Production of Biodegradable Polymeric Composites with the Addition of Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Macaúba Epicarp Fiber
2.2.1. Moisture Content
- Mu—mass of moisture lost during drying (g);
- Mi—initial mass of the residue (g);
- Mf—final mass of the residue (g).
- u—residue moisture content (%);
- Mu—mass of moisture lost during drying (g);
- Mi—initial mass of the residue (g).
2.2.2. Ash Content
- TCinzas—ash content (%);
- Mf—ash mass (g);
- Mi—sample initial mass (g).
2.2.3. Obtaining Polymer Matrices
2.2.4. Mechanical Properties
- σ—tensile strength (MPa);
- F—last maximum breaking force (N);
- A—cross-sectional area (mm).
- A—elongation at break (MPa);
- Lf—final elongation of the sample (mm);
- Lo—initial sample size (mm).
- E—modulus of elasticity or Young’s modulus (MPa);
- σ—tension (MPa);
- ε—deformation (dimensionless);
- m—sample mass (g);
- v—sample volume (cm3);
- A—sample area (cm2);
- E—sample thickness (mm).
2.2.5. Scanning Electron Microscopy—SEM
2.2.6. Fourier Transform Infrared Spectroscopy—FTIR
2.2.7. Biodegradability
- PM—mass loss (g);
- MI—initial mass (g);
- MF—final mass (g).
- PM—mass loss (%);
- m—initial mass (g);
- m′—final mass (g).
2.2.8. Experimental Design
3. Results
3.1. Moisture Content of Macaúba Epicarp
3.2. Polymer Matrices
3.3. Mechanical Properties
3.4. Biopolymers
3.4.1. Thickness
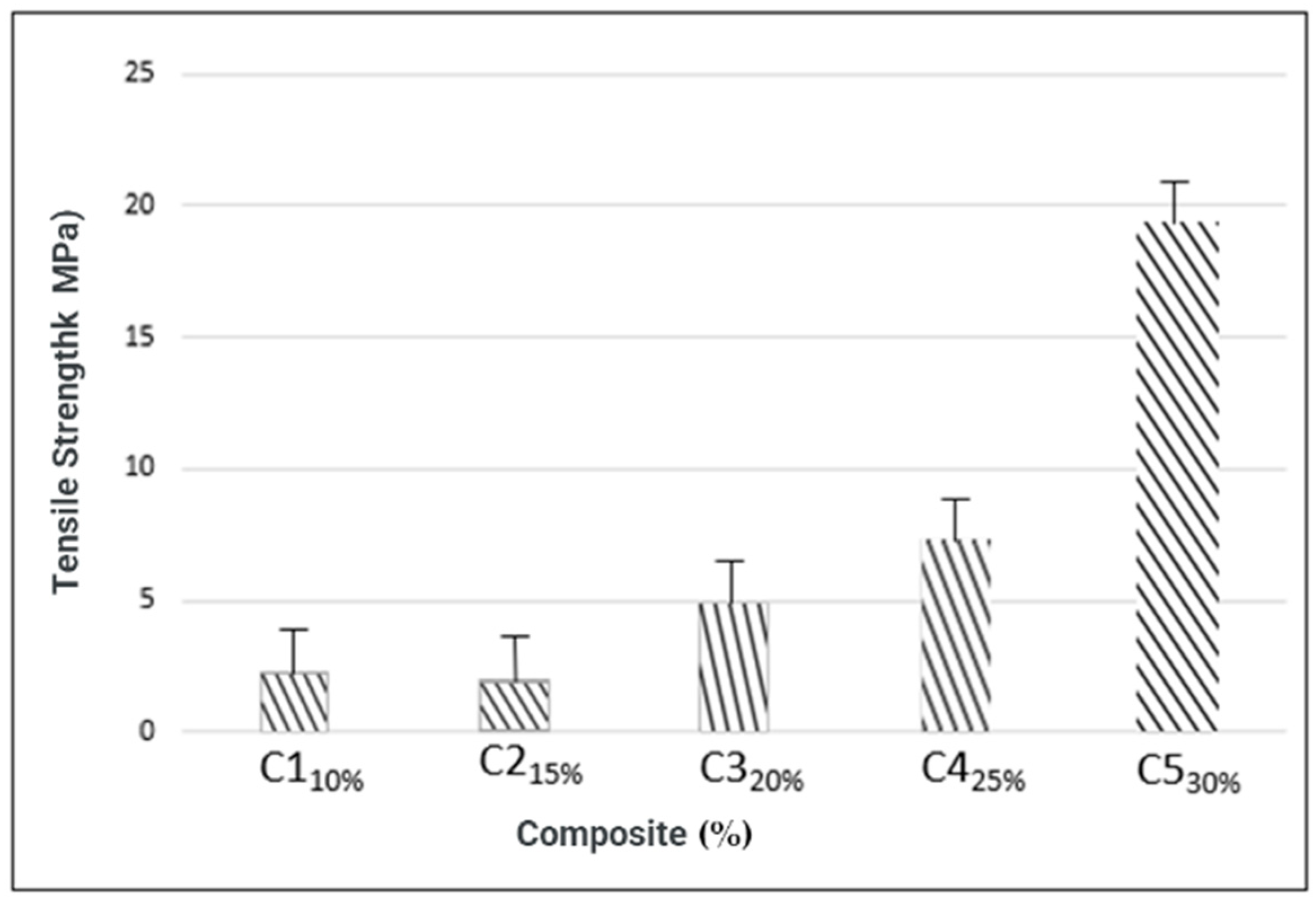
3.4.2. Tensile Strength
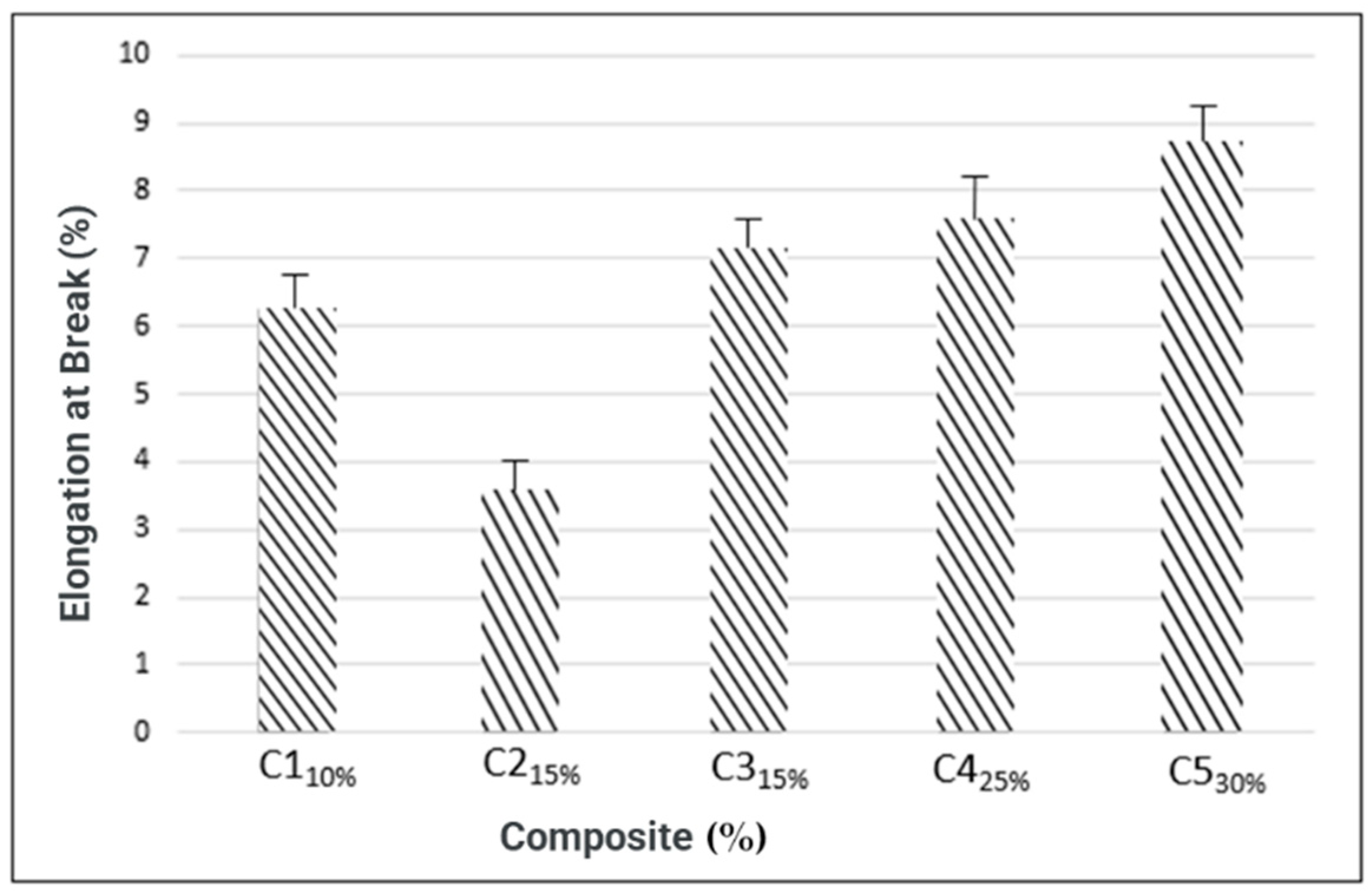
3.4.3. Stretching Resistance (Rupture)
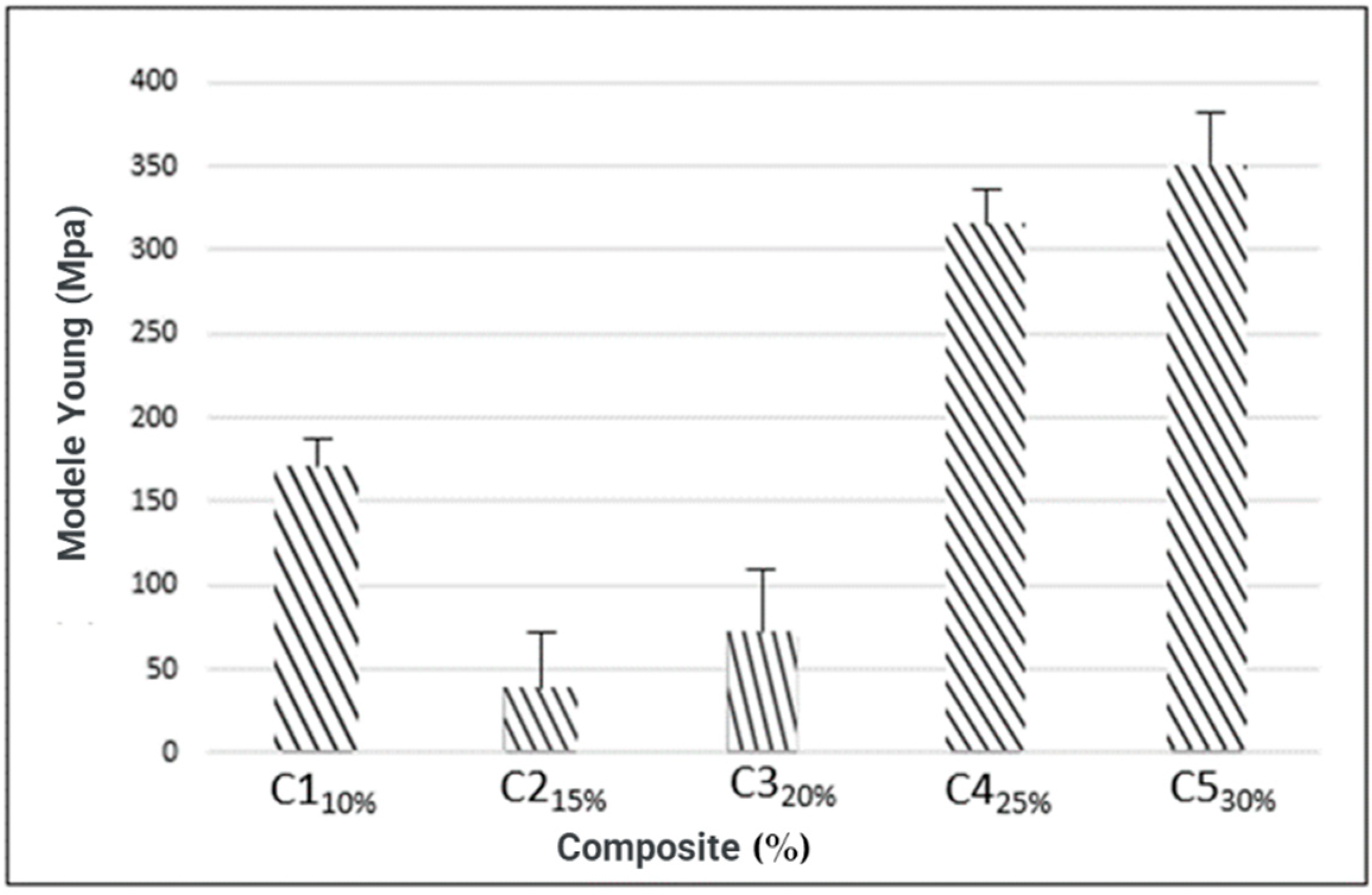
3.4.4. Young’s Modulus
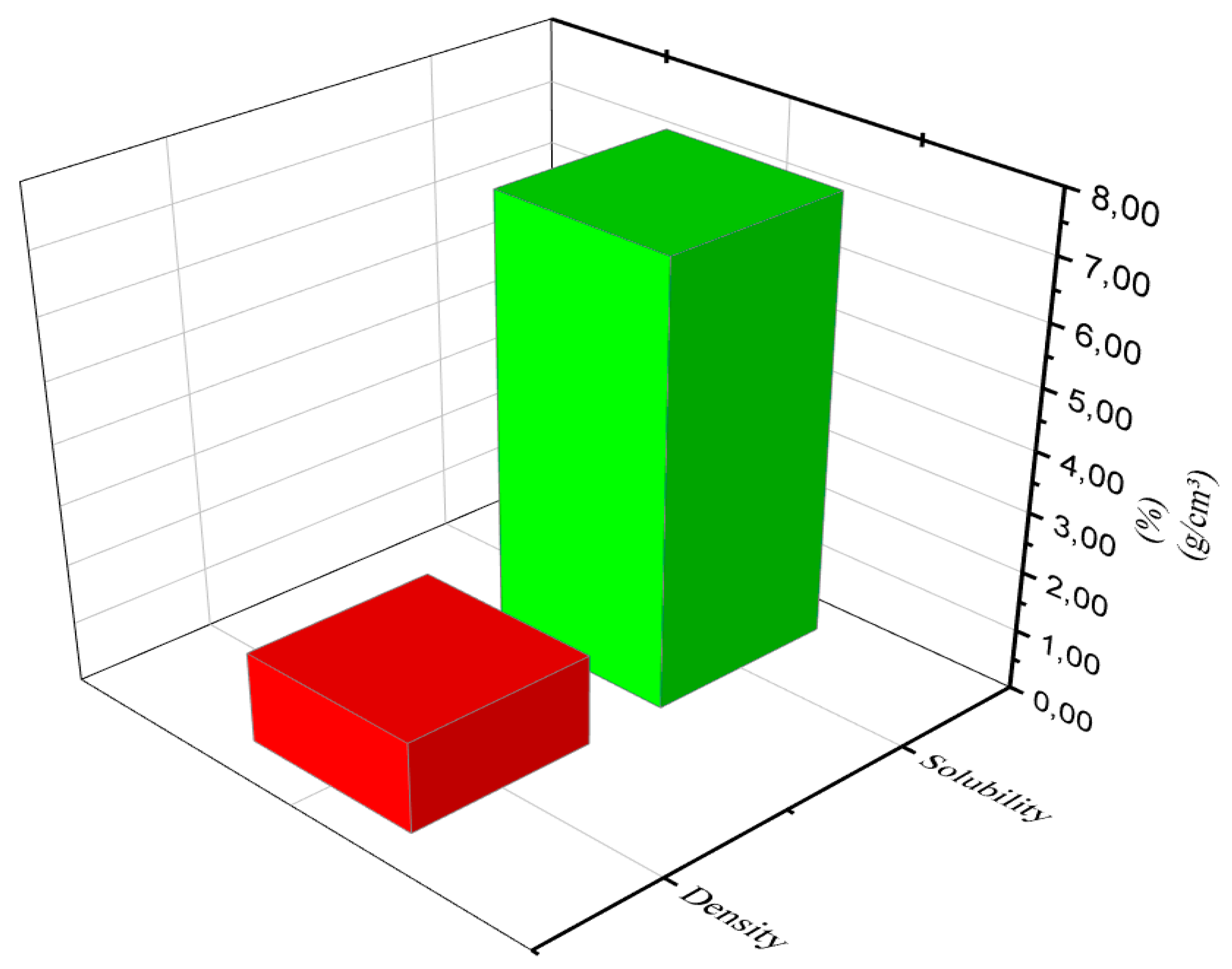
3.4.5. Density
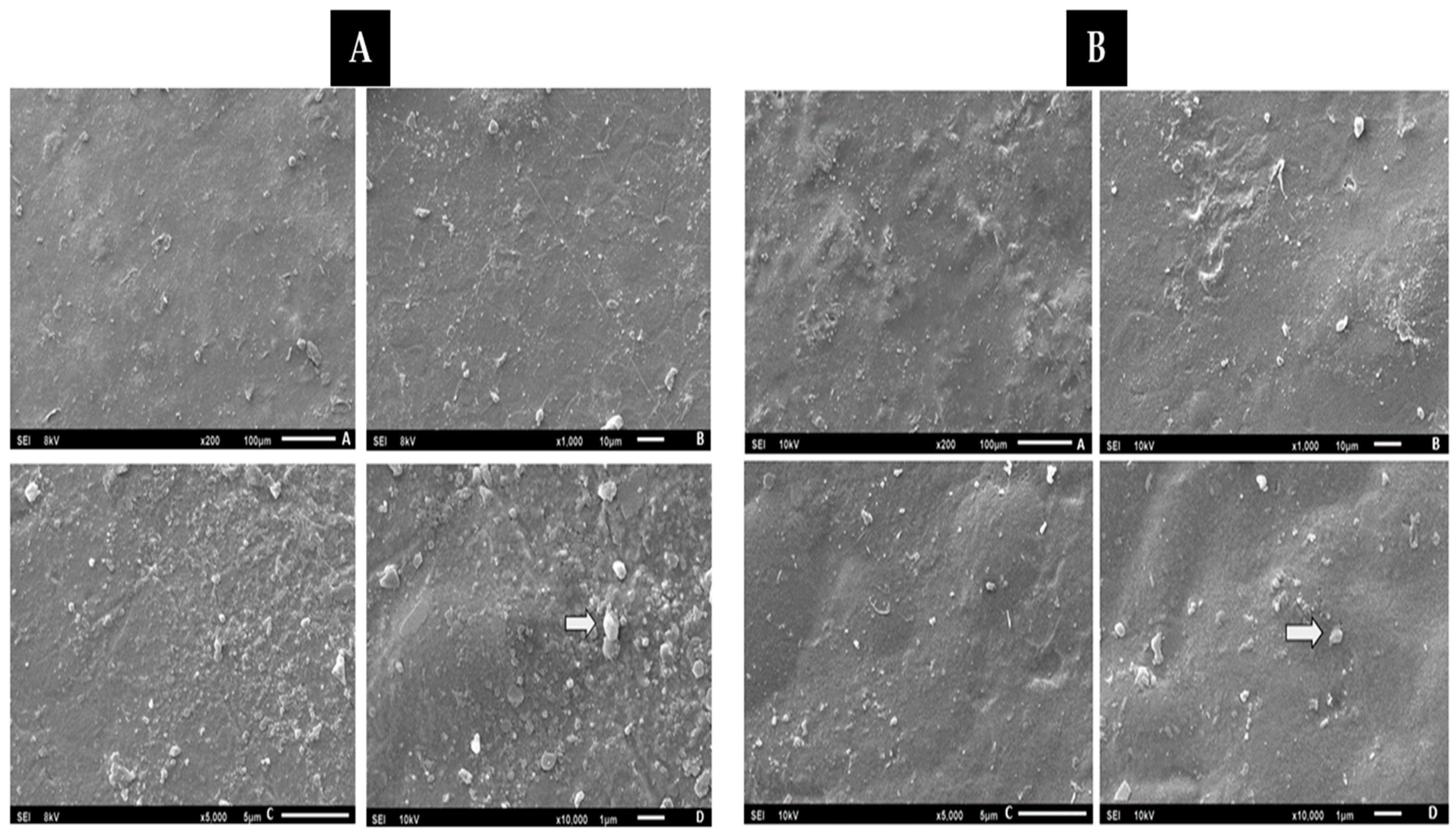
3.4.6. Scanning Electron Microscopy (SEM)
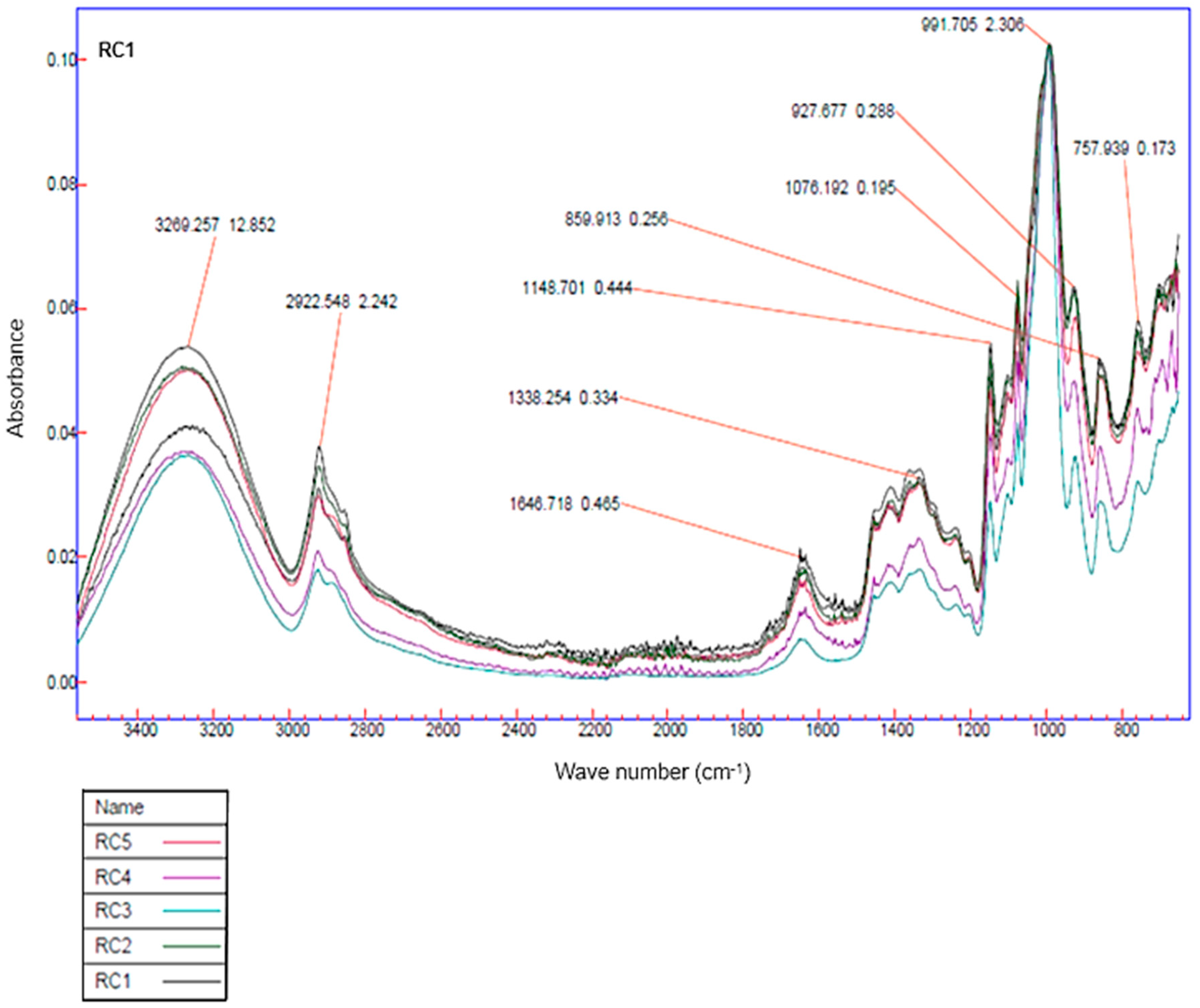
3.4.7. Spectroscopy (FTIR)
3.4.8. Biodegradability
4. Conclusions
- Replacement of glycerol by fatty acid, a residue from the biodiesel production chain;
- Since the macaúba epicarp fiber acted as a good reinforcement, promoting an improvement in the mechanical properties of the composite, an investigation with concentrations greater than 30% is therefore suggested to evaluate the influence on the technological properties;
- High concentrations of glycerol and acetic acid originate fragile and gelatinous polymeric matrices, so a new reagent that acts with glycerol must be investigated to favor its use;
- More research is underway to understand the chemical and physical changes that directly influence biopolymer characteristics and properties (mechanical, physical).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, T.D.; Hasselbalch, J.; Holmberg, K.; Stripple, J. Politics and the Plastic Crisis: A Review throughout the Plastic Life Cycle. Wiley Interdiscip. Rev. Energy Environ. 2020, 9, e360. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Sorrentino, A.; Sorrentino, L.; Notarnicola, M.; Kuchta, K.; Picuno, C.; Oliviero, M. Effect of Cellulose-Based Bioplastics on Current LDPE Recycling. Materials 2023, 16, 4869. [Google Scholar] [CrossRef]
- Yang, J.; Xu, S.; Wang, W.; Ran, X.; Ching, Y.C.; Sui, X.; Wei, Y.; Wang, R.; Al-Hada, N.M. Preparation and Characterization of Bioplastics from Silylated Cassava Starch and Epoxidized Soybean Oils. Carbohydr. Polym. 2023, 300, 120253. [Google Scholar] [CrossRef]
- Baekeland, L.H. The Synthesis, Constitution, and Uses of Bakelite. J. Ind. Eng. Chem. 1909, 1, 149–161. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Siengchin, S.; Shin, G.H.; Kim, J.T. Recent Progress of Bioplastics in Their Properties, Standards, Certifications and Regulations: A Review. Sci. Total Environ. 2023, 878, 163156. [Google Scholar] [CrossRef]
- Haque, M.K.; Uddin, M.; Kormoker, T.; Ahmed, T.; Zaman, M.R.U.; Rahman, M.S.; Rahman, M.A.; Hossain, M.Y.; Rana, M.M.; Tsang, Y.F. Occurrences, Sources, Fate and Impacts of Plastic on Aquatic Organisms and Human Health in Global Perspectives: What Bangladesh Can Do in Future? Environ. Geochem. Health 2023, 45, 5531–5556. [Google Scholar] [CrossRef]
- Ahmad, F.; Jamal, A.; Mazher, K.M.; Umer, W.; Iqbal, M. Performance Evaluation of Plastic Concrete Modified with E-Waste Plastic as a Partial Replacement of Coarse Aggregate. Materials 2021, 15, 175. [Google Scholar] [CrossRef]
- Šaravanja, A.; Pušić, T.; Dekanić, T. Microplastics in Wastewater by Washing Polyester Fabrics. Materials 2022, 15, 2683. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Analysis of Selected Properties of Injection Moulded Sustainable Biocomposites from Poly(Butylene Succinate) and Wheat Bran. Materials 2021, 14, 7049. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lu, J.; Zhang, Y.; Liang, H.; Liang, D.; Jiang, J.; Lu, Q.; Quirino, R.L.; Zhang, C. Thermosetting Polyurethanes Prepared with the Aid of a Fully Bio-Based Emulsifier with High Bio-Content, High Solid Content, and Superior Mechanical Properties. Green Chem. 2019, 21, 526–537. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Bergel, B.F.; Leite Araujo, L.; dos Santos da Silva, A.L.; Campomanes Santana, R.M. Effects of Silylated Starch Structure on Hydrophobization and Mechanical Properties of Thermoplastic Starch Foams Made from Potato Starch. Carbohydr. Polym. 2020, 241, 116274. [Google Scholar] [CrossRef]
- Garcia, P.S.; Grossmann, M.V.E.; Shirai, M.A.; Lazaretti, M.M.; Yamashita, F.; Muller, C.M.O.; Mali, S. Improving Action of Citric Acid as Compatibiliser in Starch/Polyester Blown Films. Ind. Crops Prod. 2014, 52, 305–312. [Google Scholar] [CrossRef]
- Yang, G.; Park, M.; Park, S.-J. Recent Progresses of Fabrication and Characterization of Fibers-Reinforced Composites: A Review. Compos. Commun. 2019, 14, 34–42. [Google Scholar] [CrossRef]
- Cheng, W. Preparation and Properties of Lignocellulosic Fiber/CaCO3/Thermoplastic Starch Composites. Carbohydr. Polym. 2019, 211, 204–208. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- da Silva Fernandes, F.A.; de Oliveira Costa, D.d.S.; Rossignolo, J.A. Influence of Sintering on Thermal, Mechanical and Technological Properties of Glass Foams Produced from Agro-Industrial Residues. Materials 2022, 15, 6669. [Google Scholar] [CrossRef]
- de Moura, C.V.R.; Sousa, D.d.C.; de Moura, E.M.; de Araújo, E.C.E.; Sittolin, I.M. New Biodegradable Composites from Starch and Fibers of the Babassu Coconut. Polímeros 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Ishak, M.R.; Mohammad Taha, M.; Mustapha, F.; Leman, Z. Investigation of ABS–Oil Palm Fiber (Elaeis guineensis) Composites Filament as Feedstock for Fused Deposition Modeling. Rapid Prototyp. J. 2023, 29, 897–909. [Google Scholar] [CrossRef]
- Antony, S.; Cherouat, A.; Montay, G. Fabrication and Characterization of Hemp Fibre Based 3D Printed Honeycomb Sandwich Structure by FDM Process. Appl. Compos. Mater. 2020, 27, 935–953. [Google Scholar] [CrossRef]
- Cardoso, A.N.; Santos, G.S.; Favaro, S.P.; Diniz, C.B.; Sousa, H.U. Extrativismo Da Macaúba Na Região Do Cariri Cearense: Comercialização e Oportunidades. Brazilian J. Dev. 2020, 6, 25261–25279. [Google Scholar] [CrossRef]
- Moreira, V.E.; Veroneze, R.; Saraiva, A.; Duarte, M.d.S.; Guimaraes, S.E.F.; Lopes, M.S.; Bastiaansen, J.W.M.; Santos, G.A.; Campos, L.D.; Campos, P.H.R.F. Growth Performance, Carcass and Pork Quality Traits of Growing-Finishing Pigs with High and Low Breeding Values for Residual Feed Intake Fed Diets with Macauba (Acrocomia aculeata) Pulp as Alternative Raw Material. Agriculture 2022, 12, 1860. [Google Scholar] [CrossRef]
- Calvani, C.C.; Goncalves, A.-M.B.; Silva, M.J.; Oliveira, S.L.; Marangoni, B.S.; dos Reis, D.D.; Cena, C. Portland Cement/Acrocomia aculeata Endocarp Bricks: Thermal Insulation and Mechanical Properties. Materials 2020, 13, 2081. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and Properties of Natural Fiber Polymer Composites: A Comprehensive Review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
- Vieira, A.P.; Santana, S.A.; Silva, H.A.S.; Airoldi, C. Epicarp and Mesocarp of Babassu (Orbignya speciosa): Characterization and Application. J. Braz. Chem. Soc. 2011, 22, 21–29. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Jorge, N. Proximate Composition of Guariroba (Syagrus oleracea), Jerivá (Syagrus romanzoffiana) and Macaúba (Acrocomia aculeata) Palm Fruits. Food Res. Int. 2011, 44, 2139–2142. [Google Scholar] [CrossRef]
- Benshlomo, O. Acoplamento Da Desoxigenação de Óleos Vegetais Com a Reforma de Glicerol Em Reator Multifuncional. Ph.D. Thesis, Centro Universitário FEI, São Bernardo do Campo, Brazil, 2023. [Google Scholar]
- Chaharmahali, M.; Tajvidi, M.; Najafi, S.K. Mechanical Properties of Wood Plastic Composite Panels Made from Waste Fiberboard and Particleboard. Polym. Compos. 2008, 29, 606–610. [Google Scholar] [CrossRef]
- Fernandes, F.A.d.S.; Costa, D.d.S.d.O.; Martin, C.A.G.; Rossignolo, J.A. Vitreous Foam with Thermal Insulating Property Produced with the Addition of Waste Glass Powder and Rice Husk Ash. Sustainability 2023, 15, 796. [Google Scholar] [CrossRef]
- Madsen, B.; Thygesen, A.; Lilholt, H. Plant Fibre Composites—Porosity and Stiffness. Compos. Sci. Technol. 2009, 69, 1057–1069. [Google Scholar] [CrossRef]
- Oliveira, L.M.d.R. Membranas Poliméricas Híbridas Policarbonato/Grafeno: Correlação Estrutura e Propriedades de Transporte; Universidade Federal de São Carlos: São Paulo, Brazil, 2018. [Google Scholar]
- ASTM D638-08; Standard Test Method for Tensile Properties of Plastics. Available online: https://webstore.ansi.org/standards/astm/astmd63808 (accessed on 14 September 2023).
- ASTM D882-00; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2017.
- Gontard, N.; Duchez, C.; Cuq, J.-L.; Guilbert, S. Edible Composite Films of Wheat Gluten and Lipids: Water Vapour Permeability and Other Physical Properties. Int. J. Food Sci. Technol. 2007, 29, 39–50. [Google Scholar] [CrossRef]
- ASTM G160-03; Standard Practice for Evaluating Microbial Susceptibility of Nonmetallic Materials by Laboratory Soil Burial. Available online: https://webstore.ansi.org/standards/astm/astmg16003 (accessed on 14 September 2023).
- Chandra, R.; Rustgi, R. Biodegradation of Maleated Linear Low-Density Polyethylene and Starch Blends. Polym. Degrad. Stab. 1997, 56, 185–202. [Google Scholar] [CrossRef]
- Oliveira da Silva, A.; Cortez-Vega, W.R.; Prentice, C.; Fonseca, G.G. Development and Characterization of Biopolymer Films Based on Bocaiuva (Acromonia aculeata) Flour. Int. J. Biol. Macromol. 2020, 155, 1157–1168. [Google Scholar] [CrossRef]
- Yan, L.; Kasal, B.; Huang, L. A Review of Recent Research on the Use of Cellulosic Fibres, Their Fibre Fabric Reinforced Cementitious, Geo-Polymer and Polymer Composites in Civil Engineering. Compos. Part B Eng. 2016, 92, 94–132. [Google Scholar] [CrossRef]
- Zau, M.D.L.; de Vasconcelos, R.P.; Giacon, V.M.; Lahr, F.A.R. Avaliação Das Propriedades Química, Física e Mecânica de Painéis Aglomerados Produzidos Com Resíduo de Madeira Da Amazônia—Cumaru (Dipteryx odorata) e Resina Poliuretana à Base de Óleo de Mamona. Polímeros 2014, 24, 726–732. [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, C.; Cui, B.; Sha, H.; Liu, P.; Lu, L.; Wu, Z. High-Amylose Corn Starch/Konjac Glucomannan Composite Film: Reinforced by Incorporating β-Cyclodextrin. J. Agric. Food Chem. 2021, 69, 2493–2500. [Google Scholar] [CrossRef]
- Narváez-Gómez, G.; Figueroa-Flórez, J.; Salcedo-Mendoza, J.; Pérez-Cervera, C.; Andrade-Pizarro, R. Development and Characterization of Dual-Modified Yam (Dioscorea rotundata) Starch-Based Films. Heliyon 2021, 7, e06644. [Google Scholar] [CrossRef]
- Tian, H.; Wang, K.; Liu, D.; Yan, J.; Xiang, A.; Rajulu, A.V. Enhanced Mechanical and Thermal Properties of Poly (Vinyl Alcohol)/Corn Starch Blends by Nanoclay Intercalation. Int. J. Biol. Macromol. 2017, 101, 314–320. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Effect of the Incorporation of Surfactants on the Physical Properties of Corn Starch Films. Food Hydrocoll. 2014, 38, 66–75. [Google Scholar] [CrossRef]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple Peel Extract Incorporated Poly(Vinyl Alcohol)-Corn Starch Film for Active Food Packaging: Preparation, Characterization and Antioxidant Activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef]
- Yurong, G.; Dapeng, L. Preparation and Characterization of Corn Starch/PVA/Glycerol Composite Films Incorporated with ε-Polylysine as a Novel Antimicrobial Packaging Material. e-Polymers 2020, 20, 154–161. [Google Scholar] [CrossRef]
- Barros Nunes, R.S.; Albuquerque do Nascimento, A.; Valdés Serra, J.C. Obtenção e caracterização de compósitos poliméricos biodegradáveis produzidos com resíduos agroenergéticos (bagaço da cana-de-açúcar, amido de milho e glicerol). Rev. Acta Ambient. Catarin. 2021, 18, 156–168. [Google Scholar] [CrossRef]
- Lim, R.; Kiew, P.L.; Lam, M.K.; Yeoh, W.M.; Ho, M.Y. Corn Starch/PVA Bioplastics—The Properties and Biodegradability Study Using Chlorella vulgaris Cultivation. Asia-Pacific J. Chem. Eng. 2021, 16, e2622. [Google Scholar] [CrossRef]
- Silva, M.L.T.; Brinques, G.B.; Gurak, P.D. Desenvolvimento e Caracterização de Bioplásticos de Amido de Milho Contendo Farinha de Subproduto de Broto. Braz. J. Food Technol. 2020, 23, 1–13. [Google Scholar] [CrossRef]
- Gomes, Á.V.R.; Gonçalves, F.C.P.; da Silva Júnior, M.Q.; Leite, R.H.d.L.; dos Santos, F.K.G.; Aroucha, E.M.M. Effect of Carnauba Wax and Coconut Fiber Contents on Tensile Properties of Corn Starch-Based Biocomposites. Mater. Res. 2019, 22, 1–7. [Google Scholar] [CrossRef]
- Schoeler, M.; Scremin, F.; de Mendonça, N.; Benetti, V.; de Jesus, J.; Basso, R.; Bittencourt, P. Cellulose nanofibers from cassava agro-industrial waste as reinforcement in pva films. Quim. Nova 2020, 43, 711–717. [Google Scholar] [CrossRef]
- Díaz, O.; Ferreiro, T.; Rodríguez-Otero, J.; Cobos, Á. Characterization of Chickpea (Cicer arietinum L.) Flour Films: Effects of PH and Plasticizer Concentration. Int. J. Mol. Sci. 2019, 20, 1246. [Google Scholar] [CrossRef]
- Zimmermann, M.V.; Turella, T.C.; Santana, R.M.C.; Zattera, A.J. Influência Do Tratamento Químico Da Fibra de Bananeira Em Compósitos de Poli(Etileno-Co-Acetato de Vinila) Com e Sem Agente de Expansão. Polímeros Ciência Tecnol. 2014, 24, 58–64. [Google Scholar] [CrossRef]
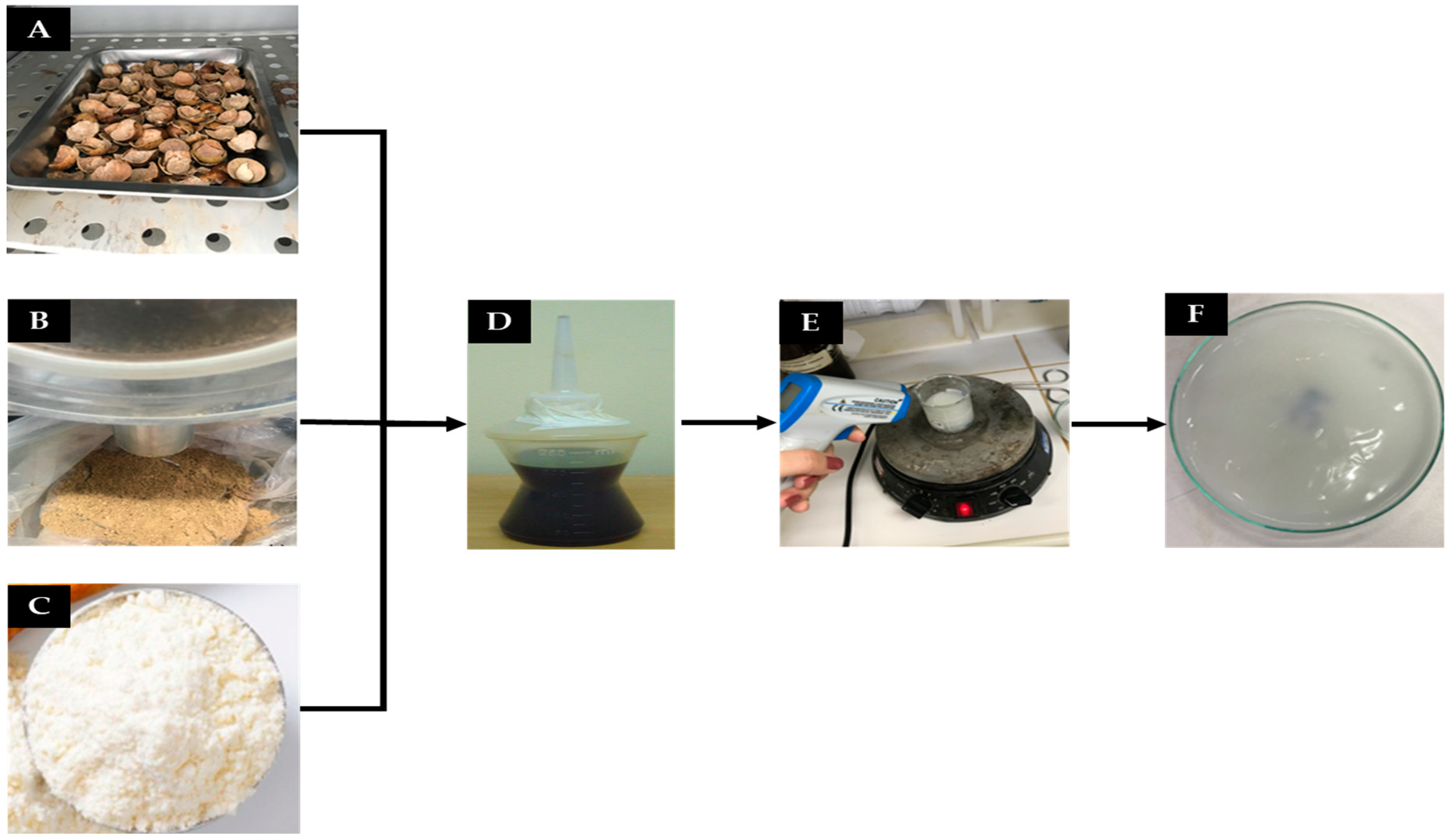

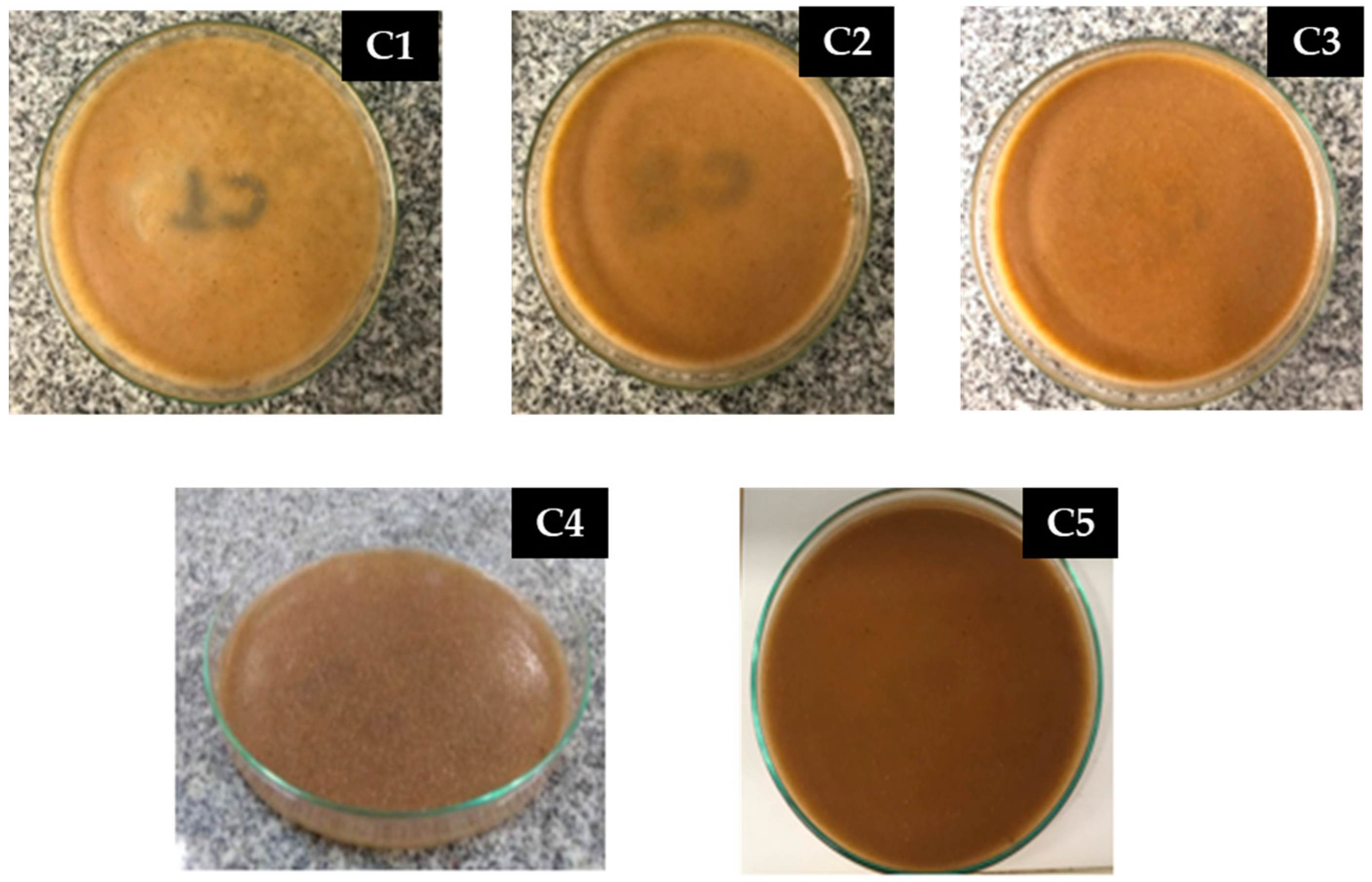


| Matrix | Corn Starch (g) | Water (mL) | Glycerol (mL) | Acetic Acid (mL) |
|---|---|---|---|---|
| M1 M2 M3 M4 M5 | 3.00 3.00 3.00 5.00 5.00 | 50.00 | 1.00 3.00 1.00 1.00 2.00 | 1.00 1.00 2.00 1.00 1.00 |
| Composite | Matrix Sample (%) | Macaúba Epicarp Fiber (%) |
|---|---|---|
| C1 C2 C3 C4 C5 | 90 85 80 75 70 | 10 15 20 25 30 |
| Sample | Moisture (%) | Ash Content (%) |
|---|---|---|
| 1 2 3 4 5 6 7 8 9 10 | 3.31 4.43 4.89 3.88 4.97 4.30 4.77 3.79 3.00 4.51 | 5.12 4.48 4.74 3.89 5.02 5.66 4.23 3.98 4.09 4.78 |
| Average mean | 4.20 | 4.60 |
| Standard deviation | ±0.67 | ±0.57 |
| Matrix | Thickness (mm) | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| M1 M2 M3 M4 M5 | 0.43 ± 0.02 0.37 ± 0.02 0.36 ± 0.02 0.65 ± 0.03 0.46 ± 0.04 | 4.90 ± 0.41 1.266 ± 0.04 1.645 ± 0.16 15.470 ± 2.09 6.977 ± 0.77 | 18.839 ± 3.9 17.414 ± 1.69 24.278 ± 1.04 7.24 ± 0.62 15.882 ± 2.12 | 71.934 ± 1.16 11.614 ± 0.11 15.06 ± 1.52 439.49 ± 3.20 160.27 ± 4.73 |
| VS | DF | SM | |||
|---|---|---|---|---|---|
| Thickness (mm) | Tensile Strength (MPa) | Elongation at Break (MPa) | Young’s Modulus (MPa) | ||
| Matrix | 4 | 0.053 | 151.07 | 401.84 | 150.56 |
| Error | 21 | 0.002 | 0.882 | 4.153 | 190.06 |
| Total | 25 | - | - | - | - |
| CV (%) | - | 14.17 | 18.56 | 11.40 | 12.10 |
| Composite | Thickness (mm) | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| C1 C2 C3 C4 C5 | 0.54 ± 0.04 0.58 ± 0.04 0.60 ± 0.02 0.62 ± 0.04 0.65 ± 0.12 | 2.259 ± 0.41 1.998 ± 0.04 4.8764 ± 0.16 7.286 ± 2.09 19.168 ± 0.77 | 6.238 ± 3.9 3.545 ± 1.69 7.127 ± 1.04 7.567 ± 0.62 8.679 ± 2.12 | 168.07 ± 15.08 37.08 ± 1.11 68.76 ± 3.52 310.7 ± 37.4 348.12 ± 13.73 |
| VS | DF | MS | |||
|---|---|---|---|---|---|
| Thickness (mm) | Tensile Strength (MPa) | Elongation at Break (MPa) | Young’s Modulus (MPa) | ||
| Composite | 4 | 0.007 | 187.61 | 13.45 | 939.23 |
| Error | 21 | 0.002 | 0.56 | 1.22 | 845.23 |
| Total | 25 | - | - | - | - |
| CV (%) | - | 11.28 | 17.78 | 17.62 | 19.34 |
| Time (days) | Description | |
|---|---|---|
| Average Weight (g) | Mass Loss (%) | |
| 0 30 60 90 | 0.52 ± 0.04 0.48 ± 0.12 0.39 ± 0.03 0.31 ± 0.06 | - 7.69 ± 0.18 18.75 ± 0.21 20.51 ± 0.27 |
| Total | 0.21 ± 0.03 | 40.38 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Fernandes, F.A.; Serra, J.C.V.; de Oliveira Costa, D.d.S.; Martin, C.A.G. Production of Biodegradable Polymeric Composites with the Addition of Waste. Materials 2023, 16, 6305. https://doi.org/10.3390/ma16186305
da Silva Fernandes FA, Serra JCV, de Oliveira Costa DdS, Martin CAG. Production of Biodegradable Polymeric Composites with the Addition of Waste. Materials. 2023; 16(18):6305. https://doi.org/10.3390/ma16186305
Chicago/Turabian Styleda Silva Fernandes, Fernando Antonio, Juan Carlos Valdés Serra, Dayriane do Socorro de Oliveira Costa, and Camilo Andrés Guerrero Martin. 2023. "Production of Biodegradable Polymeric Composites with the Addition of Waste" Materials 16, no. 18: 6305. https://doi.org/10.3390/ma16186305
APA Styleda Silva Fernandes, F. A., Serra, J. C. V., de Oliveira Costa, D. d. S., & Martin, C. A. G. (2023). Production of Biodegradable Polymeric Composites with the Addition of Waste. Materials, 16(18), 6305. https://doi.org/10.3390/ma16186305









