Investigation of the Degradation Mechanism of the Tensile Mechanical Properties of Sandstone under the Corrosion of Various pH Solutions
Abstract
:1. Introduction
2. Test Materials and Test Methods
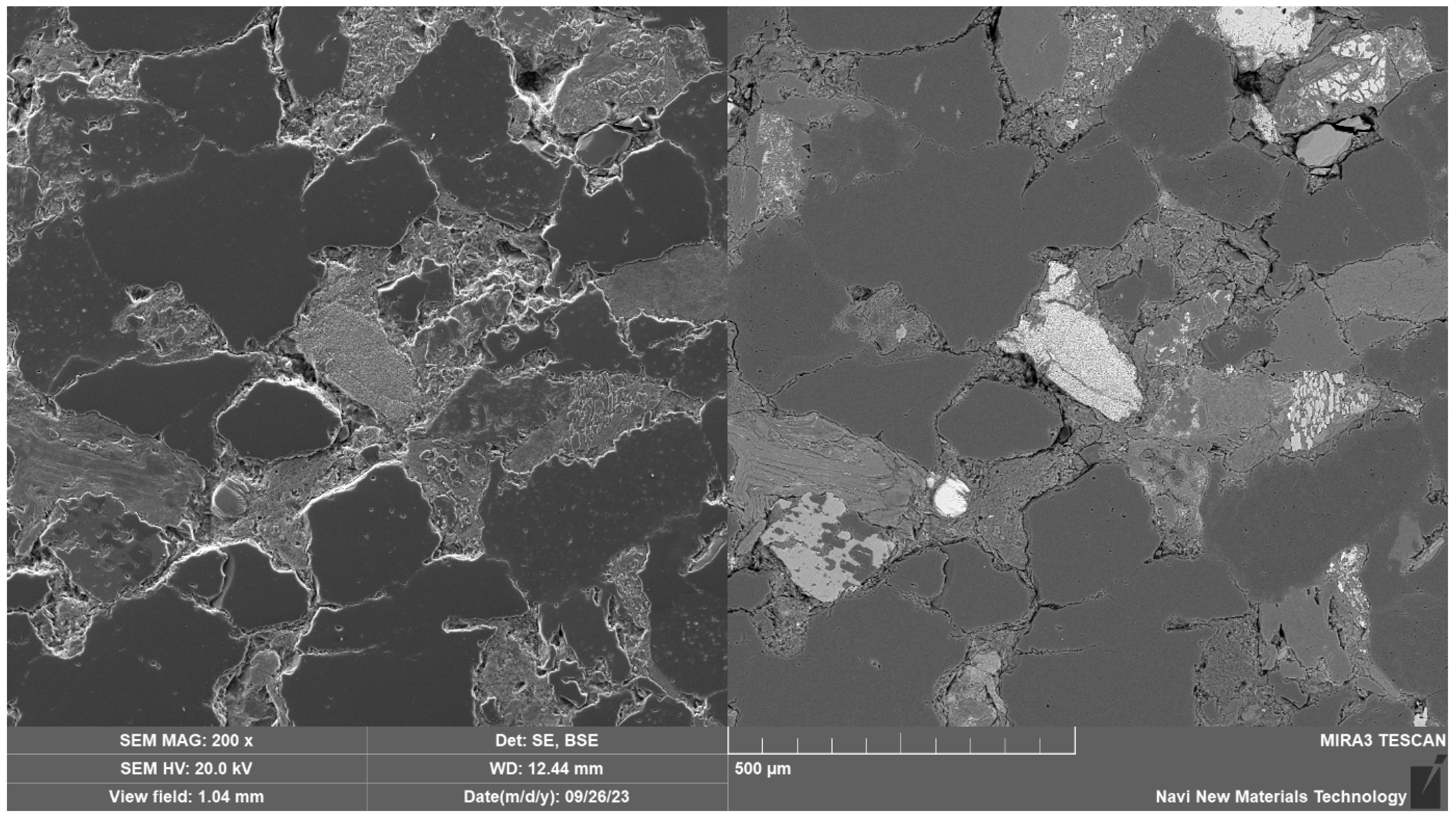
2.1. Petrographic Study
2.2. Sample Preparation
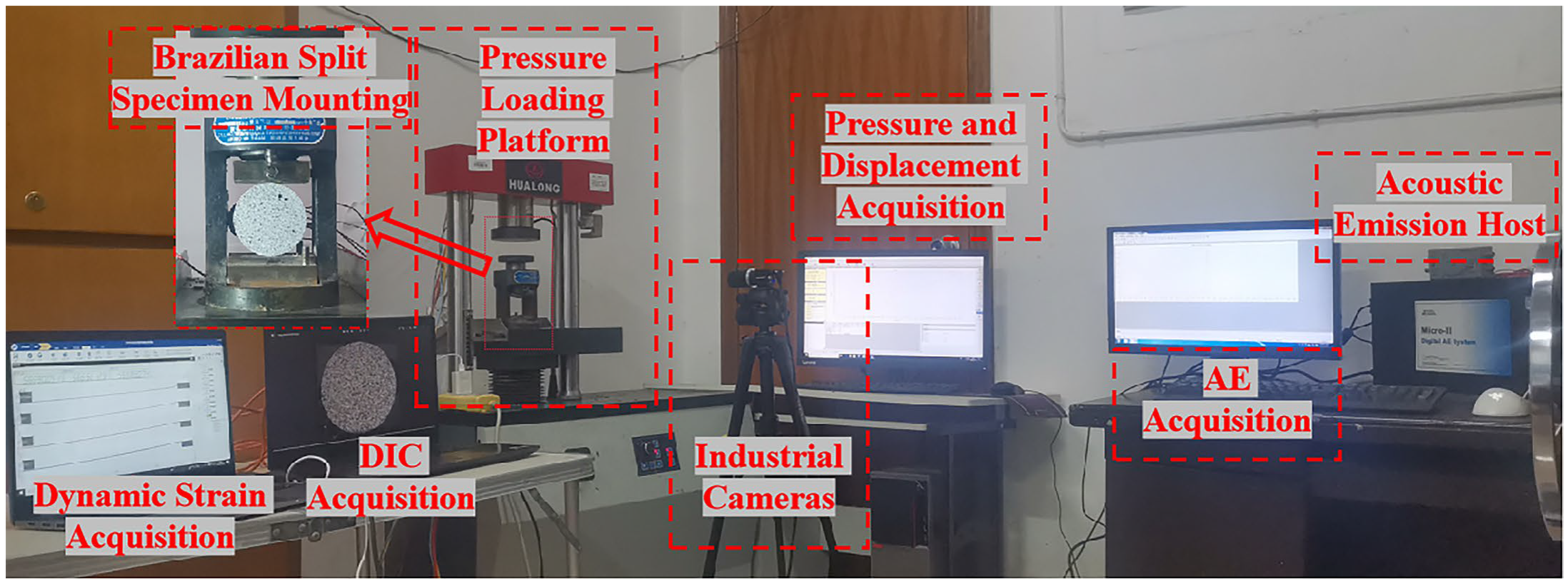
2.3. Introduction of Specimen Equipment
2.4. Pre-Treatment of Specimen
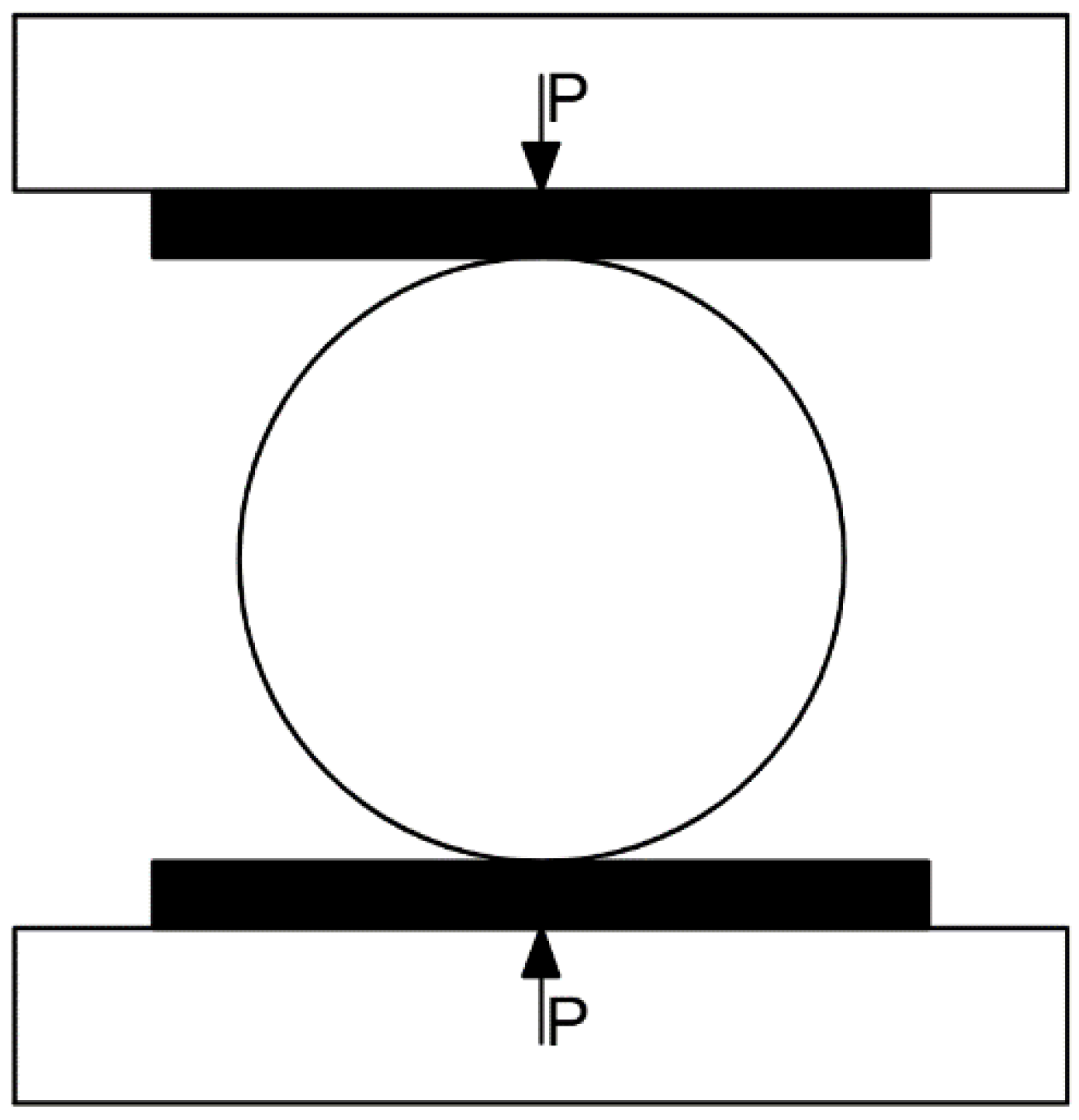
2.5. Test Loading Process and Method
3. Results and Discussion
3.1. Destruction Pattern
3.2. Mechanical Properties
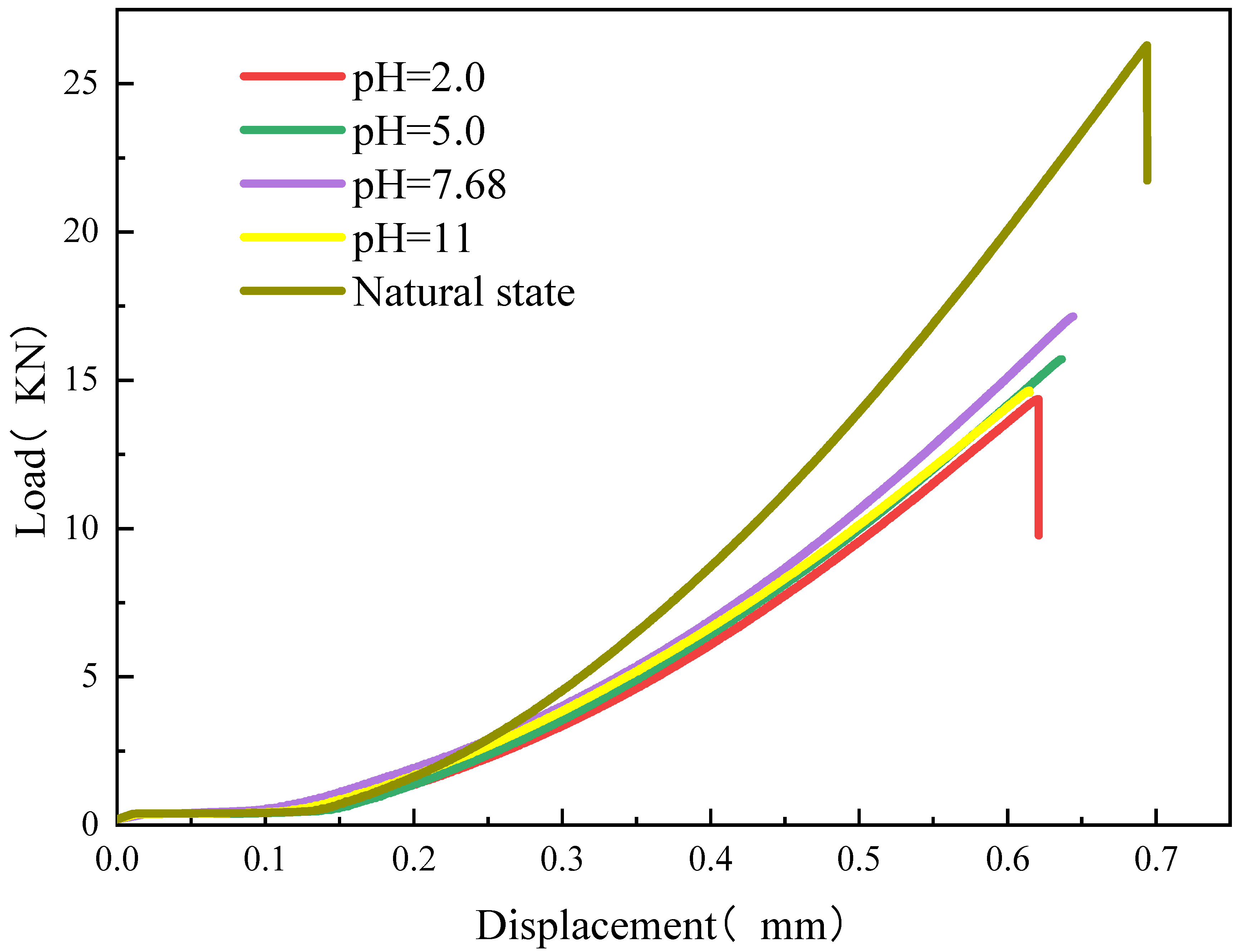
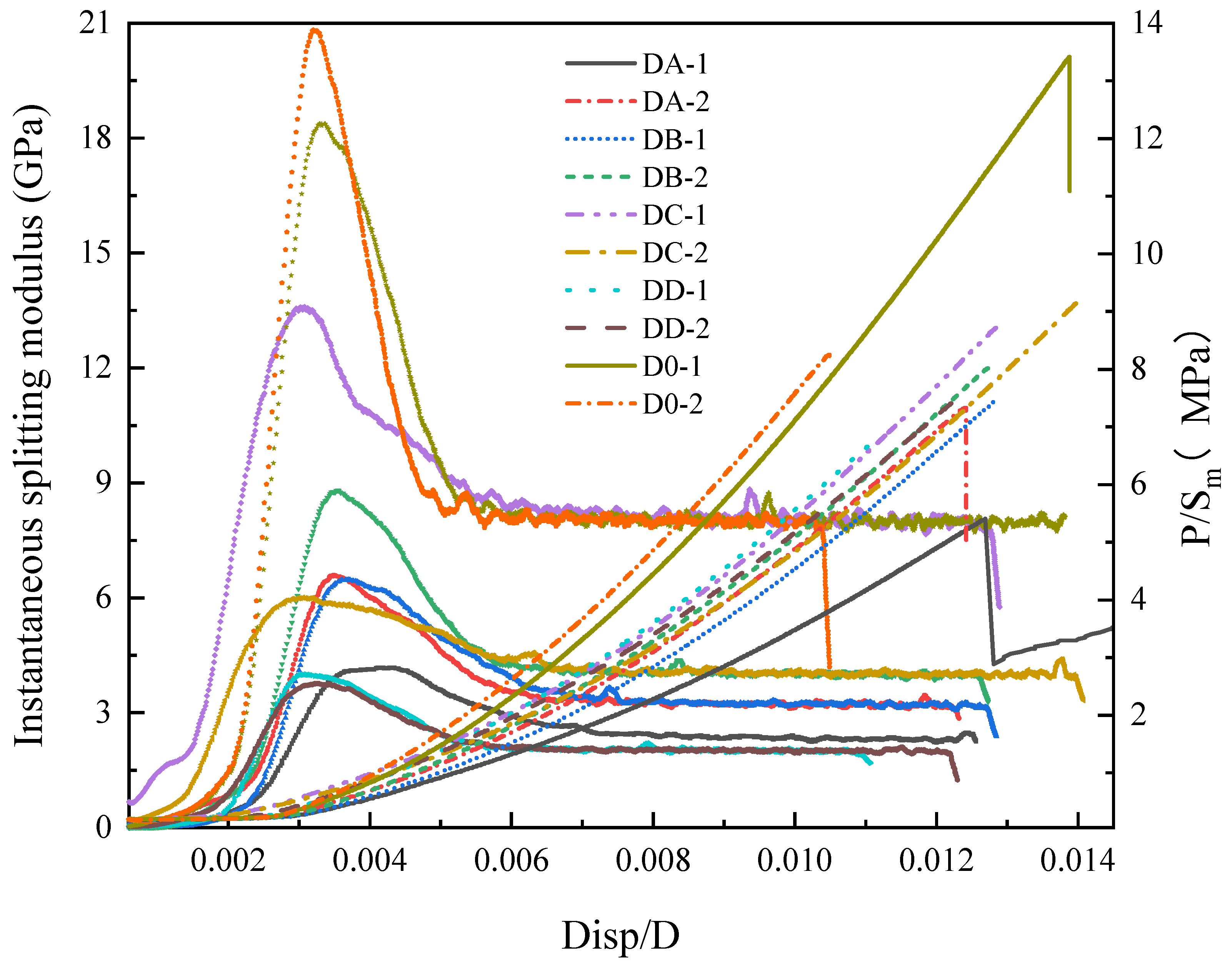
3.2.1. Displacement-Load Curve
3.2.2. Analysis of Tensile Mechanical Parameters
- (1)
- Tensile strength
- (2)
- Splitting modulus
- (3)
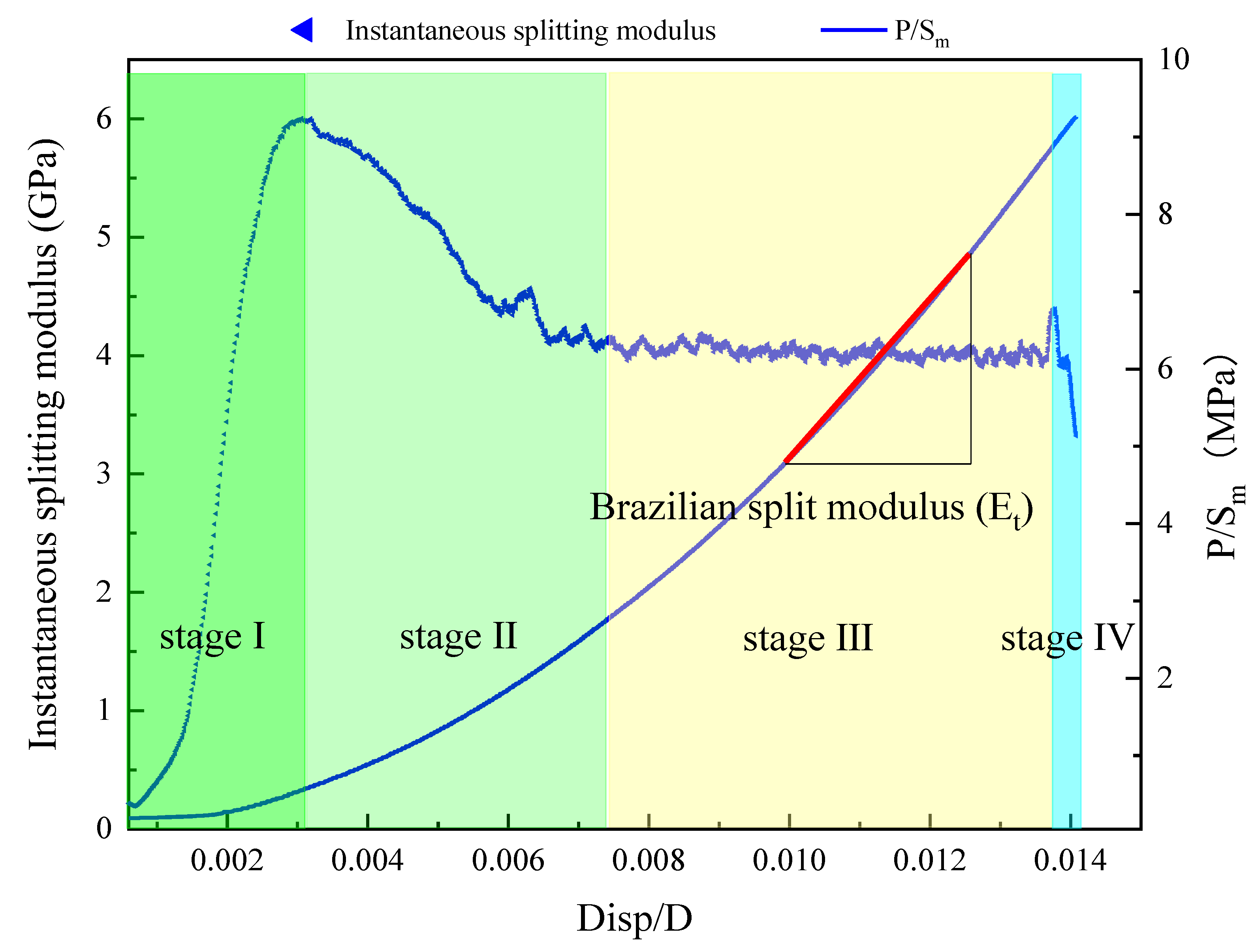
- Instantaneous splitting modulus
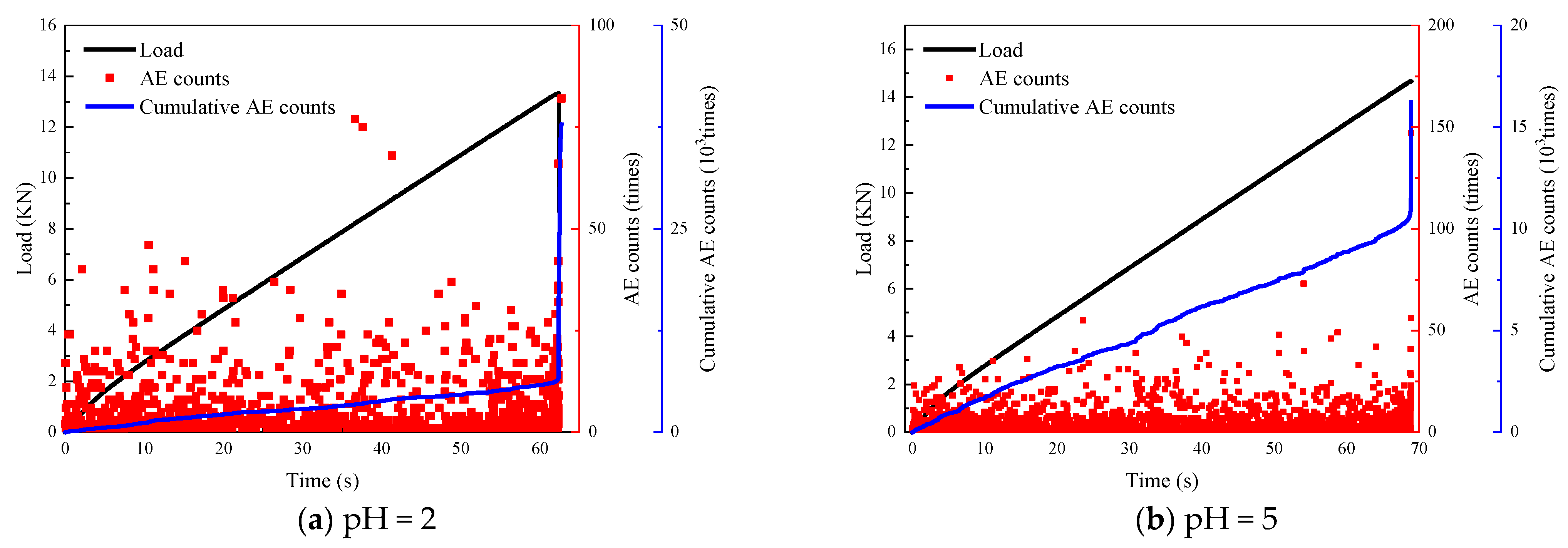
3.2.3. Acoustic Emission
4. Damage Mechanisms
4.1. Corrosion Mechanism Study
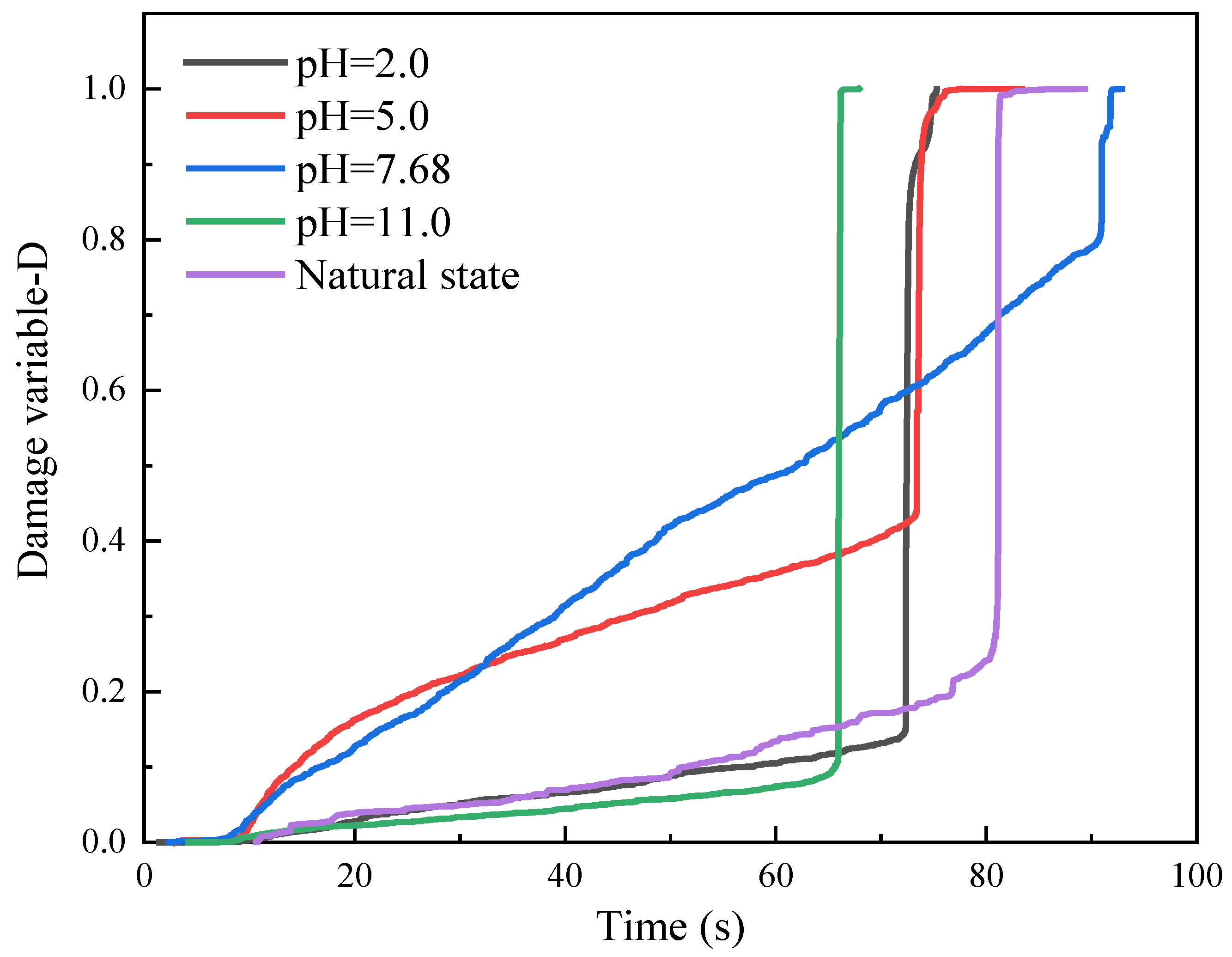
4.2. Damage Variables
5. Conclusions
- (1)
- The average tensile strength and splitting tensile modulus of the overall rock samples decreased gradually with the polarization of the solution pH. The highest point of the curve threshold is weakly alkaline. The decrease in tensile strength under acidic conditions is greater than the decrease in tensile strength under alkaline conditions.
- (2)
- The solution pH on the sandstone Brazilian splitting compaction point will have an impact. As pH increases, the sandstone compaction point and peak load ratio is smaller, but the compaction load for the peak load is between 22% and 36%.
- (3)
- The chemically eroded sandstone is characterized by an increase in acoustic emission ringing counts during the compaction phase. The rock samples, after chemical corrosion, showed a short duration of the compaction phase in the damage variable study and a steep damage evolution curve in the crack extension phase, with a rapid accumulation of the damage variable up to 1.0. These features suggest that chemical corrosion leads to an increase in pore fissures and deterioration of the internal structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutchinson, A.J.; Johnson, J.B.; Thompson, G.E. Stone degradation due to wet deposition of pollutants. Corros. Sci. 1993, 34, 1881–1898. [Google Scholar] [CrossRef]
- Yue, Z.; Ni, P.; Zhao, P.; An, K.C. Experimental Study on Mechanical Parameters of Sandstone under Water-Chemical Environment. Jiangxi Build. Mater. 2021, 41, 18–23. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A. Salinity-dependent strength and stress–strain characteristics of reservoir rocks in deep saline aquifers: An experimental study. Fuel 2014, 122, 1–11. [Google Scholar] [CrossRef]
- Shukla, R.; Ranjith, P.G.; Choi, S.K.; Haque, A.; Yellishetty, M.; Hong, L. Mechanical Behaviour of Reservoir Rock Under Brine Saturation. Rock Mech. Rock Eng. 2013, 46, 83–93. [Google Scholar] [CrossRef]
- Wang, Y.L.; Tang, J.X.; Jiang, J.; Dai, Z.Y.; Shu, G.J. Mechanical properties and parametric damage effects of grey sandstone under water-rock chemistry. J. China Coal Soc. 2017, 42, 227–235. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, C.; Xiong, Y.; Wu, W.; Zhang, Y.; Li, N. Kinetics Study of Surface Reaction Between Acid and Sandstone Based on the Rotation Disk Instrument. Chem. Technol. Fuels Oils 2020, 55, 765–777. [Google Scholar] [CrossRef]
- Ivanishin, I.B.; Nasr-El-Din, H.A. Effect of calcium content on the dissolution rate of dolomites in HCl acid. J. Pet. Sci. Eng. 2021, 202, 108463. [Google Scholar] [CrossRef]
- Ding, W.X.; Feng, X.T. Study on the damage effect and fracture criterion of fissured rocks under chemical corrosion. J. Geotech. Eng. 2009, 31, 899–904. [Google Scholar]
- Nasvi, M.C.M.; Ranjith, P.G.; Sanjayan, J.; Hague, A.; Li, X. Mechanical behaviour of wellbore materials saturated in brine water with different salinity levels. Energy 2014, 66, 239–249. [Google Scholar] [CrossRef]
- Kuva, J.; Sammaljarvi, J.; Parkkonen, J.; Siitari-Kauppi, M.; Lehtonen, M.; Turpeinen, T.; Timonen, J.; Voutilainen, M. Imaging connected porosity of crystalline rock by contrast agent-aided X-ray microtomography and scanning electron microscopy. J. Microsc. 2018, 270, 98–109. [Google Scholar] [CrossRef]
- Chen, S.L.; Feng, X.T.; Li, S.J. Effects of chemical corrosion on the mechanical properties of Xiaolangdi sandstone in the Yellow River. Rock Soil Mech. 2002, 24, 284–287, 296. [Google Scholar] [CrossRef]
- Chen, S.L.; Feng, X.T.; Li, S.J. Chemical corrosion effects on uniaxial compressive strength and fracture characteristics of rocks. J. Rock Mech. Eng. 2003, 22, 547–551. [Google Scholar] [CrossRef]
- Singh, T.N.; Sharma, P.K.; Khandelwal, M. Effect of pH on the physico-mechanical properties of marble. Bull. Eng. Geol. Environ. 2007, 66, 81–87. [Google Scholar] [CrossRef]
- Wang, W.; Liu, T.G.; Li, X.H.; Wang, R.B.; Xu, W.Y. Triaxial compression mechanical property test of granite under chemical corrosion. J. Cent. South Univ. Sci. Technol. 2015, 46, 3801–3807. [Google Scholar] [CrossRef]
- Ding, W.X.; Feng, X.T. Experimental Study on Mechanical Effects of Chert under Chemical Corrosion. Chin. J. Rock Mech. Eng. 2004, 23, 3571–3576. [Google Scholar] [CrossRef]
- Ding, W.X.; Chen, J.P.; Xu, T.; Chen, H.J.; Wang, H.Y. Mechanical and Chemical Dissolution Characterization of Tuffs under Chemical Solution Erosion. Rock Soil Mech. 2015, 36, 1825–1830. [Google Scholar] [CrossRef]
- Li, S.; Huo, R.; Wang, B.; Ren, Z.; Ding, Y.; Qian, M.; Qiu, T. Experimental Study on Physicomechanical Properties of Sandstone under Acidic Environment. Adv. Civ. Eng. 2018, 2018, 5784831. [Google Scholar] [CrossRef]
- Han, T.L.; Shi, J.P.; Chen, Y.S.; Ma, W.T. Experimental Study on the Effect of Chemical Corrosion on the Physical and Mechanical Properties of Sandstone. J. Xi’an Univ. Technol. 2014, 30, 34–39. [Google Scholar] [CrossRef]
- Li, X.N.; Wu, X.Y.; Gao, S.M.; Zhu, B.L. Experimental Study on Uniaxial Compression and Acoustic Emission Characteristics of Red Layer Soft Rock under Chemical Corrosion. J. Railw. Sci. Eng. 2015, 12, 1336–1340. [Google Scholar] [CrossRef]
- Wu, Q.; Qiang, S.; Xu, L.Q.; Liu, D.T.; Deng, Y. Experimental Study on the Deterioration of Physical and Mechanical Properties of Mortar under Chemical Corrosion. Bull. Chin. Ceram. Soc. 2019, 38, 2033–2043. [Google Scholar] [CrossRef]
- Wu, S.C.; Sun, W.; Cheng, Z.Q. Acoustic Emission Characteristics of Brazilian Splitting Tests on Sandstone with Low Porosity under Different Loading Conditions. Chin. J. Eng. 2020, 42, 988–998. [Google Scholar] [CrossRef]
- Xu, J.P.; Dong, X.L.; Fu, Y.Q.; Yu, X.F.; Zhou, F.H. Dic experimental analysis of the splitting process and strength of concrete Brazil under different loading boundaries. Acta Mech. Sin. 2020, 52, 864–876. [Google Scholar] [CrossRef]
- Sun, W.J.; Jin, A.B.; Wang, S.L.; Zhao, Y.Q.; Wei, L.C.; Jia, Y.C. Mechanical characterization of high-temperature sandstone splitting based on dic. Rock Soil Mech. 2021, 42, 511–518. [Google Scholar] [CrossRef]
- Peng, S.J.; Chen, C.C.; Xu, J.; Zhang, H.L.; Tang, Y.; Nie, W.; Zhao, K. Study on load rate dependence of rock stress-strain curves based on Brazilian splitting test. Chin. J. Rock Mech. Eng. 2018, 37, 3247–3252. [Google Scholar] [CrossRef]
- Dan, D.Q.; Konietzky, H.; Herbst, M. Brazilian tensile strength tests on some anisotropic rocks. Int. J. Rock Mech. Min. Sci. 2013, 58, 1–7. [Google Scholar] [CrossRef]
- Basu, A.; Mishra, D.A.; Roychowdhury, K. Rock failure modes under uniaxial compression, Brazilian, and point load tests. Bull. Eng. Geol. Environ. 2013, 72, 457–475. [Google Scholar] [CrossRef]
- Vervoort, A.; Min, K.; Konietzky, H. Failure of transversely isotropic rock under Brazilian test conditions. Int. J. Rock Mech. Min. Sci. 2014, 70, 343–352. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, L.; Tang, Y.; Chen, Y.; Hu, H.; Lu, G.; Chen, C.; Lin, H. Progressive formation of retrogressive landslide and the lateral length of instability. Appl. Sci. 2023, 13, 799. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Hu, H.H.; Cheng, Y.H.; Zhang, W.Y. A nonlinear rheological shear constitutive model of bolted joints considering initial damage and damage evolution. Int. J. Damage Mech. 2023, 32, 1077–1098. [Google Scholar] [CrossRef]
- Li, D.Y.; Wong, L.N.Y. The Brazilian Disc Test for Rock Mechanics Applications: Review and New Insights. Rock Mech. Rock Eng. 2013, 46, 269–287. [Google Scholar] [CrossRef]
- Lin, H.; Xiong, W.; Yan, Q. Three-Dimensional Effect of Tensile Strength in the Standard Brazilian Test Considering Contact Length. Geotech. Test. J. 2016, 39, 137–143. [Google Scholar] [CrossRef]
- Lin, H.; Xiong, W.; Yan, Q. Modified Formula for the Tensile Strength as Obtained by the Flattened Brazilian Disk Test. Rock Mech. Rock Eng. 2016, 49, 1579–1586. [Google Scholar] [CrossRef]
- Hou, P.; Gao, F.; Yang, Y.G.; Zhang, Z.Z.; Gao, Y.N.; Zhang, X.X.; Zhang, J. Experimental study on Brazilian splitting and acoustic emission of shale considering the effect of lamination. Rock Soil Mech. 2016, 37, 1603–1612. [Google Scholar] [CrossRef]
- Liu, Y.S.; Fu, H.L.; Rao, J.Y.; Dong, H.; Cao, Q. Experimental study of anisotropic Brazilian disk splitting of slate under the influence of different layer orientations. Chin. J. Rock Mech. Eng. 2012, 31, 785–791. [Google Scholar] [CrossRef]
- Yang, T.; Wang, B.X.; Sun, L.; Gao, Q.H. Analysis of the effect of bedding method on rock splitting test. Site Investig. Sci. Technol. 2002, 20, 3–7. [Google Scholar] [CrossRef]
- He, M.C.; Hu, J.C.; Xiong, W.; Liu, C.Y. Splitting test analysis of tensile strength properties of rocks. Min. Res. Dev. 2005, 25, 12–16. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, H.; Cao, R.; Sun, S.; Zha, W. Role of Rock Sections in Intermittent Joints in Controlling Rock Mass Strength and Failure Modes. Rock Mech. Rock Eng. 2023, 56, 5203–5221. [Google Scholar] [CrossRef]
- Zhuo, B.; Wang, F.; Fang, Y.; Zhou, K.; Zhu, M.; Li, S. Model test research on progressive failure mechanism of high-filled cut-and-cover tunnel and its load reduction structure. Zhongnan Daxue Xuebao Ziran Kexue Ban J. Cent. South Univ. Sci. Technol. 2022, 53, 610–619. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Wei, L.Y.; Li, G.L.; Su, H.J.; Jing, H.W. Experimental study on dynamic tensile mechanical properties of greywacke after chemical corrosion. J. Geotech. Eng. 2020, 42, 7635. [Google Scholar] [CrossRef]
- Huang, W.G.; Ouyang, M.; Huo, L.; Shu, C.-X.; Yang, P. Effect of Acidic Solution on Mechanical Properties and Mesoscopic Structure of Hard Sandstone. Geotech. Geol. Eng. 2021, 39, 783–797. [Google Scholar] [CrossRef]
- Parvizpour, S.; Jamshidi, A.; Sarikhani, R.; Dehnavi, A.G. The pH effect of sulfuric acid on the physico-mechanical properties of Atashkuh travertine, Central Iran. Environ. Earth Sci. 2022, 81, 159. [Google Scholar] [CrossRef]
- Dubey, R.K.; Singh, T.N. Influence of pH of water on mechanical properties of sandstone—An experimental approach. J. Sci. Ind. Res. 2000, 59, 583–586. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, W.; Liu, X.R.; Miao, L.L.; Xie, W.B. Deterioration rules of strength parameters of sandstone under cyclical wetting and drying in acid-based environment. Rock Soil Mech. 2018, 39, 3331–3339. [Google Scholar] [CrossRef]
- Ye, J.H.; Wu, F.Q.; Sun, J.Z. Estimation of the Tensile Elastic Modulus Using Brazilian Disc by Applying Diametrically Opposed Concentrated Loads. Int. J. Rock Mech. Min. Sci. 2009, 46, 568–576. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.B. Experimental Determination of Dynamic Tensile Properties of a Granite. Int. J. Rock Mech. Min. Sci. 2000, 37, 861–866. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, T.X. Study on the Relationship between Splitting Tensile Characteristics and Modulus of Elasticity of Granite from Three Gorges. Chin. J. Rock Mech. Eng. 2004, 23, 3258–3261. [Google Scholar] [CrossRef]
- Liu, H.; Lin, J.H.; Yang, G.S. Acoustic emission tests on tensile damage characteristics of sandstone under freeze-thaw cycle. J. Min. Saf. Eng. 2020, 38, 1–11. [Google Scholar] [CrossRef]
- Fu, L.; Zuo, S.Y.; Wang, L.; Chen, S.W.; Li, H.Y. Study on the evolution mechanism of acid-etched chemical-mechanical damage in layered tuffs. J. Eng. Geol. 2022, 30, 1–11. [Google Scholar] [CrossRef]
- Nikoudel, M.R.; Jamshidi, A.; Hafezi, M.N. An Investigation on Dissolution and Deterioration of Building Stones in Sulfuric and Nitric Acids Solutions. Sci. Q. J. Geosci. 2011, 20, 135–142. [Google Scholar]
- Arman, H.; Paramban, S. Evaporitic Rocks Disintegration under Various pH Levels as Slaking Fluid. Arab. J. Sci. Eng. 2023, 37. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, K.; Gao, F.; Li, J. Damage evolution behavior and constitutive model of sandstone subjected to chemical corrosion. Bull. Eng. Geol. Environ. 2019, 78, 5991–6002. [Google Scholar] [CrossRef]
- Yagiz, S. The Effect of pH of the Testing Liquid on the Degradability of Carbonate Rocks. Geotech. Geol. Eng. 2018, 36, 2351–2363. [Google Scholar] [CrossRef]






| Research Focus | Main Points |
|---|---|
| Degree of pH influence | The effect of pH on the uniaxial mechanical properties of sandstone is greater than the concentration [11,12]. |
| pH saline solutions and damage patterns | The brine solution has some softening effects on the rock but has little effect on the rock damage pattern [8]. |
| pH of acids and bases | The reduction in strength under acidic conditions is greater than that under alkaline conditions [13]. The peak strength of granite specimens under alkaline conditions shows varying degrees of intensification [14]. |
| Salt effect | The dissolution rates in Na2SO4, Na2CO3 and NaCl solutions are all greater than their dissolution rates in pure (distilled) water, indicating that the salt effect has a significant impact on rock dissolution [15]. |
| The same ion effect: the greater | The concentration of calcium and magnesium ions, preventing the dissolution of minerals, directly determines the rate and intensity of the dissolution of rock samples [16]. |
| Ion concentration | The higher the concentration of dissolved ions in the solution, the greater the decrease in the mechanical parameters of the sandstone [17]. |
| Main Frequency | Filter | Sampling Frequency | Parameters/Waveform Thresholds | Interval Parameter | Sampling Point | Trigger Mode |
|---|---|---|---|---|---|---|
| 120 kHz | 20–100 kHz | 1000 kHz | 40 DB | 50 μs | 2048 kHz | Internal trigger |
| Specimen | Diameter (mm) | Height (mm) | Mass (g) | Density (g/cm3) | Wave Velocity (m/s) | pH |
|---|---|---|---|---|---|---|
| DA-1 | 49.20 | 25.20 | 124.55 | 2.60 | 21.73 | 2.0 |
| DA-2 | 50.06 | 25.05 | 128.10 | 2.60 | 21.73 | |
| DA-3 | 50.00 | 25.04 | 128.06 | 2.60 | 21.60 | |
| DB-1 | 49.99 | 25.05 | 127.82 | 2.60 | 21.73 | 5.0 |
| DB-2 | 50.01 | 25.03 | 128.01 | 2.60 | 21.73 | |
| DB-3 | 50.01 | 25.03 | 128.01 | 2.60 | 21.73 | |
| DC-1 | 50.10 | 25.02 | 129.49 | 2.63 | 21.73 | 7.68 |
| DC-2 | 49.20 | 25.20 | 124.34 | 2.60 | 21.73 | |
| DC-3 | 49.98 | 25.04 | 127.95 | 2.60 | 21.73 | |
| DD-1 | 50.00 | 25.03 | 129.42 | 2.63 | 21.73 | 11 |
| DD-2 | 50.01 | 25.03 | 128.19 | 2.61 | 21.73 | |
| DD-3 | 49.99 | 25.06 | 128.02 | 2.60 | 21.73 | |
| D0-1 | 50.00 | 25.01 | 128.02 | 2.61 | 21.73 | Distilled water |
| D0-2 | 50.01 | 25.01 | 128.06 | 2.61 | 21.73 | |
| D0-3 | 50.10 | 25.02 | 129.00 | 2.62 | 21.73 |
| Immersion Solution pH | pH = 2.0 | pH = 5.0 | pH = 7.68 | pH = 11.0 |
|---|---|---|---|---|
| 7.53 | 8.01 | 8.74 | 9.48 | |
| −5.64 | −2.91 | −1.11 | 1.33 | |
| b | 0.085 | 0.087 | 0.085 | 0.036 |
| R2(COD) | 0.995 | 0.983 | 0.979 | 0.969 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Lin, H.; Cao, P.; Zhao, Q.; Pang, Y.; Yong, W. Investigation of the Degradation Mechanism of the Tensile Mechanical Properties of Sandstone under the Corrosion of Various pH Solutions. Materials 2023, 16, 6536. https://doi.org/10.3390/ma16196536
Huang L, Lin H, Cao P, Zhao Q, Pang Y, Yong W. Investigation of the Degradation Mechanism of the Tensile Mechanical Properties of Sandstone under the Corrosion of Various pH Solutions. Materials. 2023; 16(19):6536. https://doi.org/10.3390/ma16196536
Chicago/Turabian StyleHuang, Luyi, Hang Lin, Ping Cao, Qingxiong Zhao, Yongkang Pang, and Weixun Yong. 2023. "Investigation of the Degradation Mechanism of the Tensile Mechanical Properties of Sandstone under the Corrosion of Various pH Solutions" Materials 16, no. 19: 6536. https://doi.org/10.3390/ma16196536





