Effect of Olive Pit Reinforcement in Polylactic Acid Biocomposites on Environmental Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Conditioning and Characterization of OP
2.2. Manufacture of Biocomposites
2.3. Characterization of the Resulting Biocomposites
2.3.1. Manufacture of Specimens
2.3.2. Mechanical Properties
2.3.3. Structural and Morphological Analysis
2.3.4. Water Absorption
2.3.5. Differential Scanning Calorimetry (DSC)
3. Results
3.1. OP Characterization
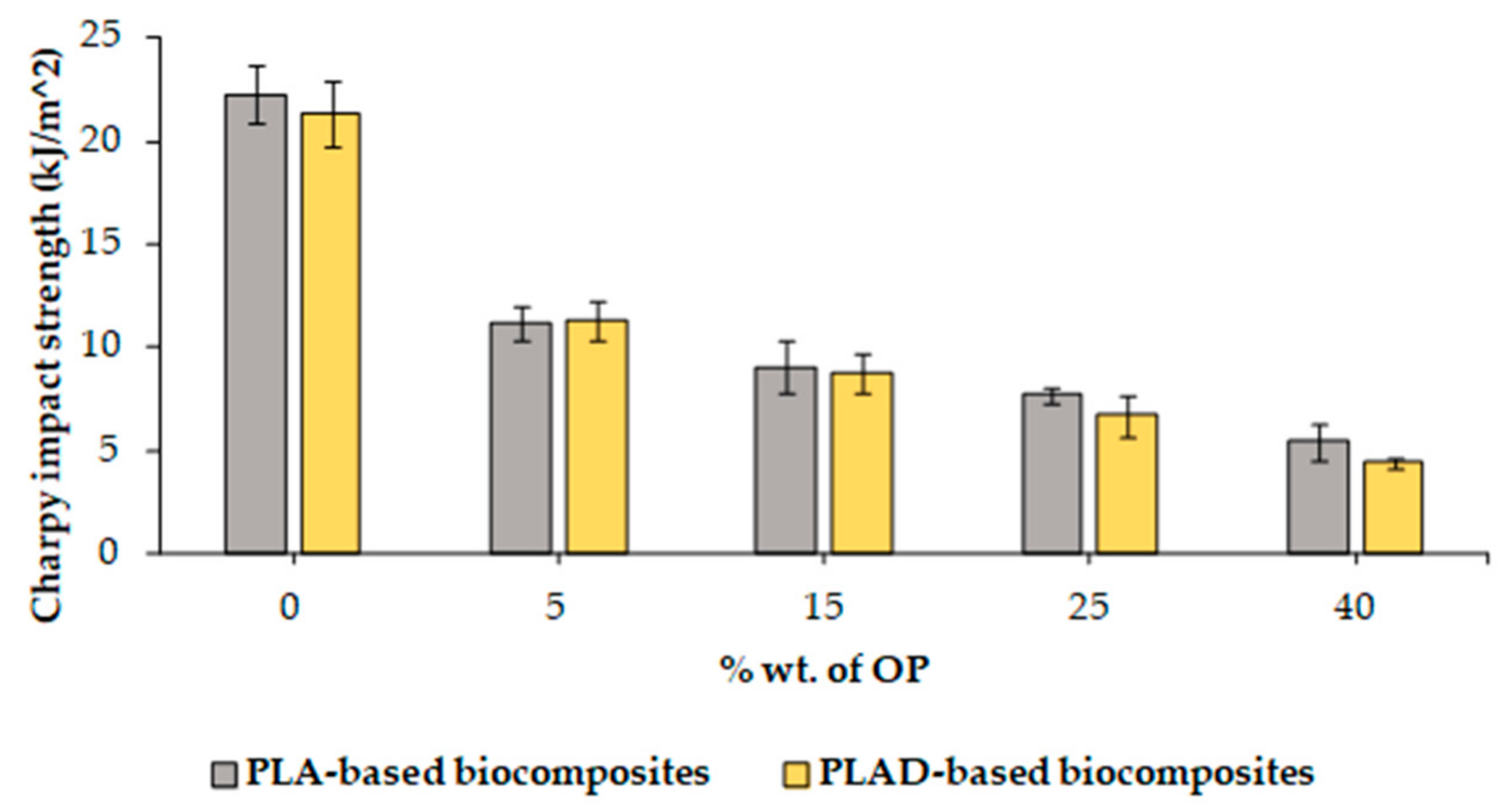
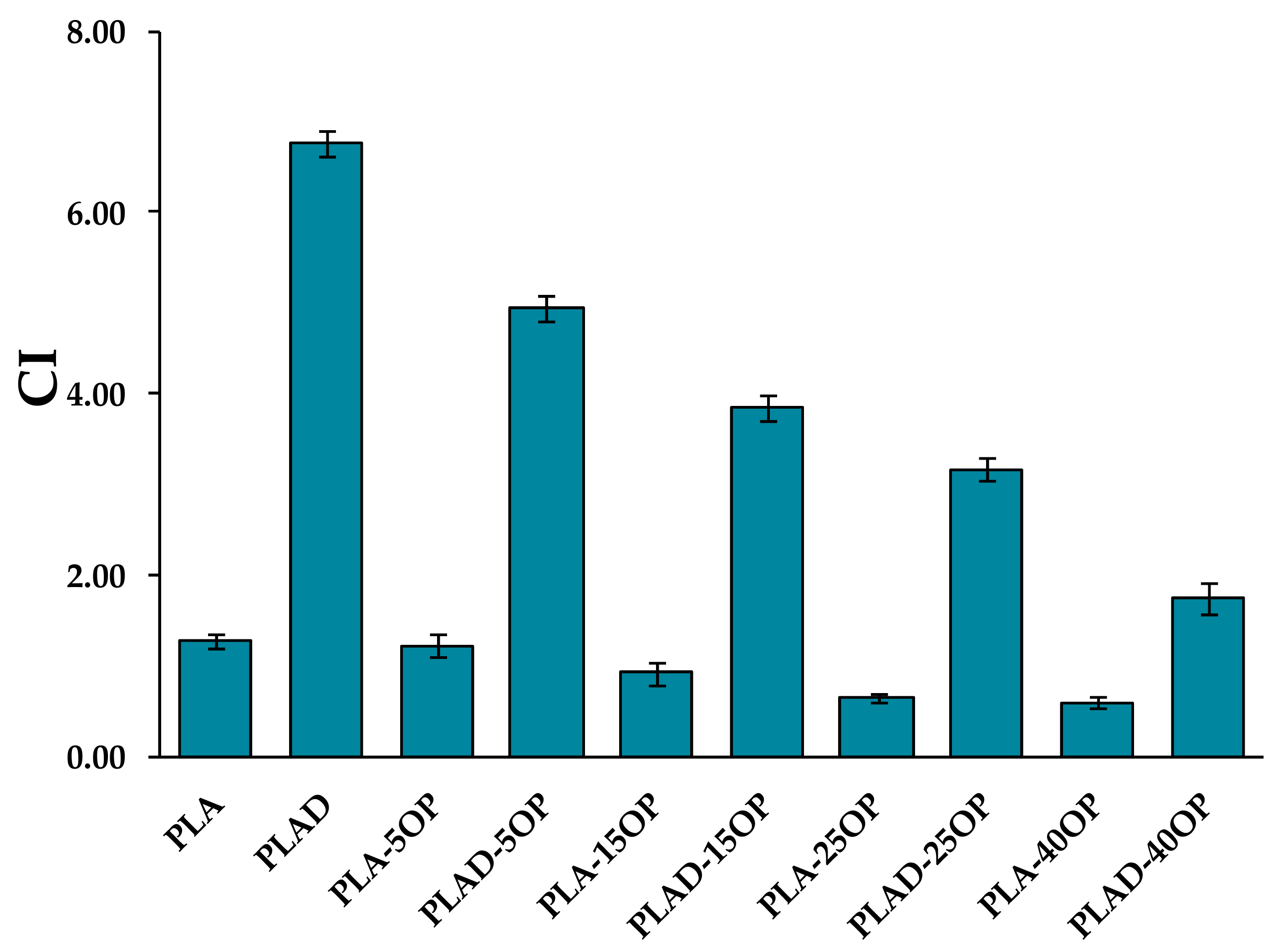
3.2. Mechanical Properties of Biocomposites
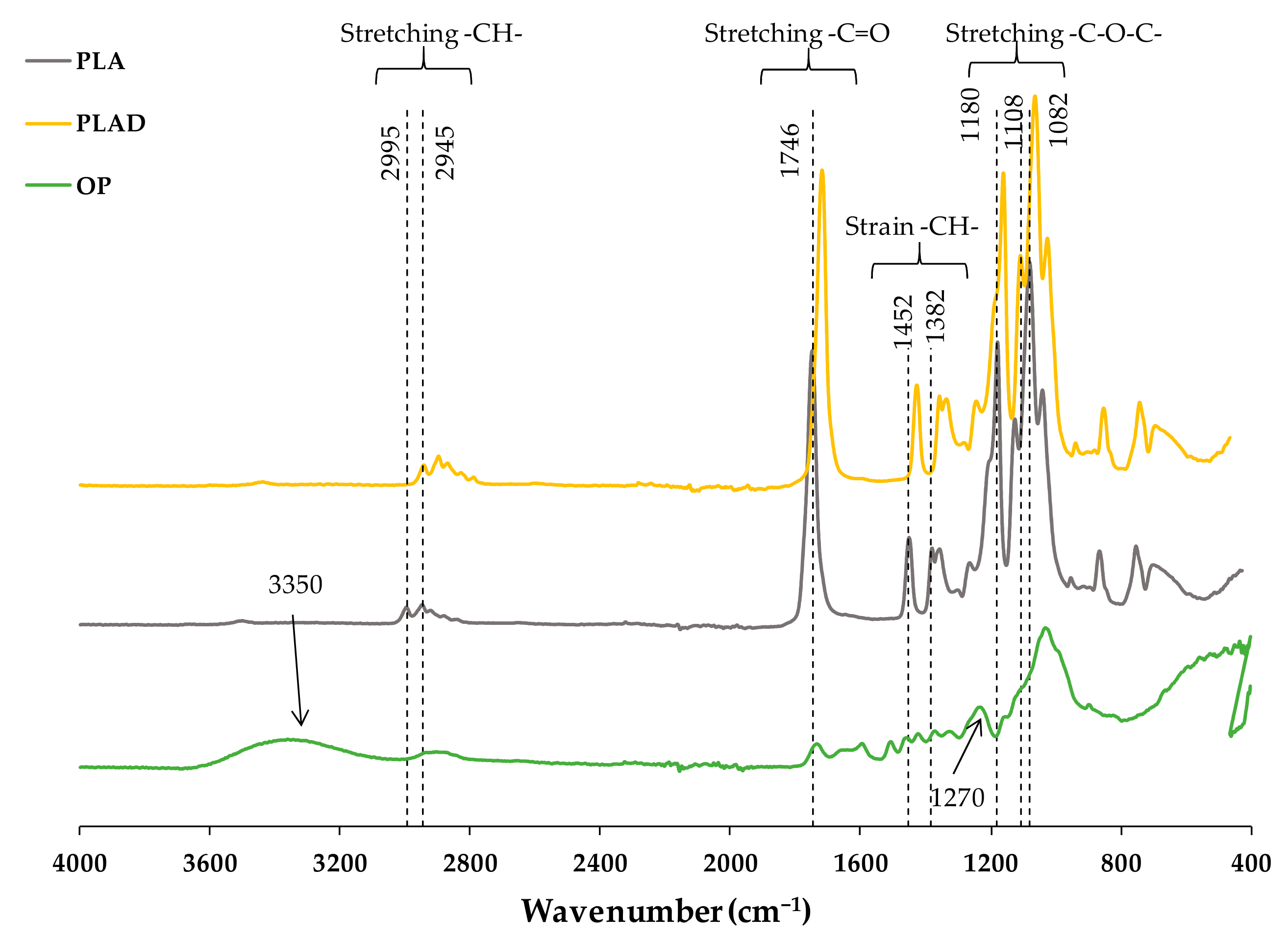
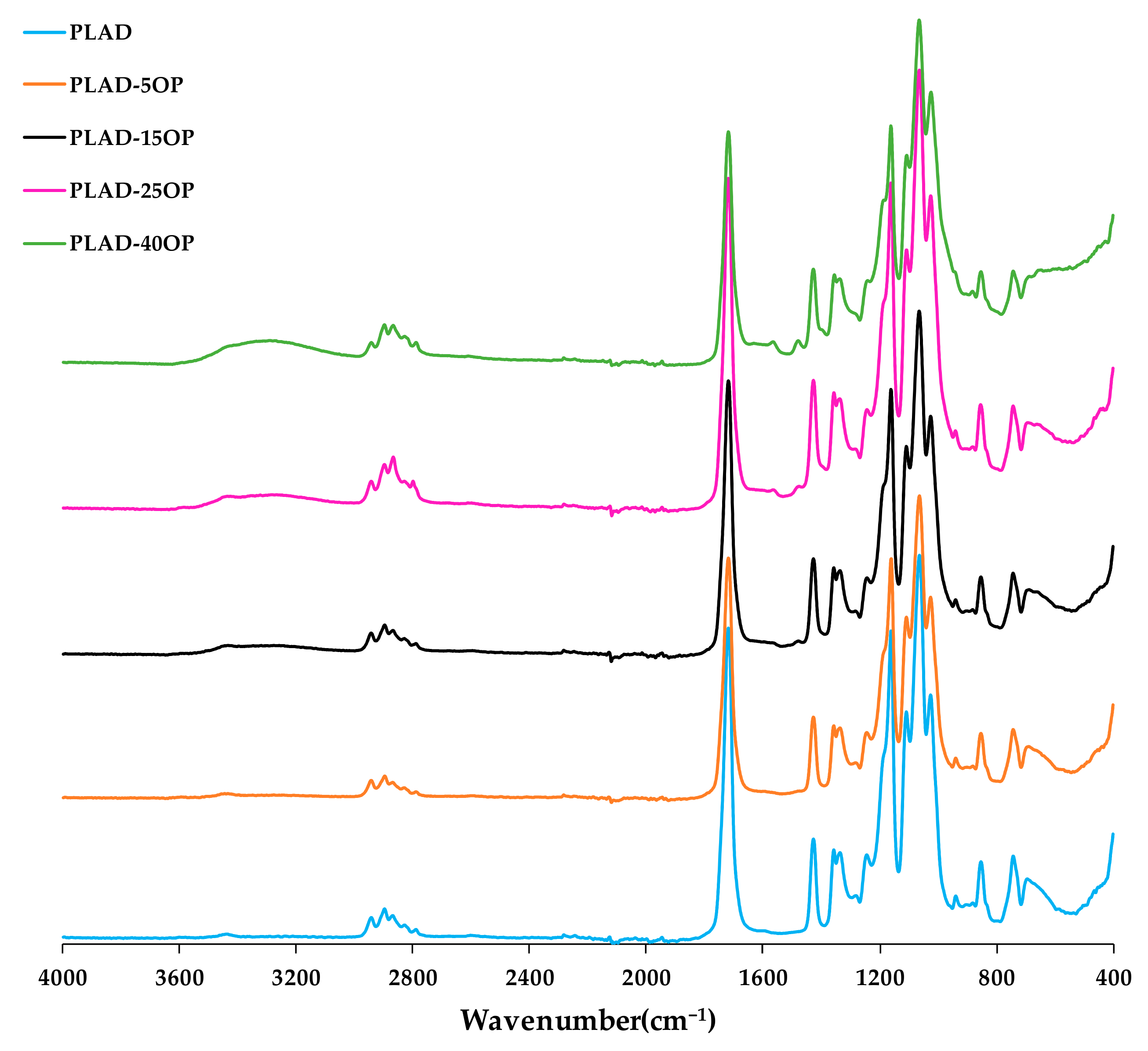
3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
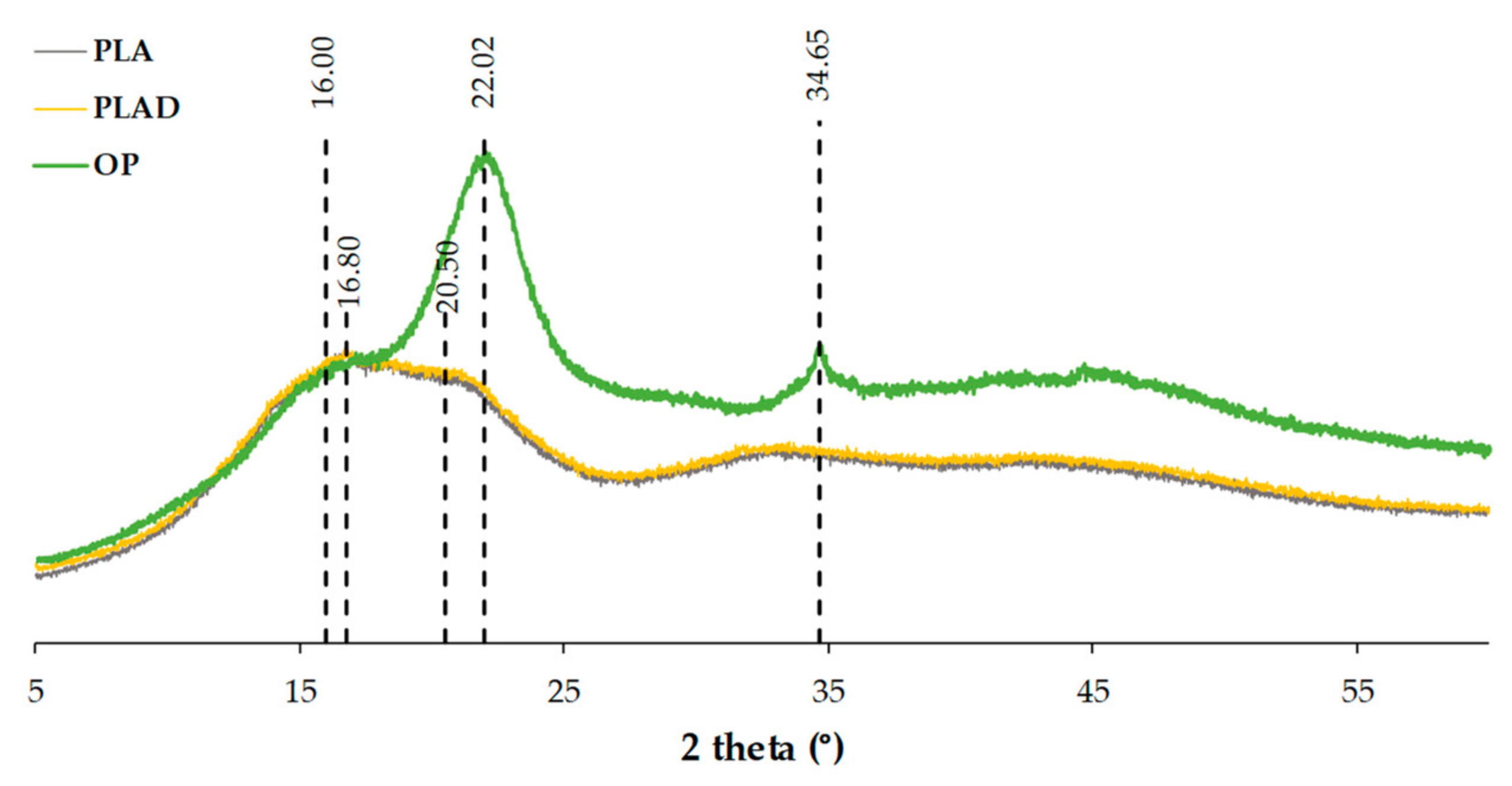
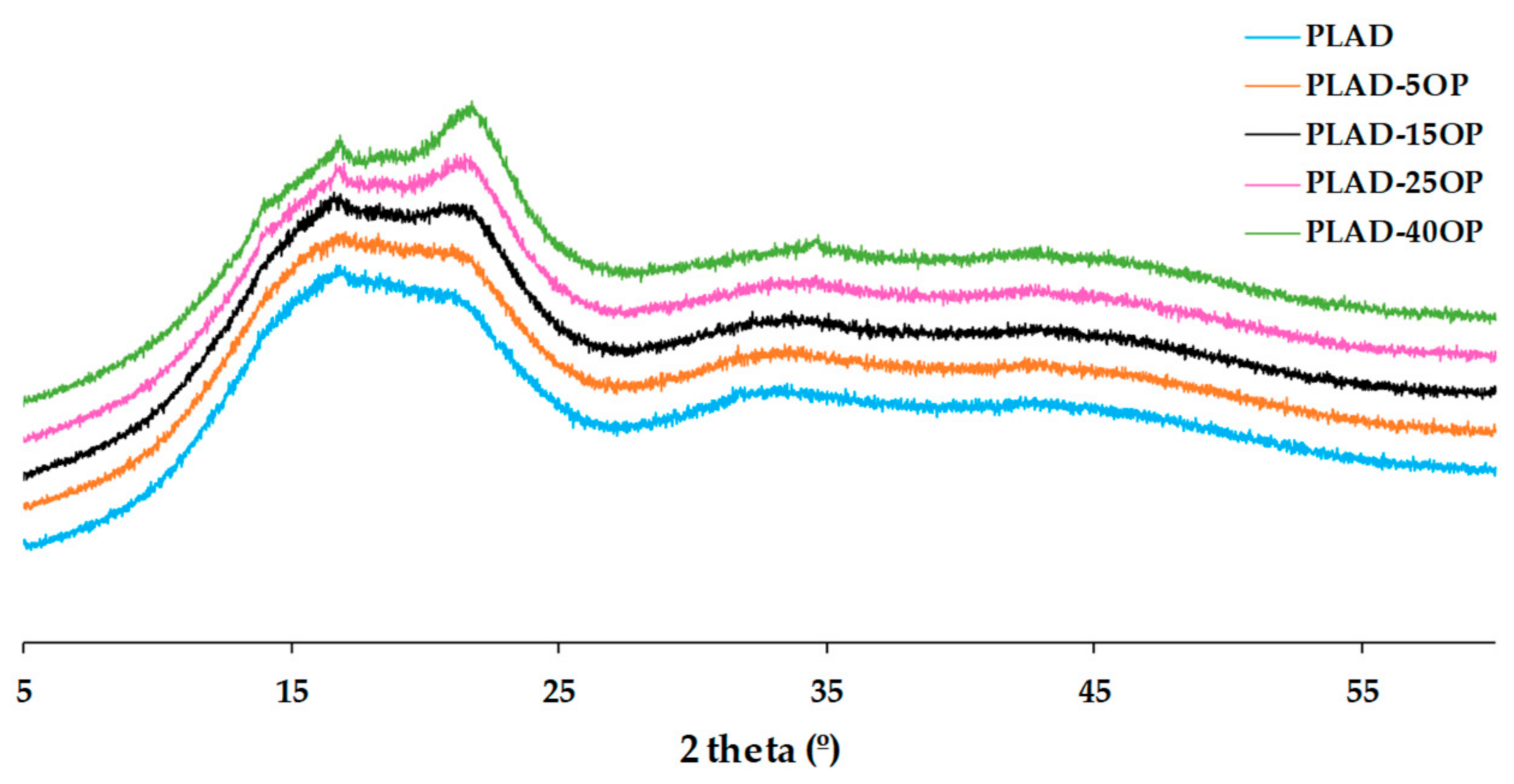
3.4. X-ray Diffraction (XRD)
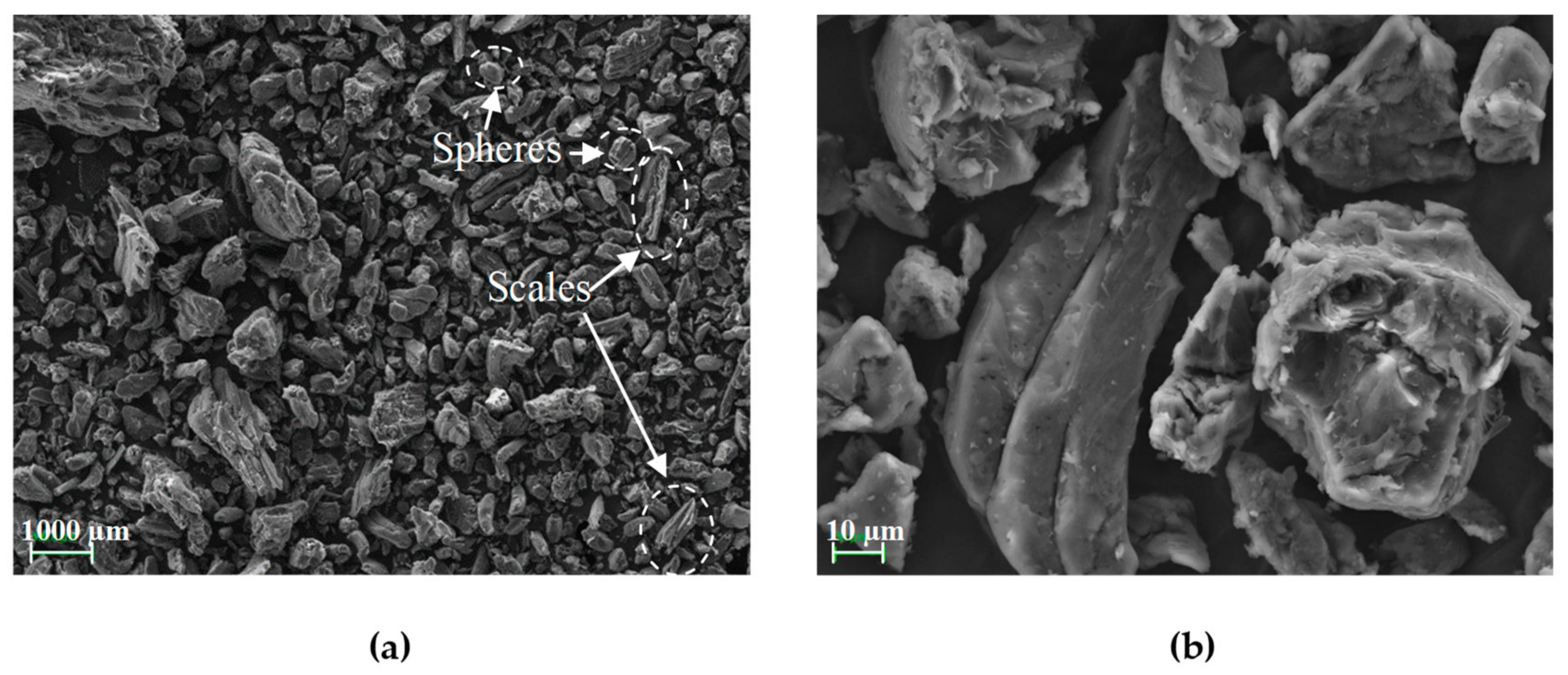
3.5. Scanning Electron Microscopy (SEM)
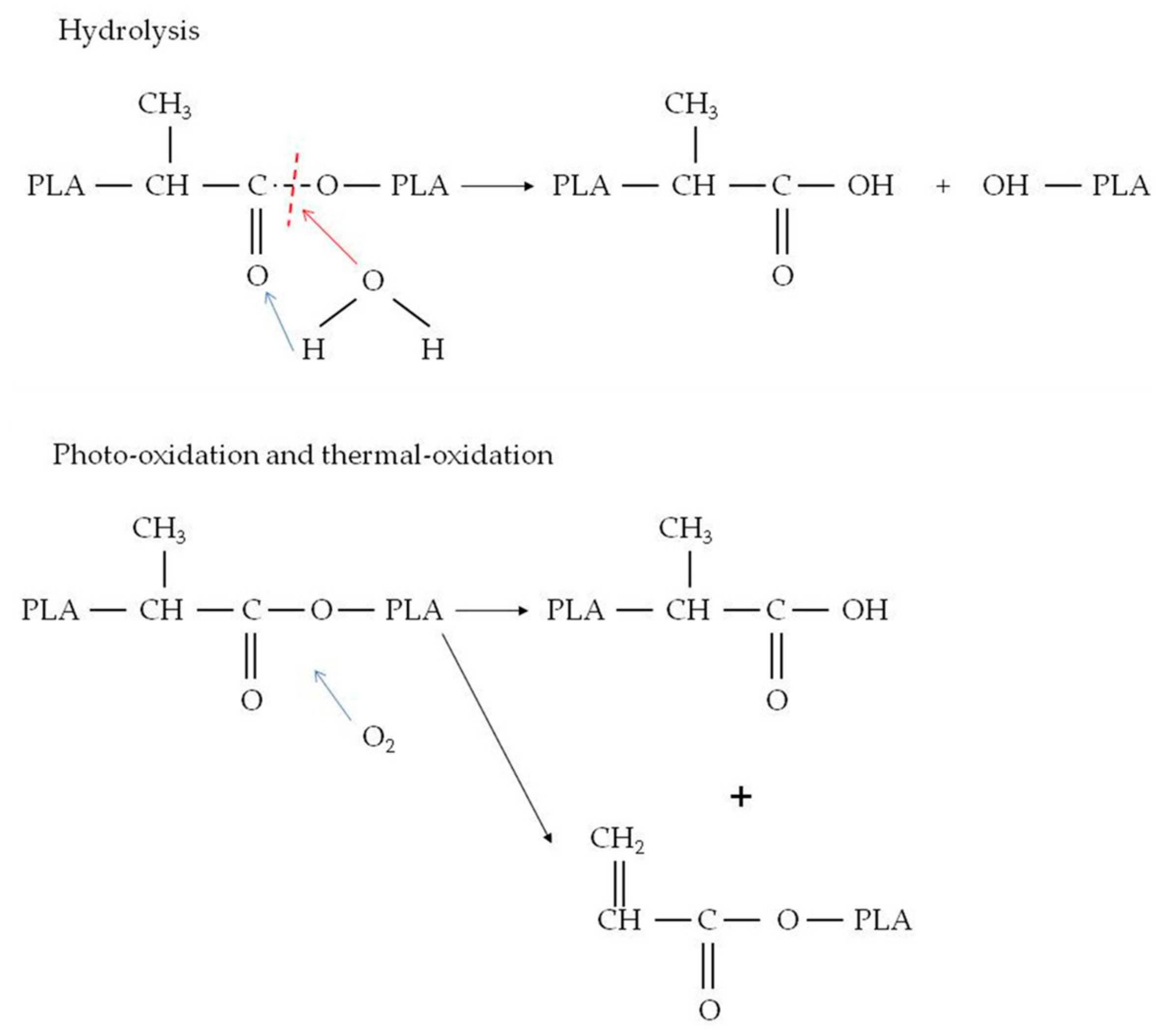
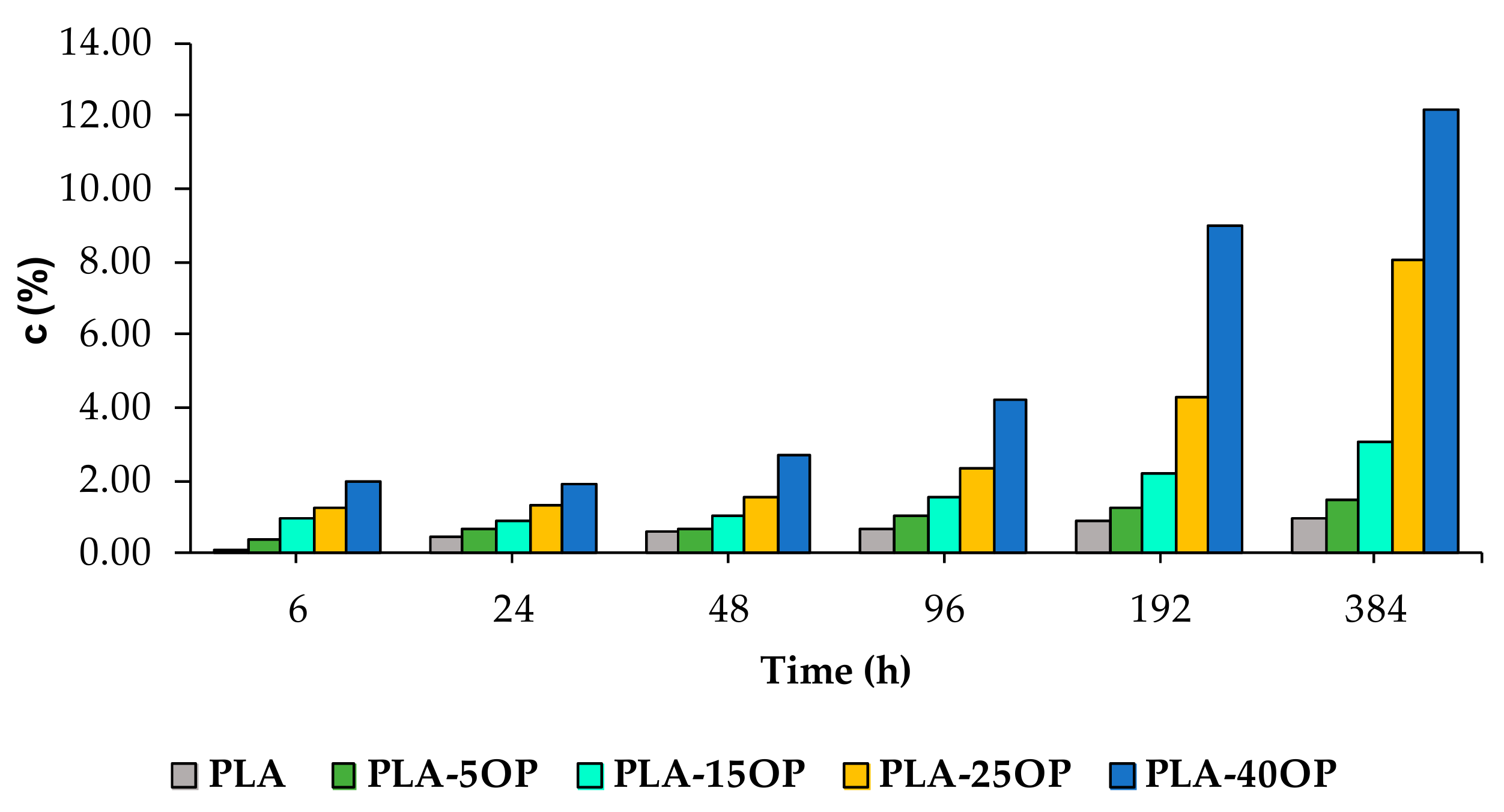
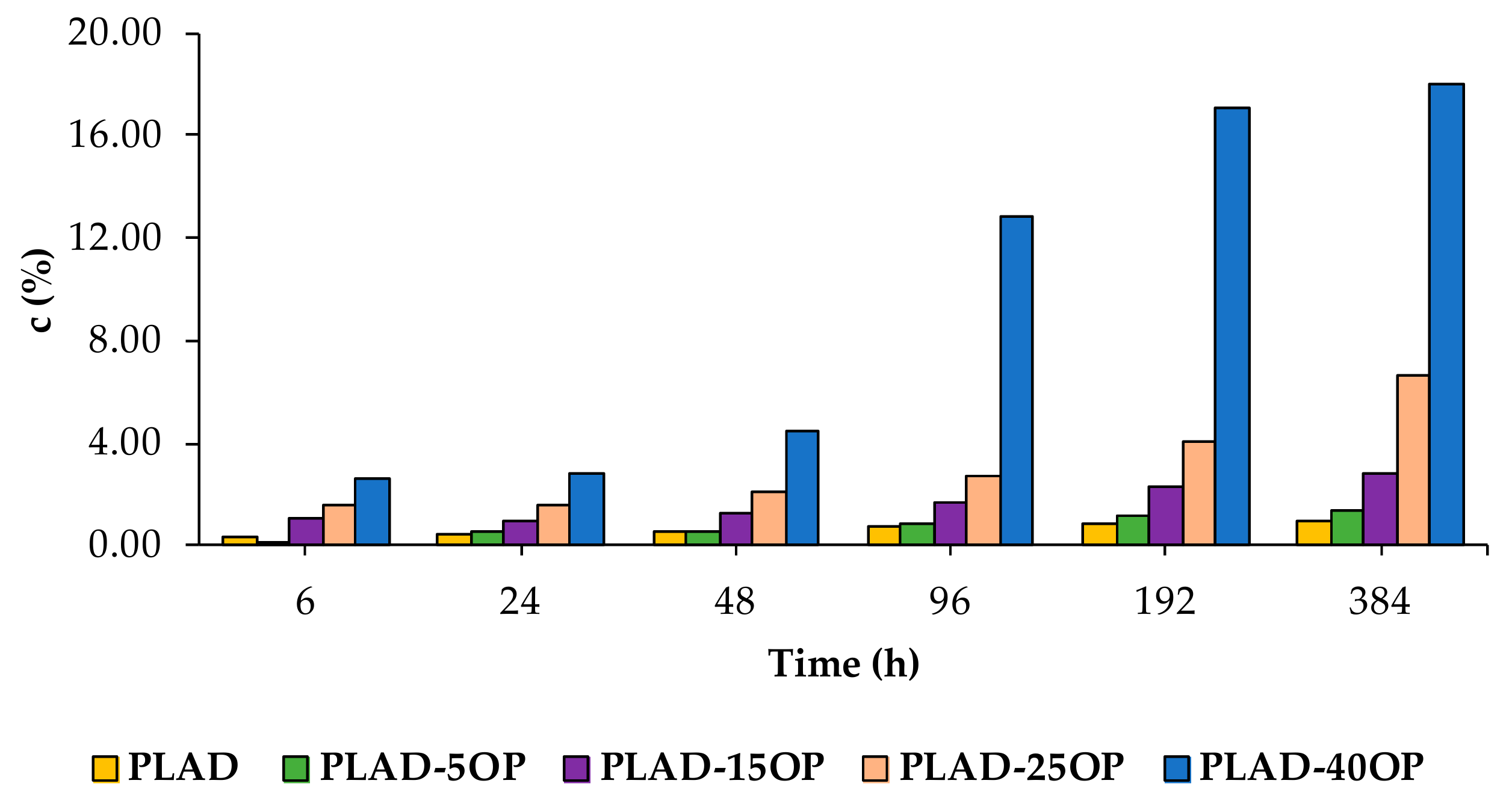
3.6. Water Absorption
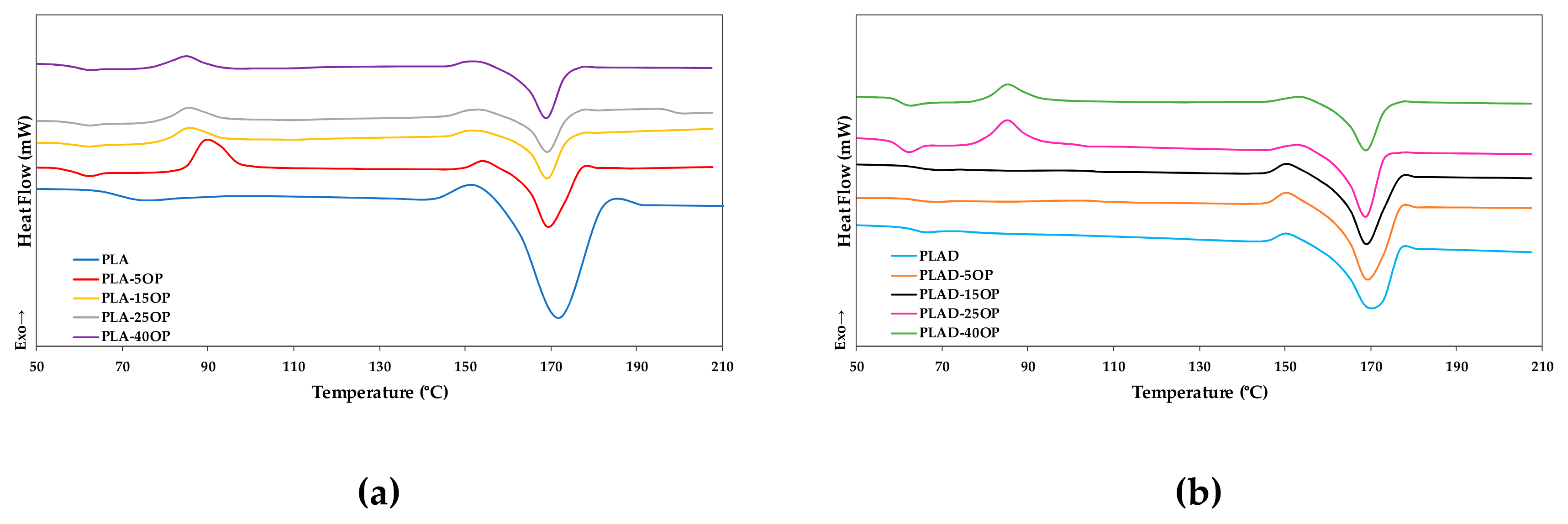
3.7. Differential Scanning Calorimetry (DSC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swetha, A.T.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste—Its applications and degradation. Int. J. Biol. Macromol. 2023, 234, 123703. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Gulino, E.F. Hydrolytic degradation of PLA/Posidonia Oceanica green composites: A simple model based on starting morpho-chemical properties. Compos. Sci. Technol. 2021, 213, 108930. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Rakesh, P.K. Study on the degradation behavior of natural fillers based PLA composites. In Advances in Bio-Based Fiber; Rangappa, S.M., Puttegowda, M., Parameswaranpillai, J., Siengchin, S., Gorbatyuk, S., Eds.; The Textile Institute Book Series; Woodhead Publishing: Swaston, UK, 2022; pp. 499–509. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, D.; Yu, T.; Yang, W.; Li, Q.; Li, Y. Enzymatic degradation behaviors and kinetics of bio-degradable jute/poly (lactic acid) (PLA) composites. Compos. Commun. 2022, 33, 101227. [Google Scholar] [CrossRef]
- Tan, J.Y.; Tey, W.Y.; Panpranot, J.; Lim, S.; Lee, K.M. Valorization of Oil Palm Empty Fruit Bunch for Cellulose Fibers: A Reinforcement Material in Polyvinyl Alcohol Biocomposites for Its Application as Detergent Capsules. Sustainability 2022, 14, 11446. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Olive pit powder as multifunctional pigment for waterborne paint: Influence of the bio-based filler on the aesthetics, durability and mechanical features of the polymer matrix. Ind. Crops Prod. 2023, 194, 116326. [Google Scholar] [CrossRef]
- Koutsomitopoulou, A.F.; Bénézet, J.C.; Bergeret, A.; Papanicolaou, G.C. Preparation and characterization of olive pit powder as a filler to PLA-matrix bio-composites. Powder Technol. 2014, 255, 10–16. [Google Scholar] [CrossRef]
- Hamida, B.; Ahmed, M.; Nadia, N.; Samira, M. Mechanical properties of polystyrene/olive stone flour composites. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 127–132. [Google Scholar]
- La Mantia, F.; Dintcheva, N.T.; Morreale, M.; Vaca-Garcia, C. Green composites of organic materials and recycled post-consumer polyethylene. Polym. Int. 2004, 53, 1888–1891. [Google Scholar] [CrossRef]
- Naghmouchi, I.; Mutjé, P.; Boufi, S. Olive stones flour as reinforcement in polypropylene composites: A step forward in the valorization of the solid waste from the olive oil industry. Ind. Crops Prod. 2015, 72, 183–191. [Google Scholar] [CrossRef]
- Naghmouchi, I.; Espinach, F.X.; Mutjé, P.; Boufi, S. Polypropylene composites based on lignocellulosic fillers: How the filler morphology affects the composite properties. Mater. Des. 2015, 65, 454–461. [Google Scholar] [CrossRef]
- Hejna, A.; Sulyman, M.; Przybysz, M.; Saeb, M.R.; Klein, M.; Formela, K. On the Correlation of Lignocellulosic Filler Composition with the Performance Properties of Poly(ε-Caprolactone) Based Biocomposites. Waste Biomass Valorization 2020, 11, 1467–1479. [Google Scholar] [CrossRef]
- Perinović, S.; Andričić, B.; Erceg, M. Thermal properties of poly(l-lactide)/olive stone flour composites. Thermochim. Acta 2010, 510, 97–102. [Google Scholar] [CrossRef]
- Haffiez, N.; Zakaria, B.S.; Mirsoleimani, S.M.; Dhar, B.R. Antibiotic resistance genes proliferation under anaerobic degradation of polylactic acid and polyhydroxy butyrate bioplastics. Environ. Int. 2023, 175, 107938. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Franco, F.; Auras, R.; Rubino, M.; Dolan, K.; Soto-Valdez, H.; Selke, S. Effect of nanoparticles on the hydrolytic degradation of PLA-nanocomposites by water-ethanol solutions. Polym. Degrad. Stab. 2017, 146, 287–297. [Google Scholar] [CrossRef]
- Copinet, A.; Bertrand, C.; Govindin, S.; Coma, V.; Couturier, Y. Effects of ultraviolet light (315 nm), temperature and relative humidity on the degradation of polylactic acid plastic films. Chemosphere 2004, 55, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Szaraniec, B.; Goryczka, T. Degradation of PLA/hemp and PLA/jute composities. Mod. Polym. Mater. Environ. Appl. 2010, 4, 215–224. [Google Scholar]
- Browning, B.L. (Ed.) Determination of lignin. In Methods of Wood Chemistry; Wiley & Sons, Interscience Publishers: New York, NY, USA, 1967; Volume 1, pp. 785–823. [Google Scholar]
- Jurado-Contreras, S.; Navas-Martos, F.J.; Rodríguez-Liébana, J.A.; Moya, A.J.; La Rubia, M.D. Manufacture and Characterization of Recycled Polypropylene and Olive Pits Biocomposites. Polymers 2022, 14, 4206. [Google Scholar] [CrossRef]
- ISO 527-2:2012; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Molding and Extrusion Plastics. ISO: Geneva, Switzerland, 2012.
- ISO 178:2019; Plastics—Determination of Flexural Properties. ISO: Geneva, Switzerland, 2020.
- ISO 179-1:2023; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. ISO: Geneva, Switzerland, 2023.
- Kǒsiḱova, B.; Slávikova, E. Use of lignin and pla as renewable additives of polypropylene films. Wood Res. 2011, 56, 393–402. [Google Scholar]
- UNE-EN ISO 62:2008; Plastics—Determination of Water Absorption. ISO: Geneva, Switzerland, 2011.
- Fehri, S.; Cinelli, P.; Coltelli, M.; Anguillesi, I.; Lazzeri, A. Thermal Properties of Plasticized Poly (Lactic Acid) (PLA) Containing Nucleating Agent. Int. J. Chem. Eng. Appl. 2016, 7, 85–88. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef] [PubMed]
- Valvez, S.; Maceiras, A.; Santos, P.; Reis, P.N.B. Olive Stones as Filler for Polymer-Based Composites: A Review. Materials 2021, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R. Fernández-Bolaños. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, Y. Jointly modified mechanical properties and accelerated hydrolytic degradation of PLA by interface reinforcement of PLA-WF. J. Mech. Behav. Biomed. Mater. 2018, 88, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Karamanlioglu, M.; Alkan, Ü. Influence of time and room temperature on mechanical and thermal degradation of PLA. Therm. Sci. 2019, 23, 51. [Google Scholar] [CrossRef]
- Perez, A.; Ramírez-Herrera, V.; Fuentes, F.; Rodrigue, D.; Silva, J.; Robledo-Ortíz, J. Crystallinity and impact strength improvement of wood-polylactic acid biocomposites produced by rotational and compression molding. Maderas Cienc. Tecnol. 2021, 23, 1–16. [Google Scholar] [CrossRef]
- Hegyesi, N.; Zhang, Y.; Kohári, A.; Polyák, P.; Sui, X.; Pukánszky, B. Enzymatic degradation of PLA/cellulose nanocrystal composites. Ind. Crops Prod. 2019, 141, 111799. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Yang, K.; Yu, W.; Shi, J.; Zheng, Q. Preparation of graphene-modified PLA/PBAT composite monofilaments and its degradation behavior. J. Mater. Res. Technol. 2022, 20, 3784–3795. [Google Scholar] [CrossRef]
- Ramle, S.F.M.; Ahmad, N.; Fazita, M.; Zahidan, N.; Boon, J.G. Physical properties and soil degradation of PLA/PBAT blends film reinforced with bamboo cellulose. IOP Conf. Ser. Earth Environ. Sci. 2020, 596, 012021. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Feng, H.; Zhang, M.; Lei, L.; Wu, X. Improvement of tensile and thermal properties of poly(lactic acid) composites with admicellar-treated rice straw fiber. Chem. Eng. J. 2011, 173, 659–666. [Google Scholar] [CrossRef]
- Bhiogade, A.; Kannan, M. Studies on thermal and degradation kinetics of cellulose micro/nanoparticle filled polylactic acid (PLA) based nanocomposites. Polym. Polym. Compos. 2021, 29, 85–98. [Google Scholar] [CrossRef]
- Wang, F.; Nan, Z.; Sun, X.; Liu, C.; Zhuang, Y.; Zan, J.; Dai, C.; Liu, Y. Characterization of degradation behaviors of PLA biodegradable plastics by infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121376. [Google Scholar] [CrossRef]
- Fernández-Barranco, C.; Yebra-Rodríguez, A.; Jiménez-Millán, J.; Navas-Martos, F.J.; Yebra, A.; Koziol, A.E.; La Rubia, M.D. Photo-oxidative degradation of injection molded sepiolite/polyamide66 nanocomposites. Appl. Clay Sci. 2020, 189, 105545. [Google Scholar] [CrossRef]
- Hong, H.; Xiao, R.; Guo, Q.; Liu, H.; Zhang, H. Quantitively Characterizing the Chemical Composition of Tailored Bagasse Fiber and Its Effect on the Thermal and Mechanical Properties of Polylactic Acid-Based Composites. Polymers 2019, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calderón, C.; Romero-Guzmán, D.; Ferrández-Montero, A.; Pérez-Giraldo, C.; González-Carrasco, J.L.; Lieblich, M.; Benavente, R.; Ferrari, B.; González-Martín, M.L.; Gallardo-Moreno, A.M. Impact of PLA/Mg films degradation on surface physical properties and biofilm survival. Colloids Surf. B Biointerfaces 2020, 185, 110617. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K.; Mishra, A.K.; Kumar, A. Mechanical Degradation of 3D Printed PLA in Simulated Marine Environment. Surf. Interfaces 2020, 21, 100778. [Google Scholar] [CrossRef]
- Podkościelna, B.; Gargol, M.; Goliszek, M.; Klepka, T.; Sevastyanova, O. Degradation and flammability of bioplastics based on PLA and lignin. Polym. Test. 2022, 111, 107622. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L. Preparation, characterization and hydrolytic degradation of PLA/PCL co-mingled nanofibrous mats prepared via dual-jet electrospinning. Eur. Polym. J. 2017, 96, 266–277. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.; Park, J.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent solvent induced crystallization and hydrolytic degradation of PLA by water-ethanol solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef]
- Wolf, M.; Gil-Castell, Ó.; Cea, J.; Carrasco, J.; Ribes, A. Degradation of Plasticised Poly(lactide) Composites with Nanofibrillated Cellulose in Different Hydrothermal Environments. J. Polym. Environ. 2022, 31, 2055–2072. [Google Scholar] [CrossRef]






| Reference | PLA (% wt.) | OP (% wt.) | BYK (% wt.) | PO1020 (% wt.) |
|---|---|---|---|---|
| PLA | 94.5 | - | 1.5 | 4 |
| PLA-5OP | 89.5 | 5 | 1.5 | 4 |
| PLA-15OP | 79.5 | 15 | 1.5 | 4 |
| PLA-25OP | 69.5 | 25 | 1.5 | 4 |
| PLA-40OP | 54.5 | 40 | 1.5 | 4 |
| Reference | PLA (% wt.) | OP (% wt.) | BYK (% wt.) | PO1020 (% wt.) |
|---|---|---|---|---|
| PLAD | 94.5 | - | 1.5 | 4 |
| PLAD-5OP | 89.5 | 5 | 1.5 | 4 |
| PLAD-15OP | 79.5 | 15 | 1.5 | 4 |
| PLAD-25OP | 69.5 | 25 | 1.5 | 4 |
| PLAD-40OP | 54.5 | 40 | 1.5 | 4 |
| Temperature (°C) | Screw Rotation Speed (rpm) | ||||||
|---|---|---|---|---|---|---|---|
| Zone I | Zone II | Zone III | Zone IV | Zone V | Zone VI | Nozzle | |
| 155 | 160 | 170 | 170 | 170 | 165 | 165 | 175 |
| Moisture (% wt.) | Ash (% wt.) | Cellulose (% wt.) | Hemicellulose (% wt.) | Lignin (%wt.) |
|---|---|---|---|---|
| 5.11 | 0.00 | 21.79 | 23.60 | 33.78 |
| Reference | σm (MPa) | σb (MPa) | εm (%) | εb (%) | Et (MPa) |
|---|---|---|---|---|---|
| PLA | 53.16 ± 0.67 | 37.47 ± 2.08 | 6.45 ± 0.22 | 13.12 ± 0.64 | 3523.96 ± 166.61 |
| PLA-5OP | 29.45 ± 0.94 | 23.88 ± 0.79 | 3.37 ± 0.21 | 6.56 ± 0.34 | 3525.85 ± 231.96 |
| PLA-15OP | 27.83 ± 0.77 | 24.90 ± 0.72 | 2.53 ± 0.14 | 3.91 ± 0.20 | 4578.21 ± 160.75 |
| PLA-25OP | 20.89 ± 0.42 | 19.22 ± 0.50 | 2.59 ± 0.14 | 2.59 ± 0.14 | 3491.67 ± 293.07 |
| PLA-40OP | 11.92 ± 0.06 | 10.65 ± 0.42 | 1.66 ± 0.12 | 1.66 ± 0.12 | 2244.43 ± 73.63 |
| PLAD | 51.59 ± 0.71 | 43.92 ± 2.08 | 5.96 ± 0.21 | 7.53 ± 0.82 | 3464.00 ± 315.02 |
| PLAD-5OP | 44.30 ± 0.36 | 41.37 ± 1.05 | 4.41 ± 0.21 | 5.39 ± 0.52 | 2995.36 ± 298.66 |
| PLAD-15OP | 32.06 ± 1.52 | 32.08 ± 1.30 | 3.08 ± 0.18 | 3.20 ± 0.19 | 3197.40 ± 156.35 |
| PLAD-25OP | 22.80 ± 2.32 | 22.99 ± 2.34 | 2.29 ± 0.48 | 2.39 ± 0.49 | 2807.83 ± 134.40 |
| PLAD-40OP | 13.30 ± 0.43 | 13.27 ± 0.40 | 1.09 ± 0.19 | 1.19 ± 0.20 | 2754.06 ± 111.40 |
| Reference | σfm (MPa) | σfb (MPa) | εfm (%) | εfb (%) | Ef (MPa) |
|---|---|---|---|---|---|
| PLA | 70.36 ± 0.56 | 68.82 ± 0.92 | 3.72 ± 0.19 | 5.00 ± 0.00 | 2667.99 ± 8.37 |
| PLA-5OP | 56.34 ± 0.82 | 45.10 ± 0.67 | 2.24 ± 0.09 | 3.02 ± 0.50 | 2901.59 ± 74.94 |
| PLA-15OP | 52.23 ± 1.27 | 42.75 ± 1.68 | 1.90 ± 0.12 | 2.16 ± 0.30 | 3324.44 ± 114.80 |
| PLA-25OP | 43.69 ± 2.78 | 35.49 ± 2.41 | 1.80 ± 0.29 | 2.16 ± 0.50 | 3348.53 ± 111.36 |
| PLA-40OP | 30.66 ± 0.46 | 24.65 ± 0.19 | 1.34 ± 0.05 | 1.47 ± 0.10 | 3349.44 ± 68.92 |
| PLAD | 71.24 ± 0.71 | 57.05 ± 0.75 | 3.70 ± 0.16 | 4.47 ± 0.13 | 2855.94 ± 20.74 |
| PLAD-5OP | 62.80 ± 0.58 | 52.10 ± 4.25 | 3.69 ± 0.29 | 4.85 ± 0.17 | 2913.71 ± 46.15 |
| PLAD-15OP | 49.47 ± 1.13 | 39.74 ± 1.02 | 2.34 ± 0.11 | 2.67 ± 0.13 | 3131.80 ± 39.62 |
| PLAD-25OP | 36.61 ± 0.92 | 29.54 ± 0.79 | 1.70 ± 0.07 | 1.95 ± 0.13 | 3205.85 ± 35.07 |
| PLAD-40OP | 25.76 ± 0.22 | 13.52 ± 3.11 | 1.10 ± 0.00 | 1.55 ± 0.22 | 3477.69 ± 51.67 |
| Reference | Tg (°C) | Tc (°C) | Tm (°C) | ΔHm (J/g) | Wc (%) |
|---|---|---|---|---|---|
| PLA | 65.07 | - | 171.42 | 41.62 | 47.00 |
| PLA-5OP | 61.05 | 90.47 | 170.69 | 31.10 | 37.09 |
| PLA-15OP | 61.42 | 86.13 | 170.56 | 22.66 | 30.42 |
| PLA-25OP | 61.40 | 85.85 | 170.54 | 25.23 | 38.74 |
| PLA-40OP | 62.09 | 84.53 | 170.16 | 26.23 | 51.37 |
| PLAD | 61.47 | - | 171.02 | 47.43 | 53.56 |
| PLAD-5OP | 60.23 | - | 170.56 | 41.02 | 48.91 |
| PLAD-15OP | 62.33 | - | 170.41 | 39.16 | 52.57 |
| PLAD-25OP | 58.39 | 85.04 | 169.98 | 33.44 | 51.34 |
| PLAD-40OP | 60.38 | 85.54 | 170.33 | 24.62 | 48.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado-Contreras, S.; Navas-Martos, F.J.; Rodríguez-Liébana, J.A.; La Rubia, M.D. Effect of Olive Pit Reinforcement in Polylactic Acid Biocomposites on Environmental Degradation. Materials 2023, 16, 5816. https://doi.org/10.3390/ma16175816
Jurado-Contreras S, Navas-Martos FJ, Rodríguez-Liébana JA, La Rubia MD. Effect of Olive Pit Reinforcement in Polylactic Acid Biocomposites on Environmental Degradation. Materials. 2023; 16(17):5816. https://doi.org/10.3390/ma16175816
Chicago/Turabian StyleJurado-Contreras, Sofía, Francisco J. Navas-Martos, José A. Rodríguez-Liébana, and M. Dolores La Rubia. 2023. "Effect of Olive Pit Reinforcement in Polylactic Acid Biocomposites on Environmental Degradation" Materials 16, no. 17: 5816. https://doi.org/10.3390/ma16175816
APA StyleJurado-Contreras, S., Navas-Martos, F. J., Rodríguez-Liébana, J. A., & La Rubia, M. D. (2023). Effect of Olive Pit Reinforcement in Polylactic Acid Biocomposites on Environmental Degradation. Materials, 16(17), 5816. https://doi.org/10.3390/ma16175816







