Co(II)-Based Metal-Organic Framework Derived CA-CoNiMn-CLDHs with Peroxidase-like Activity for Colorimetric Detection of Phenol
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Characterization
2.3. Synthesis
2.3.1. Synthesis of ZIF-67 Templates
2.3.2. Synthesis of CA-CoNiMn-CLDHs
2.4. Peroxidase-like Catalytic Activity and Steady-State Kinetics of CA-CoNiMn-CLDHs
2.5. Colorimetric Detection of Phenol
2.6. Stability and Reusability of CA-CoNiMn-CLDHs
3. Results and Discussion
3.1. Synthesis and Characterization of CA-CoNiMn-CLDHs
3.2. Peroxidase-like Activity and Mechanism of CA-CoNiMn-CLDHs
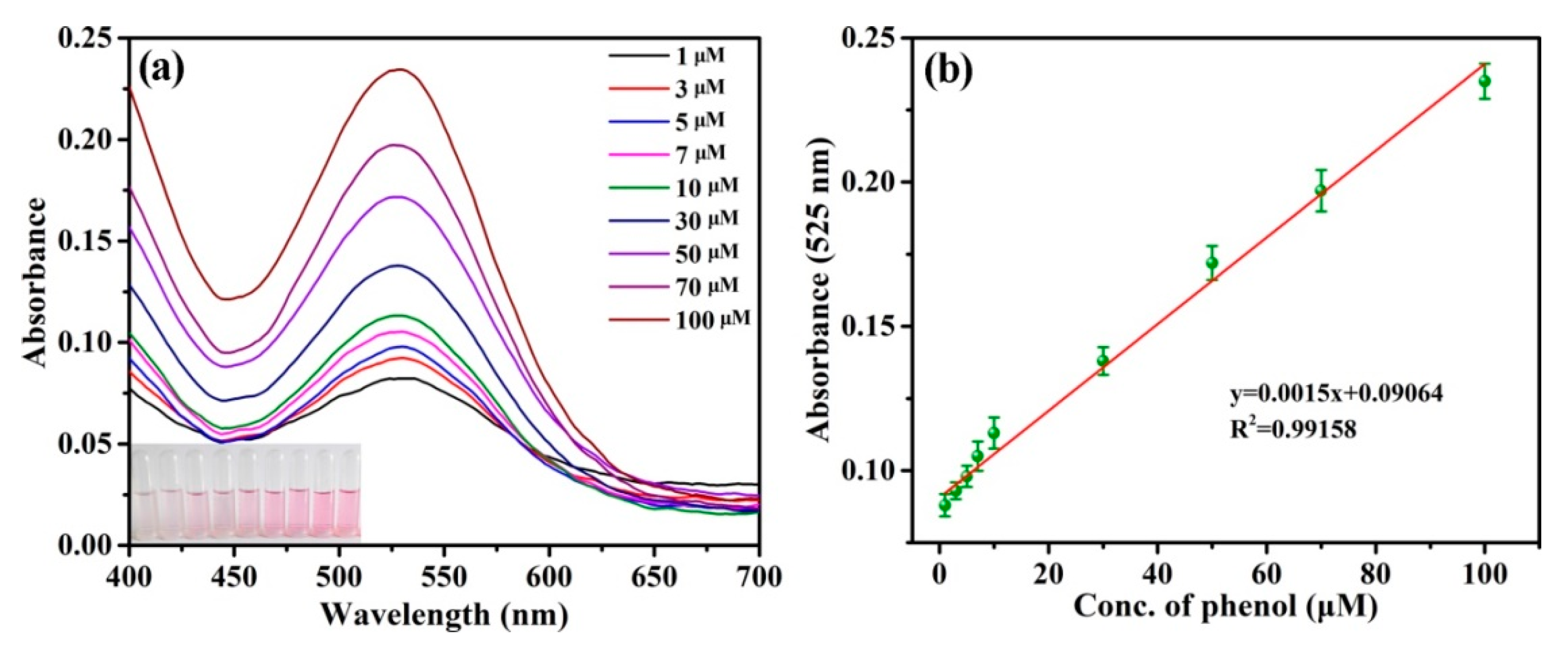
3.3. Detection of Phenol by CA-CoNiMn-CLDHs
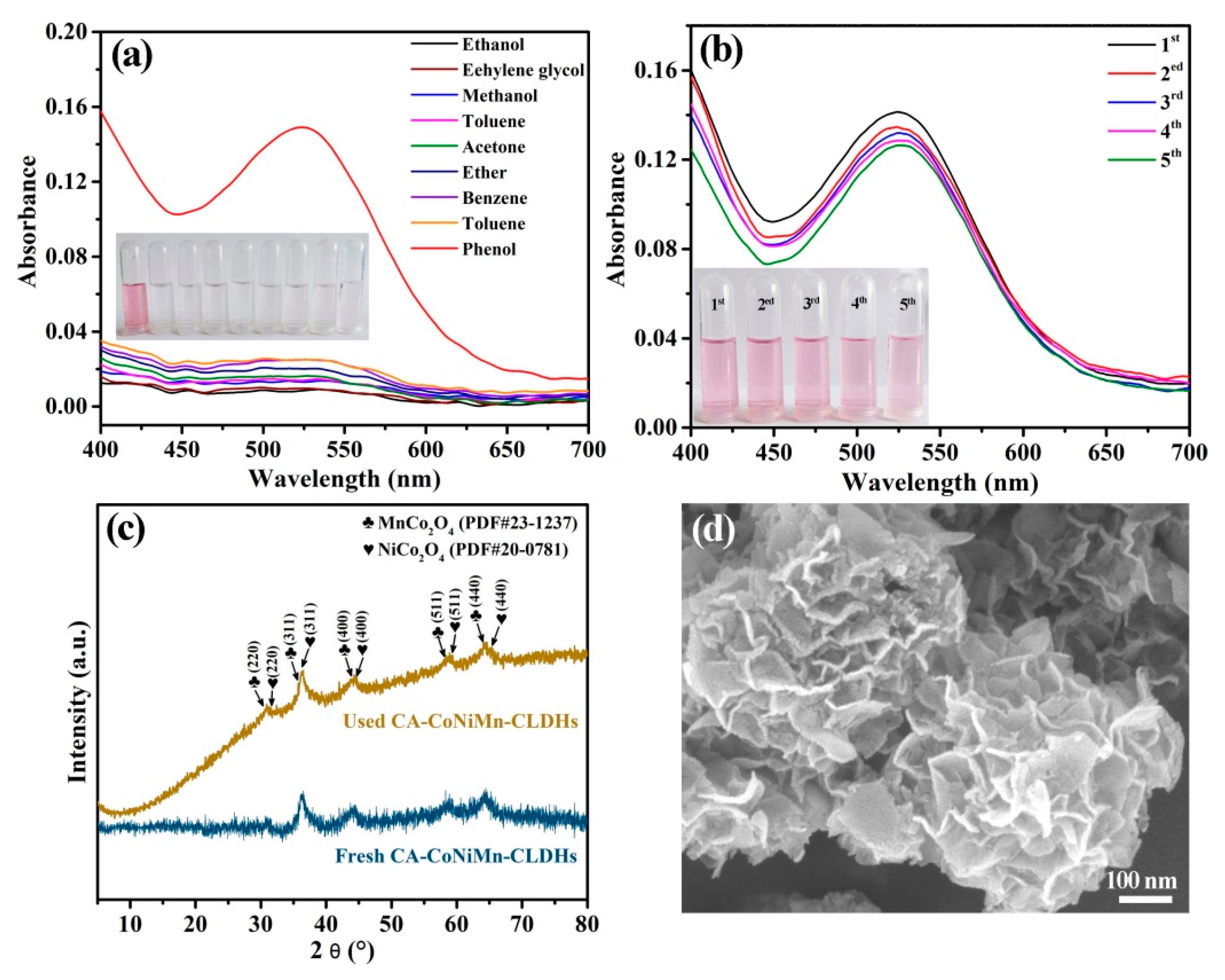
3.4. Evaluation of Selectivity, Reusability and Stability of CA-CoNiMn-CLDHs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Zeng, M.; Zhao, Y.; Zuo, X.; Meng, F.; Jie, H.; Lv, F.; Lu, Y.; Hou, J. Synergetic integration of catalase and Fe3O4 magnetic nanoparticles with metal organic framework for colorimetric detection of phenol. Environ. Res. 2022, 206, 112580. [Google Scholar] [CrossRef]
- Xu, X.; Wu, S.; Guo, D.; Niu, X. Construction of a recyclable oxidase-mimicking Fe3O4@MnOx-based colorimetric sensor array for quantifying and identifying chlorophenols. Anal. Chim. Acta 2020, 1107, 203–212. [Google Scholar] [CrossRef]
- Hou, C.; Fu, L.; Wang, Y.; Chen, W.; Chen, F.; Zhang, S.; Wang, J. Co-MOF-74 based Co3O4/cellulose derivative membrane as dual-functional catalyst for colorimetric detection and degradation of phenol. Carbohydr. Polym. 2021, 273, 118548. [Google Scholar] [CrossRef]
- Xu, L.; Wong, P.K.; Jiang, Z.; Yu, J.C. Iodide-mediated selective photocatalytic treatment of phenolic pollutants. Appl. Catal. B Environ. 2023, 338, 123080. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H.; Lin, Y.; Wang, G.; Wu, J. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef]
- Sarmento, A.P.; Borges, A.C.; de Matos, A.T.; Romualdo, L.L. Phenol degradation by Fenton-like process. Environ. Sci. Pollut. Res. 2016, 23, 18429–18438. [Google Scholar] [CrossRef]
- Kamel, R.M.; Shahat, A.; Anwar, Z.M.; El-Kady, H.A.; Kilany, E.M. Efficient dual sensor of alternate nanomaterials for sensitive and rapid monitoring of ultra-trace phenols in sea water. J. Mol. Liq. 2020, 297, 111798. [Google Scholar] [CrossRef]
- Darabdhara, G.; Das, M.R. Dual responsive magnetic Au@Ni nanostructures loaded reduced grapheme oxide sheets for colorimetric detection and photocatalytic degradation of toxic phenolic compounds. J. Hazard. Mater. 2019, 368, 365–377. [Google Scholar] [CrossRef]
- Liu, F.; Piao, Y.; Choi, J.S.; Seo, T.S. Three-dimensional graphene micropillar based electrochemical sensor for phenol detection. Biosens. Bioelectron. 2013, 50, 387–392. [Google Scholar] [CrossRef]
- Zhu, L.; Gong, L.; Zhang, Y.; Wang, R.; Ge, J.; Liu, Z.; Zare, R.N. Rapid detection of phenol using a membrane containing laccase nanoflowers. Chem. Asian J. 2013, 8, 2358–2360. [Google Scholar] [CrossRef]
- Farahbakhsh, F.; Heydari-Bafrooei, E.; Ahmadi, M.; Hekmatara, S.H.; Sabet, M. A novel aptasensing method for detecting bisphenol A using the catalytic effect of the Fe3O4/Au nanoparticles on the reduction reaction of the silver ions. Food Chem. 2021, 355, 129666. [Google Scholar] [CrossRef] [PubMed]
- Jović-Jovičić, N.; Mojović, Z.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E.; Banković, P.; Jovanović, D.; Milutinović-Nikolić, A. Smectite-chitosan-based electrodes in electrochemical detection of phenol and its derivatives. Appl. Clay Sci. 2016, 124, 62–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, K.L. Quantitative detection of phenol in wastewater using square wave voltammetry with pre-concentration. Anal. Chim. Acta 2021, 1178, 338788. [Google Scholar]
- Luo, X.; Zheng, H.; Zhang, Z.; Wang, M.; Yang, B.; Huang, L.; Wang, M. Cloud point extraction for simultaneous determination of 12 phenolic compounds by high performance liquid chromatography with fluorescence detection. Microchem. J. 2018, 137, 148–154. [Google Scholar] [CrossRef]
- Razavi, M.; Barras, A.; Ifires, M.; Swaidan, A.; Khoshkam, M.; Szunerits, S.; Kompany-Zareh, M.; Boukherroub, R. Colorimetric assay for the detection of dopamine using bismuth ferrite oxide (Bi2Fe4O9) nanoparticles as an efficient peroxidase-mimic nanozyme. J. Colloid Interface Sci. 2022, 613, 384–395. [Google Scholar] [CrossRef]
- Ma, H.; Li, M.; Yu, T.; Zhang, H.; Xiong, M.; Li, F. Magnetic ZIF-8-Based Mimic Multi-enzyme System as a Colorimetric Biosensor for Detection of Aryloxyphenoxypropionate Herbicides. ACS Appl. Mater. Interfaces 2021, 13, 44329–44338. [Google Scholar] [CrossRef]
- Lu, D.; Li, J.; Wu, Z.; Yuan, L.; Fang, W.; Zou, P.; Ma, L.; Wang, X. High-activity daisy-like zeolitic imidazolate framework-67/reduced grapheme oxide-based colorimetric biosensor for sensitive detection of hydrogen peroxide. J. Colloid Interface Sci. 2022, 608, 3069–3078. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.S.; Mirkin, C.A. A gold-nanoparticle-based real-time colorimetric screening method for endonuclease activity and inhibition. Angew. Chem. Int. Ed. 2007, 46, 3468–3470. [Google Scholar] [CrossRef]
- Wang, G.; Xu, X.; Qiu, L.; Dong, Y.; Li, Z.; Zhang, C. Dual Responsive Enzyme Mimicking Activity of AgX (X = Cl, Br, I) Nanoparticles and Its Application for Cancer Cell Detection. ACS Appl. Mater. Interfaces 2014, 6, 6434–6442. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, M.; Meng, X.; Xiang, J.; Wang, L.; Ren, Q.; Guo, S. Graphene/intermetallic PtPb nanoplates composites for boosting electrochemical detection of H2O2 released from cells. Anal. Chem. 2017, 89, 3761–3767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xin, J.; Shao, C.; Li, X.; Li, X.; Liu, S.; Liu, Y. Direct Z-scheme heterostructure of p-CuAl2O4/n-Bi2WO6 composite nanofibers for efficient overall water splitting and photodegradation. J. Colloid Interface Sci. 2019, 550, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, J.; Kim, H.S.; Cho, A.; Shim, K.H.; Le, T.N.; An, S.S.A.; Han, J.W.; Kim, M.I.; Lee, J. Heme Cofactor-Resembling Fe-N Single Site Embedded Graphene as Nanozymes to Selectively Detect H2O2 with High Sensitivity. Adv. Funct. Mater. 2019, 30, 1905410. [Google Scholar] [CrossRef]
- Li, Z.; Song, M.; Zhu, W.; Zhuang, W.; Du, X.; Tian, L. MOF-derived hollow heterostructures for advanced electrocatalysis. Coord. Chem. Rev. 2021, 439, 213946. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Wu, G.; Wang, S.; Guo, X.; Wang, L.; Wang, X.; Li, J.; Chen, L. Preparation of mixed-matrix membranes from metal organic framework (MIL-53) and poly (vinylidene fluoride) for use in determination of sulfonylurea herbicides in aqueous environments by high performance liquid chromatography. J. Colloid Interface Sci. 2019, 553, 834–844. [Google Scholar] [CrossRef]
- Bai, W.; Li, S.; Ma, J.; Cao, W.; Zheng, J. Ultrathin 2D metal- organic framework (nanosheets and nanofilms)-based xD-2D hybrid nanostructures as biomimetic enzymes and supercapacitors. J. Mater. Chem. A 2019, 7, 9086–9098. [Google Scholar] [CrossRef]
- Li, C.; Wu, R.; Zou, J.; Zhang, T.; Zhang, S.; Zhang, Z.; Hu, X.; Yan, Y.; Ling, X. MNPs@anionic MOFs/ERGO with the size selectivity for the electrochemical determination of H2O2 released from living cells. Biosens. Bioelectron. 2018, 116, 81–88. [Google Scholar] [CrossRef]
- Li, S.; Hou, Y.; Chen, Q.; Zhang, X.; Cao, H.; Huang, Y. Promoting Active Sites in MOF-Derived Homobimetallic Hollow Nanocages as a High-Performance Multifunctional Nanozyme Catalyst for Biosensing and Organic Pollutant Degradation. ACS Appl. Mater. Interfaces 2020, 12, 2581–2590. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Li, S.; Tan, J.; Xu, C.; Huang, Y. MOF-derived Co3O4@Co-Fe oxide double-shelled nanocages as multi-functional specific peroxidase-like nanozyme catalysts for chemo/biosensing and dye degradation. Chem. Eng. J. 2020, 395, 125130. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Y.; Liang, L.; Zhao, S.; Ye, F. Rational design of ZIF-67 derived hollow nanozyme through a general strategy for biosensing. Microchem. J. 2023, 190, 108601. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhua, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Zhao, X.; Li, H.; Zhang, M.; Pan, W.; Luo, Z.; Sun, X. Hierarchical Nanocages Assembled by NiCo-Layered Double Hydroxide Nanosheets for a High-Performance Hybrid Supercapacitor. ACS Appl. Mater. Interfaces 2022, 14, 34781–34792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ai, J.; Gao, H.; Zhang, W.; Wang, D. Removal of arsenic in groundwater using Slag based calcined layered double hydroxides (CLDHs) with dual functions of adsorption and photo-catalysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125300. [Google Scholar] [CrossRef]
- Ghosh, D.; Basak, S.; Wunsch, B.H.; Kumar, R.; Stellacci, F. Effect of Composition on the Catalytic Properties of Mixed-Ligand-Coated Gold Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 7900–7905. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, X.J.; Zhu, Y.; Muhammad, F.; Tan, S.; Cao, W.; Lin, S.; Jin, Z.; Gao, X.; Wei, H. Nitrogen-Doped Carbon Nanomaterials as Highly Active and Specific Peroxidase Mimics. Chem. Mater. 2018, 30, 6431–6439. [Google Scholar] [CrossRef]
- Chen, R.; Xue, J.; Gao, X.; Yu, C.; Chen, Q.; Zhou, J.; Sun, G.; Huang, W. Jahn-Teller distortions boost the ultrahigh areal capacity and cycling robustness of holey NiMn-hydroxide nanosheets for flexible energy storage devices. Nanoscale 2020, 12, 22075–22081. [Google Scholar] [CrossRef]
- Hu, H.; Guan, B.; Xia, B.; Wen, X. Designed formation of Co3O4/NiCo2O4 double-shelled nanocages with enhanced pseudocapacitive and electrocatalytic properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Ge, R.; Li, W.; Zhang, Y.; Feng, L.; Che, R. 3D freestanding flower-like nickel-cobalt layered double hydroxides enriched with oxygen vacancies as efficient electrocatalysts for water oxidation. Sustain. Mater. Technol. 2020, 25, 00170. [Google Scholar] [CrossRef]
- Jia, X.; Gao, S.; Liu, T.; Li, D.; Tang, P.; Feng, Y. Fabrication and Bifunctional Electrocatalytic Performance of Ternary CoNiMn Layered Double Hydroxides/Polypyrrole/Reduced Graphene Oxide Composite for Oxygen Reduction and Evolution Reactions. Electrochim. Acta 2017, 245, 59–68. [Google Scholar] [CrossRef]
- Mikhaylov, V.I.; Kryuchkova, A.V.; Sitnikov, P.A.; Koval, L.A.; Zemskaya, N.V.; Krivoshapkina, E.F.; Krivoshapkin, P.V. Magnetite Hydrosols with Positive and Negative Surface Charge of Nanoparticles: Stability and Effect on the Lifespan of Drosophila melanogaster. Langmuir 2020, 36, 4405–4415. [Google Scholar] [CrossRef]
- Liu, G.; Liao, L.; Dai, Z.; Qi, Q.; Wu, J.; Ma, L.Q.; Tang, C.; Xu, J. Organic adsorbents modified with citric acid and Fe3O4 enhance the removal of Cd and Pb in contaminated solutions. Chem. Eng. J. 2020, 395, 125108. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Simple rapid stabilization method through citric acid modification for magnetite nanoparticles. Sci. Rep. 2020, 10, 10793. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Gong, X.; Zeng, L.; Wang, J.; Zhu, Y. Magnetic Zr-Based Metal-Organic Frameworks: A Highly Efficient Au (III) Trapper for Gold Recycling. Materials 2022, 15, 6531. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.S.; Lima, E.C.; Sampaio, C.H.; Rodembusch, F.S.; Petter, C.O.; Cazacliu, B.G.; Dotto, G.L.; Hidalgo, G.E.N. Novel kaolin/polysiloxane based organic-inorganic hybrid materials: Sol-gel synthesis, characterization and photocatalytic properties. J. Solid State Chem. 2018, 260, 106–116. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Wu, J.; Gao, H.; Yang, W.; Hu, X. Enhanced Heterogeneous Peroxymonosulfate Activation by MOF-Derived Magnetic Carbonaceous Nanocomposite for Phenol Degradation. Materials 2023, 16, 3325. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, N.; Liu, J.; Yang, F.; Li, C.; Wang, S. Newly Constructed NiCo2O4 Derived from ZIF-67 with Dual Mimic Enzyme Properties for Colorimetric Detection of Biomolecules and Metal Ions. ACS Appl. Mater. Interfaces 2021, 13, 25044–25052. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shang, J.; Guo, C.; Zhang, J.; Zhu, F.; Han, A.; Liu, J. A General Method to Ultrathin Bimetal-MOF Nanosheets Arrays via in Situ Transformation of Layered Double Hydroxides Arrays. Small 2019, 15, 1804761. [Google Scholar] [CrossRef]
- Cui, S.; Sun, L.; Kong, F.; Huo, L.; Zhao, H. Carbon-coated MnCo2O4 nanowire as bifunctional oxygen catalysts for rechargeable Zn-air batteries. J. Power Sources 2019, 430, 25–31. [Google Scholar]
- Li, X.; Fang, Y.; Lin, X.; Tian, M.; An, X.; Fu, Y.; Li, R.; Jin, J.; Ma, J. MOF derived Co3O4 nanoparticles embedded in N-doped mesoporous carbon layer/MWCNT hybrids: Extraordinary bi-functional electrocatalysts for OER and ORR. J. Mater. Chem. 2015, 3, 17392–17402. [Google Scholar] [CrossRef]
- Li, J.; Xiong, D.; Wang, L.; Hirbod, M.K.S.; Li, X. High-performance self-assembly MnCo2O4 nanosheets for asymmetric supercapacitors. J. Energy Chem. 2019, 37, 66–72. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Tsang, S.C. Cr-MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Li, H.; Cai, S.; Hu, H.; Fang, C.; Shi, L.; Zhang, D. Rational design and in situ fabrication of MnO2@NiCo2O4 nanowire arrays on Ni foam as high-performance monolith de-NOx catalysts. J. Mater. Chem. A 2015, 3, 11543–11553. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, G.; Zhang, Z.; Guo, Y.; Guo, Y.; Wang, Y. Highly Active and Stable Co3O4/ZSM-5 Catalyst for Propane Oxidation: Effect of the Preparation Method. ACS Catal. 2013, 3, 1154–1164. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Q.; Chen, X.; Fan, B.; Xiao, K.; Zhang, S.; Deng, W.; Hu, A. Self-assembled synthesis of diamond-like MnCo2O4 as anode active material for lithium-ion batteries with high cycling stability. J. Alloys Comp. 2017, 722, 387–393. [Google Scholar] [CrossRef]
- Shi, J.; Yin, T.; Shen, W. Effect of surface modification on the peroxidase-like behaviors of carbon dots. Colloids Surf. B Biointerfaces 2019, 178, 163–169. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Z.; Lian, Q.; Liu, H.; Chen, L.; Zhou, L.; Jiang, Y.; Gao, J. Preparation of Flower-like NiMnO3 as Oxidase Mimetics for Colorimetric Detection of Hydroquinone. ACS Sustain. Chem. Eng. 2021, 9, 12766–12778. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, M.; He, Q.; Zhao, M.; Cui, H. Photoenhanced Oxidase-Peroxidase-like NiCo2O4@MnO2 Nanozymes for Colorimetric Detection of Hydroquinone. ACS Sustain. Chem. Eng. 2022, 10, 5651–5658. [Google Scholar] [CrossRef]
- Wu, S.; Guo, D.; Xu, X.; Pan, J.; Niu, X. Colorimetric quantification and discrimination of phenolic pollutants based on peroxidase-like Fe3O4 nanoparticles. Sens. Actuators B Chem. 2020, 303, 127225. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, L.; Liu, Z.; Cao, X.; Lv, L.; Xia, J.; Wang, Z. Metal-organic frameworks-derived bimetallic oxide composite nanozyme fiber membrane and the application to colorimetric detection of phenol. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129662. [Google Scholar] [CrossRef]
- Du, J.; Qi, S.; Fan, T.; Yang, Y.; Wang, C.; Shu, Q.; Zhuo, S.; Zhu, C. Nitrogen and copper-doped carbon quantum dots with intrinsic peroxidase-like activity for double-signal detection of phenol. Analyst 2021, 146, 4280–4289. [Google Scholar] [CrossRef]
- Cai, S.; Han, Q.; Qi, C.; Wang, X.; Wang, T.; Jia, X.; Yang, R.; Wang, C. MoS2-Pt3Au1 nanocomposites with enhanced peroxidase-like activities for selective colorimetric detection of phenol. Chin. J. Chem. 2017, 35, 605–612. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, Y.; Yin, Y.; Hu, W.; Liu, W.; Yang, H. Facile synthesis of enzyme-inorganic hybrid nanoflowers and its application as a colorimetric platform for visual detection of hydrogen peroxide and phenol. ACS Appl. Mater. Interfaces 2014, 6, 10775–10782. [Google Scholar] [CrossRef] [PubMed]

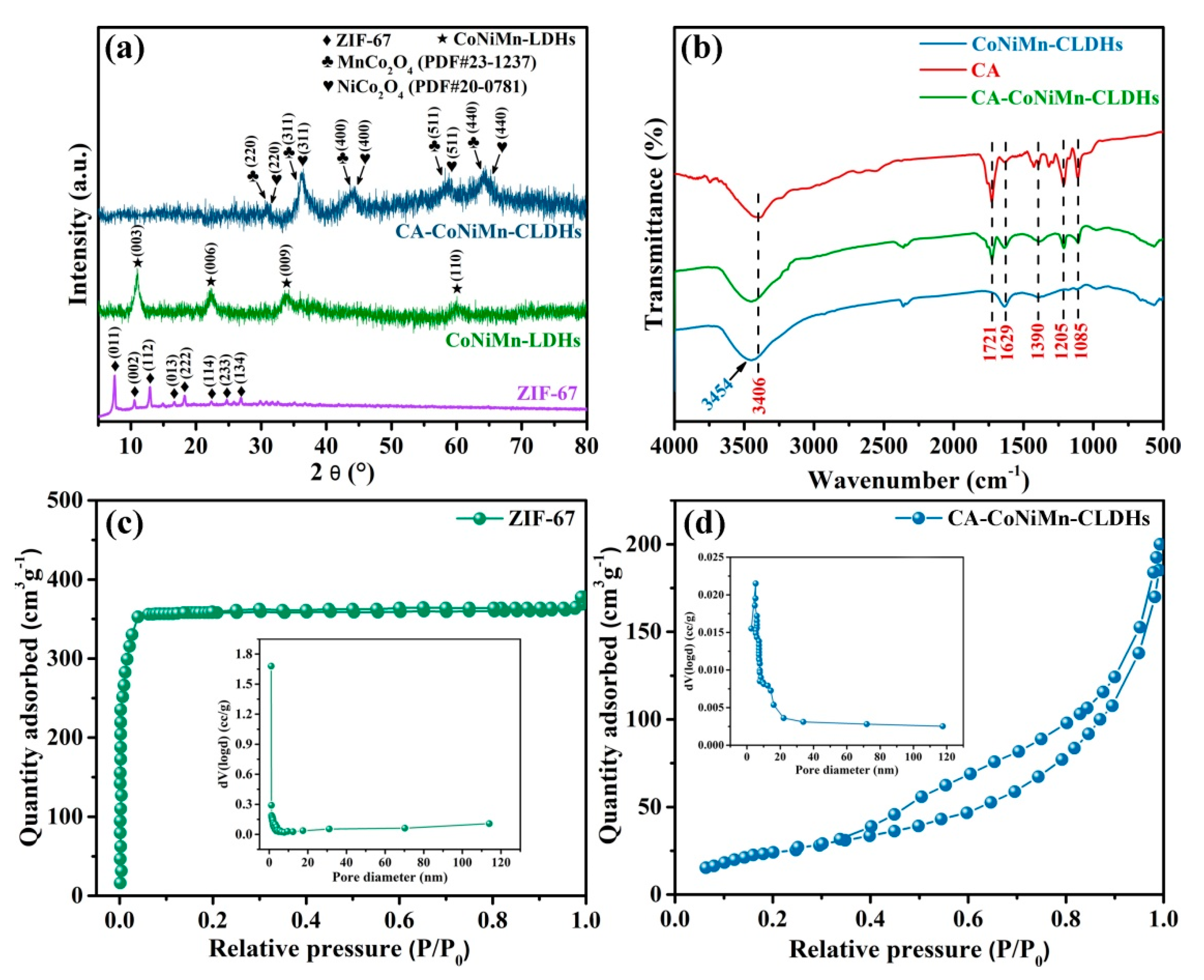
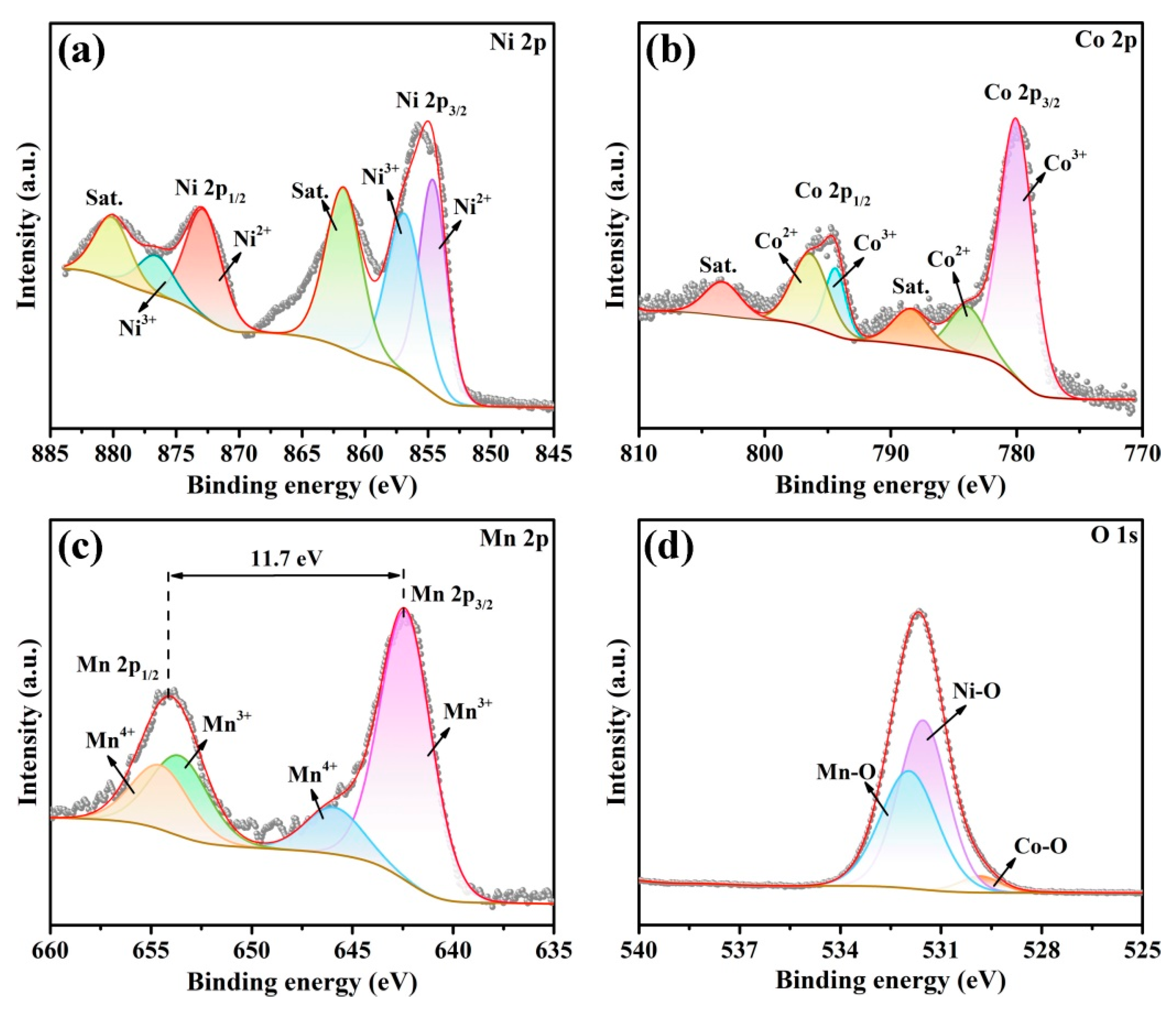
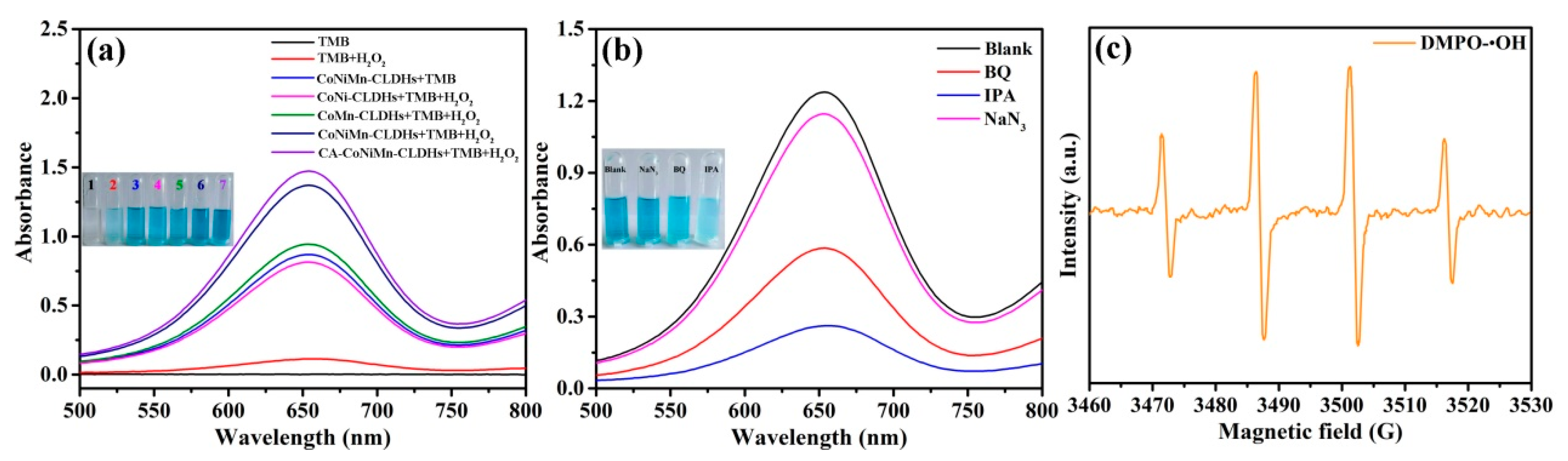


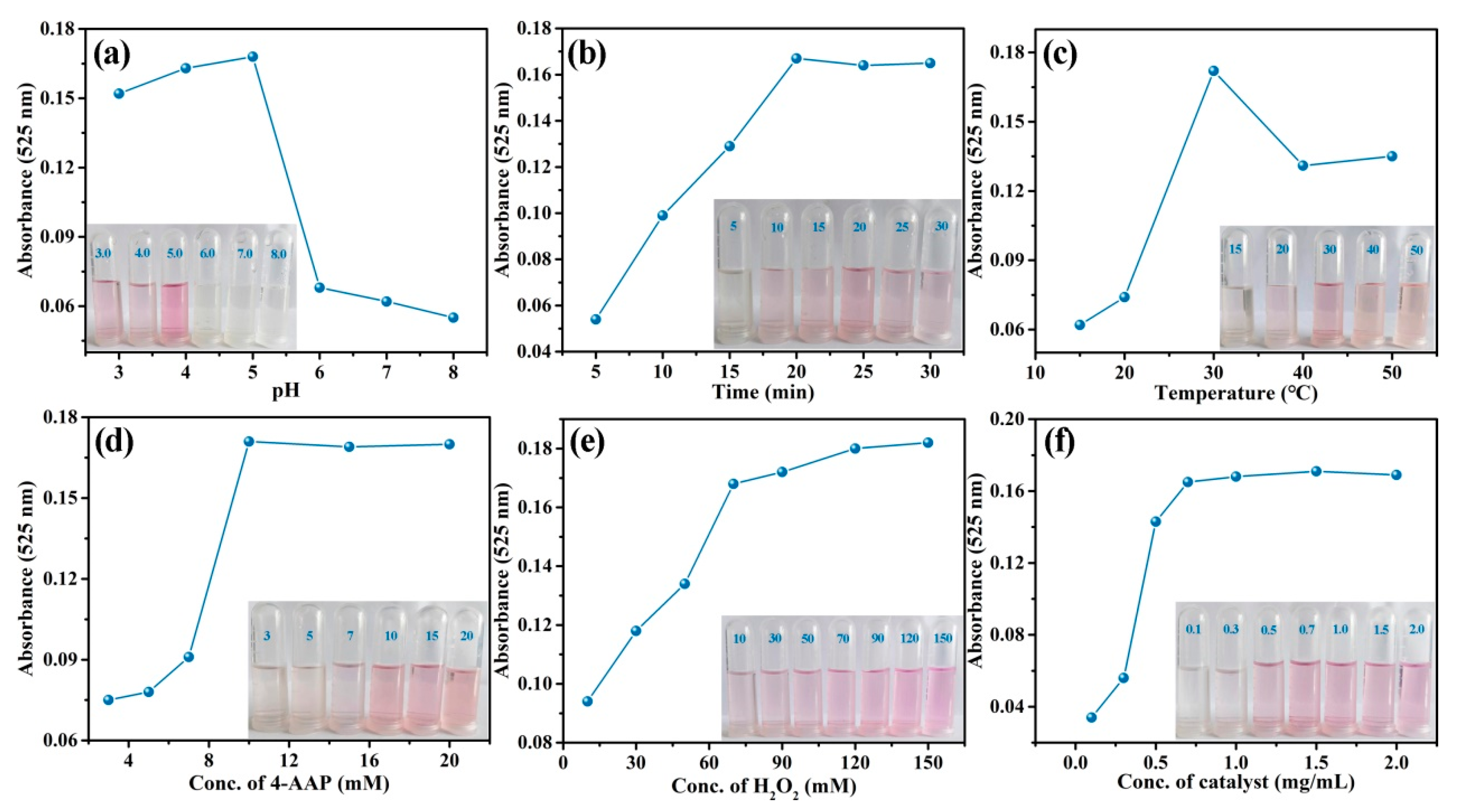
| Sample | Ni(NO3)2·6H2O (mg) | MnCl2·4H2O (mg) | Solvent |
|---|---|---|---|
| CoNi-LDHs | 160 | 0 | DMF/EtOH/DIW |
| CoMn-LDHs | 0 | 160 | DMF/EtOH/DIW |
| Sample | SBET (m2g−1) | VTotal (cm3g−1) | Dp (nm) |
|---|---|---|---|
| ZIF-67 | 892.32 | 0.83 | 2.02 |
| CA-CoNiMn-CLDHs | 181.61 | 0.52 | 8.41 |
| Materials | Km (mM) | Vmax (10−8 M s−1) | ||
|---|---|---|---|---|
| H2O2 | TMB | H2O2 | TMB | |
| HRP | 3.7 | 0.43 | 8.71 | 10 |
| CA-CoNiMn-CLDHs | 0.53 | 0.21 | 0.71 | 0.83 |
| System | LOD (μM) | Linear Range | Ref. |
|---|---|---|---|
| CAT-Fe3O4@ZIF-8 | 0.7 | 5–100 μM | [1] |
| Co3O4/CDM | 1.02 | 0.5–300 μM | [3] |
| Au@Ni13/rGO | 1.68 | 1–300 μM | [8] |
| Co/Mn-MOF-74 | 0.2 | 0.5–500 μM | [59] |
| MoS2-Pt3Au1 | 0.2 | 4–1000 μM | [61] |
| N, Cu-CQDs | 0.12 | 1–100 μM | [60] |
| HRP-Cu3(PO4)2·3H2O | 1 | 0–100 μM | [62] |
| CA-CoNiMn-CLDHs | 0.163 | 1–100 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, W.; Xin, R.; Zhang, J.; Yang, L.; Jing, M.; Ma, F.; Yang, J. Co(II)-Based Metal-Organic Framework Derived CA-CoNiMn-CLDHs with Peroxidase-like Activity for Colorimetric Detection of Phenol. Materials 2023, 16, 6212. https://doi.org/10.3390/ma16186212
Tan W, Xin R, Zhang J, Yang L, Jing M, Ma F, Yang J. Co(II)-Based Metal-Organic Framework Derived CA-CoNiMn-CLDHs with Peroxidase-like Activity for Colorimetric Detection of Phenol. Materials. 2023; 16(18):6212. https://doi.org/10.3390/ma16186212
Chicago/Turabian StyleTan, Wenjie, Rui Xin, Jiarui Zhang, Lilin Yang, Min Jing, Fukun Ma, and Jie Yang. 2023. "Co(II)-Based Metal-Organic Framework Derived CA-CoNiMn-CLDHs with Peroxidase-like Activity for Colorimetric Detection of Phenol" Materials 16, no. 18: 6212. https://doi.org/10.3390/ma16186212





