.; Ma, L.; Li, M. Porous construction and surface modification of titanium-based materials for osteogenesis: A review. Front. Bioeng. Biotechnol. 2022, 10, 973297. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef]
- Ferraris, S.; Warchomicka, F.; Iranshahi, F.; Rimondini, L.; Cochis, A.; Spriano, S. Electron Beam Structuring of Ti6Al4V: New Insights on the Metal Surface Properties Influencing the Bacterial Adhesion. Materials 2020, 13, 409. [Google Scholar] [CrossRef]
- Ghimire, A.; Song, J. Anti-Periprosthetic Infection Strategies: From Implant Surface Topographical Engineering to Smart Drug-Releasing Coatings. ACS Appl. Mater. Interfaces 2021, 13, 20921–20937. [Google Scholar] [CrossRef]
- Tang, H.; Cao, T.; Liang, X.; Wang, A.; Salley, S.O.; McAllister, J.; Ng, K.Y.S. Influence of silicone surface roughness and hydrophobicity on adhesion and colonization of Staphylococcus epidermidis. J. Biomed. Mater. Res. Part A 2009, 88, 454–463. [Google Scholar] [CrossRef]
- Maher, S.; Linklater, D.; Rastin, H.; Liao, S.T.; Martins de Sousa, K.; Lima-Marques, L.; Kingshott, P.; Thissen, H.; Ivanova, E.P.; Losic, D. Advancing of 3D-Printed Titanium Implants with Combined Antibacterial Protection Using Ultrasharp Nanostructured Surface and Gallium-Releasing Agents. ACS Biomater. Sci. Eng. 2022, 8, 314–327. [Google Scholar] [CrossRef]
- Ge, X.; Ren, C.; Ding, Y.; Chen, G.; Lu, X.; Wang, K.; Ren, F.; Yang, M.; Wang, Z.; Li, J.; et al. Micro/nano-structured TiO2 surface with dual-functional antibacterial effects for biomedical applications. Bioact. Mater. 2019, 4, 346–357. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Ghadirinejad, K.; Oskouei, H.R. An Overview on the Tribological Performance of Titanium Alloys with Surface Modifications for Biomedical Applications. Lubricants 2019, 7, 65. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio-Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Jain, S.; Scott Williamson, R.; Roach, M.D. Surface characterization, shear strength, and bioactivity of anodized titanium prepared in mixed-acid electrolytes. Surf. Coat. Technol. 2017, 325, 594–603. [Google Scholar] [CrossRef]
- Maher, S.; Kaur, G.; Lima-Marques, L.; Evdokiou, A.; Losic, D. Engineering of Micro- to Nanostructured 3D-Printed Drug-Releasing Titanium Implants for Enhanced Osseointegration and Localized Delivery of Anticancer Drugs. ACS Appl. Mater. Interfaces 2017, 9, 29562–29570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, Y.; Li, T.; Zhou, Y.; Leeflang, S.; Chen, L.; Wu, C.; Zhou, J.; Huan, Z. 3D printed titanium scaffolds with ordered TiO2 nanotubular surface and mesoporous bioactive glass for bone repair. Prog. Nat. Sci. Mater. Int. 2020, 30, 502–509. [Google Scholar] [CrossRef]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cenedese, P.; Dubot, P. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef]
- He, F.; Li, L.; Chen, L.; Li, F.; Huang, Y. Self-organizing evolution of anodized oxide films on Ti-25Nb-3Mo-2Sn-3Zr alloy and hydrophilicity. Trans. Tianjin Univ. 2014, 20, 97–102. [Google Scholar] [CrossRef]
- Ouyang, P.; Dong, H.; He, X.; Cai, X.; Wang, Y.; Li, J.; Li, H.; Jin, Z. Hydromechanical mechanism behind the effect of pore size of porous titanium scaffolds on osteoblast response and bone ingrowth. Mater. Des. 2019, 183, 108151. [Google Scholar] [CrossRef]
- Lv, J.; Jia, Z.; Li, J.; Wang, Y.; Yang, J.; Xiu, P.; Zhang, K.; Cai, H.; Liu, Z. Electron Beam Melting Fabrication of Porous Ti6Al4V Scaffolds: Cytocompatibility and Osteogenesis. Adv. Eng. Mater. 2015, 17, 1391–1398. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Pan, C.C.; Moeinzadeh, S.; Storaci, H.W.; Guzman, R.A.; Lui, E.; Ueno, M.; Utsunomiya, T.; Zhang, N.; Rhee, C.; et al. Effect of porosity of a functionally-graded scaffold for the treatment of corticosteroid-associated osteonecrosis of the femoral head in rabbits. J. Orthop. Transl. 2021, 28, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Rubshtein, A.P.; Makarova, E.B.; Bliznets, D.G.; Vladimirov, A.B. Properties of biocomposites based on titanium scaffolds with a different porosity. Bull. Mater. Sci. 2017, 40, 453–457. [Google Scholar] [CrossRef]
- Cao, W.; Chen, K.; Xue, D. Highly Ordered TiO2 Nanotube Arrays with Engineered Electrochemical Energy Storage Performances. Materials 2021, 14, 510. [Google Scholar] [CrossRef]
- Atmani, D.; Saoula, N.; Abdi, A.; Azzaz, M.; Wang, Y.; Mohamedi, M. Structural, Morphological, and Electrochemical Corrosion Properties of TiO2. Formed on Ti6Al4V Alloys by Anodization. Cryst. Res. Technol. 2018, 53, 1800138. [Google Scholar] [CrossRef]
- Rosales-Leal, J.I.; Rodríguez-Valverde, M.A.; Mazzaglia, G.; Ramón-Torregrosa, P.J.; Díaz-Rodríguez, L.; García-Martínez, O.; Vallecillo-Capilla, M.; Ruiz, C.; Cabrerizo-Vílchez, M.A. Effect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesion. Colloids Surf. A Physicochem. Eng. Asp. 2010, 365, 222–229. [Google Scholar] [CrossRef]
- Wu, S.; Wang, S.; Liu, W.; Yu, X.; Wang, G.; Chang, Z.; Wen, D. Microstructure and properties of TiO2 nanotube coatings on bone plate surface fabrication by anodic oxidation. Surf. Coat. Technol. 2019, 374, 362–373. [Google Scholar] [CrossRef]
- Kopf, B.S.; Ruch, S.; Berner, S.; Spencer, N.D.; Maniura-Weber, K. The role of nanostructures and hydrophilicity in osseointegration: In-vitro protein-adsorption and blood-interaction studies. J. Biomed. Mater. Res. Part A 2015, 103, 2661–2672. [Google Scholar] [CrossRef]
- Trampuz, A.; Windmer, A.F. Infections associated with orthopedic implants. Nosocom. Hosp. Relat. Infect. 2006, 19, 349–356. [Google Scholar]
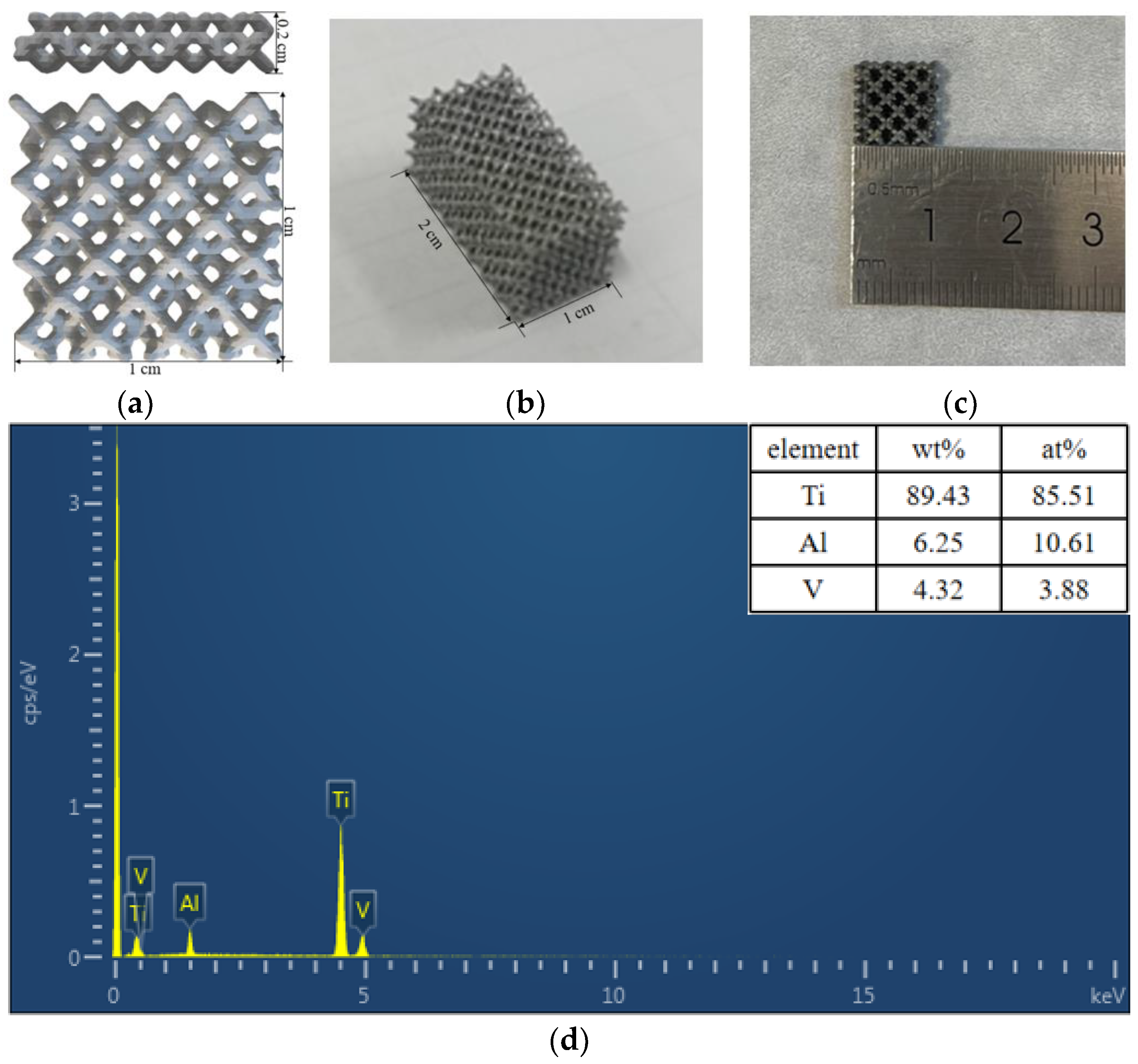

| Samples | 3D-60 | 3D-75 | 3D-85 |
|---|---|---|---|
| Pore sizes (μm) | 353 ± 23 | 563 ± 38 | 915 ± 41 |
| Samples | Max Load KN | Yield Strength MPa | Compressive Strength MPa | Compression Modulus GPa |
|---|---|---|---|---|
| 3D-60 | 11.86 ± 0.54 | 97.79 ± 5.43 | 112.48 ± 5.23 | 2.72 ± 0.2 |
| 3D-75 | 3.6 ± 0.27 | 30.4 ± 1.66 | 33.56 ± 2.25 | 1.16 ± 0.04 |
| 3D-85 | 1.35 ± 0.1 | 10.28 ± 0.74 | 12.37 ± 0.94 | 0.33 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Liu, H.; Li, A.; Liu, T.; Lu, Q.; He, F. Antibacterial Structure Design of Porous Ti6Al4V by 3D Printing and Anodic Oxidation. Materials 2023, 16, 5206. https://doi.org/10.3390/ma16155206
Yang G, Liu H, Li A, Liu T, Lu Q, He F. Antibacterial Structure Design of Porous Ti6Al4V by 3D Printing and Anodic Oxidation. Materials. 2023; 16(15):5206. https://doi.org/10.3390/ma16155206
Chicago/Turabian StyleYang, Guijun, Houjiang Liu, Ang Li, Tiansheng Liu, Qiqin Lu, and Fang He. 2023. "Antibacterial Structure Design of Porous Ti6Al4V by 3D Printing and Anodic Oxidation" Materials 16, no. 15: 5206. https://doi.org/10.3390/ma16155206
APA StyleYang, G., Liu, H., Li, A., Liu, T., Lu, Q., & He, F. (2023). Antibacterial Structure Design of Porous Ti6Al4V by 3D Printing and Anodic Oxidation. Materials, 16(15), 5206. https://doi.org/10.3390/ma16155206







