Preparation, Features, and Efficiency of Nanocomposite Fertilisers Based on Glauconite and Ammonium Dihydrogen Phosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
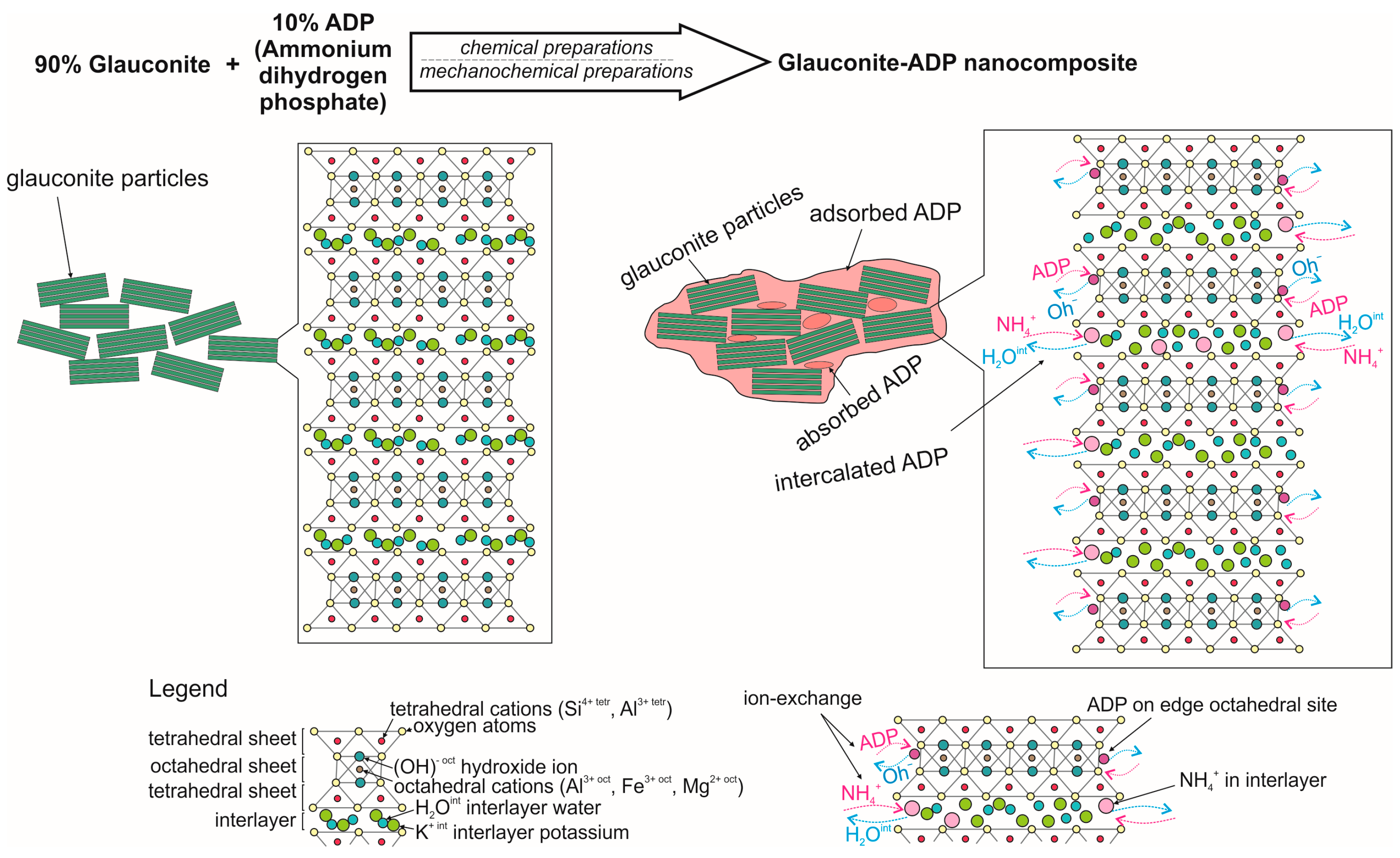
2.2. Preparation of Nanocomposites
2.3. Nanocomposite Analysis
2.4. Leach Testing
2.5. Plant Cultivation Test
3. Results
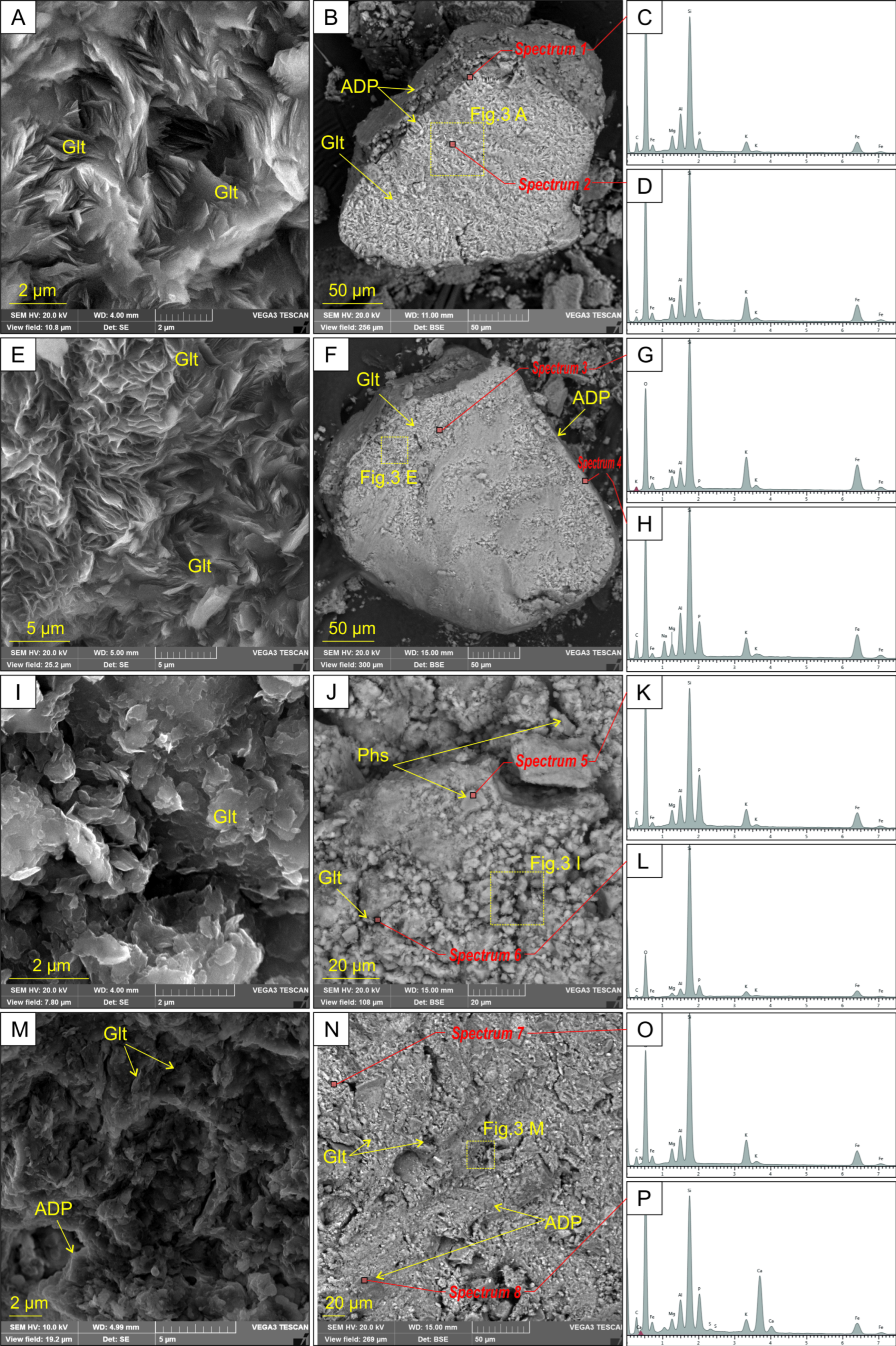
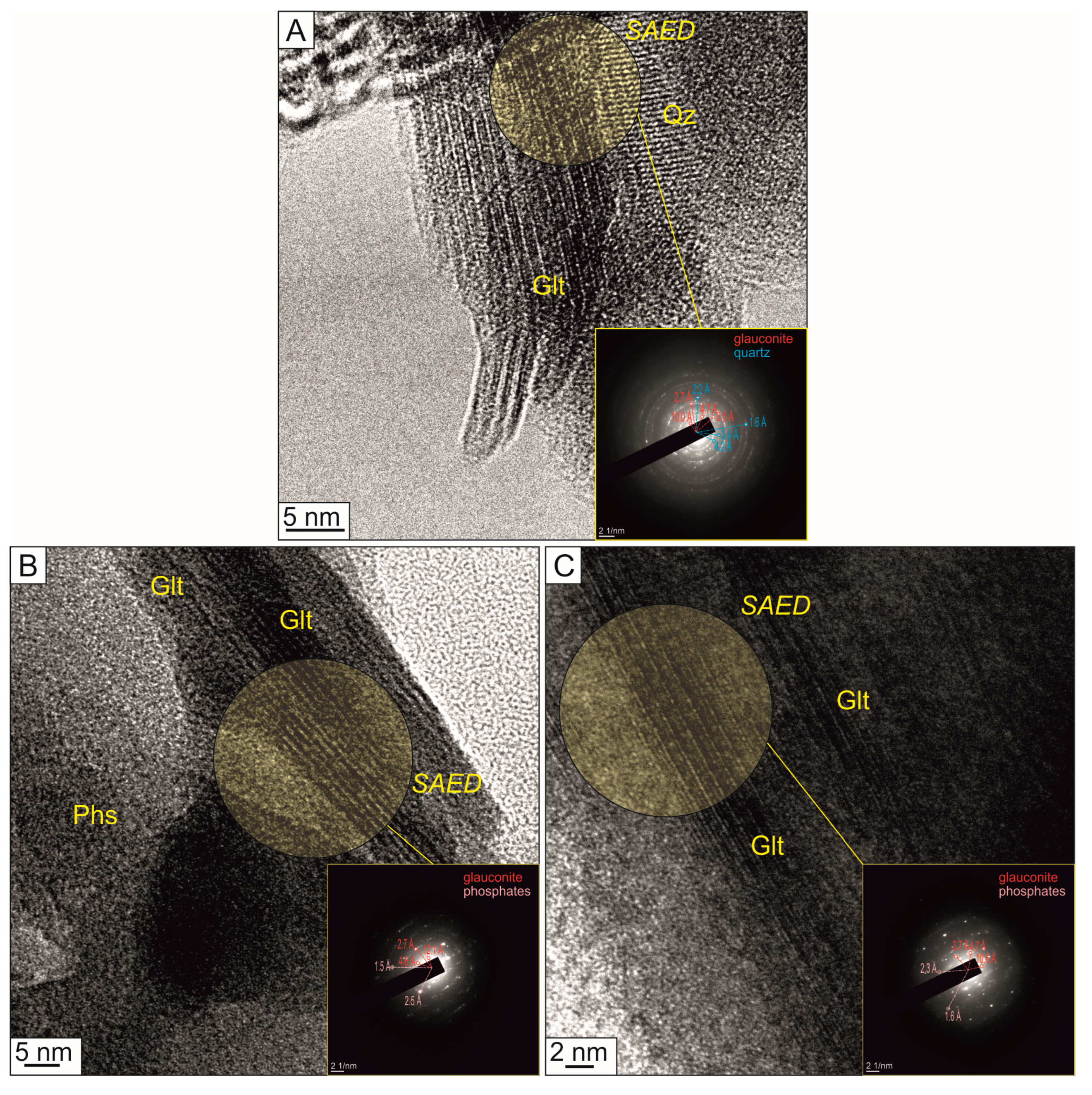
3.1. Mineralogical and Morphological Characterisations of Nanocomposites
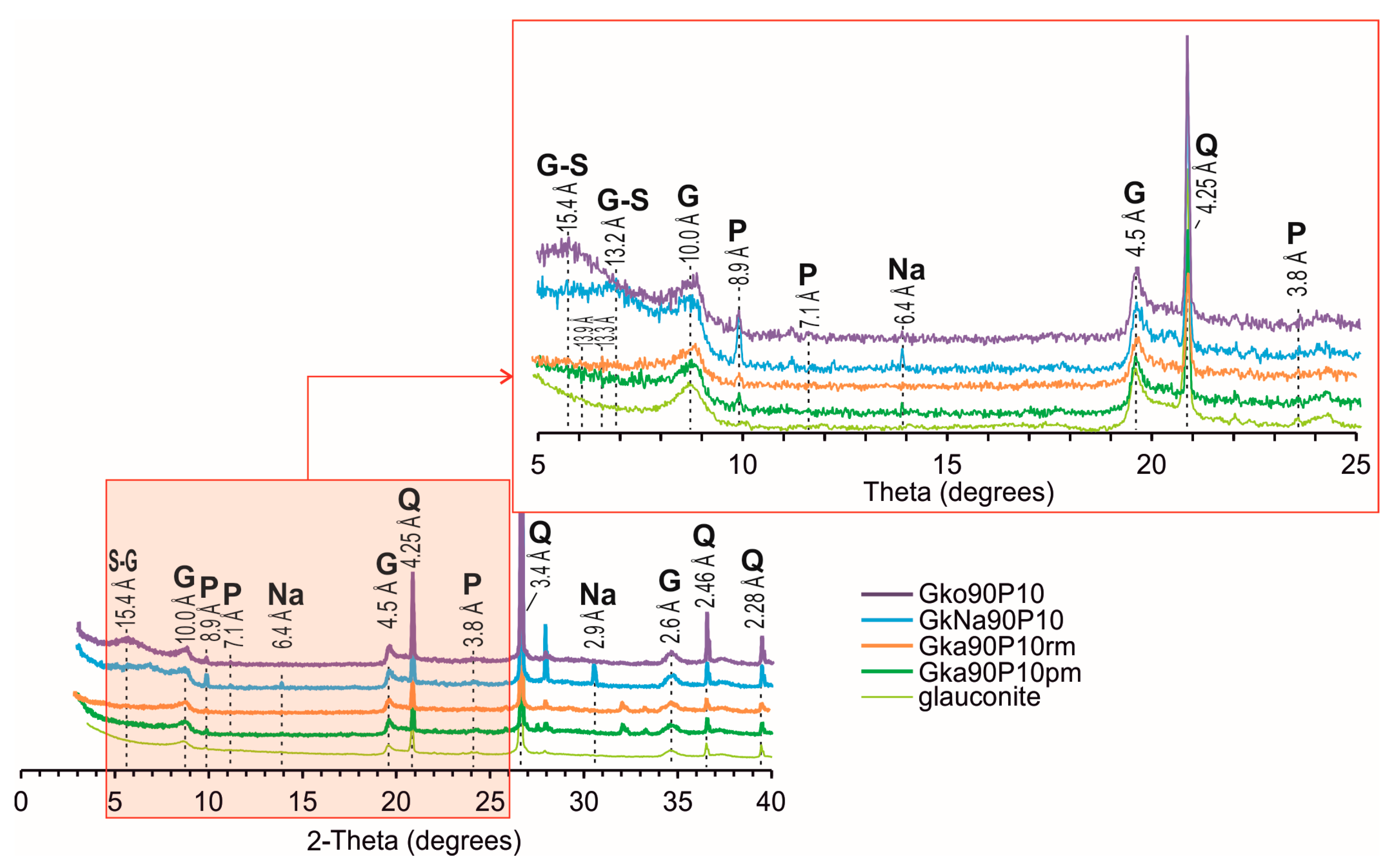
3.2. Crystal Structure Characteristics of Nanocomposites
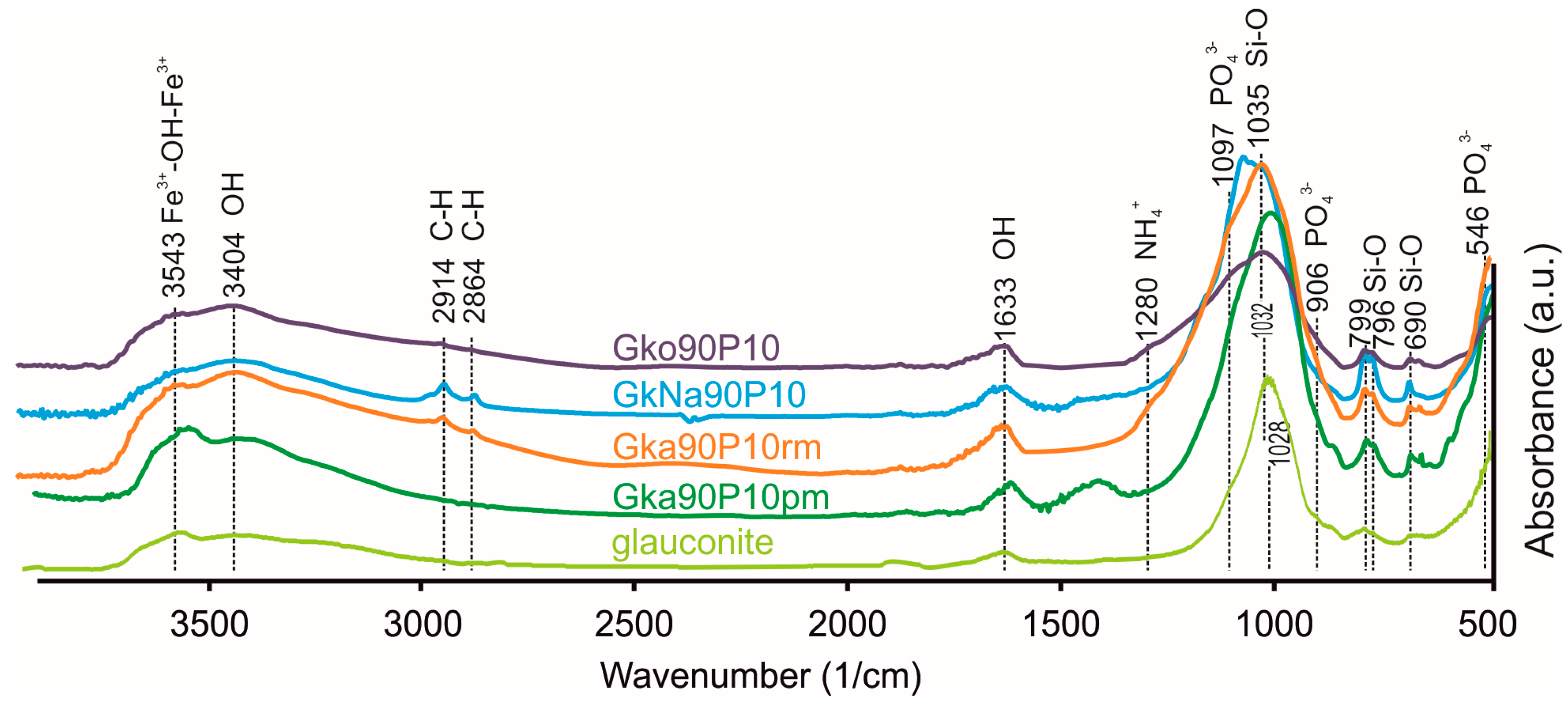
3.3. Chemical Characterisations of Nanocomposites
3.4. Kinetic Nutrients Releases
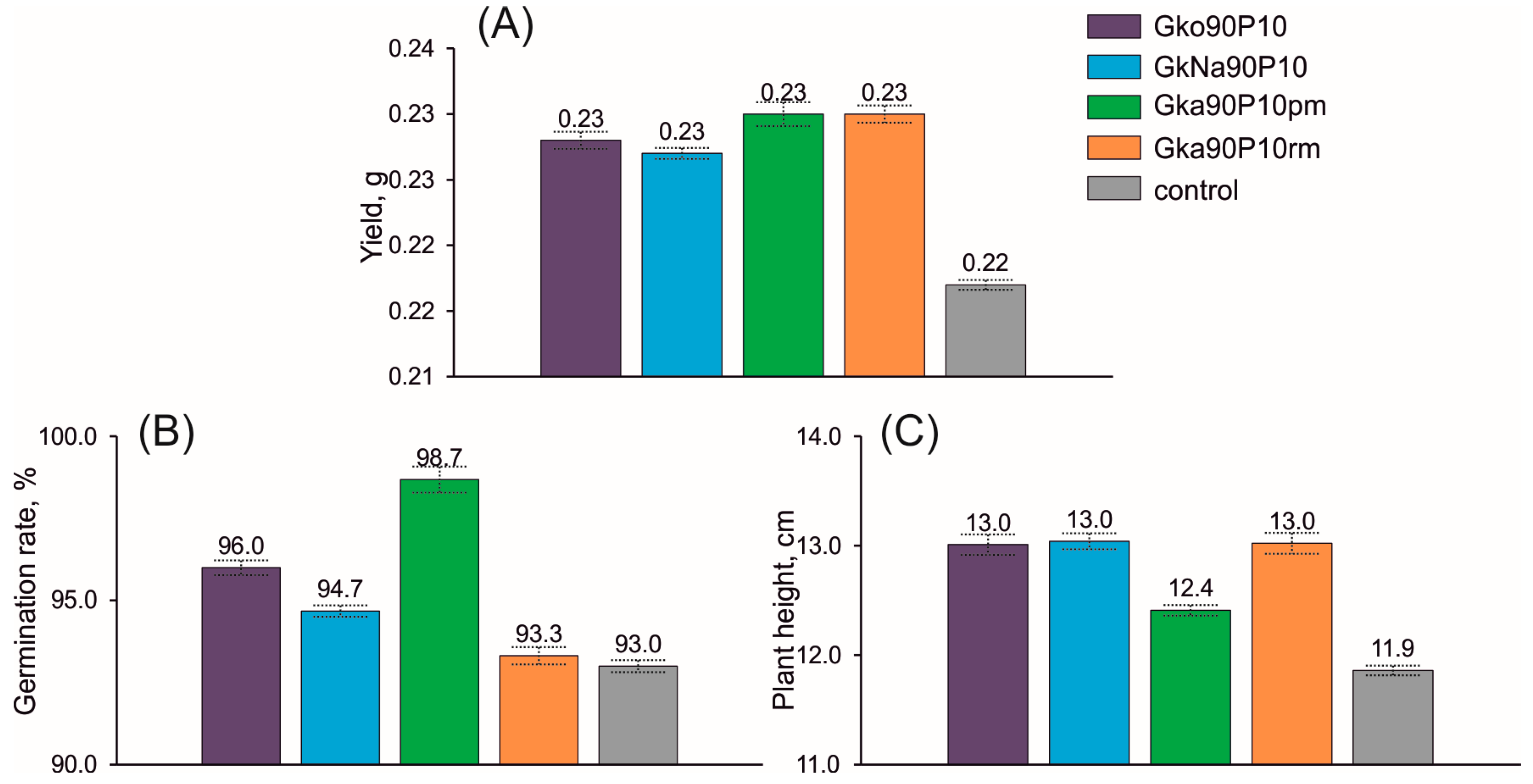
3.5. Influence of Nanocomposite on Plant Growth
4. Discussion
4.1. Characteristics of Glauconite–ADP Nanocomposites
4.2. Advantages of Glauconite–ADP Nanocomposites
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J.; Sutherland, W.J.; Ashby, J.; Auburn, J.; Baulcombe, D.; Bell, M.; Bentley, J.; Bickersteth, S.; Brown, K.; Burke, J.; et al. The top 100 questions of importance to the future of global agriculture. Int. J. Agric. Sustain. 2010, 8, 219–236. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.; Wolff, B.; D’Antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef]
- Fiorella, K.J.; Chen, R.L.; Milner, E.M.; Fernald, L.C.H. Agricultural interventions for improved nutrition: A review of livelihood and environmental dimensions. Glob. Food Secur. 2016, 8, 39–47. [Google Scholar] [CrossRef]
- Trenkel, M.E. Controlled-Release and Stabilized Fertilizers in Agriculture; International Fertilizer Industry Association: Paris, France, 1997. [Google Scholar]
- Matson, P.A. Agricultural Intensification and Ecosystem Properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Price, S.; Bennett, G.; Maxwell, T.M.R.; Zhao, C.; Walker, G.; Bunt, C. A landscape review of controlled release urea products: Patent objective, formulation and technology. J. Control. Release 2022, 348, 612–630. [Google Scholar] [CrossRef]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Chang. Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, F.; Zhang, Y.; Wang, J.; Zhuge, Y.; Zhang, S.; Gao, H. Effect of bag-controlled release fertilizer on nitrogen loss, greenhouse gas emissions, and nitrogen applied amount in peach production. J. Clean. Prod. 2019, 234, 258–274. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef]
- Lasheen, E.S.R.; Zakaly, H.M.H.; Alotaibi, B.M.; Saadawi, D.A.; Ene, A.; Fathy, D.; Awad, H.A.; El Attar, R.M. Radiological Risk Parameters of the Phosphorite Deposits, Gebel Qulu El Sabaya: Natural Radioactivity and Geochemical Characteristics. Minerals 2022, 12, 1385. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Mao, S.; Han, Y.; Chen, F.; Zhang, L.; Li, Y.; Li, C. Comparison of greenhouse gas emissions of chemical fertilizer types in China’s crop production. J. Clean. Prod. 2017, 141, 1267–1274. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Moraes, P.I.R.; Tavares, S.R.; Vaiss, V.S.; Leitão, A.A. Investigation on sustainable phosphate release in agriculture: Structural and thermodynamic study of stability, dehydration and anionic exchange of Mg-Al-HPO4 layered double hydroxide by DFT calculations. Appl. Clay Sci. 2018, 162, 428–434. [Google Scholar] [CrossRef]
- Basak, B.B.B.; Gajbhiye, N.A.A. Phosphorus enriched organic fertilizer, an effective P source for improving yield and bioactive principle of Senna (Cassia angustifolia Vhal.). Ind. Crops Prod. 2018, 115, 208–213. [Google Scholar] [CrossRef]
- Hermassi, M.; Valderrama, C.; Font, O.; Moreno, N.; Querol, X.; Batis, N.H.; Cortina, J.L. Phosphate recovery from aqueous solution by K-zeolite synthesized from fly ash for subsequent valorisation as slow release fertilizer. Sci. Total Environ. 2020, 731, 139002. [Google Scholar] [CrossRef]
- Pogorzelski, D.; Filho, J.F.L.; Matias, P.C.; Santos, W.O.; Vergütz, L.; Melo, L.C.A. Biochar as composite of phosphate fertilizer: Characterization and agronomic effectiveness. Sci. Total Environ. 2020, 743, 140604. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lv, X.; Fu, M.; Wang, Z.; Zhang, D.; Sun, Q. Risk assessment of Artemia egg shell-Mg-P composites as a slow-release phosphorus fertilizer during its formation and application in typical heavy metals contaminated environment. J. Environ. Manag. 2023, 329, 117092. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Xie, X. Chitosan-Montmorillonite-Fe Nanocomposite Hydrogel for Phosphate Recovery and Reuse. ACS ES T Eng. 2022, 3, 682–689. [Google Scholar] [CrossRef]
- Jariwala, H.; Santos, R.M.; Lauzon, J.D.; Dutta, A.; Wai Chiang, Y. Controlled release fertilizers (CRFs) for climate-smart agriculture practices: A comprehensive review on release mechanism, materials, methods of preparation, and effect on environmental parameters. Environ. Sci. Pollut. Res. 2022, 29, 53967–53995. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, C.; Chen, X.; Huang, Z.; Chen, D. Classification research and types of slow controlled release fertilizers (SRFs) used—A review. Commun. Soil Sci. Plant Anal. 2018, 49, 2219–2230. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Rashid, M.; Hussain, Q.; Khan, K.S.; Alwabel, M.I.; Hayat, R.; Akmal, M.; Ijaz, S.S.; Alvi, S.; Rehman, O.U. Carbon-Based Slow-Release Fertilizers for Efficient Nutrient Management: Synthesis, Applications, and Future Research Needs. J. Soil Sci. Plant Nutr. 2021, 21, 1144–1169. [Google Scholar] [CrossRef]
- Saraiva, R.; Ferreira, Q.; Rodrigues, G.C.; Oliveira, M. Phosphorous Nanofertilizers for Precise Application in Rice Cultivation as an Adaptation to Climate Change. Climate 2022, 10, 183. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Li, C.; Cheng, C.; Zheng, Y. Adsorption of Ammonium by Graphene Oxide-Based Composites Prepared by UV Irradiation and Using as Slow-Release Fertilizer. J. Polym. Environ. 2018, 26, 4311–4320. [Google Scholar] [CrossRef]
- Oertli, J.J. Controlled-release fertilizers. Fertil. Res. 1980, 1, 103–123. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, H.; Xing, L.; Ding, W.; Geng, Z.; Xu, C. Cellulose-based slow-release nitrogen fertilizers: Synthesis, properties, and effects on pakchoi growth. Int. J. Biol. Macromol. 2023, 244, 125413. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Gao, B.; Li, Y.C.; Xie, J. Bio-based elastic polyurethane for controlled-release urea fertilizer: Fabrication, properties, swelling and nitrogen release characteristics. J. Clean. Prod. 2019, 209, 528–537. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Kazantseva, E.A.; Varygina, D.E.; Volova, T.G. Constructing Slow-Release Formulations of Ammonium Nitrate Fertilizer Based on Degradable Poly(3-hydroxybutyrate). J. Agric. Food Chem. 2017, 65, 6745–6752. [Google Scholar] [CrossRef] [PubMed]
- Atia, A.A.; Donia, A.M.; Hussin, R.A.; Rashad, R.T. Swelling and metal ion uptake characteristics of kaolinite containing poly [(acrylic acid)-co-acrylamide] hydrogels. Desalination Water Treat. 2009, 3, 73–82. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly(AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr. Polym. 2014, 114, 269–278. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environ. Sci. Ecotechnol. 2022, 10, 100167. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K. Hydrogel-biochar composite for agricultural applications and controlled release fertilizer: A step towards pollution free environment. Energy 2022, 242, 122977. [Google Scholar] [CrossRef]
- Abhiram, G.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C.E.; McCurdy, M. Iron-rich sand promoted nitrate reduction in a study for testing of lignite based new slow-release fertilisers. Sci. Total Environ. 2023, 864, 160949. [Google Scholar] [CrossRef]
- Borges, R.; Brunatto, S.F.; Leitão, A.A.; De Carvalho, G.S.G.; Wypych, F. Solid-state mechanochemical activation of clay minerals and soluble phosphate mixtures to obtain slow-release fertilizers. Clay Miner. 2015, 50, 153–162. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Hu, C.; He, Z.-Q.; Abbas, Y.; Li, Y.; Lv, L.-F.; Hao, X.-Y.; Gai, G.-S.; Huang, Z.-H.; Yang, Y.-F.; et al. Microcrystalline Apatite Minerals: Mechanochemical Activation for Agricultural Application. Minerals 2019, 9, 211. [Google Scholar] [CrossRef]
- Rudmin, M.; Abdullayev, E.; Ruban, A.; Buyakov, A.; Soktoev, B. Mechanochemical Preparation of Slow Release Fertilizer Based on Glauconite–Urea Complexes. Minerals 2019, 9, 507. [Google Scholar] [CrossRef]
- Pimsen, R.; Porrawatkul, P.; Nuengmatcha, P.; Ramasoot, S.; Chanthai, S. Efficiency enhancement of slow release of fertilizer using nanozeolite–chitosan/sago starch-based biopolymer composite. J. Coat. Technol. Res. 2021, 18, 1321–1332. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Alrbaihat, M.; Moosa, I.; Abu-Afifeh, Q.; Al-Fayyad, H.; Hamadneh, I.; Al-Rawajfeh, A. Mechanochemical Preparation of a Novel Slow-Release Fertilizer Based on K2SO4-kaolinite. Agronomy 2022, 12, 3016. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Makarov, B.; Belousov, P.; Kurovsky, A.; Ibraeva, K.; Buyakov, A. Glauconite-Urea Nanocomposites As Polyfunctional Controlled-Release Fertilizers. J. Soil Sci. Plant Nutr. 2022, 22, 4035–4046. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.L.; Zhou, C.H.; Yang, H.M.; Ji, S.F.; Tong, D.S.; Zhong, Z.K.; Yu, W.H.; Chu, M.Q. Environmental-friendly montmorillonite-biochar composites: Facile production and tunable adsorption-release of ammonium and phosphate. J. Clean. Prod. 2017, 156, 648–659. [Google Scholar] [CrossRef]
- Maghsoodi, M.R.; Najafi, N.; Reyhanitabar, A.; Oustan, S. Hydroxyapatite nanorods, hydrochar, biochar, and zeolite for controlled-release urea fertilizers. Geoderma 2020, 379, 114644. [Google Scholar] [CrossRef]
- Silva, I.A.; Silva, D.S.; Sousa, M.U.; Menezes, R.R.; Ferreira, H.S.; Neves, G.A. Encapsulation of nitrogen fertilizers in mixtures with organoclays for controlled release. Clean Technol. Environ. Policy 2020, 1, 3. [Google Scholar] [CrossRef]
- McQuillan, R.V.; Stevens, G.W.; Mumford, K.A. Implementation of Biodegradable Liquid Marbles as a Novel Controlled Release Fertilizer. ACS Sustain. Chem. Eng. 2022, 11, 122–132. [Google Scholar] [CrossRef]
- Souza, J.L.; de Campos, A.; França, D.; Faez, R. PHB and Montmorillonite Clay Composites as KNO3 and NPK Support for a Controlled Release. J. Polym. Environ. 2019, 27, 2089–2097. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Makarov, B.; Ibraeva, K.; Konstantinov, A. Mechanical Activation of Smectite-Based Nanocomposites for Creation of Smart Fertilizers. Appl. Sci. 2022, 12, 809. [Google Scholar] [CrossRef]
- Rukchonlatee, S.; Siriphannon, P. Facile Preparation of Montmorillonite/Crosslinked Chitosan Containing Potassium Nitrate Nanocomposites as Eco-Friendly Slow Release Fertilizers. J. Polym. Environ. 2023, 31, 3454–3465. [Google Scholar] [CrossRef]
- Slomp, C.P. Vivianite blues. Nat. Geosci. 2023, 16, 394. [Google Scholar] [CrossRef]
- Fatimah, I.; Yudha, S.P.; Rubiyanto, D.; Widodo, I.D. Methenamine-smectite clay as slow release fertiliser: Physicochemical and kinetics study. Chem. Eng. Trans. 2017, 56, 1639–1644. [Google Scholar] [CrossRef]
- Borges, R.; Baika, L.M.; Grassi, M.T.; Wypych, F. Mechanochemical conversion of chrysotile/K2HPO4 mixtures into potential sustainable and environmentally friendly slow-release fertilizers. J. Environ. Manag. 2018, 206, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Rudmin, M.; Banerjee, S.; Yakich, T.; Tabakaev, R.; Ibraeva, K.; Buyakov, A.; Soktoev, B.; Ruban, A. Formulation of a slow-release fertilizer by mechanical activation of smectite/glauconite and urea mixtures. Appl. Clay Sci. 2020, 196, 105775. [Google Scholar] [CrossRef]
- Makó, É.; Kristóf, J.; Horváth, E.; Vágvölgyi, V. Kaolinite-urea complexes obtained by mechanochemical and aqueous suspension techniques-A comparative study. J. Colloid Interface Sci. 2009, 330, 367–373. [Google Scholar] [CrossRef]
- Al-Rawajfeh, A.E.; AlShamaileh, E.M.; Alrbaihat, M.R. Clean and efficient synthesis using mechanochemistry: Preparation of kaolinite–KH2PO4 and kaolinite–(NH4)2HPO4 complexes as slow released fertilizer. J. Ind. Eng. Chem. 2019, 73, 336–343. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S.; Xie, L.; Wang, Y. Environmentally Friendly Slow-Release Nitrogen Fertilizer. J. Agric. Food Chem. 2011, 59, 10169–10175. [Google Scholar] [CrossRef]
- Lei, Z.; Cagnetta, G.; Li, X.; Qu, J.; Li, Z.; Zhang, Q.; Huang, J. Enhanced adsorption of potassium nitrate with potassium cation on H3PO4 modified kaolinite and nitrate anion into Mg-Al layered double hydroxide. Appl. Clay Sci. 2018, 154, 10–16. [Google Scholar] [CrossRef]
- Rutkai, G.; Makó, É.; Kristóf, T. Simulation and experimental study of intercalation of urea in kaolinite. J. Colloid Interface Sci. 2009, 334, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Ma, L.; Tao, Q. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Ali, S.M.; Nasr, E.A.; Mahmoud, H.A.A.; Awwad, E.M. Effective Sequestration of Phosphate and Ammonium Ions by the Bentonite/Zeolite Na-P Composite as a Simple Technique to Control the Eutrophication Phenomenon: Realistic Studies. ACS Omega 2020, 5, 14656–14668. [Google Scholar] [CrossRef]
- Manna, S.; Das, P.; Basak, P.; Sharma, A.K.; Singh, V.K.; Patel, R.K.; Pandey, J.K.; Ashokkumar, V.; Pugazhendhi, A. Separation of pollutants from aqueous solution using nanoclay and its nanocomposites: A review. Chemosphere 2021, 280, 130961. [Google Scholar] [CrossRef] [PubMed]
- Odin, G.S.; Matter, A. De glauconiarum origine. Sedimentology 1981, 28, 611–641. [Google Scholar] [CrossRef]
- Guggenheim, S.; Adams, J.M.; Bain, D.C.; Bergaya, F.; Brigatti, M.F.; Drits, V.A.; Formoso, M.L.L.; Galan, E.; Kogure, T.; Stanjek, H. Summary of recommendations of nomenclature committees relevant to clay mineralogy: Report of the Association International pour l’Etude des Argiles (AIPEA) Nomenclature Committee for 2006 (Clays and Clay Minerals). Clays Clay Miner. 2007, 55, 761–772. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Mazurov, A.; Makarov, B.; Martemyanov, D. Economic potential of glauconitic rocks in Bakchar deposit (S-E Western Siberia) for alternate potash fertilizer. Appl. Clay Sci. 2017, 150, 225–233. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Makarov, B.; Mazurov, A.; Ruban, A.; Oskina, Y.; Tolkachev, O.; Buyakov, A.; Shaldybin, M. An investigation of plant growth by the addition of glauconitic fertilizer. Appl. Clay Sci. 2019, 180, 105178. [Google Scholar] [CrossRef]
- Karimi, E.; Abdolzadeh, A.; Sadeghipour, H.R.; Aminei, A. The potential of glauconitic sandstone as a potassium fertilizer for olive plants. Arch. Agron. Soil Sci. 2012, 58, 983–993. [Google Scholar] [CrossRef]
- Castro, L.; Tourn, S. Direct Application of Phosphate Rocks and Glauconite as Alternative Sources of Fertilizer in Argentina. Explor. Min. Geol. 2003, 12, 71–78. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Makarov, B. Evaluation of the Effects of the Application of Glauconitic Fertilizer on Oat Development: A Two-Year Field-Based Investigation. Agronomy 2020, 10, 872. [Google Scholar] [CrossRef]
- Franzosi, C.; Castro, L.N.; Celeda, A.M. Technical Evaluation of Glauconies as Alternative Potassium Fertilizer from the Salamanca Formation, Patagonia, Southwest Argentina. Nat. Resour. Res. 2014, 23, 311–320. [Google Scholar] [CrossRef]
- McRae, S.G. Glauconite. Earth-Sci. Rev. 1972, 8, 397–440. [Google Scholar] [CrossRef]
- Rudmin, M.; Maximov, P.; Dasi, E.; Kurovsky, A.; Gummer, Y.; Ibraeva, K.; Kutugin, V.; Soktoev, B.; Ponomarev, K.; Tararushkin, E.; et al. Intercalation of carbamide to globular glauconite by chemical processing for the creation of slow-release nanocomposites. Appl. Clay Sci. 2023, 243, 107075. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Hillier, S. Quantitative Analysis of Clay and other Minerals in Sandstones by X-ray Powder Diffraction (XRPD). In Clay Mineral Cements in Sandstones; Blackwell Publishing Ltd.: Oxford, UK, 2003; pp. 213–251. ISBN 9781444304336. [Google Scholar]
- Bish, D.L.; Post, J.E. Quantitative mineralogical analysis using the Rietveld full-pattern fitting method. Am. Mineral. 1993, 78, 932–940. [Google Scholar]
- Taylor, J.C. Computer Programs for Standardless Quantitative Analysis of Minerals Using the Full Powder Diffraction Profile. Powder Diffr. 1991, 6, 2–9. [Google Scholar] [CrossRef]
- Khan, A.Z.; Khan, Z.S.; Patle, S.; Rewatkar, K.G.; Zodape, S. FTIR spectral analysis of glycine doped ammonium dihydrogen phosphate (ADP) crystal. J. Phys. Conf. Ser. 2021, 1913, 012028. [Google Scholar] [CrossRef]
- Yang, S.S.; Chen, Z.B. The Study on Aging and Degradation Mechanism of Ammonium Polyphosphate in Artificial Accelerated Aging. Procedia Eng. 2018, 211, 906–910. [Google Scholar] [CrossRef]
- Emmerich, K.; Madsen, F.T.; Kahr, G. Dehydroxylation behavior of heat-treated and steam-treated homoionic cis-vacant montmorillonites. Clays Clay Miner. 1999, 47, 591–604. [Google Scholar] [CrossRef]
- Alrbaihat, M.R.; Al-Rawajfeh, A.E.; Alshamaileh, E. A mechanochemical preparation, properties and kinetic study of kaolin-N, P fertilizers for agricultural applications. J. Mech. Behav. Mater. 2021, 30, 265–271. [Google Scholar] [CrossRef]
- Mortland, M.M.; Fripiat, J.J.; Chaussidon, J.; Uytterhoeven, J. Interaction between ammonia and the expanding lattices of montmorillonite and vermiculite. J. Phys. Chem. 1963, 67, 248–258. [Google Scholar] [CrossRef]
- Solihin; Zhang, Q.; Tongamp, W.; Saito, F. Mechanochemical synthesis of kaolin–KH2PO4 and kaolin–NH4H2PO4 complexes for application as slow release fertilizer. Powder Technol. 2011, 212, 354–358. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, A.; Dutta, R.K. Biodegradable fertilizer nanocomposite hydrogel based on poly(vinyl alcohol)/kaolin/diammonium hydrogen phosphate (DAhP) for controlled release of phosphate. Polym. Bull. 2021, 78, 2933–2950. [Google Scholar] [CrossRef]



| Nanocomposites | Weight Loss Intervals (°C) | ||||||
|---|---|---|---|---|---|---|---|
| 0–110 | 110–240 | 240–302 | 302–365 | 365–590 | 590–1000 | ||
| Weight loss (%) | Gko90P10 | 99.1 | 96.3 | 95.5 | 95.0 | 93.0 | 91.8 |
| GkNa90P10 | 99.6 | 98.2 | 97.9 | 97.4 | 95.2 | 94.4 | |
| Gka90P10rm | 99.1 | 95.9 | 94.9 | 94.4 | 92.3 | 91.3 | |
| Gka90P10pm | 99.8 | 98.7 | 98.3 | 97.8 | 95.0 | 92.3 | |
| Glauconite | 99.7 | 98.6 | 98.3 | 97.9 | 95.8 | 95.2 | |
| Final residue (%) | Gko90P10 | 0.9 | 2.8 | 0.8 | 0.5 | 1.9 | 1.2 |
| GkNa90P10 | 0.4 | 1.4 | 0.4 | 0.4 | 2.3 | 0.7 | |
| Gka90P10rm | 0.9 | 3.2 | 0.9 | 0.6 | 2.0 | 1.1 | |
| Gka90P10pm | 0.2 | 1.1 | 0.4 | 0.5 | 2.7 | 2.7 | |
| Glauconite | 0.3 | 1.1 | 0.3 | 0.4 | 2.1 | 0.6 | |
| Features | Nanocomposites | |||
|---|---|---|---|---|
| Gko90P10 | GkNa90P10 | Gka90P10rm | Gka90P10pm | |
| A shift of the basal reflection 001 on the bulk XRD | Up to 15.4 Å | Up to 13.2 Å | Up to 13.3 Å | Up to 13.9 Å |
| Morphology of elementary particles | The relic globular and pellet grains with size of 100–400 µm | Microparticles with size of 40–120 µm | ||
| ADP coating | 2–8 µm thick film | Film and aggregates | ||
| Mineral unit structure | The unit structure thickness is 9.3–14.7 Å (average: 11.9 Å) | The unit structure thickness is 9.7–13.4 Å (average: 11.2 Å) | ||
| The interlayer varies from 2.4 to 4.2 Å (average: 3.2 Å) | The interlayer varies from 1.9 to 3.6 Å (average: 3.0 Å) | |||
| Adsorbed ADF in interparticle space (macropores) | 2.8 wt.% | 1.4 wt.% | 3.2 wt.% | 1.1 wt.% |
| Adsorbed ADF on edge plane (mesopores) | 0.8 wt.% | 0.4 wt.% | 0.9 wt.% | 0.4 wt.% |
| Intercalated NH4+ | 3.7 wt.% | 3.4 wt.% | 3.7 wt.% | 6.0 wt.% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudmin, M.; Makarov, B.; López-Quirós, A.; Maximov, P.; Lokteva, V.; Ibraeva, K.; Kurovsky, A.; Gummer, Y.; Ruban, A. Preparation, Features, and Efficiency of Nanocomposite Fertilisers Based on Glauconite and Ammonium Dihydrogen Phosphate. Materials 2023, 16, 6080. https://doi.org/10.3390/ma16186080
Rudmin M, Makarov B, López-Quirós A, Maximov P, Lokteva V, Ibraeva K, Kurovsky A, Gummer Y, Ruban A. Preparation, Features, and Efficiency of Nanocomposite Fertilisers Based on Glauconite and Ammonium Dihydrogen Phosphate. Materials. 2023; 16(18):6080. https://doi.org/10.3390/ma16186080
Chicago/Turabian StyleRudmin, Maxim, Boris Makarov, Adrián López-Quirós, Prokopiy Maximov, Valeria Lokteva, Kanipa Ibraeva, Alexander Kurovsky, Yana Gummer, and Alexey Ruban. 2023. "Preparation, Features, and Efficiency of Nanocomposite Fertilisers Based on Glauconite and Ammonium Dihydrogen Phosphate" Materials 16, no. 18: 6080. https://doi.org/10.3390/ma16186080
APA StyleRudmin, M., Makarov, B., López-Quirós, A., Maximov, P., Lokteva, V., Ibraeva, K., Kurovsky, A., Gummer, Y., & Ruban, A. (2023). Preparation, Features, and Efficiency of Nanocomposite Fertilisers Based on Glauconite and Ammonium Dihydrogen Phosphate. Materials, 16(18), 6080. https://doi.org/10.3390/ma16186080









