Silk Fibroin/ZnO Coated TiO2 Nanotubes for Improved Antimicrobial Effect of Ti Dental Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
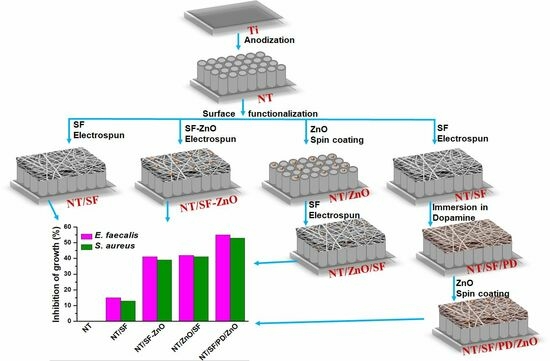
2.2. Preparation of TiO2 Nanotube on Titanium Surface
2.3. Construction of Hybrid Film TiO2 Nanotubes/Silk Fibroin/Polydopamine/ZnO Nanoparticles
2.3.1. Deposition of Silk Fibroin Fibers
2.3.2. Deposition Silk Fibroin Fibers/ZnO Nanoparticles
2.3.3. Deposition of Zn Nanoparticles and Silk Fibroin Fibers
2.3.4. Deposition Silk Fibroin Fibers, Polydopamine Films, and ZnO Nanoparticles
2.4. Samples Characterization
2.5. Antibacterial Assay
3. Results and Discussion
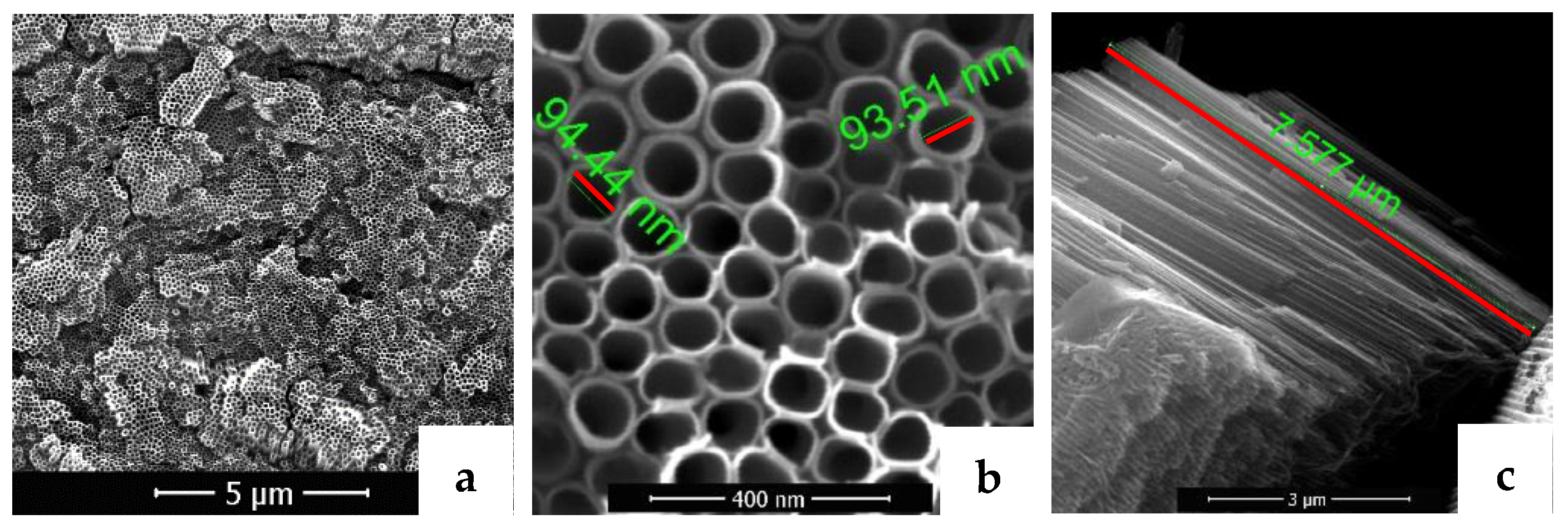
3.1. Surfaces Morphology and Composition
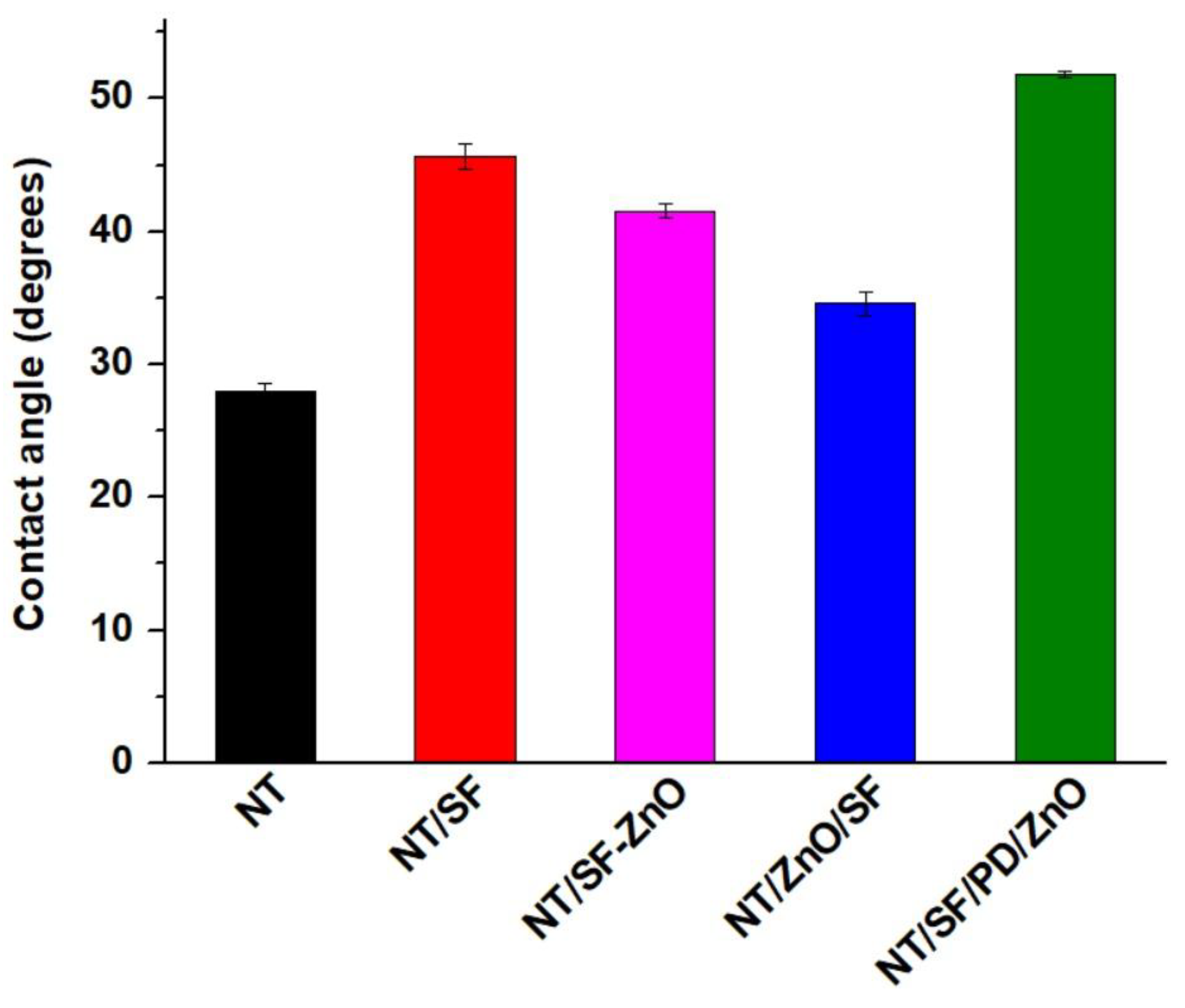
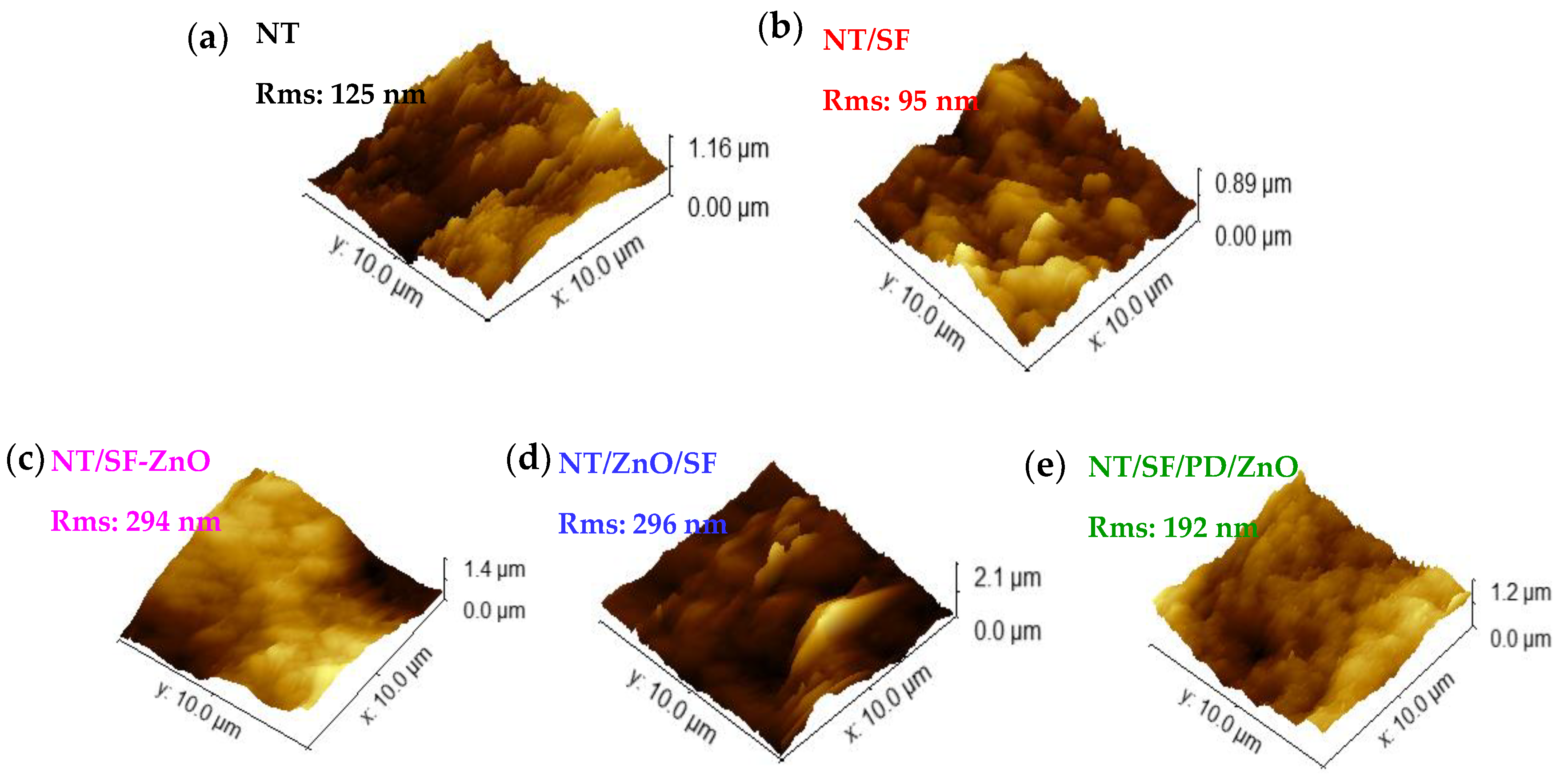
3.2. Surface Topography and Wettability
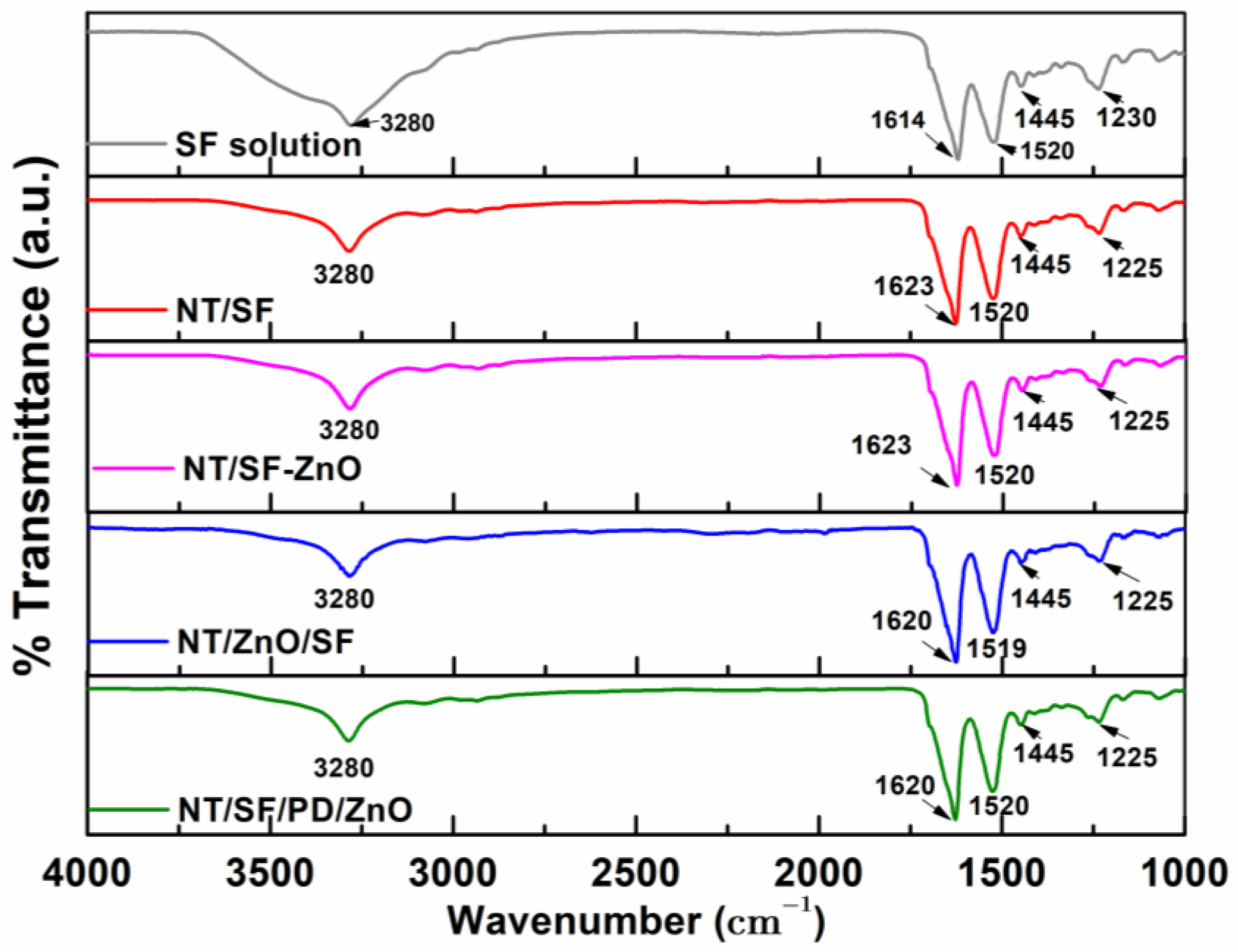
3.3. Fourier Transform Infrared Spectroscopy
3.4. Electrochemical Characterization
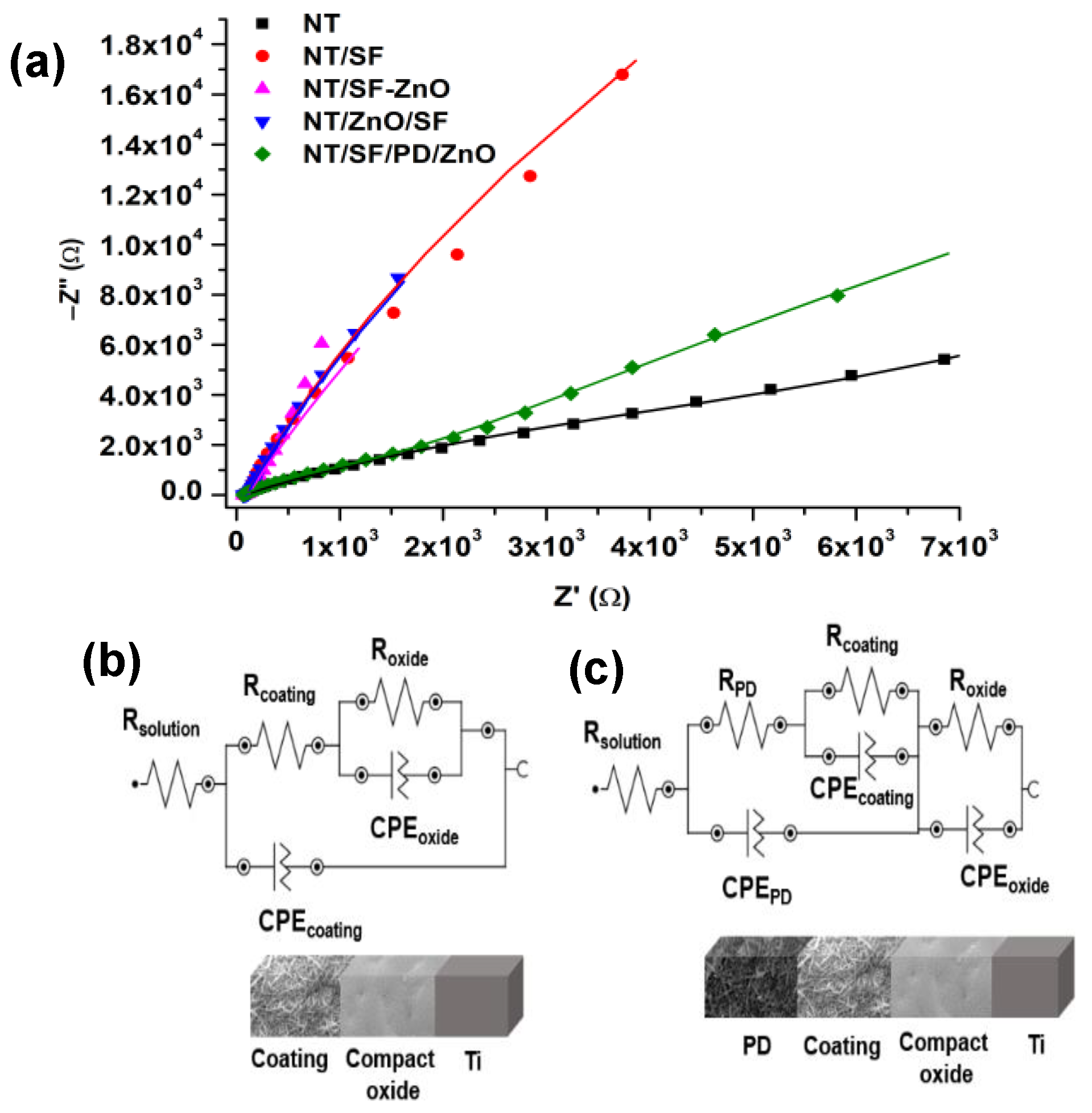
3.4.1. Electrochemical Impedance Spectroscopy (EIS)
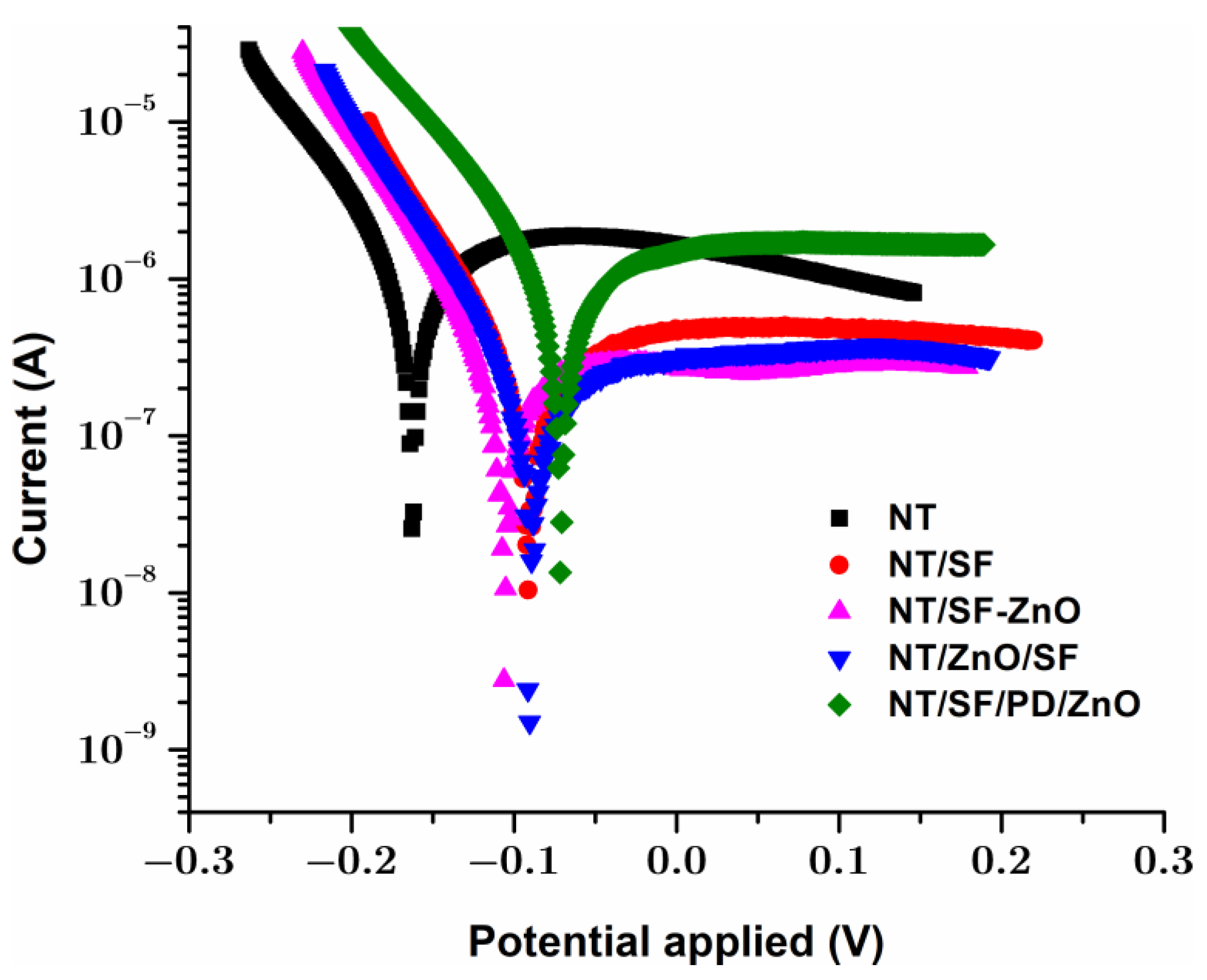
3.4.2. Tafel Analysis
3.4.3. Cyclic Voltammetry
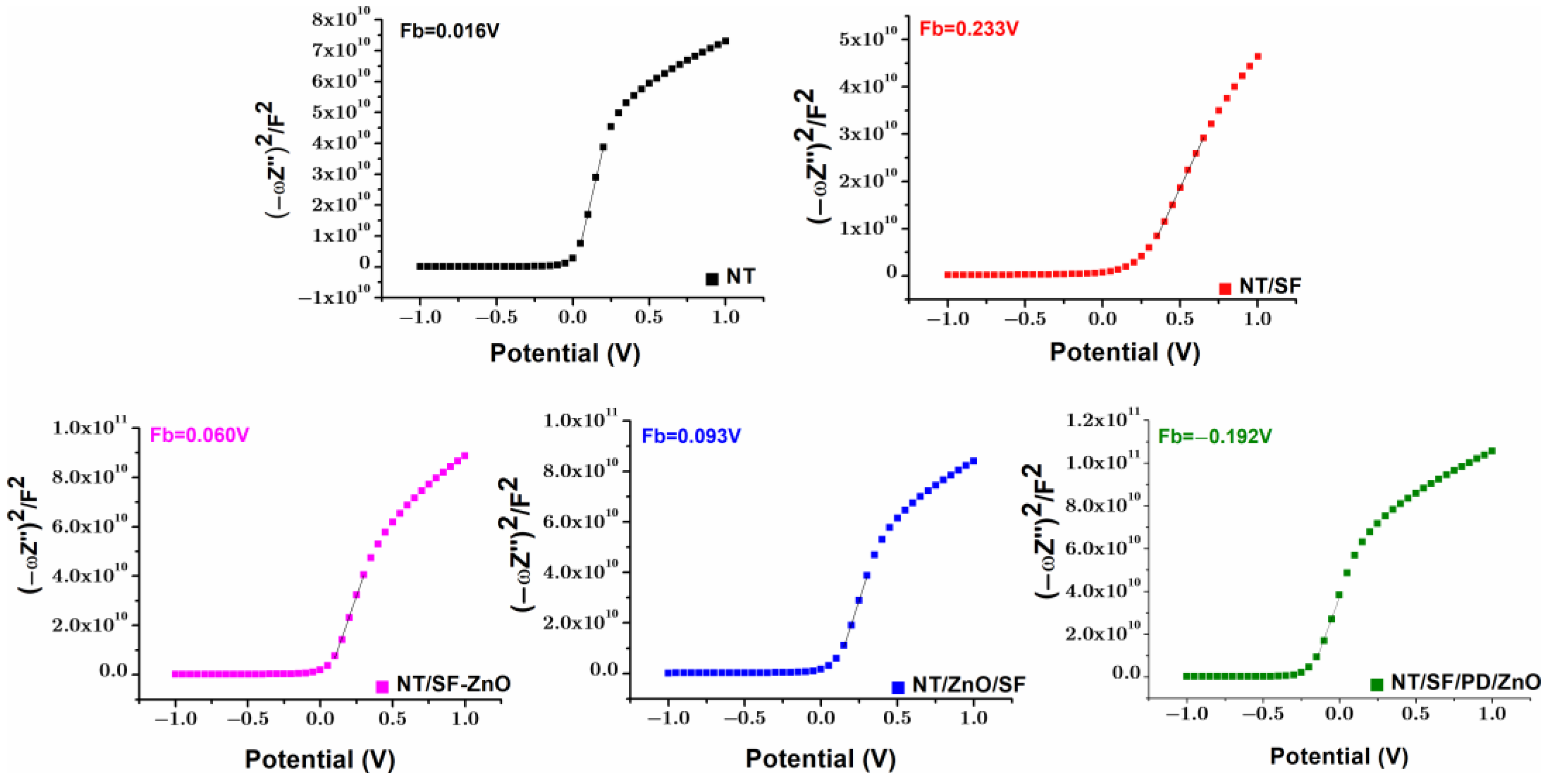
3.4.4. Mott–Schottky
3.5. In Vitro Antibacterial Assay
- (a)
- High concentrations of zinc can disrupt the balance of metal ions in the bacterial cells, interfering with essential biochemical processes [86].
- (b)
- Zinc ions can inhibit certain bacterial enzymes, impeding bacterial growth and metabolism [87]
- (c)
- (d)
- Zinc is shown to inhibit the formation of biofilms, which are communities of microorganisms that can adhere to surfaces and are often resistant to antimicrobial agents [89].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamdy, T.M. Dental Biomaterial Scaffolds in Tooth Tissue Engineering: A Review. Curr. Oral Health Rep. 2023, 10, 14–21. [Google Scholar] [CrossRef]
- Farag, M.M. Recent trends on biomaterials for tissue regeneration applications. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Wang, L.; Ding, X.; Feng, W.; Gao, Y.; Zhao, S.; Fan, Y. Biomechanical study on implantable and interventional medical devices. Acta Mech. Sin. 2021, 37, 875–894. [Google Scholar] [CrossRef]
- Romanos, G.E.; Fischer, G.A.; Rahman, Z.T.; Delgado-Ruiz, R. Spectrometric Analysis of the Wear from Metallic and Ceramic Dental Implants following Insertion: An In Vitro Study. Materials 2022, 15, 1200. [Google Scholar] [CrossRef]
- Ou, P.; Zhang, T.; Wang, J.; Li, C.; Shao, C.; Ruan, J. Bone response in vivo of Ti-45Zr alloy as dental implant material. J. Mater. Sci. Mater. Med. 2022, 33, 47. [Google Scholar] [CrossRef]
- Bhaskar, B.; Kasoju, N.; Sreenivasa Rao, P.; Raju Baadhe, R.; Nagarjuna, V. Biomaterials in Tissue Engineering and Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Lv, Y.; Wang, B.; Liu, G.; Tang, Y.; Lu, E.; Xie, K.; Lan, C.; Liu, J.; Qin, Z.; Wang, L. Metal material, properties and design methods of porous biomedical scaffolds for additive manufacturing: A review. Front. Bioeng. Biotechnol. 2021, 9, 641130. [Google Scholar] [CrossRef]
- Ralls, A.; Kumar, P.; Misra, M.; Menezes, P.L. Material design and surface engineering for bio-implants. JOM 2020, 72, 684–696. [Google Scholar] [CrossRef]
- Contuzzi, N.; Casalino, G.; Boccaccio, A.; Ballini, A.; Charitos, I.A.; Bottalico, L.; Santacroce, L. Metals Biotribology and Oral Microbiota Biocorrosion Mechanisms. J. Funct. Biomater. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Lijnev, A.; Elango, J.; Gómez-López, V.M.; Pérez-Albacete Martínez, C.; Granero Marín, J.M.; Maté Sánchez De Val, J.E. Antibacterial and Proliferative Effects of NaOH-Coated Titanium, Zirconia, and Ceramic-Reinforced PEEK Dental Composites on Bone Marrow Mesenchymal Stem Cells. Pharmaceutics 2022, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P. Bone tissue engineering in the treatment of bone defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef] [PubMed]
- Filip, N.; Radu, I.; Veliceasa, B.; Filip, C.; Pertea, M.; Clim, A.; Pinzariu, A.C.; Drochioi, I.C.; Hilitanu, R.L.; Serban, I.L. Biomaterials in Orthopedic Devices: Current Issues and Future Perspectives. Coatings 2022, 12, 1544. [Google Scholar] [CrossRef]
- Litak, J.; Szymoniuk, M.; Czyżewski, W.; Hoffman, Z.; Litak, J.; Sakwa, L.; Kamieniak, P. Metallic implants used in lumbar interbody fusion. Materials 2022, 15, 3650. [Google Scholar] [CrossRef]
- Gulati, K.; Chopra, D.; Kocak-Oztug, N.A.; Verron, E. Fit and forget: The future of dental implant therapy via nanotechnology. Adv. Drug Deliv. Rev. 2023, 199, 114900. [Google Scholar] [CrossRef]
- Heydariyan, Z.; Soofivand, F.; Dawi, E.A.; Al-Kahdum, S.A.A.; Hameed, N.M.; Salavati-Niasari, M. A comprehensive review: Different approaches for encountering of bacterial infection of dental implants and improving their properties. J. Drug Deliv. Sci. Technol. 2023, 84, 104401. [Google Scholar] [CrossRef]
- Hossain, N.; Islam, M.A.; Chowdhury, M.A.; Alam, A. Advances of nanoparticles employment in dental implant applications. Appl. Surf. Sci. Adv. 2022, 12, 100341. [Google Scholar] [CrossRef]
- Moghadasi, K.; Isa, M.S.M.; Ariffin, M.A.; Raja, S.; Wu, B.; Yamani, M.; bin Muhamad, M.R.; Yusof, F.; Jamaludin, M.F.; bin Ab Karim, M.S. A review on biomedical implant materials and the effect of friction stir based techniques on their mechanical and tribological properties. J. Mater. Res. Technol. 2022, 17, 1054–1121. [Google Scholar] [CrossRef]
- Pandey, A.; Sahoo, S. Progress on Medical Implant: A Review and Prospects. J. Bionic Eng. 2023, 20, 470–494. [Google Scholar] [CrossRef]
- Lü, W.; Wang, N.; Gao, P.; Li, C.; Zhao, H.; Zhang, Z. Effects of anodic titanium dioxide nanotubes of different diameters on macrophage secretion and expression of cytokines and chemokines. Cell Prolif. 2015, 48, 95–104. [Google Scholar] [CrossRef]
- Oh, S.; Jin, S. Titanium oxide nanotubes with controlled morphology for enhanced bone growth. Mater. Sci. Eng. C 2006, 26, 1301–1306. [Google Scholar] [CrossRef]
- Roguska, A.; Belcarz, A.; Zalewska, J.; Hołdyński, M.; Andrzejczuk, M.; Pisarek, M.; Ginalska, G. Metal TiO2 nanotube layers for the treatment of dental implant infections. ACS Appl. Mater. Interfaces 2018, 10, 17089–17099. [Google Scholar] [CrossRef]
- Rabiatul Basria, S.M.N.M.; Roshasnorlyza, H.; Mustafa Fadzil, F.; Srimala, S. Titanium Dioxide Nanotube Arrays for Biomedical Implant Materials and Nanomedicine Applications. In Titanium Dioxide; Dongfang, Y., Ed.; IntechOpen: Rijeka, Croatia, 2018; Ch. 23. [Google Scholar]
- Achitei, D.C.; Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Benchea, M.; Istrate, B. Ni-Cr Alloys Assessment for Dental Implants Suitability. Appl. Sci. 2022, 12, 12814. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Szałaj, U.; Fudala, D.; Zaręba, T.; Wojnarowicz, J.; Łojkowski, W.; Tyski, S.; Dowgierd, K.; Mielczarek, A. Dental implant healing screws as temporary oral drug delivery systems for decrease of infections in the area of the head and neck. Int. J. Nanomed. 2022, 17, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart dental materials for antimicrobial applications. Bioact. Mater. 2023, 24, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, W.; Zhou, W.; Zhang, S.; Li, M.; Li, N.; Pan, G.; Zhang, X.; Bai, J.; Zhu, C. Immunomodulatory biomaterials for implant-associated infections: From conventional to advanced therapeutic strategies. Biomater. Res. 2022, 26, 72. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Shirvan, A.R.; Li, Y.; Wen, C. Surface modification of additively manufactured metallic biomaterials with active antipathogenic properties. Smart Mater. Manuf. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The potential application of green-synthesized metal nanoparticles in dentistry: A comprehensive review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef]
- Allizond, V.; Comini, S.; Cuffini, A.M.; Banche, G. Current knowledge on biomaterials for orthopedic applications modified to reduce bacterial adhesive ability. Antibiotics 2022, 11, 529. [Google Scholar] [CrossRef]
- Wang, X.; Fan, H.; Zhang, F.; Zhao, S.; Liu, Y.; Xu, Y.; Wu, R.; Li, D.; Yang, Y.; Liao, L.; et al. Antibacterial Properties of Bilayer Biomimetic Nano-ZnO for Dental Implants. ACS Biomater. Sci. Eng. 2020, 6, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, S.; Gill, R. Applications of biopolymer coatings in biomedical engineering. J. Electrochem. Sci. Eng. 2023, 13, 63–81. [Google Scholar] [CrossRef]
- Nawae, S.; Meesane, J.; Muensit, N.; Daengngam, C. Layer-by-layer self-assembled films of silk fibroin/collagen/poly (diallyldimethylammonium chloride) as nucleating surface for osseointegration to design coated dental implant materials. Mater. Des. 2018, 160, 1158–1167. [Google Scholar] [CrossRef]
- Murchio, S.; Benedetti, M.; Berto, A.; Agostinacchio, F.; Zappini, G.; Maniglio, D. Hybrid Ti6Al4V/Silk Fibroin Composite for Load-Bearing Implants: A Hierarchical Multifunctional Cellular Scaffold. Materials 2022, 15, 6156. [Google Scholar] [CrossRef]
- Li, G.; Sun, S. Silk Fibroin-Based Biomaterials for Tissue Engineering Applications. Molecules 2022, 27, 2757. [Google Scholar] [CrossRef] [PubMed]
- Roxana, V.M.J.; Calenic, B.; Zaharia, C.; Maria, G. Silk fibroin and potential uses in regenerative dentistry-a systematic review. Stomatol. Educ. J. 2015, 1, 108–115. [Google Scholar]
- Karatepe, U.Y.; Ozdemir, T. Improving mechanical and antibacterial properties of PMMA via polyblend electrospinning with silk fibroin and polyethyleneimine towards dental applications. Bioact. Mater. 2020, 5, 510–515. [Google Scholar] [CrossRef]
- Sideratou, Z.; Biagiotti, M.; Tsiourvas, D.; Panagiotaki, K.N.; Zucca, M.V.; Freddi, G.; Lovati, A.B.; Bottagisio, M. Antibiotic-Loaded Hyperbranched Polyester Embedded into Peptide-Enriched Silk Fibroin for the Treatment of Orthopedic or Dental Infections. Nanomaterials 2022, 12, 3182. [Google Scholar] [CrossRef] [PubMed]
- Bottagisio, M.; Palombella, S.; Lopa, S.; Sangalli, F.; Savadori, P.; Biagiotti, M.; Sideratou, Z.; Tsiourvas, D.; Lovati, A.B. Vancomycin-nanofunctionalized peptide-enriched silk fibroin to prevent methicillin-resistant Staphylococcus epidermidis-induced femoral nonunions in rats. Front. Cell. Infect. Microbiol. 2023, 12, 1963. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Jang, Y.S.; Jang, J.H.; Bae, T.S.; Lee, S.J.; Lee, M.H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019847067. [Google Scholar] [CrossRef]
- Jia, L.; Han, F.; Wang, H.; Zhu, C.; Guo, Q.; Li, J.; Zhao, Z.; Zhang, Q.; Zhu, X.; Li, B. Polydopamine-assisted surface modification for orthopaedic implants. J. Orthop. Transl. 2019, 17, 82–95. [Google Scholar] [CrossRef]
- Du, L.; Liao, R.; Zhang, H.; Qu, X.; Hu, X. Redox-activity of polydopamine for ultrafast preparation of self-healing and adhesive hydrogels. Colloids Surf. B Biointerfaces 2022, 214, 112469. [Google Scholar] [CrossRef]
- Zhu, Y.-W.; Sun, Y.-J.; Wang, J.-L.; Yu, B.-R. Antimicrobial and antifouling surfaces through polydopamine bio-inspired coating. Rare Met. 2022, 41, 499–518. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Pardeshi, S.; Sharma, J.G.; Lee, S.H.; Choi, E.H. Biomedical and Clinical Importance of Mussel-Inspired Polymers and Materials. Mar. Drugs 2015, 13, 6792–6817. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liu, H.; Lin, M.; Xie, W.; Yang, X.; Cai, Y. Corrigendum: Controllable performance of a dopamine-modified silk fibroin-based bio-adhesive by doping metal ions (2021Biomed. Mater.16045025). Biomed. Mater. 2023, 18, 049509. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L.; Babanejad, N. Bioinspired Polymers: Transformative Applications in Biomedicine and Regenerative Medicine. Life 2023, 13, 1673. [Google Scholar] [CrossRef]
- Păun, A.G. TiO2 Surfaces Modification for Amoxicillin Release Used in Dental Implantology. U.P.B. Sci. Bull. Ser. B 2023, 85, 71–84. [Google Scholar]
- Popescu, S.; Zarif, M.-E.; Dumitriu, C.; Ungureanu, C.; Pirvu, C. Silk Fibroin-Based Hybrid Nanostructured Coatings for Titanium Implantable Surfaces Modification. Coatings 2020, 10, 518. [Google Scholar] [CrossRef]
- Zhang, X.; Baughman, C.B.; Kaplan, D.L. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials 2008, 29, 2217–2227. [Google Scholar] [CrossRef]
- Olaru, A.G.; Butculescu, V.; Dumitriu, C.; Badea, N.; Popescu, S.; Ungureanu, C.; Pirvu, C. Biopolymers as intermediate layers for amoxicillin grafting on antibacterial surface. Surf. Interfaces 2022, 33, 102224. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Q.; Shen, X.; Yin, Q.; Pan, M.; Jiang, C.; Hu, C. Enhancing the Interfacial Adhesion with Rubber Matrix by Grafting Polydopamine-Carbon Nanotubes onto Poly(p-phenylene terephthalamide) Fibers. Polymers 2019, 11, 1231. [Google Scholar] [CrossRef]
- Jaiswal, S.; Duffy, B.; Jaiswal, A.K.; Stobie, N.; McHale, P. Enhancement of the antibacterial properties of silver nanoparticles using beta-cyclodextrin as a capping agent. Int. J. Antimicrob. Agents 2010, 36, 280–283. [Google Scholar] [CrossRef]
- Saha, S.; Pramanik, K.; Biswas, A. Silk fibroin coated TiO2 nanotubes for improved osteogenic property of Ti6Al4V bone implants. Mater. Sci. Eng. C 2019, 105, 109982. [Google Scholar] [CrossRef]
- Ungureanu, C.; Dumitriu, C.; Popescu, S.; Enculescu, M.; Tofan, V.; Popescu, M.; Pirvu, C. Enhancing antimicrobial activity of TiO2/Ti by torularhodin bioinspired surface modification. Bioelectrochemistry 2016, 107, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Mezzourh, H.; Ben Moumen, S.; Amjoud, M.; Mezzane, D.; El Amraoui, Y.; Marbati, B.; Lahmar, A.; Jouiad, M.; El Marssi, M. Effect of growth time on structural and surface properties of TiO2 nanostructures deposited by single-step hydrothermal method. Mater. Today Proc. 2021, 51, 2053–2058. [Google Scholar] [CrossRef]
- Tan, T.; Zhao, Q.; Kuwae, H.; Ueno, T.; Chen, P.; Tsutsumi, Y.; Mizuno, J.; Hanawa, T.; Wakabayashi, N. Surface properties and biocompatibility of sandblasted and acid-etched titanium–zirconium binary alloys with various compositions. Dent. Mater. J. 2022, 41, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Anitha, V.; Lee, J.-H.; Lee, J.; Banerjee, A.N.; Joo, S.W.; Min, B.K. Biofilm formation on a TiO2 nanotube with controlled pore diameter and surface wettability. Nanotechnology 2015, 26, 065102. [Google Scholar] [CrossRef] [PubMed]
- Genna, S.; Giannini, O.; Guarino, S.; Ponticelli, G.S.; Tagliaferri, F. Laser texturing of AISI 304 stainless steel: Experimental analysis and genetic algorithm optimisation to control the surface wettability. Int. J. Adv. Manuf. Technol. 2020, 110, 3005–3022. [Google Scholar] [CrossRef]
- Sun, D.; Böhringer, K.F. Self-Cleaning: From Bio-Inspired Surface Modification to MEMS/Microfluidics System Integration. Micromachines 2019, 10, 101. [Google Scholar] [CrossRef]
- Kim, H.; Kumbar, S.G.; Nukavarapu, S.P. Biomaterial-directed cell behavior for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100260. [Google Scholar] [CrossRef] [PubMed]
- Cestari, M.; Caldas, B.S.; Fonseca, D.P.; Balbinot, R.B.; Lazarin-Bidóia, D.; Otsuka, I.; Nakamura, C.V.; Borsali, R.; Muniz, E.C. Silk fibroin nanofibers containing chondroitin sulfate and silver sulfadiazine for wound healing treatment. J. Drug Deliv. Sci. Technol. 2022, 70, 103221. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.L.; Dai, F.Y.; Zhang, H.H.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y.Z. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J. Transl. Med. 2012, 10, 117. [Google Scholar] [CrossRef]
- Tong, M.-H.; Chen, Y.-X.; Lin, S.-W.; Zhao, H.-P.; Chen, R.; Jiang, X.; Shi, H.-Y.; Zhu, M.-L.; Zhou, Q.-Q.; Lu, C.-Z. Synchronous electrochemical anodization: A novel strategy for preparing cerium doped TiO2 nanotube arrays toward visible-light PEC water splitting. Electrochim. Acta 2023, 463, 142793. [Google Scholar] [CrossRef]
- Chiou, S.-H.; Ho, H.-C.; Liao, H.-T.; Tsai, F.-Y.; Tsao, C.-W.; Hsu, Y.-J.; Hsueh, C.-H. Plasmonic gold nanoplates-decorated ZnO branched nanorods@TiO2 nanorods heterostructure photoanode for efficient photoelectrochemical water splitting. J. Photochem. Photobiol. A Chem. 2023, 443, 114816. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Biesold-McGee, G.V.; Huang, J.; Ng, Y.H.; Sun, H.; Wang, J.; Lai, Y.; Lin, Z. Silk fibroin-derived nitrogen-doped carbon quantum dots anchored on TiO2 nanotube arrays for heterogeneous photocatalytic degradation and water splitting. Nano Energy 2020, 78, 105313. [Google Scholar] [CrossRef]
- Kadri, Y.; Srasra, E.; Bekri-Abbes, I.; Herrasti, P. Facile and eco-friendly synthesis of polyaniline/ZnO composites for corrosion protection of AA-2024 aluminium alloy. J. Electroanal. Chem. 2021, 893, 115335. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Tian, J.; Xu, X.; Dai, J.; Wang, X. Preparation and characterization of TiO2/ZnO composite coating on carbon steel surface and its anticorrosive behavior in seawater. Compos. Part B Eng. 2013, 46, 135–144. [Google Scholar] [CrossRef]
- Zhang, C.; Huffer, J.; Sprik, M. Coupling of Surface Chemistry and Electric Double Layer at TiO2 Electrochemical Interfaces. J. Phys. Chem. Lett. 2019, 10, 3871–3876. [Google Scholar] [CrossRef] [PubMed]
- Poortinga, A.T.; Bos, R.; Norde, W.; Busscher, H.J. Electric double layer interactions in bacterial adhesion to surfaces. Surf. Sci. Rep. 2002, 47, 3–32. [Google Scholar] [CrossRef]
- Rijnaarts, H.H.M.; Norde, W.; Lyklema, J.; Zehnder, A.J.B. DLVO and steric contributions to bacterial deposition in media of different ionic strengths. Colloid Surf. B 1999, 14, 179–195. [Google Scholar] [CrossRef]
- Roessler, S.; Zimmermann, R.; Scharnweber, D.; Werner, C.; Worch, H. Characterization of oxide layers on Ti6Al4V and titanium by streaming potential and streaming current measurements. Colloid Surf. B 2002, 26, 387–395. [Google Scholar] [CrossRef]
- Thulasi, K.M.; Manikkoth, S.T.; Paravannoor, A.; Palantavida, S.; Vijayan, B.K. Supercapacitor electrodes based on modified titania nanotube arrays on flexible substrates. Int. J. Mater. Res. 2021, 112, 937–944. [Google Scholar] [CrossRef]
- Bao, W.; Wu, Y.; Xie, Y.; Yao, C. Fabrication and electrochemical performance of nickel oxide nanoparticles anchored titanium dioxide nanotube array hybrid electrode. Funct. Mater. Lett. 2020, 13, 2051017. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Dettlaff, A.; Skiba, F.; Trzcinski, K.; Szkoda, M.; Sobaszek, M.; Ficek, M.; Dec, B.; Macewicz, L.; Wyrębski, K.; et al. Enhanced Charge Storage Mechanism and Long-Term Cycling Stability in Diamondized Titania Nanocomposite Supercapacitors Operating in Aqueous Electrolytes. J. Phys. Chem. C 2020, 124, 15698–15712. [Google Scholar] [CrossRef]
- Guerra, A.; Achour, A.; Vizireanu, S.; Dinescu, G.; Messaci, S.; Hadjersi, T.; Boukherroub, R.; Coffinier, Y.; Pireaux, J.-J. ZnO/Carbon nanowalls shell/core nanostructures as electrodes for supercapacitors. Appl. Surf. Sci. 2019, 481, 926–932. [Google Scholar] [CrossRef]
- Mîndroiu, V.M.; Stoian, A.B.; Irodia, R.; Trușcă, R.; Vasile, E. Titanium Dioxide Thin Films Produced on FTO Substrate Using the Sol–Gel Process: The Effect of the Dispersant on Optical, Surface and Electrochemical Features. Materials 2023, 16, 3147. [Google Scholar] [PubMed]
- Baran Aydın, E.; Sığırcık, G. Preparations of different ZnO nanostructures on TiO2 nanotube via electrochemical method and its application in hydrogen production. Int. J. Hydrogen Energy 2019, 44, 11488–11502. [Google Scholar] [CrossRef]
- Cai, H.; Liang, P.; Hu, Z.; Shi, L.; Yang, X.; Sun, J.; Xu, N.; Wu, J. Enhanced Photoelectrochemical Activity of ZnO-Coated TiO2 Nanotubes and Its Dependence on ZnO Coating Thickness. Nanoscale Res. Lett. 2016, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gázquez, P.J.; Blasco-Tamarit, E.; Muñoz-Portero, M.J.; Solsona, B.; Fernández-Domene, R.M.; Sánchez-Tovar, R.; García-Antón, J. Influence of Zn(NO3)2 concentration during the ZnO electrodeposition on TiO2 nanosponges used in photoelectrochemical applications. Ceram. Int. 2022, 48, 14460–14472. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Sedarat, Z.; Taylor-Robinson, A.W. Biofilm Formation by Pathogenic Bacteria: Applying a Staphylococcus aureus Model to Appraise Potential Targets for Therapeutic Intervention. Pathogens 2022, 11, 388. [Google Scholar] [CrossRef]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococcal infection—Treatment and antibiotic resistance. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Lin, Y.; Zhang, L.; Yang, Y.; Yang, M.; Hong, Q.; Chang, K.; Dai, J.; Chen, L.; Pan, C.; Hu, Y.; et al. Loading Gentamicin and Zn2+ on TiO2 Nanotubes to Improve Anticoagulation, Endothelial Cell Growth, and Antibacterial Activities. Stem Cells Int. 2021, 2021, 9993247. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules 2022, 12, 1852. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, M.; Sheikh, T.M.M.; Mumtaz, M.Z.; Chohan, T.A.; Shamim, S.; Liu, Y. Zinc Essentiality, Toxicity, and Its Bacterial Bioremediation: A Comprehensive Insight. Front. Microbiol. 2022, 13, 900740. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Ramirez, M.S.; Tolmasky, M.E. Zinc: Multidimensional effects on living organisms. Biomedicines 2021, 9, 208. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Wu, S.; Zhou, R.; Zhang, K.; Zhang, Z.; Liu, J.; Qin, S.; Shi, J. Nanomaterial-based zinc ion interference therapy to combat bacterial infections. Front. Immunol. 2022, 13, 899992. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, C.; Prokocimer Yair, Z.; Reifen, R.; Shemesh, M. Antimicrobial effect of Zn2+ ions governs the microbial quality of donor human milk. Foods 2021, 10, 637. [Google Scholar] [CrossRef]
- Abdelghafar, A.; Yousef, N.; Askoura, M. Zinc oxide nanoparticles reduce biofilm formation, synergize antibiotics action and attenuate Staphylococcus aureus virulence in host; an important message to clinicians. BMC Microbiol. 2022, 22, 244. [Google Scholar] [CrossRef] [PubMed]
- Aydin Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Almoudi, M.M.; Hussein, A.S.; Abu Hassan, M.I.; Mohamad Zain, N. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J. 2018, 30, 283–291. [Google Scholar] [CrossRef]
- Tsang, P.C.; Chu, F.C.; Samaranayake, L.P. Staphylococci may indeed cause acute dental infections. BMJ Clin. Res. Ed. 2002, 325, 599. [Google Scholar] [CrossRef][Green Version]
- Ribeiro, N.F.; Cousin, G.C. Staphylococci are unlikely to cause acute dental infections. BMJ Clin. Res. Ed. 2002, 324, 1457. [Google Scholar] [CrossRef][Green Version]
- El-Telbany, M.; El-Didamony, G.; Askora, A. Bacteriophages to Control Multi-Drug Resistant Enterococcus faecalis Infection of Dental Root Canals. Microorganisms 2021, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Kayaoglu, G.; Ørstavik, D. Virulence factors of Enterococcus faecalis: Relationship to endodontic disease. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2004, 15, 308–320. [Google Scholar] [CrossRef] [PubMed]
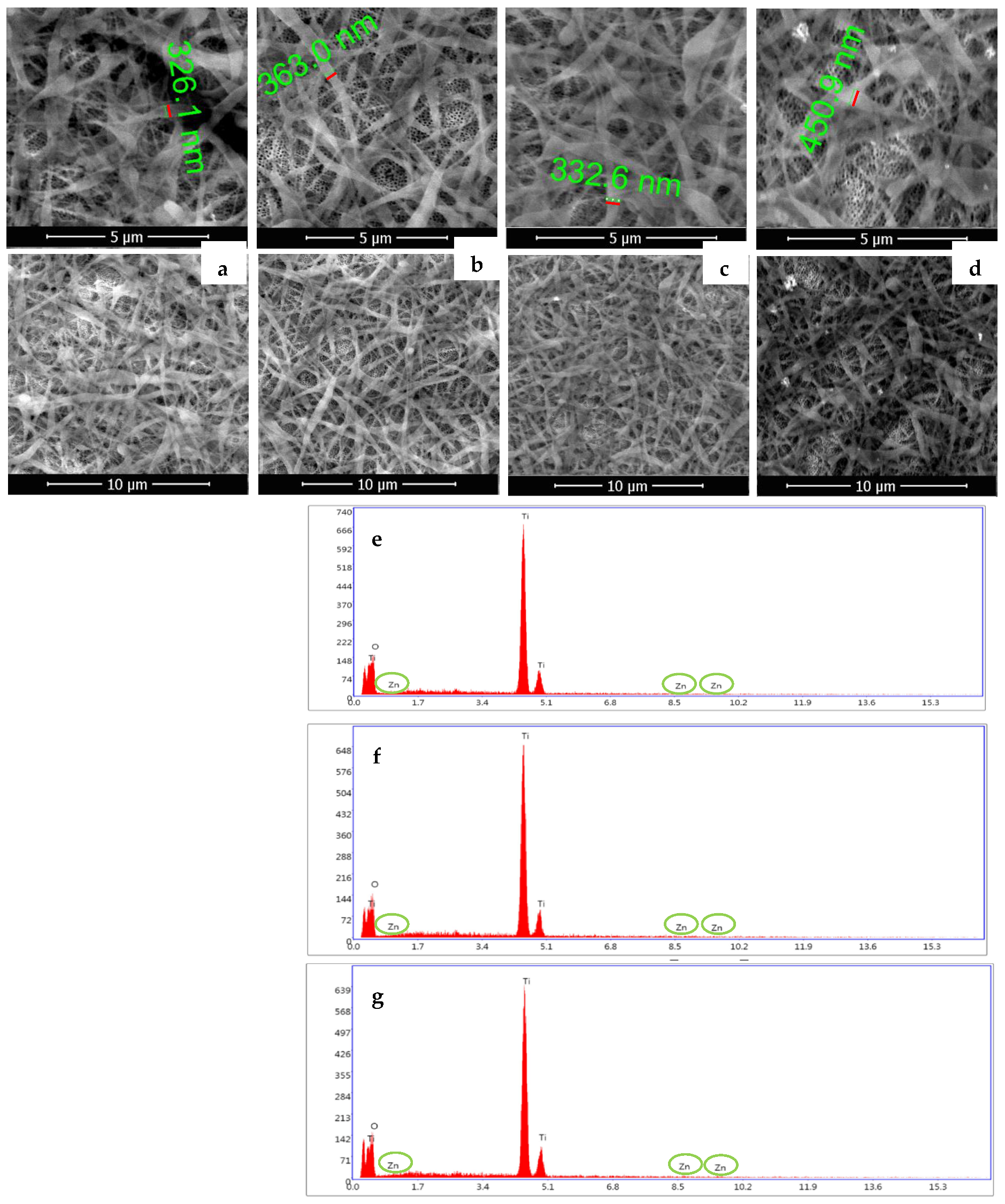

| Samples | Weight % | ||
|---|---|---|---|
| Ti | O | Zn | |
| NT/SF-ZnO | 52.46 | 47.17 | 0.37 |
| NT/ZnO/SF | 54.32 | 45.28 | 0.40 |
| NT/SF/PD/ZnO | 52.18 | 47.35 | 0.47 |
| Parameters Samples | RS (Ω) | RPD (Ω) | CPEPD | Rcoating (Ω) | CPEcoating | Roxide (Ω) | CPEoxide | X2 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Y0 (µS·sn) | N | Y0 (µS·sn) | N | Y0 (µS·sn) | N | ||||||
| NT | 99 | 4 × 103 | 62 | 0.71 | 20 × 103 | 149 | 0.68 | 0.01 | |||
| NT/SF | 91 | - | - | - | 8 × 103 | 266 | 0.92 | 136 × 103 | 65 | 0.96 | 0.07 |
| NT/SF-ZnO | 68 | 9 × 103 | 278 | 0.82 | 156 × 103 | 900 | 0.93 | 0.04 | |||
| NT/ZnO/SF | 68 | 10 × 103 | 356 | 0.87 | 184 × 103 | 897 | 0.98 | 0.03 | |||
| NT/SF/PD/ZnO | 63 | 1.7 × 103 | 79 | 0.74 | 5 × 103 | 270 | 0.61 | 92 × 103 | 297 | 0.89 | 0.01 |
| Samples |
Corrosion Potential (V) |
Corrosion Current Density (A/cm2) |
Corrosion Rate (mm/Year) |
Protection Efficiency (%) |
|---|---|---|---|---|
| NT | −0.161 | 3.896 × 10−6 | 0.0452 | - |
| NT/SF | −0.092 | 0.359 × 10−6 | 0.0042 | 91 |
| NT/SF-ZnO | −0.104 | 0.249 × 10−6 | 0.0029 | 94 |
| NT/ZnO/SF | −0.091 | 0.119 × 10−6 | 0.0014 | 97 |
| NT/SF/PD/ZnO | −0.071 | 0.568 × 10−6 | 0.0066 | 84 |
| Samples | Enterococcus faecalis | Staphylococcus aureus |
|---|---|---|
| NT | - | - |
| NT/SF | 15 | 13 |
| NT/SF-ZnO | 41 | 39 |
| NT/ZnO/SF | 42 | 41 |
| NT/SF/PD/ZnO | 55 | 53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păun, A.G.; Dumitriu, C.; Ungureanu, C.; Popescu, S. Silk Fibroin/ZnO Coated TiO2 Nanotubes for Improved Antimicrobial Effect of Ti Dental Implants. Materials 2023, 16, 5855. https://doi.org/10.3390/ma16175855
Păun AG, Dumitriu C, Ungureanu C, Popescu S. Silk Fibroin/ZnO Coated TiO2 Nanotubes for Improved Antimicrobial Effect of Ti Dental Implants. Materials. 2023; 16(17):5855. https://doi.org/10.3390/ma16175855
Chicago/Turabian StylePăun, Angela Gabriela, Cristina Dumitriu, Camelia Ungureanu, and Simona Popescu. 2023. "Silk Fibroin/ZnO Coated TiO2 Nanotubes for Improved Antimicrobial Effect of Ti Dental Implants" Materials 16, no. 17: 5855. https://doi.org/10.3390/ma16175855
APA StylePăun, A. G., Dumitriu, C., Ungureanu, C., & Popescu, S. (2023). Silk Fibroin/ZnO Coated TiO2 Nanotubes for Improved Antimicrobial Effect of Ti Dental Implants. Materials, 16(17), 5855. https://doi.org/10.3390/ma16175855