Effect of Residue Acrylic Monomers in Synthesized Solvent-Free Photoreactive Pressure-Sensitive Adhesives on the Main Properties of Transfer Tapes Applied to Joining Wooden Elements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Investigated Solvent-Free Photoreactive Acrylic Pressure-Sensitive Adhesives
2.3. Viscosity of Synthesized Solvent-Free Photoreactive Acrylic PSAs
2.4. Free Monomers Concentration in Synthesized Acrylic PSA
2.5. The Coating Weight of Transfer Acrylic PSA and Type of Cover Material
2.6. UV-Initiated Crosslinking
2.7. Conditioning
2.8. Measurement of Tack, Peel Adhesion, Shear Strength and Shrinkage
3. Results
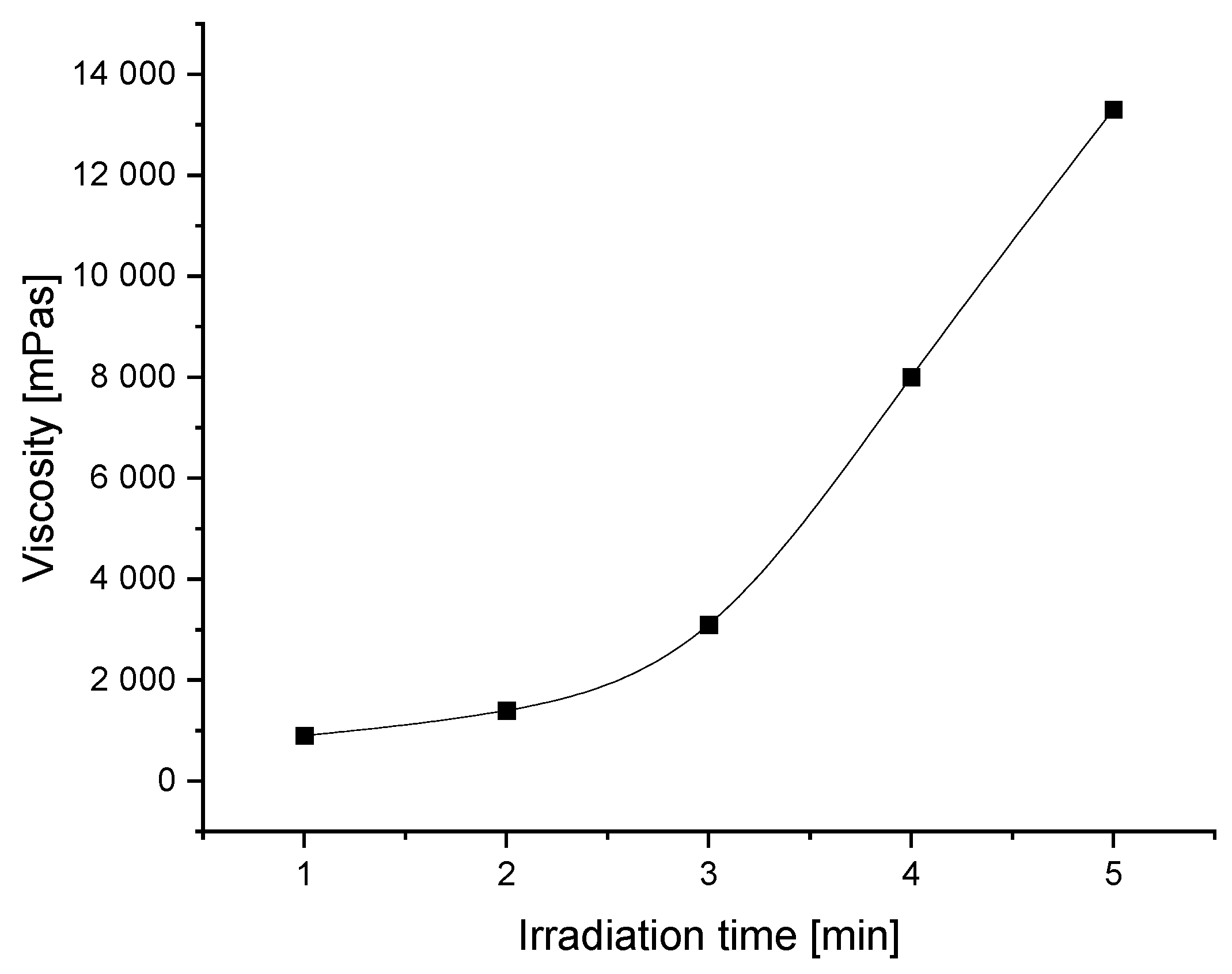
3.1. Viscosity of Synthesized Solvent-Free Photoreactive Acrylic PSA
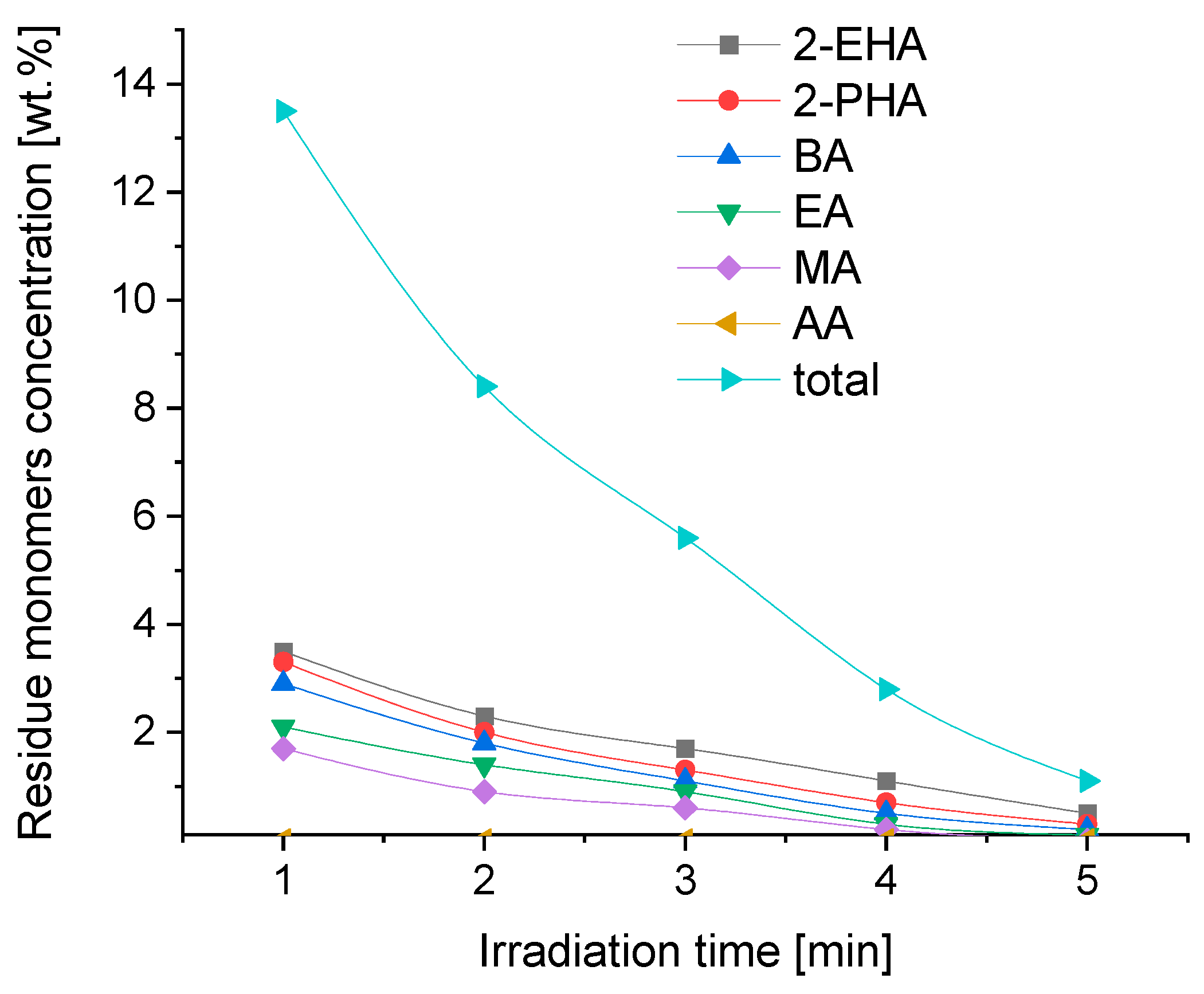
3.2. Free Monomer Concentration in Synthesized Acrylic PSA
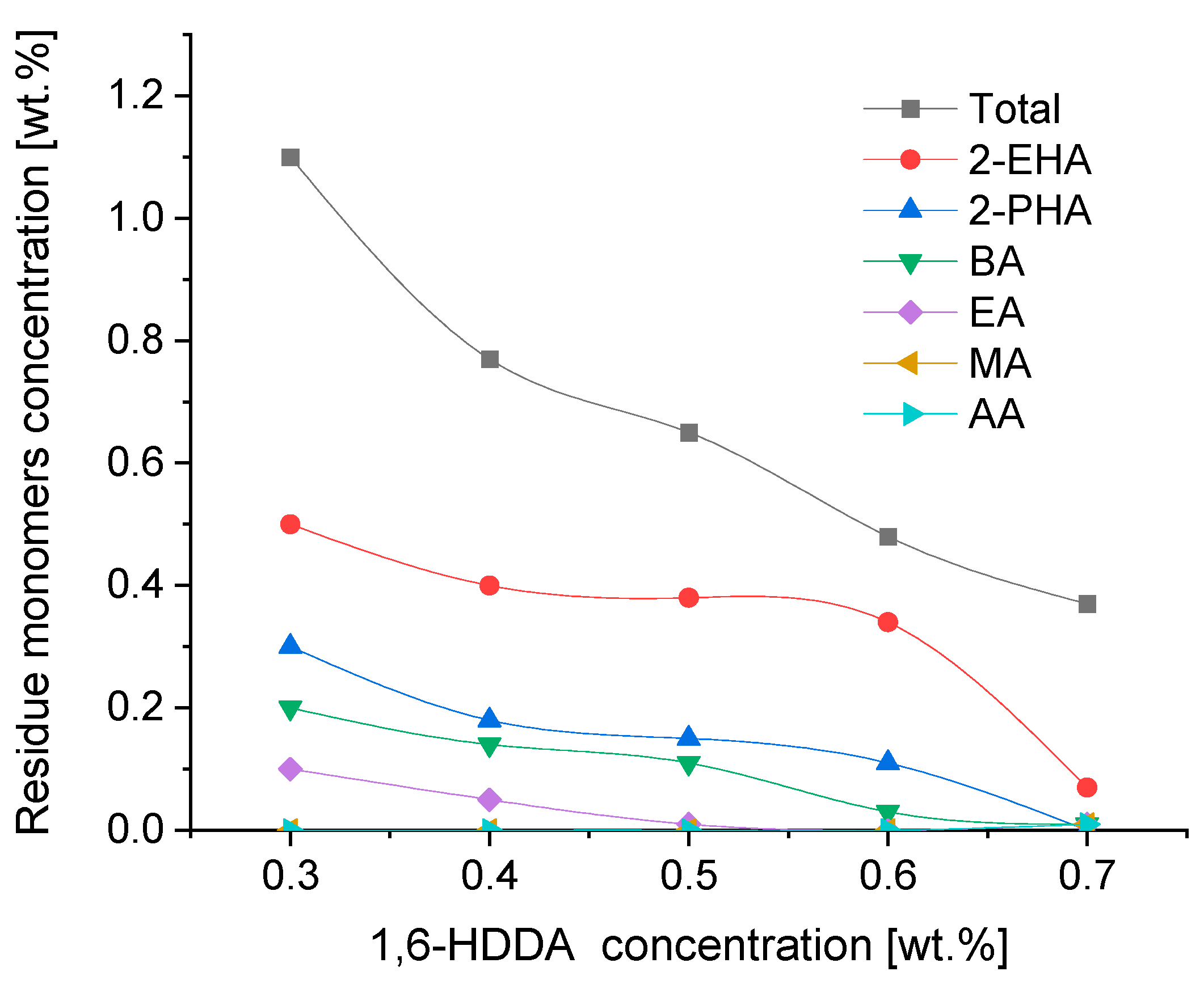
3.3. UV-Initiated Crosslinking of Photoreactive Prepolymers
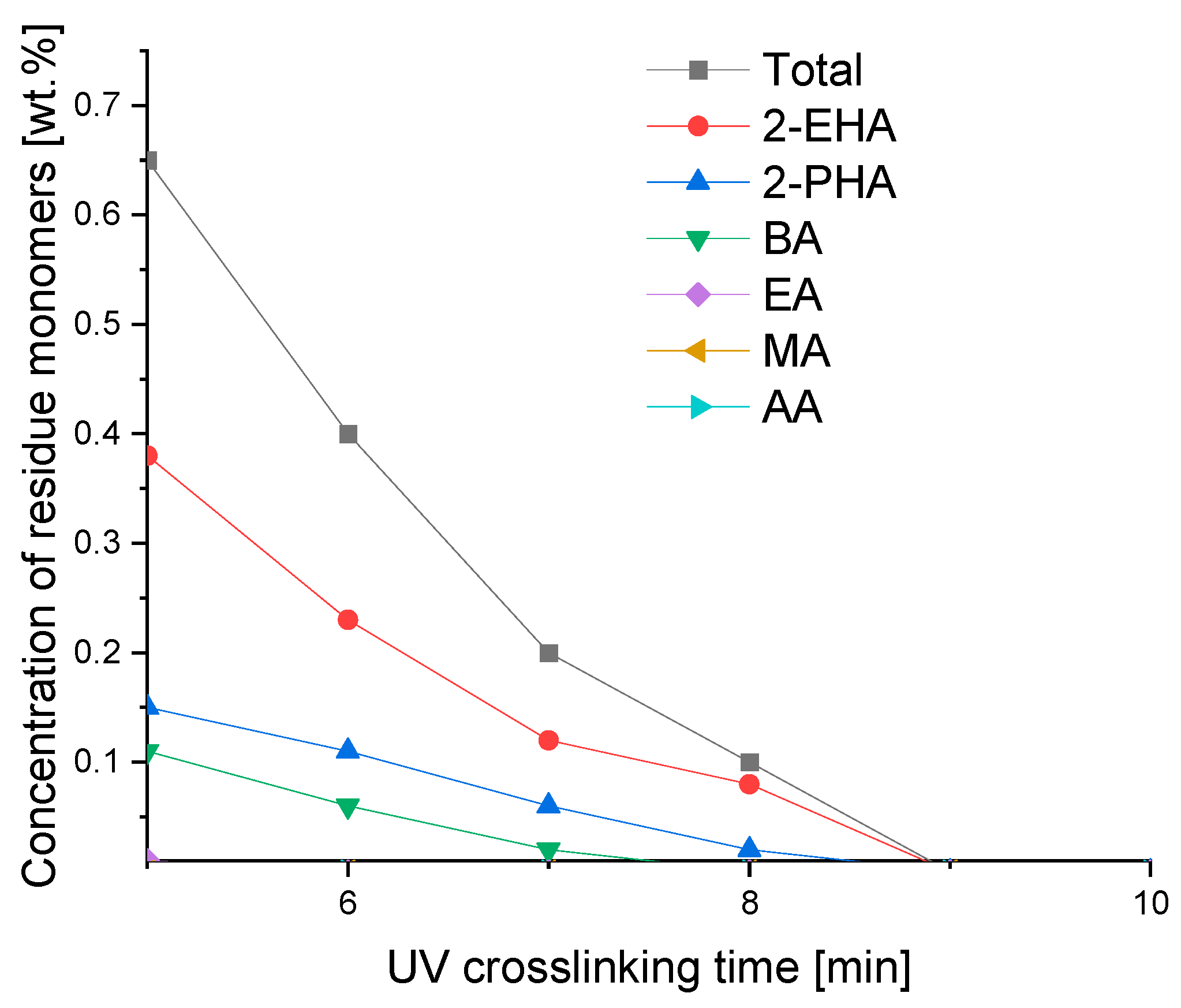
3.4. UV-Initiated Crosslinking According to Crosslinking Time
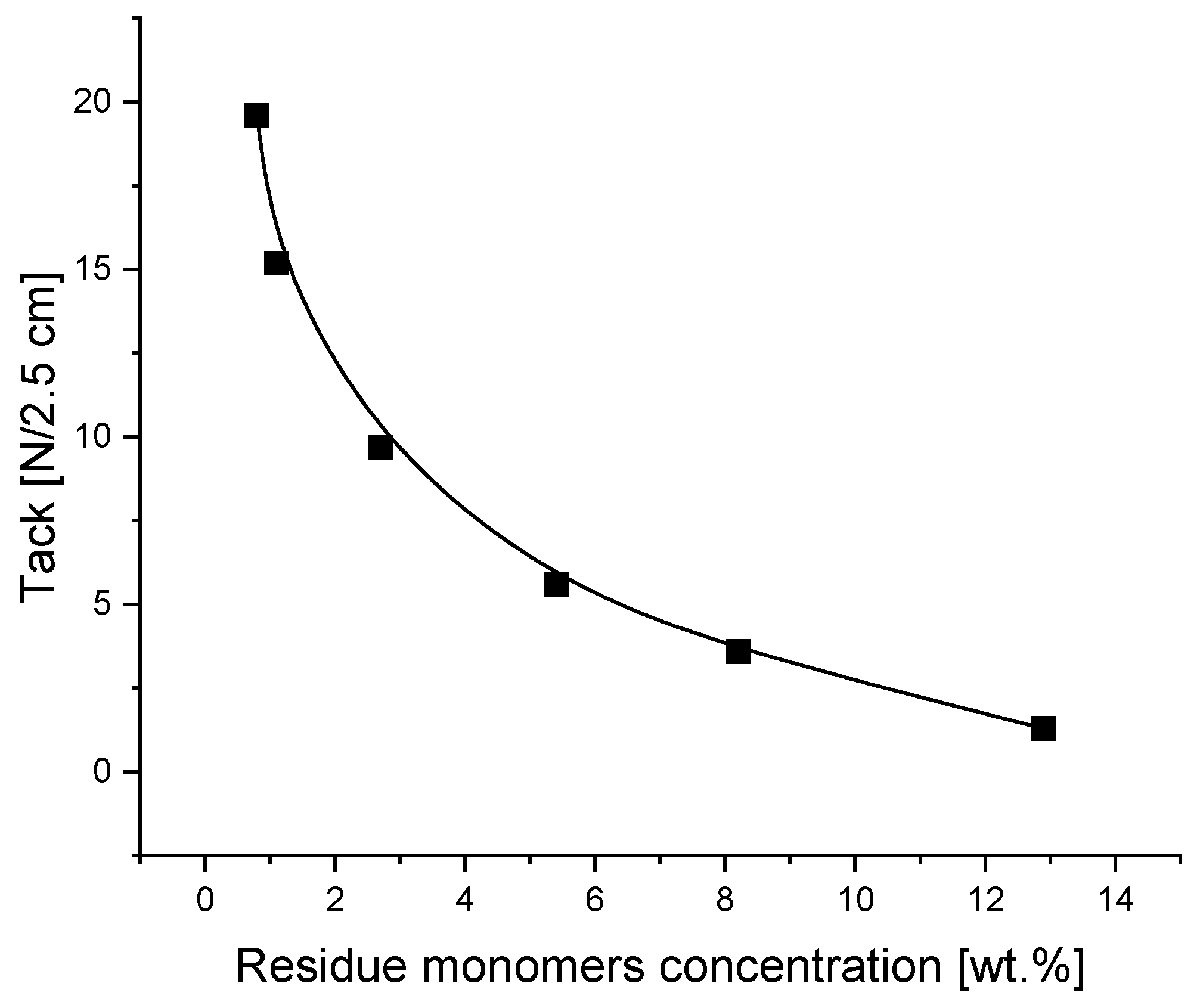
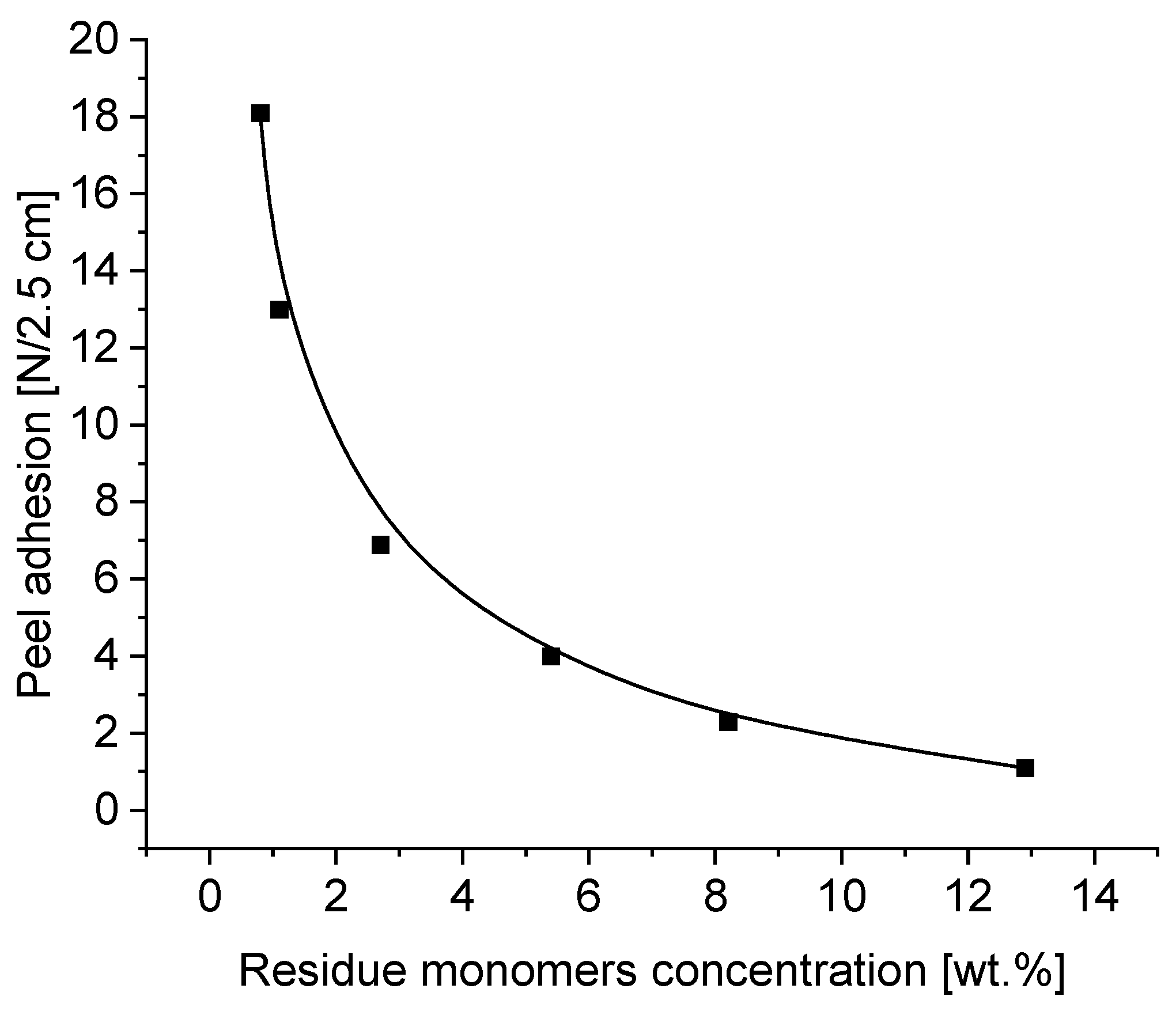
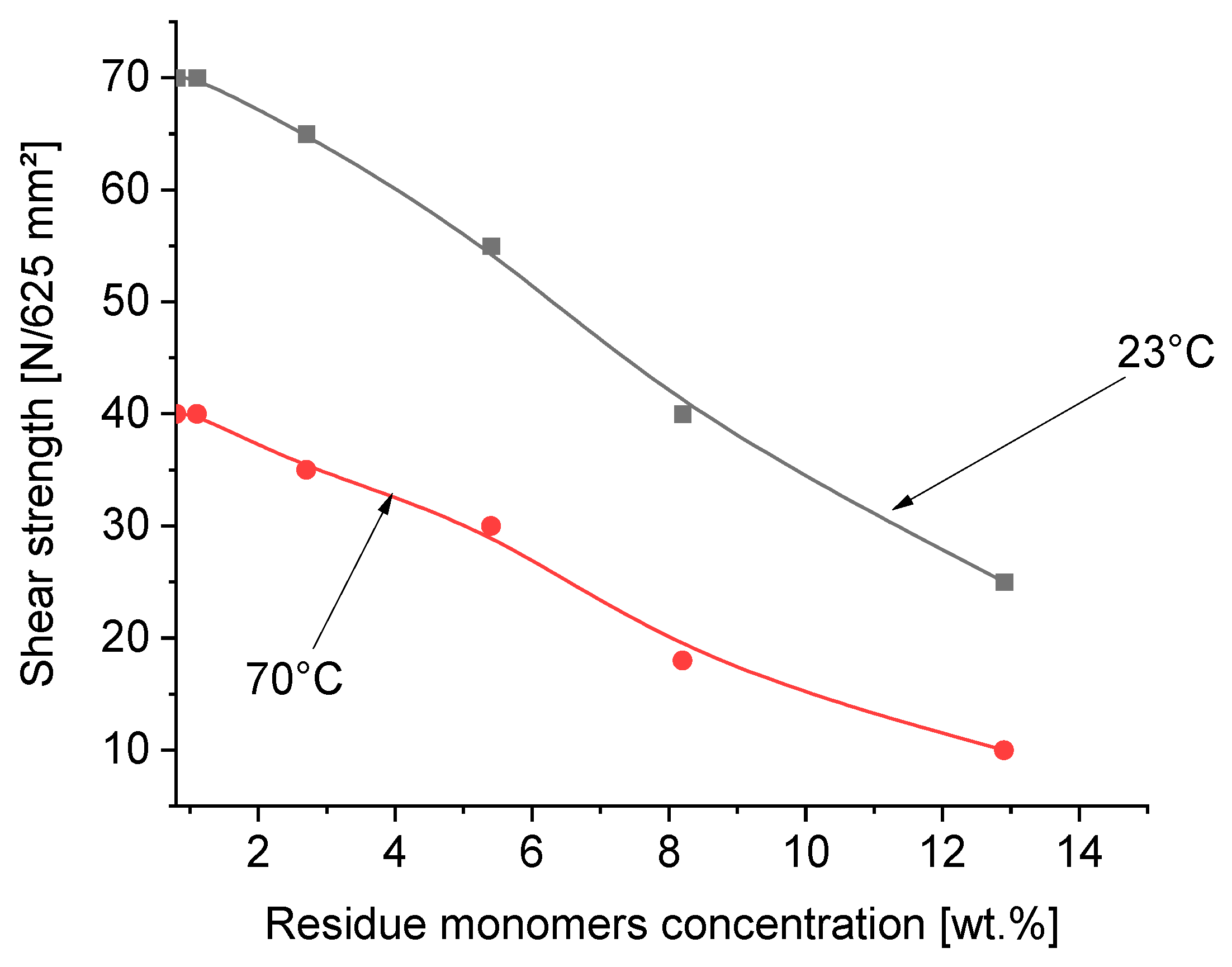
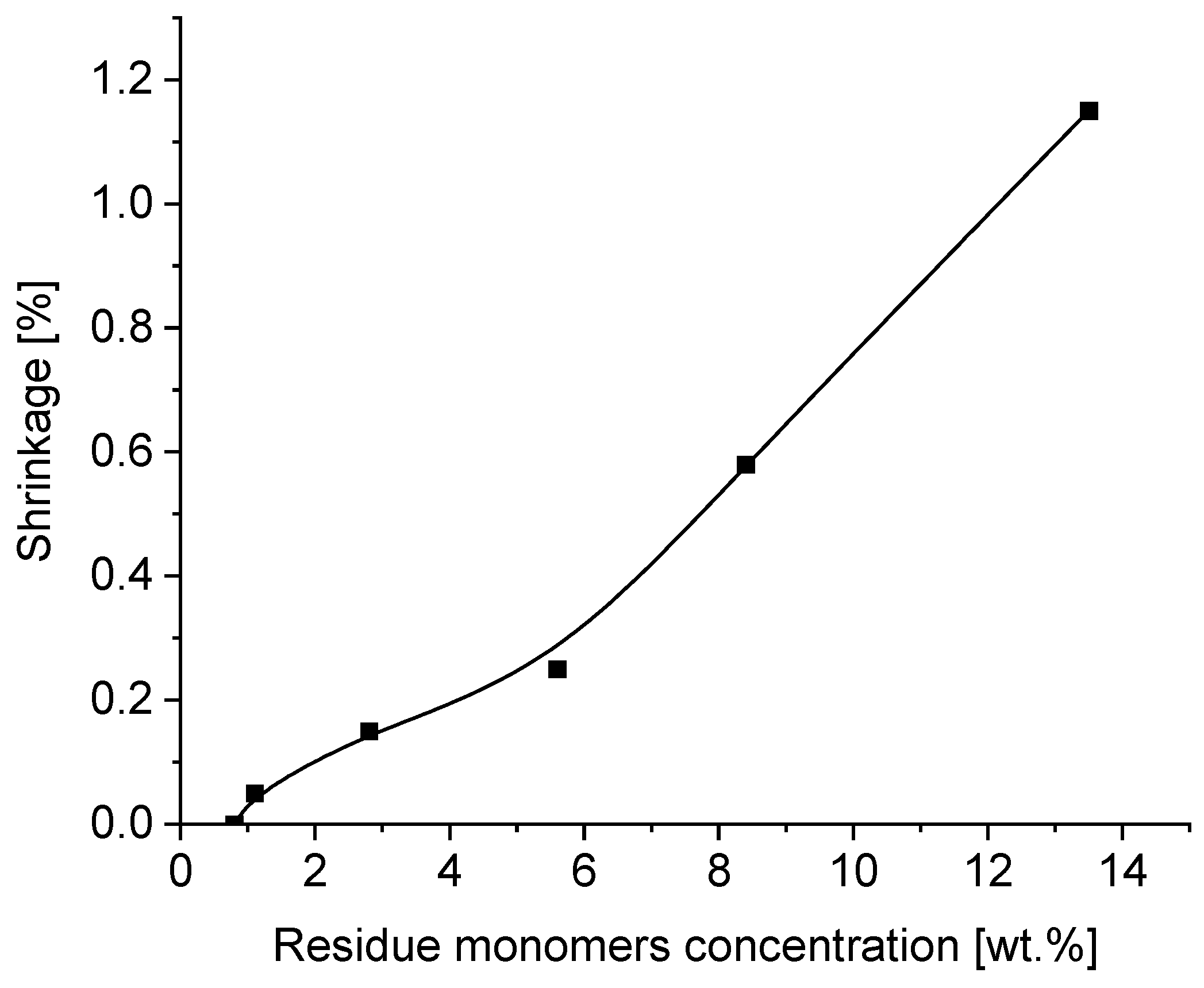
3.5. Effect of Residue Monomers on Tack, Peel Adhesion, Shear Strength and Shrinkage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elsmore, M.T.; Atkinson, R.L.; Irvine, D.J.; Howdle, S.M.; De Focatiis, D.S.A. Sustainable terpene triblock copolymers with tuneable properties for pressure sensitive adhesive applications. Polym. Test. 2022, 109, 107530. [Google Scholar] [CrossRef]
- Taghizadeh, S.M.; Ghasemi, D. Synthesis and optimization of a four-component acrylic-based copolymer as pressure sensitive adhesive. Iranian Polym. J. 2010, 19, 343–352. [Google Scholar]
- Diethert, A.; Ecker, K.; Peykova, Y.; Willenbacher, N.; Müller-Buschbaum, P. Tailoring the near-surface composition profiles of pressure-sensitive adhesive films and the resulting mechanical properties. ACS Appl. Mater. Interfaces 2011, 3, 2012–2021. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shim, G.-S.; Park, J.-W.; Kim, H.-J.; Kim, Y. Adhesion performance and recovery of acrylic pressure-sensitive adhesives thermally crosslinked with styrene–isoprene–styrene elastomer blends for flexible display applications. J. Ind. Eng. Chem. 2019, 78, 461–467. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shim, G.-S.; Kim, H.-J.; Kim, Y. Adhesion Performance and Recovery of Acrylic PSA with Acrylic Elastomer (AE) Blends via Thermal Crosslinking for Application in Flexible Displays. Polymers 2019, 11, 1959. [Google Scholar] [CrossRef]
- Karnal, P.; Jha, A.; Wen, H.; Gryska, S.; Barrios, C.; Frechette, J. Contribution of Surface Energy to pH-Dependent Underwater Adhesion of an Acrylic Pressure-Sensitive Adhesive. Langmuir 2019, 35, 5151–5161. [Google Scholar] [CrossRef]
- Márquez, I.; Paredes, N.; Alarcia, F.; Velasco, J.I. Adhesive Performance of Acrylic Pressure-Sensitive Adhesives from Different Preparation Processes. Polymers 2021, 13, 2627. [Google Scholar] [CrossRef]
- Márquez, I.; Paredes, N.; Alarcia, F.; Velasco, J.I. Influence of Acrylonitrile Content on the Adhesive Properties of Water-Based Acrylic Pressure-Sensitive Adhesives. Polymers 2022, 14, 909. [Google Scholar] [CrossRef]
- Baraghoosh, M.; Zohuri, G.H.; Behzadpour, M.; Gholami, M.; Hosseinpour, P.; Arabi, S.M. Effect of Different Tackifiers on Emulsion-Based Pressure-Sensitive Adhesive (PSA). Prog. Color Color. Coat. 2022, 15, 295–303. [Google Scholar]
- Casas-Soto, C.R.; Conejo-Dávila, A.S.; Osuna, V.; Chávez-Flores, D.; Espinoza-Hicks, J.C.; Flores-Gallardo, S.G.; Vega-Rios, A. Dibutyl itaconate and lauryl methacrylate copolymers by emulsion polymerization for development of sustainable pressure-sensitive adhesives. Polymers 2022, 14, 632. [Google Scholar] [CrossRef]
- Goulding, T.M. Pressure-Sensitive Adhesives. In Handbook of Adhesive Technology, 1st ed.; Taylor & Francis Group: Abingdon, UK, 1994. [Google Scholar]
- Khanjani, J. Pressure-Sensitive Adhesive Joints. In Adhesives and Adhesive Joints in Industry Applications; Rudawska, A., Ed.; IntechOpen: Rijeka, Croatia, 2019; p. 148. [Google Scholar] [CrossRef]
- Back, J.-H.; Kwon, Y.; Roldao, J.C.; Yu, Y.; Kim, H.-J.; Gierschner, J.; Lee, W.; Kwon, M.S. Synthesis of solvent-free acrylic pressure-sensitive adhesives via visible-light-driven photocatalytic radical polymerization without additives. Green Chem. 2020, 22, 8289–8297. [Google Scholar] [CrossRef]
- Fuensanta, M.; Martín-Martínez, J.M. Viscoelastic and Adhesion Properties of New Poly(Ether-Urethane) Pressure-Sensitive Adhesives. Front. Mech. Eng. 2020, 6, 34. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, D.Y.; Yoon, S.H.; Kim, S.H.; Han, M.S.; Jeon, S.; Kim, Y.; Lim, Y.K.; Hwang, D.-H.; Jung, S.-H.; et al. Adhesion Improvement of Solvent-Free Pressure-Sensitive Adhesives by Semi-IPN Using Polyurethanes and Acrylic Polymers. Polymers 2022, 14, 3963. [Google Scholar] [CrossRef]
- Pandey, V.; Fleury, A.; Villey, R.; Creton, C.; Ciccotti, M. Linking peel and tack performances of pressure sensitive adhesives. Soft Matter 2020, 16, 3267–3275. [Google Scholar] [CrossRef]
- Seok, W.C.; Leem, J.T.; Song, H.J. Acrylic pressure-sensitive adhesives based on ethylene glycol acrylate for flexible display application: Highly elastic and recoverable properties. Polym. Test. 2022, 108, 107491. [Google Scholar] [CrossRef]
- Czech, Z.; Witczak, M.; Kowalska, J. The influence of residue monomers on selected properties of acrylic pressure-sensitive adhesives. Drewno 2012, 188, 59–70. [Google Scholar]
- Barrios, C.A. Pressure Sensitive Adhesive Tape: A Versatile Material Platform for Optical Sensors. Sensors 2020, 20, 5303. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Gziut, K. Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2020, 13, 5661. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Kraśkiewicz, A. The Effect of Type-I Photoinitiators on the Kinetics of the UV-Induced Cotelomerization Process of Acrylate Monomers and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2021, 14, 4563. [Google Scholar] [CrossRef]
- Rudawska, A.; Wahab, M.A. Mechanical Properties of Adhesive Joints Made with Pressure-Sensitive Adhesives. Stroj. Vestn. J. Mech. Eng. 2021, 67, 380–388. [Google Scholar] [CrossRef]
- Sun, S.; Li, M.; Liu, A. A review on mechanical properties of pressure sensitive adhesives. Int. J. Adhes. Adhes. 2013, 41, 98–106. [Google Scholar] [CrossRef]
- Sancho-Querol, S.; Yáñez-Pacios, A.J.; Martín-Martínez, J.M. New binary blends of ethylene-co-n-butyl acrylate (EBA) copolymer and low molecular weight rosin ester resin with potential as pressure sensitive adhesives. Materials 2018, 11, 2037. [Google Scholar] [CrossRef]
- Fuensanta, M.; Khoshnood, A.; Rodríguez-Llansola, F.; Martín-Martínez, J.M. New Waterborne Polyurethane-Urea Synthesized with Ether-Carbonate Copolymer and Amino-Alcohol Chain Extenders with Tailored Pressure-Sensitive Adhesion Properties. Materials 2020, 13, 627. [Google Scholar] [CrossRef]
- Takahashi, K.; Yanai, F.; Inaba, K.; Kishimoto, K.; Kozone, Y.; Sugizaki, T. Sticking Effect of a Tackifier on the Fibrillation of Acrylic Pressure-Sensitive Adhesives. Langmuir 2021, 37, 11457–11464. [Google Scholar] [CrossRef]
- Bakar, R.A.; Li, Y.; Hewitson, O.P.; Roth, P.J.; Keddie, J.L. Azide Photochemistry in Acrylic Copolymers for Ultraviolet Cross-Linkable Pressure-Sensitive Adhesives: Optimization, Debonding-on-Demand, and Chemical Modification. ACS Appl. Mater. Interfaces 2022, 14, 30216–30227. [Google Scholar] [CrossRef]
- Back, J.-H.; Kwon, Y.; Kim, H.-J.; Yu, Y.; Lee, W.; Kwon, M.S. Visible-Light-Curable Solvent-Free Acrylic Pressure-Sensitive Adhesives via Photoredox-Mediated Radical Polymerization. Molecules 2021, 26, 385. [Google Scholar] [CrossRef]
- Antosik, A.K.; Bartkowiak, M.; Czech, Z. Pressure-Sensitive Adhesives Based on Acrylics; Wydawnictwo Uczelniane Zachodniopomorskiego Uniwersytetu Technologicznego w Szczecinie: Szczecin, Poland, 2022; pp. 1–67. Available online: https://hdl.handle.net/20.500.12539/1548 (accessed on 15 June 2023).
- Benedek, I.; Feldstein, M.M. Technology of Pressure-Sensitive Adhesives and Products; Taylor & Francis Group, LLC: Philadelphia, PA, USA, 2009; p. 568. [Google Scholar]
- Creton, C. Pressure-Sensitive Adhesives: An Introductory Course. MRS Bull. 2003, 28, 434–439. [Google Scholar] [CrossRef]
- Czech, Z.; Wesołowska, M. Development of solvent-free acrylic pressure-sensitive adhesives. Eur. Polym. J. 2007, 43, 3604–3612. [Google Scholar] [CrossRef]
- Ortega-Iguña, M.; Chludzinski, M.; Sánchez-Amaya, J.M. Comparative Mechanical Study of Pressure Sensitive Adhesives over Aluminium Substrates for Industrial Applications. Polymers 2022, 14, 4783. [Google Scholar] [CrossRef]
- Shim, G.-S.; Kim, H.-J.; Bartkowiak, M. Curing behaviour and impact of crosslinking agent variation in stepwise UV/UV cured acrylic pressure-sensitive adhesives. J. Mater. Res. Technol. 2021, 15, 1622–1629. [Google Scholar] [CrossRef]
- Zhu, M.; Cao, Z.; Haijun, Z.; Yijun, X.; Li, G.; Nongyue, W.; Yingchun, L.; Lianqi, H.; Xiongwei, Q. Preparation of environmentally friendly acrylic pressure-sensitive adhesives by bulk photopolymerization and their performance. RSC Adv. 2020, 10, 10277–10284. [Google Scholar] [CrossRef]
- Jacobs, W.P.V.; Dolan, J.D.; Dillard, D.A.; Ohanehi, D.C. An Evaluation of Acrylic Pressure Sensitive Adhesive Tapes for Bonding Wood in Building Construction Applications. J. Adhes. Sci. Technol. 2012, 26, 1349–1381. [Google Scholar] [CrossRef]

| Kind of Monomers | Abbreviation | Chemical Formula | Supplier |
|---|---|---|---|
| 2-ethylhexyl acrylate | 2-EHA | 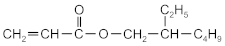 | BASF (Ludwigshafen, Germany) |
| 2-propylheptyl acrylate | 2-PHA | 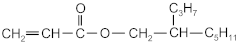 | BASF (Ludwigshafen, Germany) |
| butyl acrylate | BA | 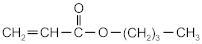 | BASF (Ludwigshafen, Germany) |
| ethyl acrylate | EA | 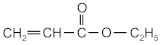 | BASF (Ludwigshafen, Germany) |
| methyl acrylate | MA |  | BASF (Ludwigshafen, Germany) |
| acrylic acid | AA | 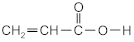 | BASF (Ludwigshafen, Germany) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czech, Z.; Bartkowiak, M.; Krystofiak, T. Effect of Residue Acrylic Monomers in Synthesized Solvent-Free Photoreactive Pressure-Sensitive Adhesives on the Main Properties of Transfer Tapes Applied to Joining Wooden Elements. Materials 2023, 16, 7563. https://doi.org/10.3390/ma16247563
Czech Z, Bartkowiak M, Krystofiak T. Effect of Residue Acrylic Monomers in Synthesized Solvent-Free Photoreactive Pressure-Sensitive Adhesives on the Main Properties of Transfer Tapes Applied to Joining Wooden Elements. Materials. 2023; 16(24):7563. https://doi.org/10.3390/ma16247563
Chicago/Turabian StyleCzech, Zbigniew, Marcin Bartkowiak, and Tomasz Krystofiak. 2023. "Effect of Residue Acrylic Monomers in Synthesized Solvent-Free Photoreactive Pressure-Sensitive Adhesives on the Main Properties of Transfer Tapes Applied to Joining Wooden Elements" Materials 16, no. 24: 7563. https://doi.org/10.3390/ma16247563
APA StyleCzech, Z., Bartkowiak, M., & Krystofiak, T. (2023). Effect of Residue Acrylic Monomers in Synthesized Solvent-Free Photoreactive Pressure-Sensitive Adhesives on the Main Properties of Transfer Tapes Applied to Joining Wooden Elements. Materials, 16(24), 7563. https://doi.org/10.3390/ma16247563







