Nanoparticles in Cancer Diagnosis and Treatment
Abstract
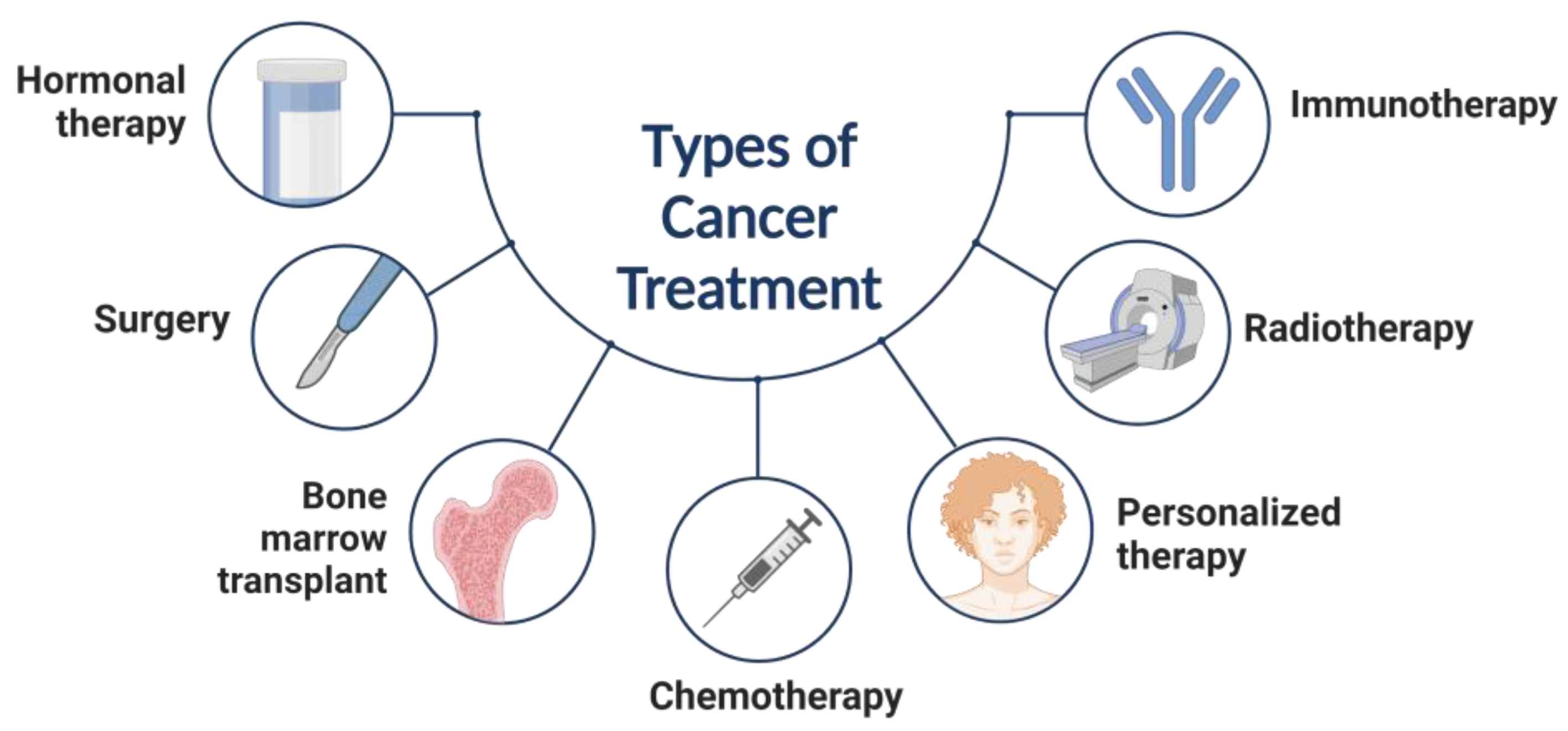
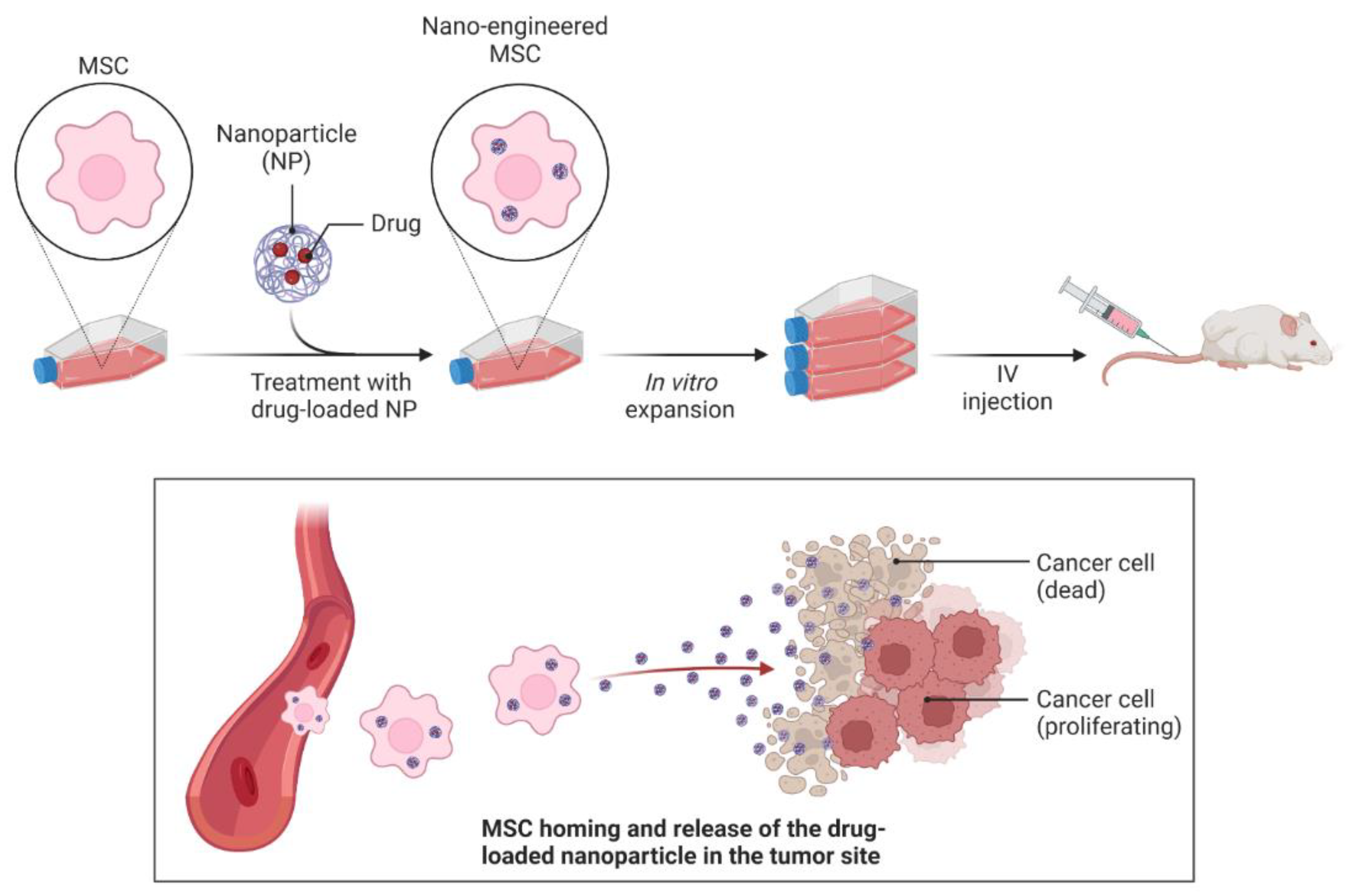
:1. Introduction
Current State of Research on Metal Nanoparticles for Cancer Diagnosis and Treatment
2. Role of Nanoparticles for Cancer, Biomedical Properties, and Therapeutics
3. Metal Nanoparticles and Their Application in Cancer
3.1. Gold-Based Nanoparticles
Photoacoustic Imaging
3.2. Silver-Based Nanoparticles
3.3. Palladium-Based Nanoparticles
3.4. Iron Oxide-Based Nanoparticles
Super Paramagnetic Iron Oxide Nanoparticles for Cancer Treatment
3.5. Copper-Based Nanoparticles
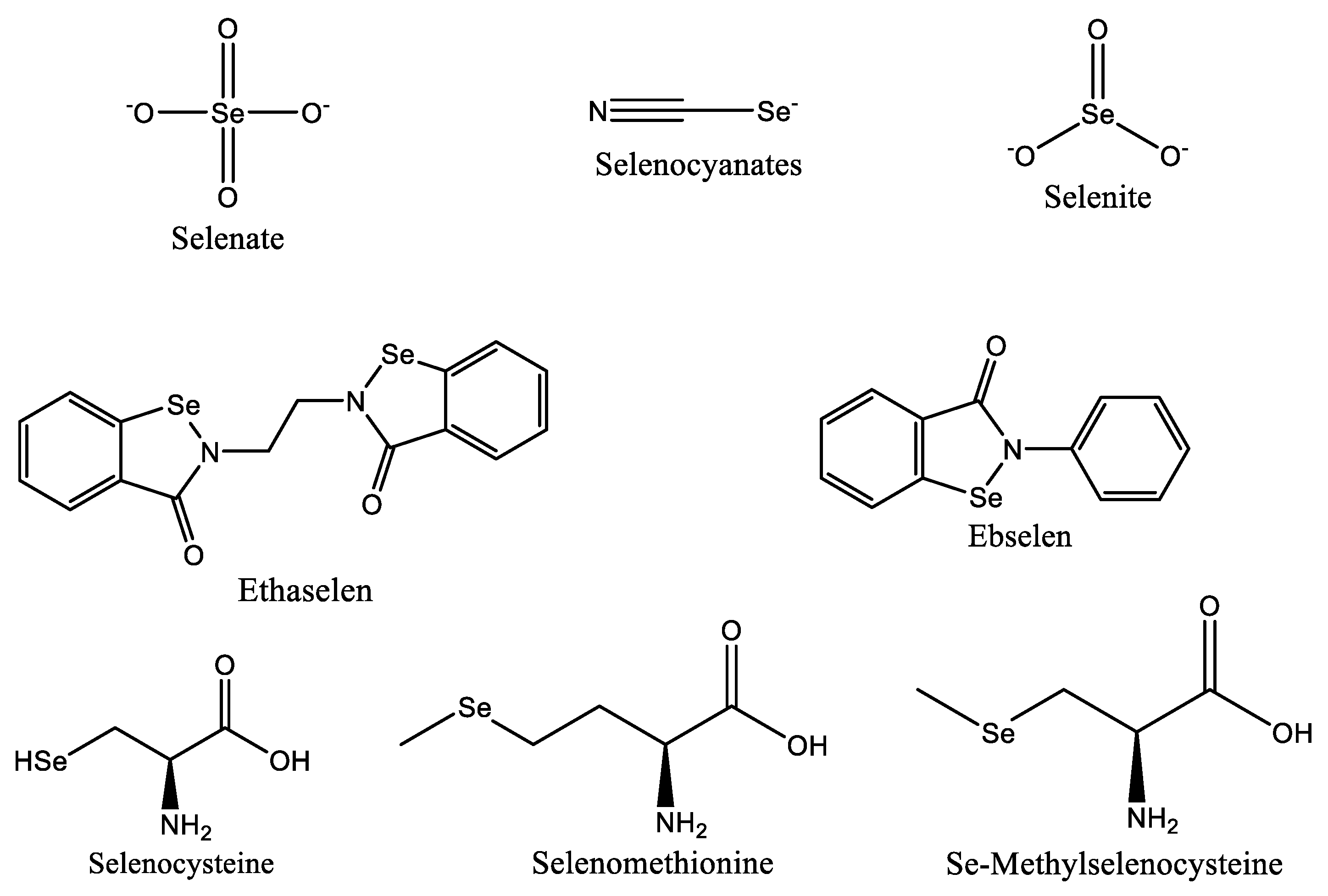
3.6. Selenium-Based Nanoparticles
4. Nanoparticles for Medical Imaging
5. Targeting of Cancer Cells by Metallic Nanoparticles
- The availability of specific targets first bullet;
- Ligands for these targets;
- Techniques for delivering the drug to its target via various delivery systems conjugated to the ligands.
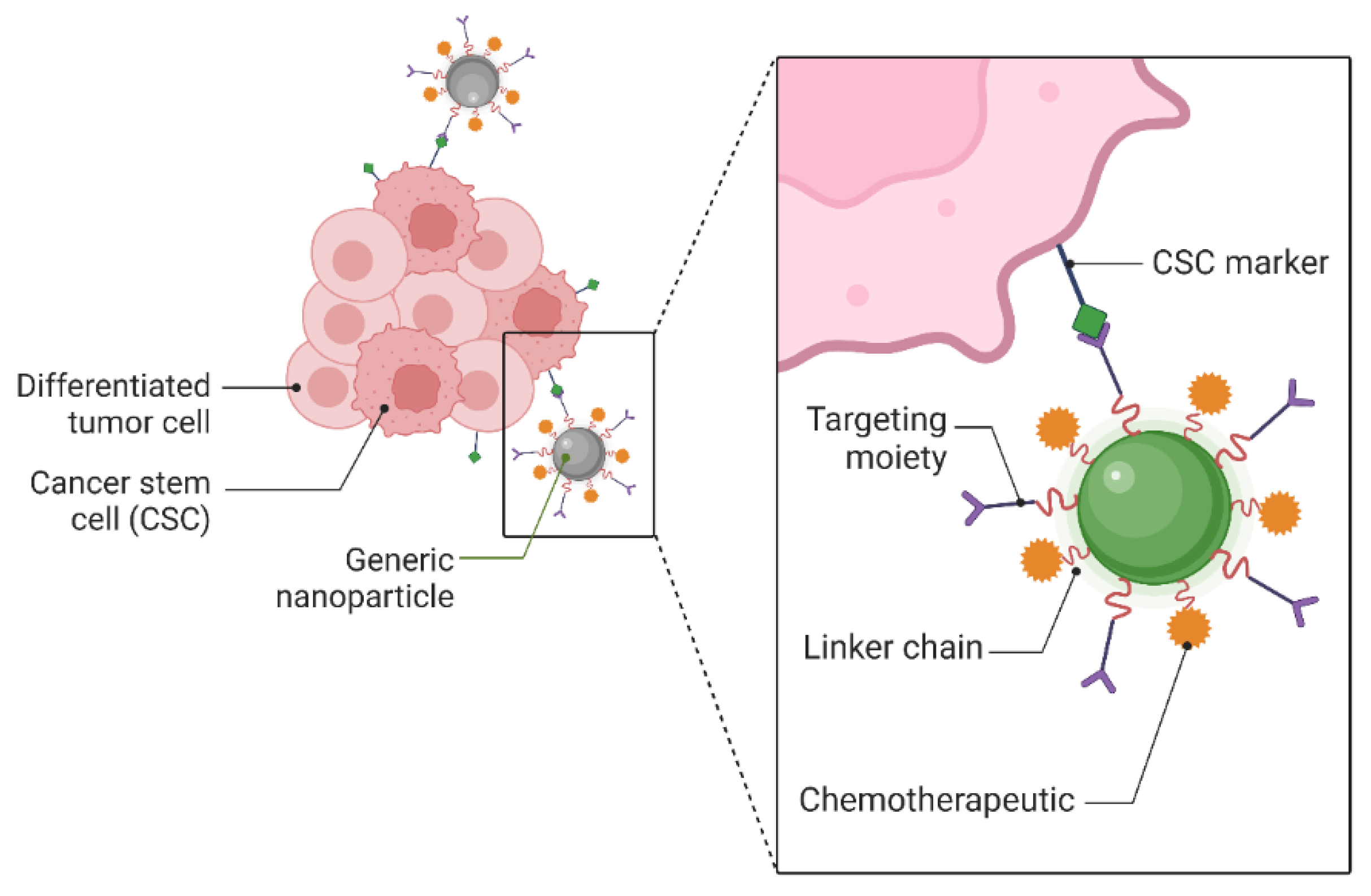
5.1. Targeting Cancer Stem Cells with Nanomaterials
5.2. Targeted Drug Delivery
5.3. Metal Nanoparticle Mediated Cryosurgery
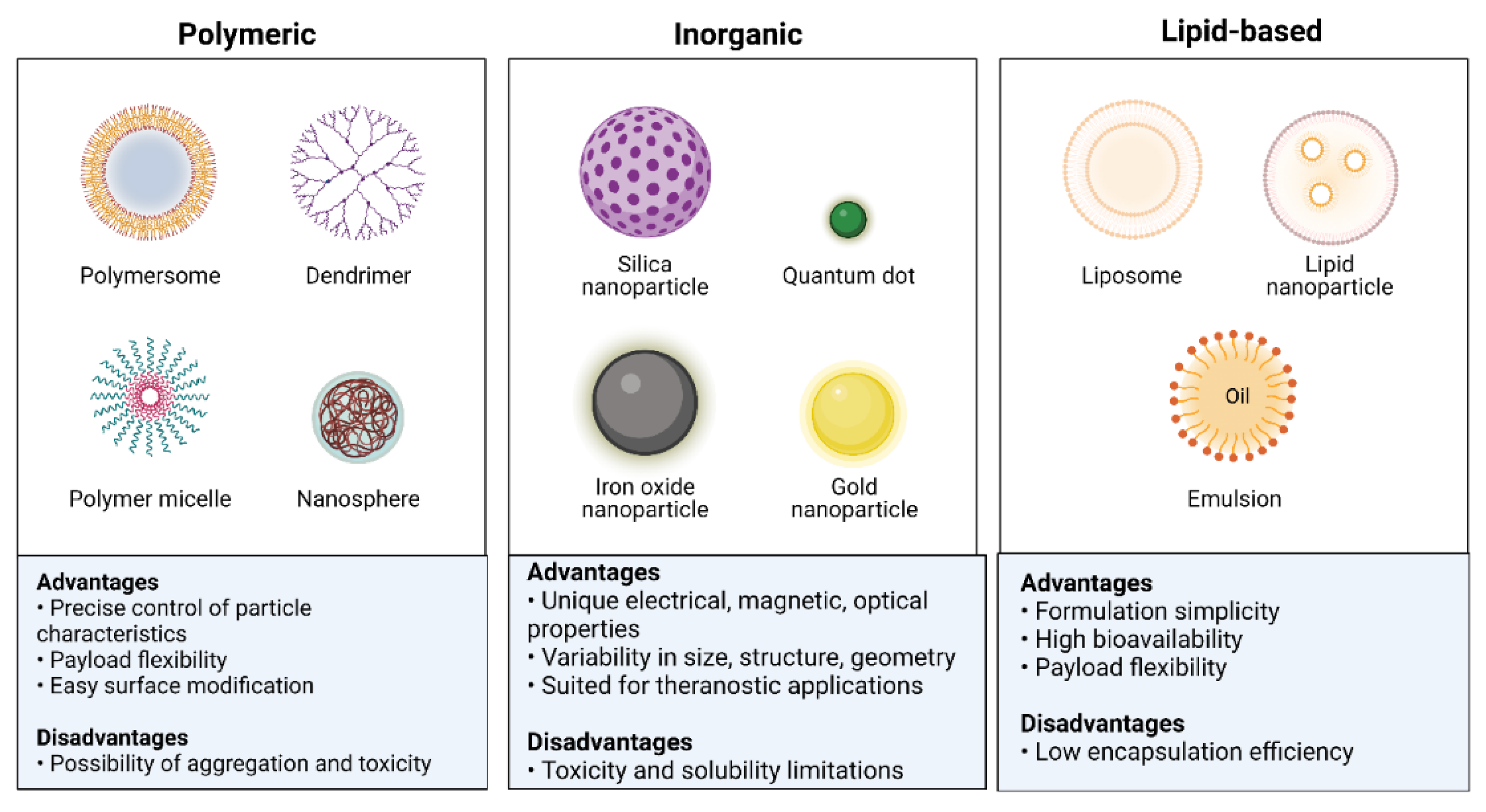
6. Types and Biological Properties of Nanoparticle Delivery Platforms
7. Current Limitations of Metal Nanoparticles and the Challenges
8. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinardell, M.; Mitjans, M. Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef] [Green Version]
- Conde, J.; Doria, G.; Baptista, P. Noble Metal Nanoparticles Applications in Cancer. J. Drug Deliv. 2012, 2012, 751075. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Centre for Traditional Medicine. Available online: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine (accessed on 15 May 2022).
- Sutradhar, K.B.; Amin, M.L. Nanotechnology in Cancer Drug Delivery and Selective Targeting. ISRN Nanotechnol. 2014, 2014, 939378. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Rodriguez, L.B. Molecular targeting of liposomal nanoparticles to tumor microenvironment. Int. J. Nanomed. 2012, 8, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.T. Targeted Nanoparticles for Cancer Therapy: Promises and Challenges. J. Nanomed. Nanotechnol. 2011, 2, 1000103e. [Google Scholar] [CrossRef] [Green Version]
- Coates, A.; Abraham, S.; Kaye, S.B.; Sowerbutts, T.; Frewin, C.; Fox, R.M.; Tattersall, M.H.N. On the receiving end—Patient perception of the side-effects of cancer chemotherapy. Eur. J. Cancer Clin. Oncol. 1983, 19, 203–208. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2017, 26, 617–632. [Google Scholar] [CrossRef]
- Ho, B.N.; Pfeffer, C.M.; Singh, A.T. Update on Nanotechnology-based Drug Delivery Systems in Cancer Treatment. Anticancer Res. 2017, 37, 5975–5981. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Pandey, R.; Varma, A.; Barman, I. Polymer-based nanoparticles for drug delivery systems and cancer therapeutics. In Natural Polymers for Drug Delivery; CABI: Wallingford, UK, 2017; pp. 53–70. [Google Scholar] [CrossRef]
- Ficai, D.; Ficai, A. New Challenges in Cancer Treatment, from Novel Agents to Innovative Administration. Anti-Cancer Agents Med. Chem. 2019, 19, 4–5. [Google Scholar] [CrossRef]
- Kumar, A.; Gatto, G.; Delogu, F.; Pilia, L. DFT study of [Pt(Cl)2L] complex (L = rubeanic acid) and its derived compounds with DNA purine bases. Chem. Phys. 2020, 530, 110646. [Google Scholar] [CrossRef]
- Sack, M.; Alili, L.; Karaman, E.; Das, S.; Gupta, A.; Seal, S.; Brenneisen, P. Combination of Conventional Chemotherapeutics with Redox-Active Cerium Oxide Nanoparticles—A Novel Aspect in Cancer Therapy. Mol. Cancer Ther. 2014, 13, 1740–1749. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Singh, S.P.; Chinde, S.; Rahman, M.F.; Mahboob, M.; Grover, P. Toxicity Study of Cerium Oxide Nanoparticles in Human Neuroblastoma Cells. Int. J. Toxicol. 2014, 33, 86–97. [Google Scholar] [CrossRef]
- Sreena, R.; Nathanael, A.J. Biodegradable Biopolymeric Nanoparticles for Biomedical Applications-Challenges and Future Outlook. Materials 2023, 16, 2364. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 20–31. [Google Scholar] [CrossRef]
- Balogh, L.P. Nano-Enabled Medical Applications; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Florence, A.T. “Targeting” nanoparticles: The constraints of physical laws and physical barriers. J. Control. Release 2012, 164, 115–124. [Google Scholar] [CrossRef]
- Du, K.; Feng, J.; Gao, X.; Zhang, H. Nanocomposites based on lanthanide-doped upconversion nanoparticles: Diverse designs and applications. Light Sci. Appl. 2022, 11, 222. [Google Scholar] [CrossRef]
- Yang, L.; Jia, J.; Li, S. Advances in the Application of Exosomes Identification Using Surface-Enhanced Raman Spectroscopy for the Early Detection of Cancers. Front. Bioeng. Biotechnol. 2022, 9, 808933. [Google Scholar] [CrossRef]
- He, H.; Zou, Z.; Wang, B.; Xu, G.; Chen, C.; Qin, X.; Yu, C.; Zhang, J. Copper Oxide Nanoparticles Induce Oxidative DNA Damage and Cell Death via Copper Ion-Mediated P38 MAPK Activation in Vascular Endothelial Cells. Int. J. Nanomed. 2020, 15, 3291–3302. [Google Scholar] [CrossRef]
- Kumar, S.; Mongia, A.; Gulati, S.; Singh, P.; Diwan, A.; Shukla, S. Emerging theranostic gold nanostructures to combat cancer: Novel probes for Combinatorial Immunotherapy and Photothermal Therapy. Cancer Treat. Res. Commun. 2020, 25, 100258. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20–37. [Google Scholar] [CrossRef] [Green Version]
- Dessale, M.; Mengistu, G.; Mengist, H.M. Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis. Int. J. Nanomed. 2022, 17, 3735–3749. [Google Scholar] [CrossRef]
- Machado, S.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Utilization of food industry wastes for the production of zero-valent iron nanoparticles. Sci. Total Environ. 2014, 496, 233–240. [Google Scholar] [CrossRef]
- Yang, Q.; Jones, S.W.; Parker, C.L.; Zamboni, W.C.; Bear, J.E.; Lai, S.K. Evading Immune Cell Uptake and Clearance Requires PEG Grafting at Densities Substantially Exceeding the Minimum for Brush Conformation. Mol. Pharm. 2014, 11, 1250–1258. [Google Scholar] [CrossRef]
- Zeineldin, R.; Syoufjy, J. Cancer Nanotechnology: Opportunities for Prevention, Diagnosis, and Therapy. In Cancer Nanotechnology: Methods and Protocols; Humana Press: New York, NY, USA, 2017; pp. 3–12. [Google Scholar] [CrossRef]
- Jani, P.; Subramanian, S.; Korde, A.; Rathod, L.; Sawant, K.K. Theranostic Nanocarriers in Cancer: Dual Capabilities on a Single Platform. In Functional Bionanomaterials: From Biomolecules to Nanoparticles; Springer: Cham, Switzerland, 2020; pp. 293–312. [Google Scholar] [CrossRef]
- Nikolova, M.; Slavchov, R.; Nikolova, G. Nanotechnology in Medicine. In Drug Discovery and Evaluation: Methods in Clinical Pharmacology; Springer: Cham, Switzerland, 2020; pp. 533–546. [Google Scholar] [CrossRef]
- Saxena, S.K.; Nyodu, R.; Kumar, S.; Maurya, V.K. Current Advances in Nanotechnology and Medicine. In NanoBioMedicine; Springer: Singapore, 2020; pp. 3–16. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bhattacharyya, S. Nanotechnology in Medicine. In Biotechnology Business—Concept Delivery; Springer: Cham, Switzerland, 2020; pp. 57–64. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 013001. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, P.; Vinhas, R.; Fernandes, A.; Baptista, P. Gold Nanotheranostics: Proof-of-Concept or Clinical Tool? Nanomaterials 2015, 5, 1853–1879. [Google Scholar] [CrossRef] [Green Version]
- De, J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Barar, J.; Omidi, Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts 2013, 3, 149–162. [Google Scholar] [CrossRef]
- Omidi, Y.; Barar, J. Targeting tumor microenvironment: Crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014, 4, 55–67. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [Green Version]
- Verissimo, T.V.; Santos, N.T.; Silva, J.R.; Azevedo, R.B.; Gomes, A.J.; Lunardi, C.N. In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater. Sci. Eng. C 2016, 65, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015, 8, 1771–1799. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, X.; Liang, X.-J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [Green Version]
- Mantri, Y.; Davidi, B.; Lemaster, J.E.; Hariri, A.; Jokerst, J.V. Iodide-doped precious metal nanoparticles: Measuring oxidative stress in vivo via photoacoustic imaging. Nanoscale 2020, 12, 10511–10520. [Google Scholar] [CrossRef]
- Vlăsceanu, G.M.; Marin, Ş.; Ţiplea, R.E.; Bucur, I.R.; Lemnaru, M.; Marin, M.M.; Grumezescu, A.M.; Andronescu, E. Silver nanoparticles in cancer therapy. In Nanobiomaterials in Cancer Therapy; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 29–56. [Google Scholar] [CrossRef]
- Kent, R.D.; Vikesland, P.J. Controlled Evaluation of Silver Nanoparticle Dissolution Using Atomic Force Microscopy. Environ. Sci. Technol. 2012, 46, 6977–6984. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, E.; Milani, M.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A.; Tayefi Nasrabadi, H.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2014, 42, 173–180. [Google Scholar] [CrossRef]
- Nedelcu, I.-A.; Ficai, A.; Sonmez, M.; Ficai, D.; Oprea, O.; Andronescu, E. Silver Based Materials for Biomedical Applications. Curr. Org. Chem. 2014, 18, 173–184. [Google Scholar] [CrossRef]
- Qureshi, A.T.; Monroe, W.T.; Dasa, V.; Gimble, J.M.; Hayes, D.J. miR-148b–Nanoparticle conjugates for light mediated osteogenesis of human adipose stromal/stem cells. Biomaterials 2013, 34, 7799–7810. [Google Scholar] [CrossRef]
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Wu, B.; Yang, J.; Zheng, N. Small Adsorbate-Assisted Shape Control of Pd and Pt Nanocrystals. Adv. Mater. 2012, 24, 862–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, S.; Wei, J.; Chen, M.; Zheng, N. Two-dimensional Pd-based nanomaterials for bioapplications. Sci. Bull. 2017, 62, 579–588. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2010, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, S.; He, C.; Mo, S.; Wang, X.; Liu, G.; Zheng, N. Safety profile of two-dimensional Pd nanosheets for photothermal therapy and photoacoustic imaging. Nano Res. 2016, 10, 1234–1248. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Fang, W.; Tang, S.; Liu, P.; Fang, X.; Gong, J.; Zheng, N. Pd Nanosheet-Covered Hollow Mesoporous Silica Nanoparticles as a Platform for the Chemo-Photothermal Treatment of Cancer Cells. Small 2012, 8, 3816–3822. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Yang, J.; Tan, Y.; Zheng, N. Etching Growth under Surface Confinement: An Effective Strategy to Prepare Mesocrystalline Pd Nanocorolla. J. Am. Chem. Soc. 2011, 133, 15946–15949. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Liu, B.; Ren, B.; Zheng, N. Enhancing the Photothermal Stability of Plasmonic Metal Nanoplates by a Core-Shell Architecture. Adv. Mater. 2011, 23, 3420–3425. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Tang, S.; Guo, Z.; Wang, X.; Mo, S.; Huang, X.; Liu, G.; Zheng, N. Core-Shell Pd@Au Nanoplates as Theranostic Agents for In-Vivo Photoacoustic Imaging, CT Imaging, and Photothermal Therapy. Adv. Mater. 2014, 26, 8210–8216. [Google Scholar] [CrossRef]
- Tang, S.; Huang, X.; Zheng, N. Silica coating improves the efficacy of Pd nanosheets for photothermal therapy of cancer cells using near infrared laser. Chem. Commun. 2011, 47, 3948–3950. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, J.; Sun, D.; Li, Q.; Ma, J.; Chen, X.; Zhu, X.; Zheng, N. A Novel Theranostic Nanoplatform Based on Pd@Pt-PEG-Ce6 for Enhanced Photodynamic Therapy by Modulating Tumor Hypoxia Microenvironment. Adv. Funct. Mater. 2018, 28, 1706310. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, X.; Zhao, Z.; Fang, W.; Chen, M.; Huang, Y.; Chen, X. Photothermally enhanced photodynamic therapy based on mesoporous Pd@Ag@mSiO2 nanocarriers. J. Mater. Chem. B 2013, 1, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shi, S.; Huang, Y.; Tang, S.; Chen, X. Simultaneous Photodynamic and Photothermal Therapy Using Photosensitizer-Functionalized Pd Nanosheets by Single Continuous Wave Laser. ACS Appl. Mater. Interfaces 2014, 6, 8878–8885. [Google Scholar] [CrossRef]
- Li, S.; Gu, K.; Wang, H.; Xu, B.; Li, H.; Shi, X.; Huang, Z.; Liu, H. Degradable Holey Palladium Nanosheets with Highly Active 1D Nanoholes for Synergetic Phototherapy of Hypoxic Tumors. J. Am. Chem. Soc. 2020, 142, 5649–5656. [Google Scholar] [CrossRef]
- Sun, D.; Huang, Y.; Zhang, X.; Peng, J.; Li, J.; Ming, J.; Wei, J.; Chen, X.; Zheng, N. A Pd corolla–human serum albumin–indocyanine green nanocomposite for photothermal/photodynamic combination therapy of cancer. J. Mater. Chem. B 2018, 6, 6969–6976. [Google Scholar] [CrossRef]
- Gil, Y.-G.; Kang, S.; Chae, A.; Kim, Y.-K.; Min, D.-H.; Jang, H. Synthesis of porous Pd nanoparticles by therapeutic chaga extract for highly efficient tri-modal cancer treatment. Nanoscale 2018, 10, 19810–19817. [Google Scholar] [CrossRef]
- Tang, S.; Chen, M.; Zheng, N. Multifunctional ultrasmall Pd nanosheets for enhanced near-infrared photothermal therapy and chemotherapy of cancer. Nano Res. 2014, 8, 165–174. [Google Scholar] [CrossRef]
- Shi, S.; Chen, X.; Wei, J.; Huang, Y.; Weng, J.; Zheng, N. Platinum(iv) prodrug conjugated Pd@Au nanoplates for chemotherapy and photothermal therapy. Nanoscale 2016, 8, 5706–5713. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Xu, T.; Xu, M.; Wen, Y.; Liu, Y.; Liu, J.; Qin, X. Targeted hexagonal Pd nanosheet combination therapy for rheumatoid arthritis via the photothermal controlled release of MTX. J. Mater. Chem. B 2019, 7, 112–122. [Google Scholar] [CrossRef]
- Song, M.; Liu, N.; He, L.; Liu, G.; Ling, D.; Su, X.; Sun, X. Porous hollow palladium nanoplatform for imaging-guided trimodal chemo-, photothermal-, and radiotherapy. Nano Res. 2018, 11, 2796–2808. [Google Scholar] [CrossRef]
- Chen, M.; Guo, Z.; Chen, Q.; Wei, J.; Li, J.; Shi, C.; Xu, D.; Zhou, D.; Zhang, X.; Zheng, N. Pd nanosheets with their surface coordinated by radioactive iodide as a high-performance theranostic nanoagent for orthotopic hepatocellular carcinoma imaging and cancer therapy. Chem. Sci. 2018, 9, 4268–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Chen, M.; Peng, C.; Mo, S.; Shi, C.; Fu, G.; Wen, X.; Zhuang, R.; Su, X.; Liu, T.; et al. pH-sensitive radiolabeled and superfluorinated ultra-small palladium nanosheet as a high-performance multimodal platform for tumor theranostics. Biomaterials 2018, 179, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.L.; Medintz, I.L.; Jewell, C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today 2019, 27, 73–98. [Google Scholar] [CrossRef]
- Zhao, P.; Jin, Z.; Chen, Q.; Yang, T.; Chen, D.; Meng, J.; Lu, X.; Gu, Z.; He, Q. Local generation of hydrogen for enhanced photothermal therapy. Nat. Commun. 2018, 9, 4241. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Wang, Y.S.; Jin, Z.; Zhao, P.; Zhang, H.; Wen, Y.; He, Q. Porphyrin–palladium hydride MOF nanoparticles for tumor-targeting photoacoustic imaging-guided hydrogenothermal cancer therapy. Nanoscale Horiz. 2019, 4, 1185–1193. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjug. Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Muthuraman, P.; Sreekanth, T.V.M.; Kim, D.H.; Shim, J. Green synthesis: In-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem. 2017, 10, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A. Copper Nanomaterials as Drug Delivery System against Infectious Agents and Cancerous Cells. J. Appl. Life Sci. Int. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef] [PubMed]
- El-Bayoumy, K.; Sinha, R. Molecular chemoprevention by selenium: A genomic approach. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 591, 224–236. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium Compounds, Apoptosis and Other Types of Cell Death: An Overview for Cancer Therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Jing, F.; Fu, X.; Li, S.; Li, B.; Zhao, J.; Wang, X.; Liu, Y.; Chen, B. Synthesis and in Vitro Antiproliferative Evaluation of Novel Hybrids from 1,3,4-Thiadiazole and Benzisoselenazolone. Chem. Pharm. Bull. 2015, 63, 431–437. [Google Scholar] [CrossRef] [Green Version]
- El-Bayoumy, K. The protective role of selenium on genetic damage and on cancer. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 475, 123–139. [Google Scholar] [CrossRef]
- Massoud, T.F.; Gambhir, S.S. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Gene Dev 2003, 17, 545–580. [Google Scholar] [CrossRef] [Green Version]
- European Society of Radiology. Medical imaging in personalised medicine: A white paper of the research committee of the European Society of Radiology (ESR). Insights Imaging 2011, 2, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials 2022, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Sun, Z.; Rao, W.; Liu, J. Nanoparticle-mediated cryosurgery for tumor therapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 493–506. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, L.; Chen, K.; Zhang, W.; Zhang, Q.; Li, Q.; Hu, K. Nanoparticles: A New Approach to Upgrade Cancer Diagnosis and Treatment. Nanoscale Res. Lett. 2021, 16, 88. [Google Scholar] [CrossRef]
- Huang, H.; Dong, Y.; Zhang, Y.; Ru, D.; Wu, Z.; Zhang, J.; Shen, M.; Duan, Y.; Sun, Y. GSH-sensitive Pt(IV) prodrug-loaded phase-transitional nanoparticles with a hybrid lipid-polymer shell for precise theranostics against ovarian cancer. Theranostics 2019, 9, 1047–1065. [Google Scholar] [CrossRef]
- Lai, J.; Wang, T.; Wang, H.; Shi, F.; Gu, W.; Ye, L. MnO nanoparticles with unique excitation-dependent fluorescence for multicolor cellular imaging and MR imaging of brain glioma. Microchim. Acta 2018, 185, 244. [Google Scholar] [CrossRef]
- Wang, D.; Lin, H.; Zhang, G.; Si, Y.; Yang, H.; Bai, G.; Yang, C.; Zhong, K.; Cai, D.; Wu, Z.; et al. Effective pH-Activated Theranostic Platform for Synchronous Magnetic Resonance Imaging Diagnosis and Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 31114–31123. [Google Scholar] [CrossRef]
- Jiang, W.; Fang, H.; Liu, F.; Zhou, X.; Zhao, H.; He, X.; Guo, D. PEG-coated and Gd-loaded fluorescent silica nanoparticles for targeted prostate cancer magnetic resonance imaging and fluorescence imaging. Int. J. Nanomed. 2019, 14, 5611–5622. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, S.; Sun, J.; Zhu, S.; Chen, C.; Xie, W.; Zheng, J.; Zhu, Y.; Xiao, L.; Hao, L.; et al. Folate-Targeted and Oxygen/Indocyanine Green-Loaded Lipid Nanoparticles for Dual-Mode Imaging and Photo-sonodynamic/Photothermal Therapy of Ovarian Cancer in Vitro and in Vivo. Mol. Pharm. 2019, 16, 4104–4120. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makela, A.V.; Gaudet, J.M.; Schott, M.A.; Sehl, O.C.; Contag, C.H.; Foster, P.J. Magnetic Particle Imaging of Macrophages Associated with Cancer: Filling the Voids Left by Iron-Based Magnetic Resonance Imaging. Mol. Imaging Biol. 2020, 22, 958–968. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, T.; Zhao, E.; Docter, D.; Yang, W.; Stauber, R.H.; Gao, M. Small is Smarter: Nano MRI Contrast Agents—Advantages and Recent Achievements. Small 2016, 12, 556–576. [Google Scholar] [CrossRef]
- Busquets, M.A.; Estelrich, J.; Sánchez-Martín, M.J. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [Green Version]
- Boyle, G.E.; Ahern, M.; Cooke, J.; Sheehy, N.P.; Meaney, J.F. An Interactive Taxonomy of MR Imaging Sequences. RadioGraphics 2006, 26, e24. [Google Scholar] [CrossRef] [PubMed]
- Recommendations for Cross-Sectional Imaging in Cancer Management, Third Edition. Available online: https://www.rcr.ac.uk/publication/recommendations-cross-sectional-imaging-cancer-management-third-edition (accessed on 12 December 2022).
- Murphy, K.J.; Brunberg, J.A.; Cohan, R.H. Adverse reactions to gadolinium contrast media: A review of 36 cases. Am. J. Roentgenol. 1996, 167, 847–849. [Google Scholar] [CrossRef] [Green Version]
- Rhee, C.M. Association Between Iodinated Contrast Media Exposure and Incident Hyperthyroidism and Hypothyroidism. Arch. Intern. Med. 2012, 172, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Lee, G.-D.; Park, S.S.; Ju, C.-S.; Lim, K.T.; Hong, S.-S. Synthesis of TiO2/SiO2 nanoparticles in a water-in-carbon-dioxide microemulsion and their photocatalytic activity. Res. Chem. Intermed. 2005, 31, 379–389. [Google Scholar] [CrossRef]
- Blasiak, B.; van Veggel, F.C.J.M.; Tomanek, B. Applications of Nanoparticles for MRI Cancer Diagnosis and Therapy. J. Nanomater. 2013, 2013, 48578. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Duan, W.; Sahoo, S.K. Multifunctional nanoparticle–EpCAM aptamer bioconjugates: A paradigm for targeted drug delivery and imaging in cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 379–389. [Google Scholar] [CrossRef]
- Jiang, Y.-W.; Gao, G.; Jia, H.-R.; Zhang, X.; Zhao, J.; Ma, N.; Liu, J.-B.; Liu, P.; Wu, F.-G. Copper Oxide Nanoparticles Induce Enhanced Radiosensitizing Effect via Destructive Autophagy. ACS Biomater. Sci. Eng. 2019, 5, 1569–1579. [Google Scholar] [CrossRef]
- Wan, C.; Tai, J.; Zhang, J.; Guo, Y.; Zhu, Q.; Ling, D.; Gu, F.; Gan, J.; Zhu, C.; Wang, Y.; et al. Silver nanoparticles selectively induce human oncogenic γ-herpesvirus-related cancer cell death through reactivating viral lytic replication. Cell Death Dis. 2019, 10, 932. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.U.; Kim, W.H.; Lee, H.S.; Park, K.H.; Sim, S.J. Quantitative and Specific Detection of Exosomal miRNAs for Accurate Diagnosis of Breast Cancer Using a Surface-Enhanced Raman Scattering Sensor Based on Plasmonic Head-Flocked Gold Nanopillars. Small 2019, 15, e1804968. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Pal, R.; Kumar, A.; Misra, G. Exploring TEAD2 as a drug target for therapeutic intervention of cancer: A multi-computational case study. Brief. Bioinf. 2021, 22, bbab007. [Google Scholar] [CrossRef]
- Ammarah, U.; Kumar, A.; Pal, R.; Bal, N.C.; Misra, G. Identification of new inhibitors against human Great wall kinase using in silico approaches. Sci. Rep. 2018, 8, 4894. [Google Scholar] [CrossRef] [Green Version]
- Au, J.L.S.; Jang, S.H.; Zheng, J.; Chen, C.T.; Song, S.; Hu, L.; Wientjes, M.G. Determinants of drug delivery and transport to solid tumors. J. Control. Release 2001, 74, 31–46. [Google Scholar] [CrossRef]
- Jain, K.K. Targeted Drug Delivery for Cancer. Technol. Cancer Res. Treat. 2016, 4, 311–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Lyakhovich, A.; Lleonart, M.E. Bypassing Mechanisms of Mitochondria-Mediated Cancer Stem Cells Resistance to Chemo- and Radiotherapy. Oxid. Med. Cell. Longev. 2016, 2016, 1716341. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Minocha, N.; Garg, V.; Dureja, H. Nanostructured materials used in drug delivery. Mater. Today Proc. 2022, 69, 174–180. [Google Scholar] [CrossRef]
- Roy, S.; Roy, S.; Kar, M.; Thakur, S.; Akhter, Y.; Kumar, A.; Delogu, F.; Padhi, S.; Saha, A.; Banerjee, B. p38 MAPK pathway and its interaction with TRF2 in cisplatin induced chemotherapeutic response in head and neck cancer. Oncogenesis 2018, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Roy, S.; Kar, M.; Chakraborty, A.; Kumar, A.; Delogu, F.; Asthana, S.; Hande, M.P.; Banerjee, B. Combined treatment with cisplatin and the tankyrase inhibitor XAV-939 increases cytotoxicity, abrogates cancer-stem-like cell phenotype and increases chemosensitivity of head-and-neck squamous-cell carcinoma cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 846, 503084. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kar, M.; Roy, S.; Padhi, S.; Kumar, A.; Thakur, S.; Akhter, Y.; Gatto, G.; Banerjee, B. Inhibition of CD44 sensitizes cisplatin-resistance and affects Wnt/β-catenin signaling in HNSCC cells. Int. J. Biol. Macromol. 2020, 149, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Asghari, F.; Khademi, R.; Esmaeili Ranjbar, F.; Veisi Malekshahi, Z.; Faridi Majidi, R. Application of Nanotechnology in Targeting of Cancer Stem Cells: A Review. Int. J. Stem Cells 2019, 12, 227–239. [Google Scholar] [CrossRef]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Ma, P.X. Host−Guest Interaction Mediated Polymeric Assemblies: Multifunctional Nanoparticles for Drug and Gene Delivery. ACS Nano 2010, 4, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877. [Google Scholar] [CrossRef] [Green Version]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef]
- Layek, B.; Sadhukha, T.; Panyam, J.; Prabha, S. Nano-Engineered Mesenchymal Stem Cells Increase Therapeutic Efficacy of Anticancer Drug through True Active Tumor Targeting. Mol. Cancer Ther. 2018, 17, 1196–1206. [Google Scholar] [CrossRef] [Green Version]
- Muldrew, K.; Rewcastle, J.; Donnelly, B.J.; Saliken, J.C.; Liang, S.; Goldie, S.; Olson, M.; Baissalov, R.; Sandison, G. Flounder Antifreeze Peptides Increase the Efficacy of Cryosurgery. Cryobiology 2001, 42, 182–189. [Google Scholar] [CrossRef]
- Liu, J.; Deng, Z.-S. Nano-Cryosurgery: Advances and Challenges. J. Nanosci. Nanotechnol. 2009, 9, 4521–4542. [Google Scholar] [CrossRef]
- Di, D.-R.; He, Z.-Z.; Sun, Z.-Q.; Liu, J. A new nano-cryosurgical modality for tumor treatment using biodegradable MgO nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1233–1241. [Google Scholar] [CrossRef]
- Chua, K.J.; Chou, S.K.; Ho, J.C. An analytical study on the thermal effects of cryosurgery on selective cell destruction. J. Biomech. 2007, 40, 100–116. [Google Scholar] [CrossRef]
- Ye, P.; Kong, Y.; Chen, X.; Li, W.; Liu, D.; Xie, Y.; Zhou, Y.; Zou, H.; Chang, Z.; Dai, H.; et al. Fe3O4 nanoparticles and cryoablation enhance ice crystal formation to improve the efficiency of killing breast cancer cells. Oncotarget 2016, 8, 11389–11399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Agarwal, P.; Liang, Y.; Xu, J.; Zhao, G.; Tkaczuk, K.H.R.; Lu, X.; He, X. Enhanced cancer therapy with cold-controlled drug release and photothermal warming enabled by one nanoplatform. Biomaterials 2018, 180, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zou, Y.; Yang, L. Uncertainty and sensitivity analysis of properties of phase change micro/nanoparticles for thermal protection during cryosurgery. Forsch. Ingenieurwes. 2012, 76, 41–50. [Google Scholar] [CrossRef]
- Jelveh, S.; Chithrani, D.B. Gold Nanostructures as a Platform for Combinational Therapy in Future Cancer Therapeutics. Cancers 2011, 3, 1081–1110. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Duncan, R. Polymer therapeutics: Top 10 selling pharmaceuticals—What next? J. Control. Release 2014, 190, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Polymer therapeutics at a crossroads? Finding the path for improved translation in the twenty-first century. J. Drug Target. 2017, 25, 759–780. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Maso, K.; Grigoletto, A.; Vicent, M.J.; Pasut, G. Molecular Platforms for Targeted Drug Delivery. Int. Rev. Cell Mol. Biol. 2019, 346, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Hung, Y.-C.; Liau, I.; Huang, G.S. Assessment of the In Vivo Toxicity of Gold Nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]



| Type of Therapy | Material | Reference |
|---|---|---|
| Photothermal therapy (PTT) | Pd NSs | [55,56] |
| Pd collora | [57] | |
| Pd@Ag nanoplates | [58] | |
| Pd@Au nanoplates | [59] | |
| Pd NS-CO Pd-TAT | [60] | |
| PTT and photodynamic therapy (PDT) | Pd@Pt-PEG-Ce6 | [61] |
| Pd@Ag@mSiO2-Ce6 | [62] | |
| Pd-PEI-Ce6 | [63] | |
| H-Pd NSs | [64] | |
| PLCs-HSA-ICG | [65] | |
| PTT and chemotherapy | Dox-loaded 8dc-Pd NPs | [66] |
| SPNS-DOX | [67] | |
| Pd@Au-PEG-Pt | [68] | |
| HMSS-NH2/DOX@Pd | [69] | |
| PTT and radiation therapy | [131I]PHPdNPs-DOX | [70] |
| 131I-Pd-PEG | [71,72] | |
| PTT and immunotherapy | Pd-CpG | [73] |
| PTT and hydrogen therapy | PdH0.2 nanocubes | [74] |
| PdH-MOF | [75] |
| Nanoparticles | Size (nm) | Targeting Material | Cell Line | Imaging Technology | Ref. |
|---|---|---|---|---|---|
| PLGA-mPEG | 151.1 ± 1.3 | cRGD | SKOV-3 cells | Ultrasound | [95] |
| MnO-TETT | 6.7 ± 1.2 | None | C6 glioma cells | Fluorescence/T1-MRI 1 | [96] |
| Ultra small MnO@mesoporous silica | 30–50 | Dox | HeLa cells | MRI-guided chemotherapy | [97] |
| PEG–coated and Gdloaded flourescent silica | 125.5 ± 9.9 | YPSMA-1 | LNCaP and PC3 prostate cancer cells | MRI/fluorescence imaging | [98] |
| Oxygen/indocyanine green-loaded (OINPs) | 300 | Folate | SKOV3 ovarian cancer cells | Ultrasound/ photoacoustic | [99] |
| NPs | Size (nm) | Thermal Conductivity (W/m K) | Heat Capacity (J/m3 K) | Benefits | Ref. |
|---|---|---|---|---|---|
| Fe3O4 | 8–14 | 7.1 | 3.2 × 106 | More intracellular ice formation, high thermal conductivity | [135] |
| MgO | 50 | 34.3 | 3.2 × 106 | Nontoxic, biodegradable, and few side-effects | [133] |
| HCPN-CG | 103.9 ± 1.5 | None | None | Cold-responsive NP for control drug release and NIR-induced photothermal effect. | [136] |
| PCM | 10–20 | 0.35 | 2.56 × 106 | Health tissue protection | [137] |
| Au | 3 | 297.7 | 2.2 × 106 | Good biological compatibility, high thermal conductivity | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranwal, J.; Barse, B.; Di Petrillo, A.; Gatto, G.; Pilia, L.; Kumar, A. Nanoparticles in Cancer Diagnosis and Treatment. Materials 2023, 16, 5354. https://doi.org/10.3390/ma16155354
Baranwal J, Barse B, Di Petrillo A, Gatto G, Pilia L, Kumar A. Nanoparticles in Cancer Diagnosis and Treatment. Materials. 2023; 16(15):5354. https://doi.org/10.3390/ma16155354
Chicago/Turabian StyleBaranwal, Jaya, Brajesh Barse, Amalia Di Petrillo, Gianluca Gatto, Luca Pilia, and Amit Kumar. 2023. "Nanoparticles in Cancer Diagnosis and Treatment" Materials 16, no. 15: 5354. https://doi.org/10.3390/ma16155354







