Antibacterial Activity and Sustained Effectiveness of Calcium Silicate-Based Cement as a Root-End Filling Material against Enterococcus faecalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of the Specimens
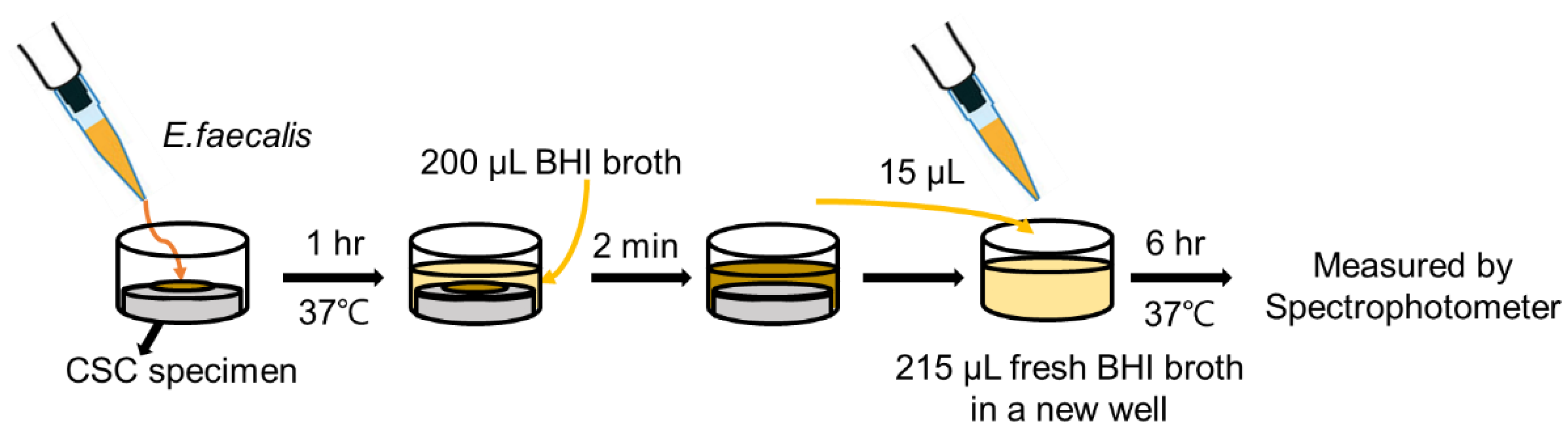
2.2. Antibacterial Activity
2.3. Sustained Antibacterial Effectiveness
2.4. Cytotoxicity on Human Periodontal Ligament Stem Cell
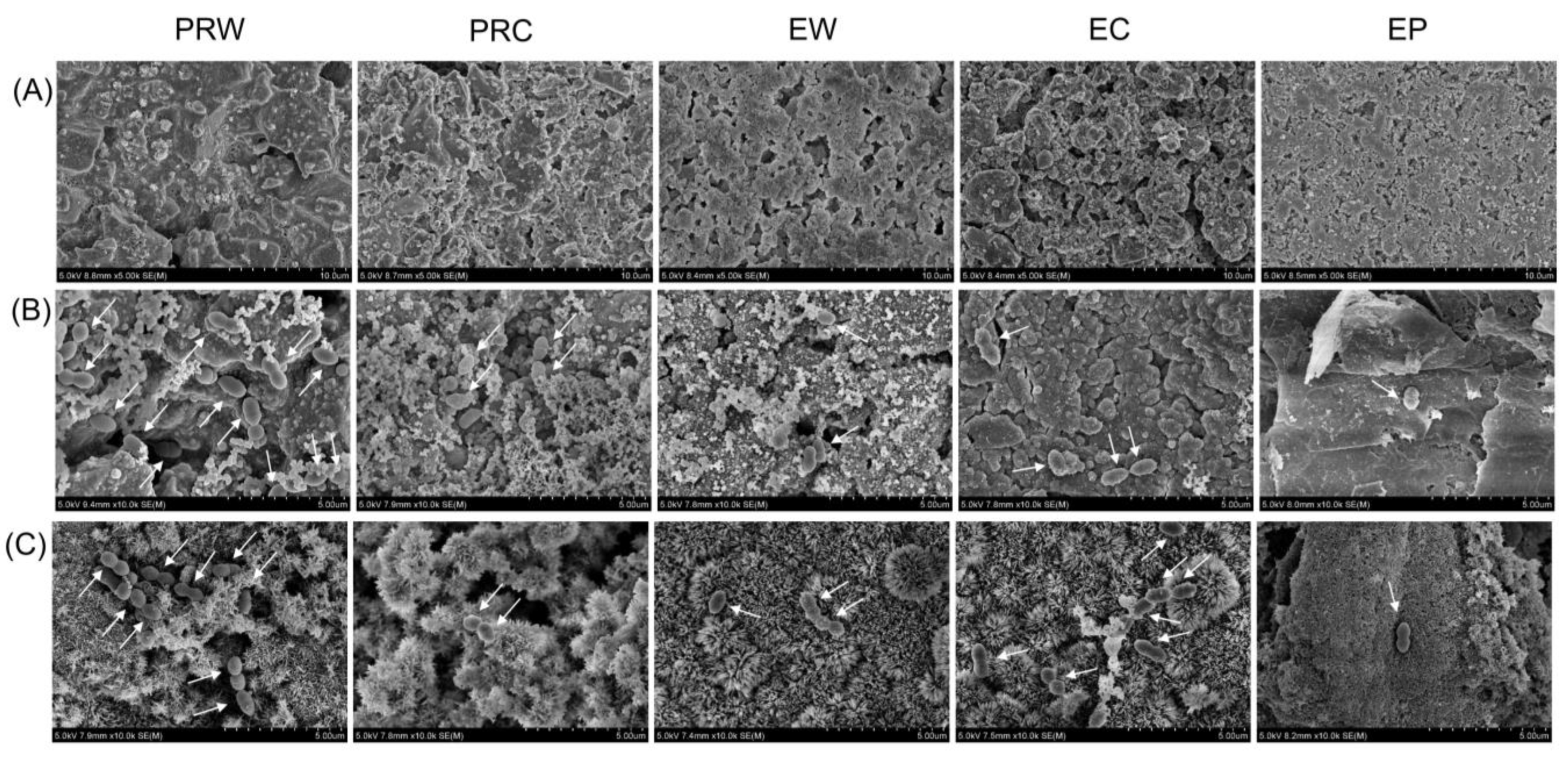
2.5. Scanning Electron Microscopic Analysis
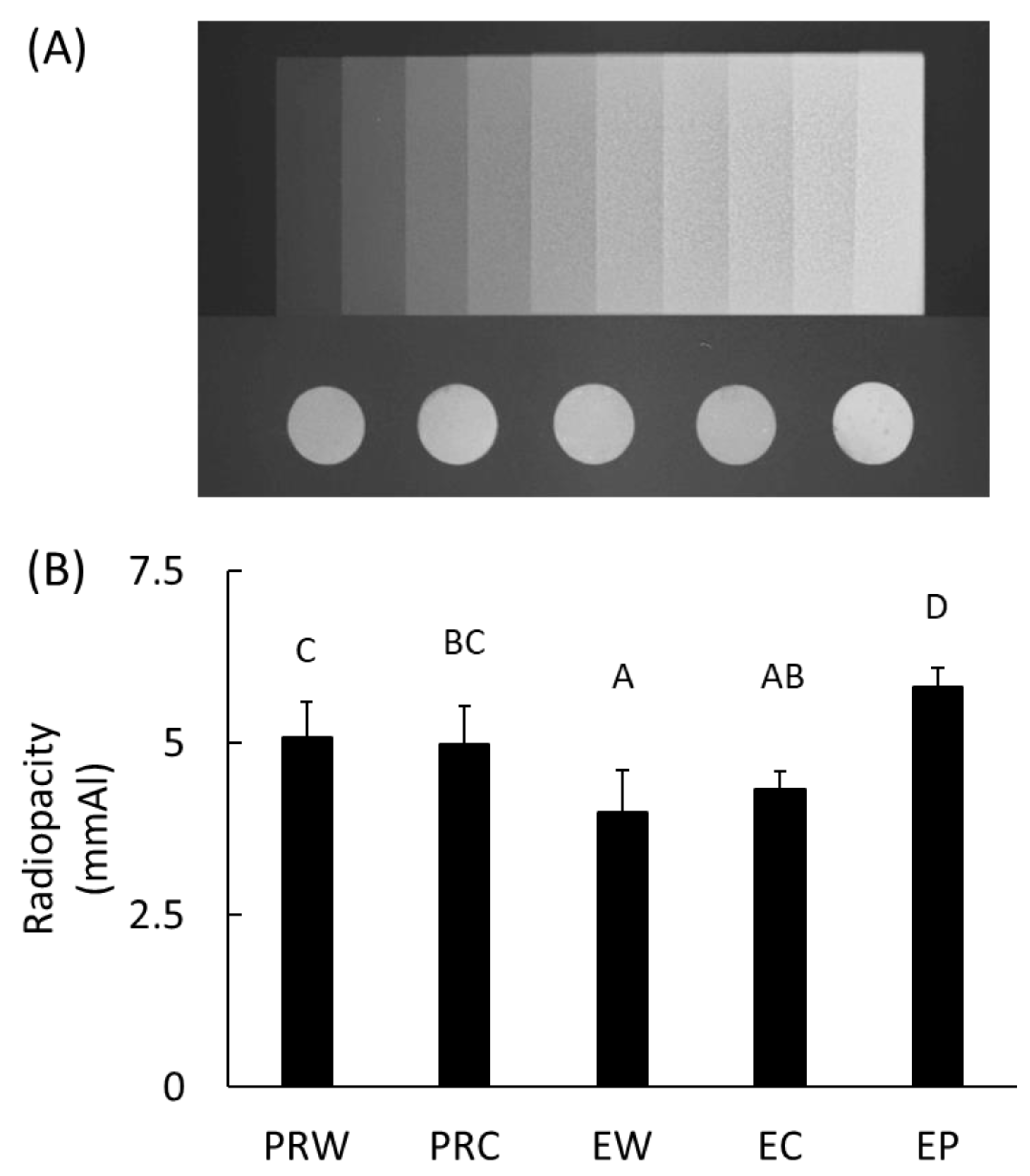
2.6. Radiopacity
2.7. Statistical Analysis
3. Results
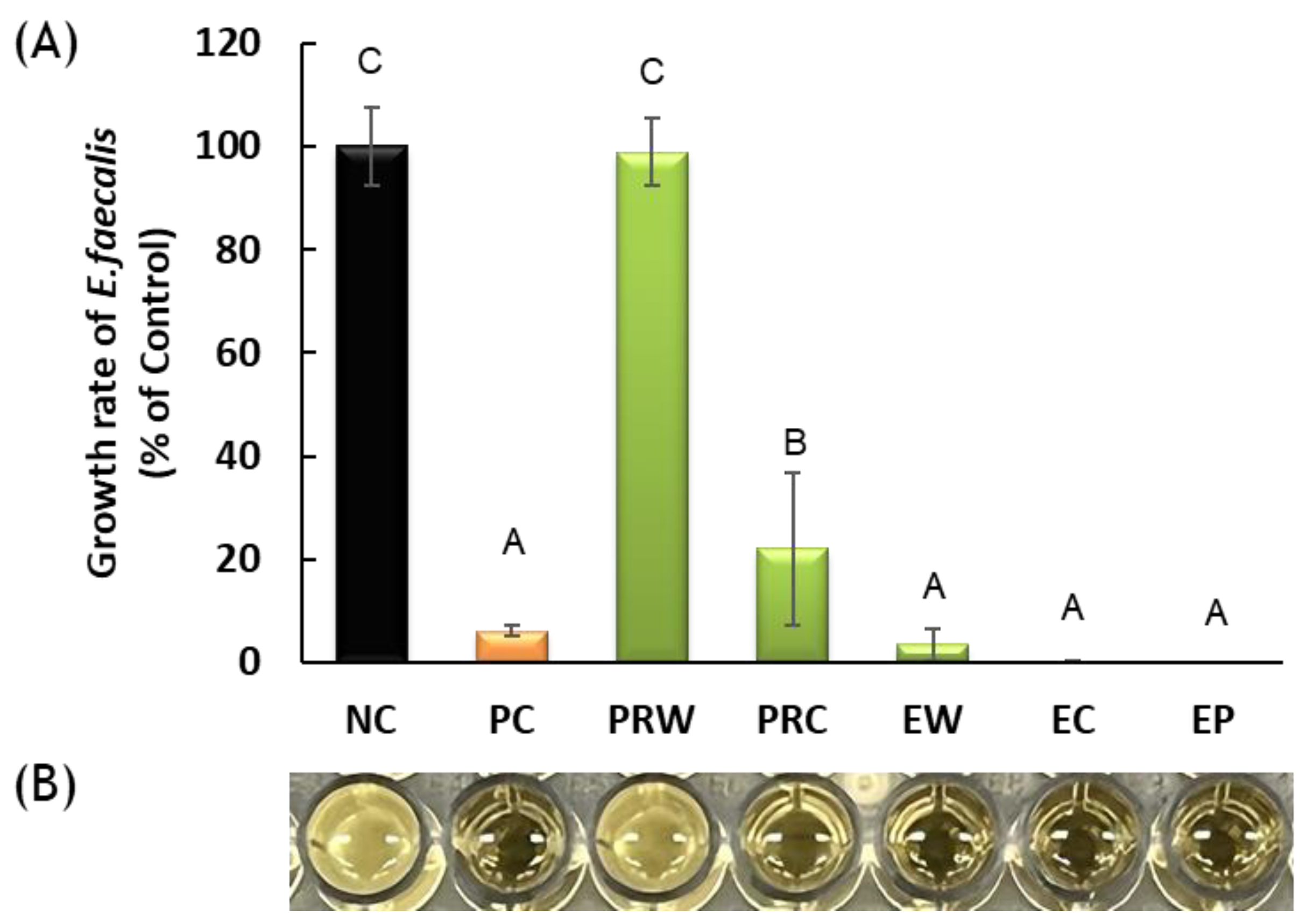
3.1. Antibacterial Activity
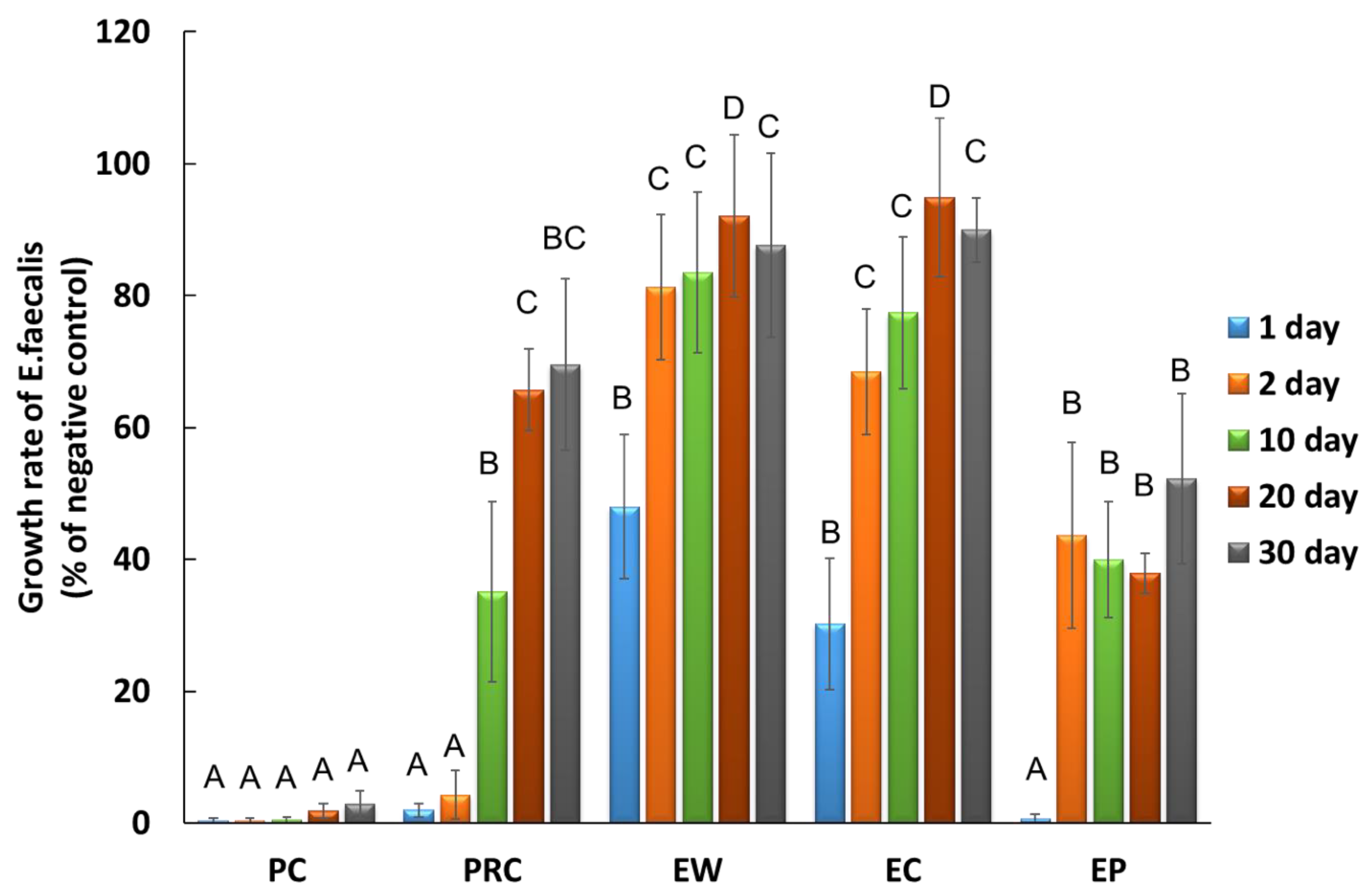
3.2. Sustained Antibacterial Effectiveness
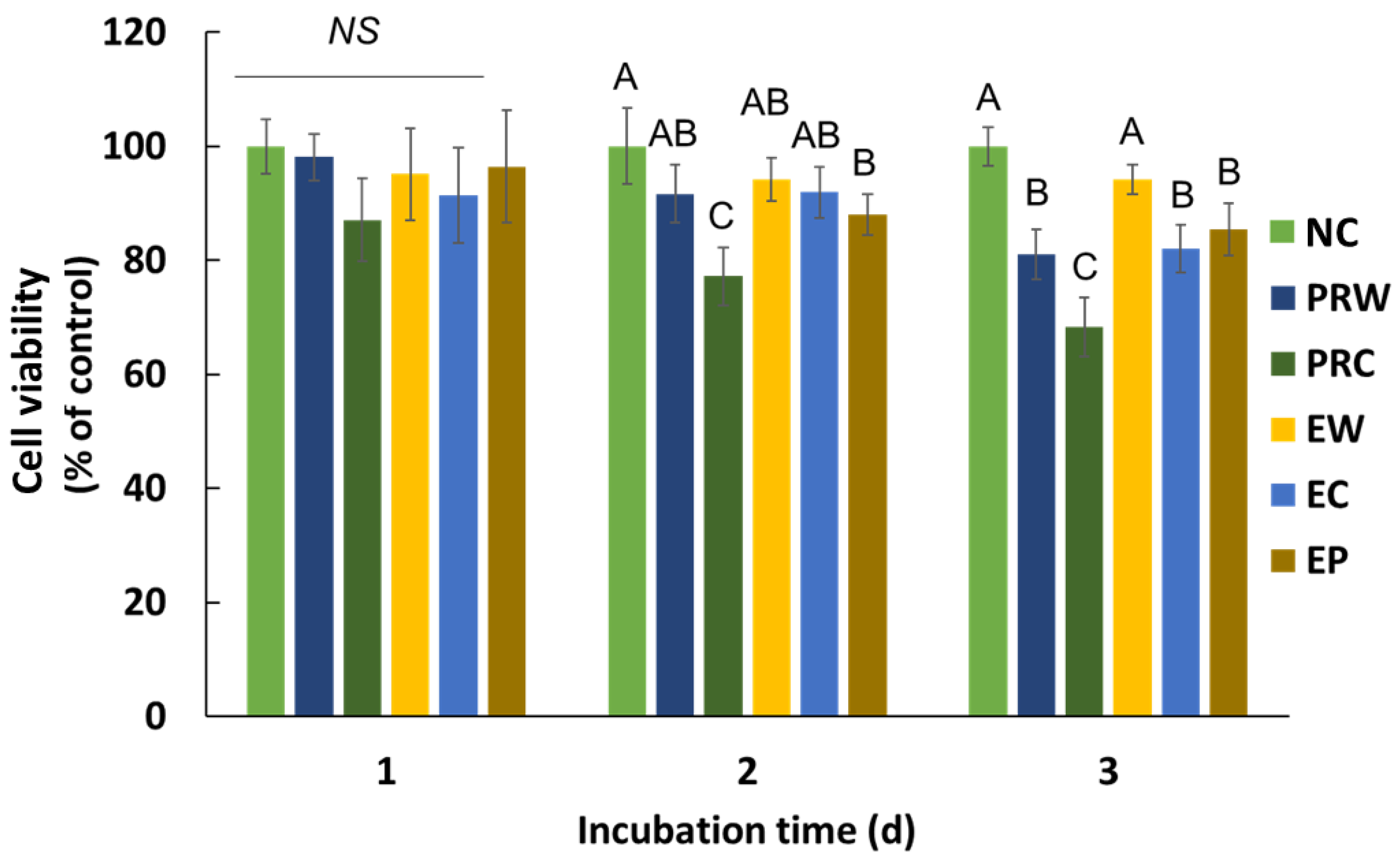
3.3. Cytotoxicity on Human Periodontal Ligament Stem Cell (hPDLSC)
3.4. Scanning Electron Microscopic Analysis
3.5. Radiopacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camilleri, J. Classification of hydraulic cements used in dentistry. Front. Dent. Med. 2020, 1, 9. [Google Scholar] [CrossRef]
- Torabinejad, M.; Watson, T.F.; Pitt Ford, T.R. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J. Endod. 1993, 19, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Pitt Ford, T.R. Root end filling materials: A review. Endod. Dent. Traumatol. 1996, 12, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Attik, G.N.; Villat, C.; Hallay, F.; Pradelle-Plasse, N.; Bonnet, H.; Moreau, K.; Colon, P.; Grosgogeat, B. In vitro biocompatibility of a dentine substitute cement on human MG 63 osteoblasts cells: B iodentine™ versus MTA®. Int. Endod. J. 2014, 47, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Cornélio, A.L.; Rodrigues, E.M.; Salles, L.P.; Mestieri, L.B.; Faria, G.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Bioactivity of MTA Plus, Biodentine and an experimental calcium silicate-based cement on human osteoblast-like cells. Int. Endod. J. 2017, 50, 39–47. [Google Scholar] [CrossRef]
- Alghamdi, F.; Shakir, M. The influence of Enterococcus faecalis as a dental root canal pathogen on endodontic treatment: A systematic review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef]
- Portenier, I.; Waltimo, T.M.T.; Haapasalo, M. Enterococcus faecalis–the root canal survivor and ‘star’ in post-treatment disease. Endod. Top. 2003, 6, 135–159. [Google Scholar] [CrossRef]
- Vazquez-Garcia, F.; Tanomaru-Filho, M.; Chávez-Andrade, G.M.; Bosso-Martelo, R.; Basso-Bernardi, M.I.; Guerreiro-Tanomaru, J.M. Effect of silver nanoparticles on physicochemical and antibacterial properties of calcium silicate cements. Braz. Dent. J. 2016, 27, 508–514. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part I: Chemical, physical, and antibacterial properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Hernandez, E.P.; Botero, T.M.; Mantellini, M.G.; McDonald, N.J.; Nör, J.E. Effect of ProRoot MTA mixed with chlorhexidine on apoptosis and cell cycle of fibroblasts and macrophages in vitro. Int. Endod. J. 2005, 38, 137–143. [Google Scholar] [CrossRef]
- Lindblad, R.M.; Lassila, L.V.J.; Vallittu, P.K.; Tjäderhane, L. The effect of chlorhexidine and dimethyl sulfoxide on long-term sealing ability of two calcium silicate cements in root canal. Dent. Mater. 2021, 37, 328–335. [Google Scholar] [CrossRef]
- Mittag, S.G.; Eissner, C.; Zabel, L.; Wrbas, K.T.; Kielbassa, A.M. The influence of chlorhexidine on the antibacterial effects of MTA. Quintessence Int. 2012, 43, 901–906. [Google Scholar] [PubMed]
- Holt, D.M.; Watts, J.D.; Beeson, T.J.; Kirkpatrick, T.C.; Rutledge, R.E. The anti-microbial effect against Enterococcus faecalis and the compressive strength of two types of mineral trioxide aggregate mixed with sterile water or 2% chlorhexidine liquid. J. Endod. 2007, 33, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Stringhini Junior, E.; Dos Santos, M.G.C.; Oliveira, L.B.; Mercadé, M. MTA and biodentine for primary teeth pulpotomy: A systematic review and meta-analysis of clinical trials. Clin. Oral Investig. 2019, 23, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yang, W.; Kim, H.; Ko, H. Comparison of the biological properties of ProRoot MTA, OrthoMTA, and Endocem MTA cements. J. Endod. 2014, 40, 1649–1653. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; Carvalho, N.K.; Guberman, M.R.D.C.L.; Prado, M.; Senna, P.M.; Souza, E.M.; De-Deus, G. Push-out Bond Strength of Fast-setting Mineral Trioxide Aggregate and Pozzolan-based Cements: ENDOCEM MTA and ENDOCEM Zr. J. Endod. 2017, 43, 801–804. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.J.; Lee, K.W.; Yu, M.K.; Min, K.S. Push-out bond strength and intratubular biomineralization of a hydraulic root-end filling material premixed with dimethyl sulfoxide as a vehicle. Restor. Dent. Endod. 2023, 48, e8. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kye, M.; Ha, Y.J.; Kim, S.Y. Biocompatible properties and mineralization potential of premixed calcium silicate-based cements and fast-set calcium silicate-based cements on human bone marrow-derived mesenchymal stem cells. Materials 2022, 15, 7595. [Google Scholar] [CrossRef]
- Eldeniz, A.U.; Hadimli, H.H.; Ataoglu, H.; Orstavik, D. Antibacterial effect of selected root-end filling materials. J. Endod. 2006, 32, 345–349. [Google Scholar] [CrossRef]
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Sen, H.G.; Helvacioglu-Yigit, D.; Yilmaz, A. Radiopacity evaluation of calcium silicate cements. BMC Oral Health 2023, 23, 491. [Google Scholar] [CrossRef]
- Paños-Crespo, A.; Sánchez-Torres, A.; Gay-Escoda, C. Retrograde filling material in periapical surgery: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e422–e429. [Google Scholar] [CrossRef] [PubMed]
- Tanomaru-Filho, M.; da Silva, G.F.; Duarte, M.A.; Gonçalves, M.; Tanomaru, J.M. Radiopacity evaluation of root-end filling materials by digitization of images. J. Appl. Oral Sci. 2008, 16, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.; Elbahary, S.; Haj-Yahya, S.; Jammal, L.; Shemesh, H.; Tsesis, I. The invasion of bacterial biofilms into the dentinal tubules of extracted teeth retrofilled with fluorescently labeled retrograde filling materials. Appl. Sci. 2020, 10, 6996. [Google Scholar] [CrossRef]
- Kang, C.M.; Hwang, J.; Song, J.S.; Lee, J.H.; Choi, H.J.; Shin, Y. Effects of three calcium silicate cements on inflammatory response and mineralization-inducing potentials in a dog pulpotomy model. Materials 2018, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, Y.; Lee, J.; Kim, J.; Lee, J.; Han, M.R.; Kim, J.; Shin, J. Evaluation of setting time, solubility, and compressive strength of four calcium silicate-based cements. J. Korean Acad. Pediatr. Dent. 2023, 50, 217–228. [Google Scholar] [CrossRef]
- Stowe, T.J.; Sedgley, C.M.; Stowe, B.; Fenno, J.C. The effects of chlorhexidine gluconate (0.12%) on the antimicrobial properties of tooth-colored ProRoot mineral trioxide aggregate. J. Endod. 2004, 30, 429–431. [Google Scholar] [CrossRef]
- Kapralos, V.; Rukke, H.V.; Ørstavik, D.; Koutroulis, A.; Camilleri, J.; Sunde, P.T. Antimicrobial and physicochemical characterization of endodontic sealers after exposure to chlorhexidine digluconate. Dent. Mater. 2021, 37, 249–263. [Google Scholar] [CrossRef]
- Shahi, S.; Rahimi, S.; Yavari, H.R.; Shakouie, S.; Nezafati, S.; Abdolrahimi, M. Sealing ability of white and gray mineral trioxide aggregate mixed with distilled water and 0.12% chlorhexidine gluconate when used as root-end filling materials. J. Endod. 2007, 33, 1429–1432. [Google Scholar] [CrossRef]
- Vitti, R.P.; Pacheco, R.R.; Silva, E.J.N.L.; Prati, C.; Gandolfi, M.G.; Piva, E.; Ogliari, F.A.; Zanchi, C.H.; Sinhoreti, M.A.C. Addition of phosphates and chlorhexidine to resin-modified MTA materials. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2195–2201. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Castelo-Baz, P.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int. Endod. J. 2017, 50, e63–e72. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Biocompatibility of new pulp-capping materials NeoMTA Plus, MTA Repair HP, and Biodentine on human dental pulp stem cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, L.T.; Cullinan, M.P.; Love, R.M. Dental stem cells and their potential role in apexogenesis and apexification. Int. Endod. J. 2009, 42, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Kotova, A.V.; Lobov, A.A.; Dombrovskaya, J.A.; Sannikova, V.Y.; Ryumina, N.A.; Klausen, P.; Shavarda, A.L.; Malashicheva, A.B.; Enukashvily, N.I. Comparative analysis of dental pulp and periodontal stem cells: Differences in morphology, functionality, osteogenic differentiation and proteome. Biomedicines 2021, 9, 1606. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, B.; Gao, Z.; Xu, J.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Regeneration characteristics of different dental derived stem cell sheets. J. Oral Rehabil. 2020, 47, 66–72. [Google Scholar] [CrossRef]
- Rebolledo, S.; Alcántara-Dufeu, R.; Luengo Machuca, L.; Ferrada, L.; Sánchez-Sanhueza, G.A. Real-time evaluation of the biocompatibility of calcium silicate-based endodontic cements: An in vitro study. Clin. Exp. Dent. Res. 2023, 9, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Odabaş, M.E.; Çinar, C.; Akça, G.; Araz, I.; Ulusu, T.; Yücel, H. Short-term antimicrobial properties of mineral trioxide aggregate with incorporated silver-zeolite. Dent. Traumatol. 2011, 27, 189–194. [Google Scholar] [CrossRef]
- Jafari, F.; Samadi Kafil, H.S.; Jafari, S.; Aghazadeh, M.; Momeni, T. Antibacterial activity of MTA fillapex and AH 26 root canal sealers at different time intervals. Iran Endod. J. 2016, 11, 192–197. [Google Scholar] [CrossRef]
- Kim, R.J.; Kim, M.O.; Lee, K.S.; Lee, D.Y.; Shin, J.H. An in vitro evaluation of the antibacterial properties of three mineral trioxide aggregate (MTA) against five oral bacteria. Arch. Oral Biol. 2015, 60, 1497–1502. [Google Scholar] [CrossRef]
- Morita, M.; Kitagawa, H.; Nakayama, K.; Kitagawa, R.; Yamaguchi, S.; Imazato, S. Antibacterial activities and mineral induction abilities of proprietary MTA cements. Dent. Mater. J. 2021, 40, 297–303. [Google Scholar] [CrossRef]
- Choi, Y.; Park, S.J.; Lee, S.H.; Hwang, Y.C.; Yu, M.K.; Min, K.S. Biological effects and washout resistance of a newly developed fast-setting pozzolan cement. J. Endod. 2013, 39, 467–472. [Google Scholar] [CrossRef]
- Ashi, T.; Mancino, D.; Hardan, L.; Bourgi, R.; Zghal, J.; Macaluso, V.; Al-Ashkar, S.; Alkhouri, S.; Haikel, Y.; Kharouf, N. Physicochemical and Antibacterial Properties of Bioactive Retrograde Filling Materials. Bioengineering 2022, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Kodama, S.; Okiji, T. Evaluation of calcium-releasing and apatite-forming abilities of fast-setting calcium silicate-based endodontic materials. Int. Endod. J. 2015, 48, 124–130. [Google Scholar] [CrossRef] [PubMed]
| Trade Name | Code | Liquid Type | Powder or Paste Composition | Manufacturer |
|---|---|---|---|---|
| Pro Root MTA | PRW | Distilled water | Tricalcium silicate, Dicalcium silicate, Bismuth oxide, Tricalcium aluminate, calcium sulfate dehydrate, tetra calcium aluminoferrite, gypsum, calcium oxide | Dentsply, Tulsa, TN, USA |
| PRC | Chlorhexidine | |||
| Endocem MTA | EW | Distilled water | Tricalcium silicate, Dicalcium silicate, Bismuth oxide, Tricalcium aluminate | Maruchi, Wonju, Republic of Korea |
| EC | Chlorhexidine | |||
| Endocem MTA Premixed Regular | EP | Injectable type paste | Zirconium dioxide, Calcium silicate, calcium aluminate, Calcium sulfate, Dimethyl sulfoxide, Lithium carbonate, Thickening agents | Maruchi, Wonju, Republic of Korea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.-H.; Shin, S.-J.; Oh, S.; Bae, J.-M. Antibacterial Activity and Sustained Effectiveness of Calcium Silicate-Based Cement as a Root-End Filling Material against Enterococcus faecalis. Materials 2023, 16, 6124. https://doi.org/10.3390/ma16186124
Moon S-H, Shin S-J, Oh S, Bae J-M. Antibacterial Activity and Sustained Effectiveness of Calcium Silicate-Based Cement as a Root-End Filling Material against Enterococcus faecalis. Materials. 2023; 16(18):6124. https://doi.org/10.3390/ma16186124
Chicago/Turabian StyleMoon, Seong-Hee, Seong-Jin Shin, Seunghan Oh, and Ji-Myung Bae. 2023. "Antibacterial Activity and Sustained Effectiveness of Calcium Silicate-Based Cement as a Root-End Filling Material against Enterococcus faecalis" Materials 16, no. 18: 6124. https://doi.org/10.3390/ma16186124
APA StyleMoon, S.-H., Shin, S.-J., Oh, S., & Bae, J.-M. (2023). Antibacterial Activity and Sustained Effectiveness of Calcium Silicate-Based Cement as a Root-End Filling Material against Enterococcus faecalis. Materials, 16(18), 6124. https://doi.org/10.3390/ma16186124









