Utilization of Plant Oils for Sustainable Polyurethane Adhesives: A Review
Abstract
1. Introduction
2. Major Types of Polyurethane Adhesive
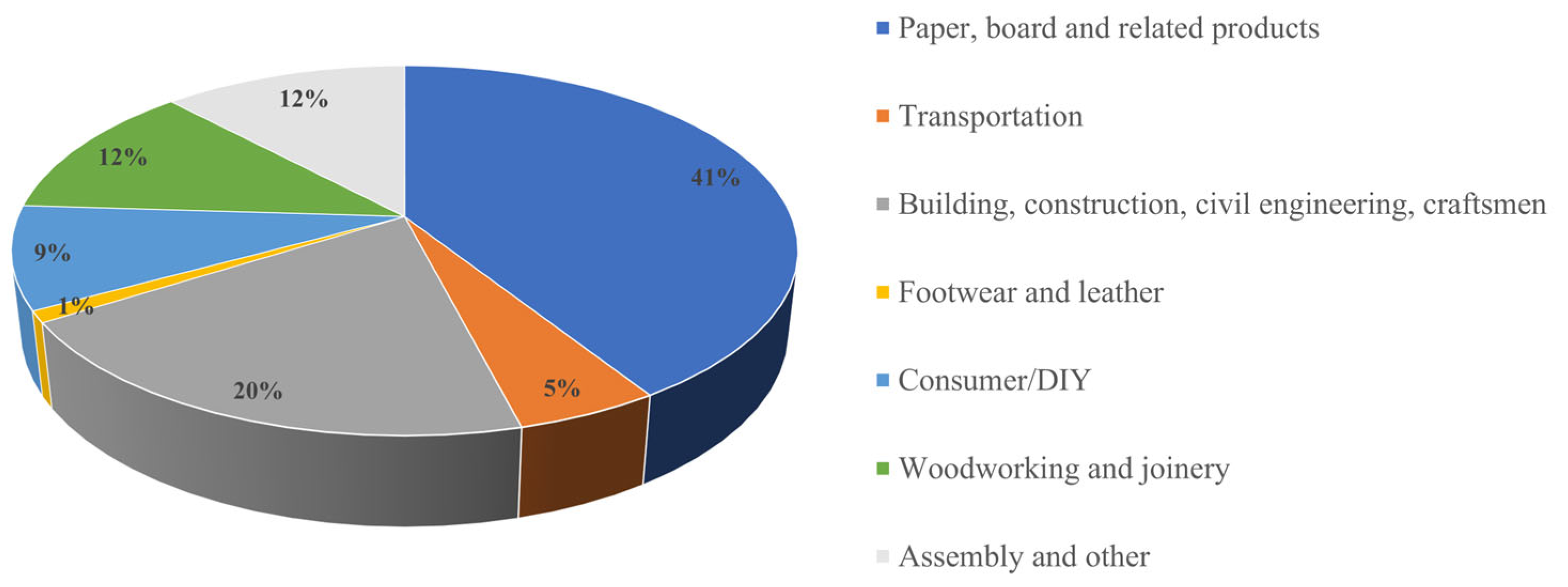
3. European Adhesive Market
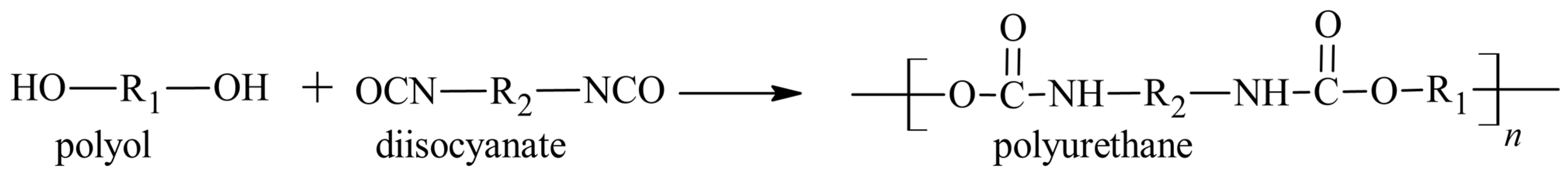
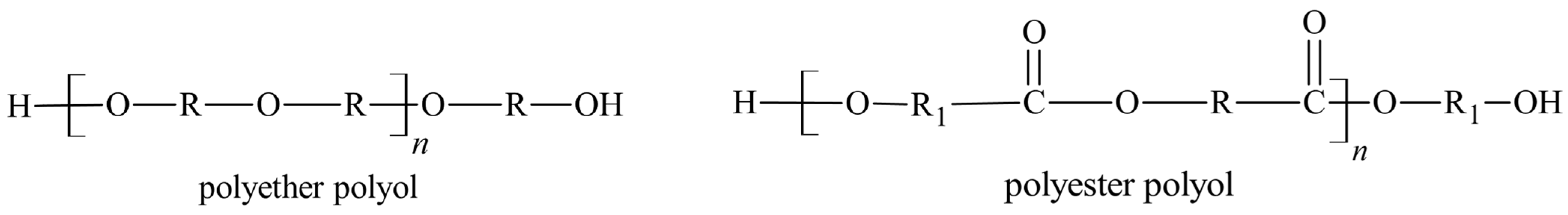
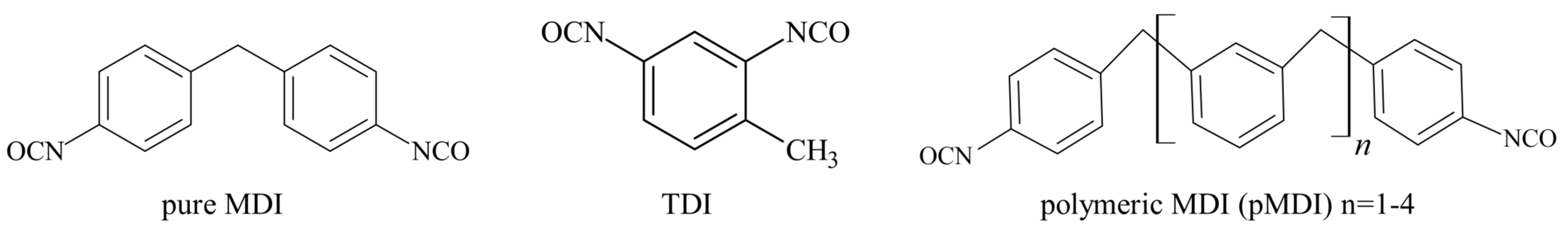
4. Raw Materials for Standard Polyurethane Adhesives
5. Environmental Issues with the Production of Polyurethane Adhesives
5.1. European Union Green Deal-General Issue
5.2. Decarbonization and the EU Green Deal in the Construction Market
- The EU aims to accelerate the renovation of existing buildings to improve their energy efficiency. The Renovation Wave initiative seeks to double the renovation rate of buildings across Europe and prioritize energy-efficient upgrades, including insulation, efficient heating and cooling systems, and the use of renewable energy sources [31].
- The Energy Performance of Buildings Directive (EPBD) sets standards for energy performance in buildings and promotes the use of renewable energy sources [32].
- Sustainable Building Standards: The European Green Deal emphasizes the development and promotion of sustainable building standards and certifications through: Leadership in Energy and Environmental Design (LEED) and BREEAM are widely recognized and applied across Europe to ensure that buildings meet high environmental performance standards [32].
- The Green Deal promotes the principles of the circular economy in the construction sector. This involves minimizing waste generation, promoting the reuse and recycling of materials, and adopting more sustainable construction practices [28].
- The EU is mobilizing significant financial resources to support the transition to greener buildings through the European Investment Bank, and various funding programs aim to provide financial incentives and support for energy-efficient building renovations and sustainable construction projects [7].
- The European Green Deal finally promotes research and innovation in the construction sector to develop new materials, technologies, and construction methods that are more sustainable and energy-efficient [27].
6. Plant Oils Resources for Eco-Friendly Polyurethanes
6.1. The Use of Plant Oil
6.2. Potential of Waste Oil Used for Polyurethane Production
- Water Pollution and Issues with Sewer Systems. Improper disposal of used oils, such as dumping in sewers or wastewater, can lead to water pollution. This poses a threat to aquatic ecosystems by affecting water quality and the survival of aquatic organisms. Additionally, if used oils are flushed into the sewer, they can contribute to the formation of fatbergs, leading to blockages in sewage pipes and system failures, incurring additional repair costs [78,79,80].
- Biobased oils are typically derived from crops. Their production is limited by the availability of suitable land, water resources, and favorable climatic conditions. Consequently, it might compete with food production, leading to concerns about food security and increased prices for agricultural commodities [83,84].
- The cultivation of crops for biobased oils can result in increased pressure on land resources. Deforestation not only reduces biodiversity but also releases significant amounts of carbon dioxide into the atmosphere, aggravating climate change [84].
- While biobased oils are often considered more environmentally friendly than fossil fuels, their production and processing can still generate greenhouse gas emissions. The cultivation, harvesting, and transportation of crops, as well as the extraction and processing of oils, can contribute to carbon dioxide emissions and other greenhouse gases [84,86].
7. Summary and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szycher, M. Szycher’s Handbook of Polyurethanes; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429108907. [Google Scholar]
- de Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry; American Chemical Society: Washington, DC, USA, 2021; pp. 1–24. [Google Scholar]
- Oertel, G. Polyurethane Handbook, 2nd ed.; Carl Hanser: Munich, Germany, 1993; pp. 594–602. [Google Scholar]
- Edward, M.P. Handbook of Adhesives and Sealants, 2nd ed.; McGraw-Hill Professional: New York, NY, USA, 2007; pp. 403–410. [Google Scholar]
- Wirpsza, Z. Poliuretany Chemia Technologia Zastosowanie; Wydawnictwa Naukowo-Techniczne: Warsaw, Poland, 1991. [Google Scholar]
- David, J. Dunn Adhesives and Sealants—Technology, Applications and Markets; Rapra Technology Limited: Shrewsbury, UK, 2003. [Google Scholar]
- Henrique Savelsberg, J.R. European Adhesives and Sealants Market—2021–2026; FEICA, Smithers: Akron, OH, USA, 2021. [Google Scholar]
- Brune, K.; Dieckhoff, S.; Fricke, H.; Groß, A.; Haag, K.; Hartwig, A.; Leite Cavalcanti, W.; Mayer, B.; Noeske, M.; Wilken, R. Circular Economy and Adhesive Bonding Technology; Life Processes Material; Fraunhofer Verlag: Stuttgart, Germany, 2020. [Google Scholar] [CrossRef]
- Marques, J.B.; Barbosa, A.Q.; da Silva, C.I.; Carbas, R.J.C.; da Silva, L.F.M. An Overview of Manufacturing Functionally Graded Adhesives—Challenges and Prospects. J. Adhes. 2021, 97, 172–206. [Google Scholar] [CrossRef]
- Brandtner-Hafner, M. Structural Safety Evaluation of Polymeric Adhesive Systems for Concrete Bonding. In Proceedings of the 7th World Congress on Civil, Structural, and Environmental Engineering (CSEE’22), Virtual Conference, 13–15 April 2022. [Google Scholar]
- Aznar, M.; Vera, P.; Canellas, E.; Nerín, C.; Mercea, P.; Störmer, A. Composition of the Adhesives Used in Food Packaging Multilayer Materials and Migration Studies from Packaging to Food. J. Mater. Chem. 2011, 21, 4358–4370. [Google Scholar] [CrossRef]
- Bandel, A. Gluing Wood; Catas: San Giovanni al Natisone, Italy, 1995. [Google Scholar]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology Limited: Shrewsbury, UK, 2005. [Google Scholar]
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780203912225. [Google Scholar]
- Arkema Technical Paper: Crayvallac Antisettle CVP; Arkema: Colombes, France, 2023.
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-Based Polyurethane Prepared from Kraft Lignin and Modified Castor Oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Sardon, H.; Mecerreyes, D.; Basterretxea, A.; Avérous, L.; Jehanno, C. From Lab to Market: Current Strategies for the Production of Biobased Polyols. ACS Sustain. Chem. Eng. 2021, 9, 10664–10677. [Google Scholar] [CrossRef]
- Alhanish, A.; Abu Ghalia, M. Developments of Biobased Plasticizers for Compostable Polymers in the Green Packaging Applications: A Review. Biotechnol. Prog. 2021, 37, e3210. [Google Scholar] [CrossRef] [PubMed]
- Mori, R. Replacing All Petroleum-Based Chemical Products with Natural Biomass-Based Chemical Products: A Tutorial Review. RSC Sustain. 2023, 1, 179–212. [Google Scholar] [CrossRef]
- PN-EN ISO 14040:2009/A1:2021-03; Wersja Angielska Zarządzanie Środowiskowe—Ocena Cyklu Życia—Zasady i Struktura. International Organization for Standardization: Geneva, Switzerland, 2009.
- PN-EN ISO 14044:2009; Wersja Polska Zarządzanie Środowiskowe—Ocena Cyklu Życia—Wymagania i Wytyczne. International Organization for Standardization: Geneva, Switzerland, 2009.
- Lesiuk, A.; Oleszczuk, P.; Kuśmierz, M. Zastosowanie Techniki LCA w Ekologicznej Ocenie Produktów, Technologii i Gospodarce Odpadami; Uniwersytet Rzeszowski: Rzeszów, Poland, 2012; pp. 453–466. [Google Scholar]
- Fridrihsone, A.; Romagnoli, F.; Kirsanovs, V.; Cabulis, U. Life Cycle Assessment of Vegetable Oil Based Polyols for Polyurethane Production. J. Clean. Prod. 2020, 266, 121403. [Google Scholar] [CrossRef]
- Helling, R.K.; Russell, D.A. Use of Life Cycle Assessment to Characterize the Environmental Impacts of Polyol Production Options. Green Chem. 2009, 11, 380–389. [Google Scholar] [CrossRef]
- Nayak, P.L. Natural Oil-Based Polymers: Opportunities and Challenges. J. Macromol. Sci. Part C Polym. Rev. 2000, 40, 1–21. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Plant Oils as Platform Chemicals for Polyurethane Synthesis: Current State-of-the-Art. Biomacromolecules 2010, 11, 2825–2835. [Google Scholar] [CrossRef]
- Sustainable Chemicals Strategy of the Union: Time to Deliver—Council Conclusions. 2021. Available online: https://data.consilium.europa.eu/doc/document/ST-5245-2021-REV-2/en/pdf (accessed on 1 February 2024).
- A New Circular Economy Action Plan: For a Cleaner and More Competitive Europe. 2021. Available online: https://www.oneplanetnetwork.org/knowledge-centre/resources/new-circular-economy-action-plan-cleaner-and-more-competitive-europe (accessed on 1 February 2024).
- European Climate Law—Council’s General Approach; European Union: Maastricht, The Netherlands, 2020.
- Balaras, C.A.; Dascalaki, E.G.; Patsioti, M.; Droutsa, K.G.; Kontoyiannidis, S.; Cholewa, T. Carbon and Greenhouse Gas Emissions from Electricity Consumption in European Union Buildings. Buildings 2023, 14, 71. [Google Scholar] [CrossRef]
- European Commission. Intelligent Cities Challenge Renovation Wave In Practice Legal Notice; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Olasolo-Alonso, P.; López-Ochoa, L.M.; Las-Heras-Casas, J.; López-González, L.M. Energy Performance of Buildings Directive Implementation in Southern European Countries: A Review. Energy Build. 2023, 281, 112751. [Google Scholar] [CrossRef]
- Kaikade, D.S.; Sabnis, A.S. Polyurethane Foams from Vegetable Oil-Based Polyols: A Review. Polym. Bull. 2023, 80, 2239–2261. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Bio-Renewable Sources for Synthesis of Eco-Friendly Polyurethane Adhesives—Review. Open J. Polym. Chem. 2017, 7, 57–75. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.-F.; Guerin, C.; Raoul, Y.; Mouloungui, Z. From Petrochemical Polyurethanes to Biobased Polyhydroxyurethanes. Macromolecules 2013, 46, 3771–3792. [Google Scholar] [CrossRef]
- Zakwan, A.N.; Hirzin, R.S.F.N. Comparative Study on Optimization of Factors Affecting Epoxidation-Hydroxylation Reaction for The Production of Waste Cooking Oil Based-Polyol. Sci. Res. J. 2022, 19, 66401. [Google Scholar]
- Mudhaffar, B.; Salimon, J. Epoxidation of Vegetable Oils and Fatty Acids: Catalysts, Methods and Advantages. J. Appl. Sci. 2010, 10, 1545–1553. [Google Scholar] [CrossRef]
- Campanella, A.; Baltanás, M.A.; Capel-Sánchez, M.C.; Campos-Martín, J.M.; Fierro, J.L.G. Soybean Oil Epoxidation with Hydrogen Peroxide Using an Amorphous Ti/SiO2 Catalyst. Green Chem. 2004, 6, 330–334. [Google Scholar] [CrossRef]
- Goud, V.V.; Patwardhan, A.V.; Dinda, S.; Pradhan, N.C. Kinetics of Epoxidation of Jatropha Oil with Peroxyacetic and Peroxyformic Acid Catalysed by Acidic Ion Exchange Resin. Chem. Eng. Sci. 2007, 62, 4065–4076. [Google Scholar] [CrossRef]
- Gerbase, A.E.; Gregório, J.R.; Martinelli, M.; Brasil, M.C.; Mendes, A.N.F. Epoxidation of Soybean Oil by the Methyltrioxorhenium-CH2Cl2/H2O2 Catalytic Biphasic System. J. Am. Oil Chem. Soc. 2002, 79, 179–181. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Maungchareon, A.; Saravari, O. Preparation and Properties of Palm Oil-Based Rigid Polyurethane Nanocomposite Foams. J. Reinf. Plast. Compos. 2010, 29, 218–225. [Google Scholar] [CrossRef]
- Valero, M.F.; Gonzalez, A. Polyurethane Adhesive System from Castor Oil Modified by a Transesterification Reaction. J. Elastomers Plast. 2012, 44, 433–442. [Google Scholar] [CrossRef]
- Moghadam, P.N.; Yarmohamadi, M.; Hasanzadeh, R.; Nuri, S. Preparation of Polyurethane Wood Adhesives by Polyols Formulated with Polyester Polyols Based on Castor Oil. Int. J. Adhes. Adhes. 2016, 68, 273–282. [Google Scholar] [CrossRef]
- Malewska, E.; Polaczek, K.; Kurańska, M. Impact of Various Catalysts on Transesterification of Used Cooking Oil and Foaming Processes of Polyurethane Systems. Materials 2022, 15, 7807. [Google Scholar] [CrossRef] [PubMed]
- Mohd Tahir, S.; Wan Salleh, W.N.; Nor Hadid, N.S.; Enderus, N.F.; Ismail, N.A. Synthesis of Waste Cooking Oil-Based Polyol Using Sugarcane Bagasse Activated Carbon and Transesterification Reaction for Rigid Polyurethane Foam. Mater. Sci. Forum 2016, 846, 690–696. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zhang, W.; Javni, I. Structure and Properties of Polyurethanes Prepared from Triglyceride Polyols by Ozonolysis. Biomacromolecules 2005, 6, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Desroches, M.; Caillol, S.; Lapinte, V.; Auvergne, R.; Boutevin, B. Synthesis of Biobased Polyols by Thiol-Ene Coupling from Vegetable Oils. Macromolecules 2011, 44, 2489–2500. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Guo, A.; Javni, I.; Cvetković, I.; Hong, D.P. Polyurethane Networks from Polyols Obtained by Hydroformylation of Soybean Oil. Polym. Int. 2008, 57, 275–281. [Google Scholar] [CrossRef]
- Hong, J.; Radojčić, D.; Yang, X.; Wan, X.; Petrović, Z.S. Tough Thermosetting Polyurethanes and Adhesives from Rubber Seed Oil by Hydroformylation. J. Appl. Polym. Sci. 2020, 137, 48509. [Google Scholar] [CrossRef]
- Samadi, A.; Martínez, L.A.; Miranda, M.A.; Morera, I.M. Mechanism of Lipid Peroxidation Photosensitized by Tiaprofenic Acid: Product Studies Using Linoleic Acid and 1,4-Cyclohexadienes as Model Substrates. Photochem. Photobiol. 2007, 73, 359–365. [Google Scholar] [CrossRef]
- Silva, B.B.R.; Santana, R.M.C.; Forte, M.M.C. A Solventless Castor Oil-Based PU Adhesive for Wood and Foam Substrates. Int. J. Adhes. Adhes. 2010, 30, 559–565. [Google Scholar] [CrossRef]
- Orgilés-Calpena, E.; Arán-Aís, F.; Torró-Palau, A.M.; Orgilés-Barceló, C. Synthesis and Characterisation of Potentially Biodegradable Polyurethane Adhesives from Soybased Polyols. Polym. Renew. Resour. 2014, 5, 99–113. [Google Scholar] [CrossRef]
- Fridrihsone-Girone, A.; Stirna, U.; Misāne, M.; Lazdiņa, B.; Deme, L. Spray-Applied 100% Volatile Organic Compounds Free Two Component Polyurethane Coatings Based on Rapeseed Oil Polyols. Prog. Org. Coat. 2016, 94, 90–97. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Kośny, J.; Strąkowska, A.; Masłowski, M.; Strzelec, K. Linseed Oil as a Natural Modifier of Rigid Polyurethane Foams. Ind. Crops Prod. 2018, 115, 40–51. [Google Scholar] [CrossRef]
- Jabar, J.M. Production of Sustainable Rigid Polyurethane Foam from Chemically Modified Underutilized Jatropha curcas L. Seed Oil: Influence of Polyol Chemical Structure on Properties of Polymer. Curr. Res. Green Sustain. Chem. 2022, 5, 100331. [Google Scholar] [CrossRef]
- Khoon Poh, A.; Choy Sin, L.; Sit Foon, C.; Cheng Hock, C. Polyurethane Wood Adhesive from Palm Oil-Based Polyester Polyol. J. Adhes. Sci. Technol. 2014, 28, 1020–1033. [Google Scholar] [CrossRef]
- Leng, X.; Li, C.; Cai, X.; Yang, Z.; Zhang, F.; Liu, Y.; Yang, G.; Wang, Q.; Fang, G.; Zhang, X. A Study on Coconut Fatty Acid Diethanolamide-Based Polyurethane Foams. RSC Adv. 2022, 12, 13548–13556. [Google Scholar] [CrossRef]
- Das, B.; Konwar, U.; Mandal, M.; Karak, N. Sunflower Oil Based Biodegradable Hyperbranched Polyurethane as a Thin Film Material. Ind. Crops Prod. 2013, 44, 396–404. [Google Scholar] [CrossRef]
- Ramanujam, S.; Zequine, C.; Bhoyate, S.; Neria, B.; Kahol, P.; Gupta, R. Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams. C 2019, 5, 13. [Google Scholar] [CrossRef]
- Polaczek, K.; Kurańska, M. Hemp Seed Oil and Oilseed Radish Oil as New Sources of Raw Materials for the Synthesis of Bio-Polyols for Open-Cell Polyurethane Foams. Materials 2022, 15, 8891. [Google Scholar] [CrossRef]
- Kurańska, M.; Polaczek, K.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-Cell Rigid Polyurethane Bio-Foams Based on Modified Used Cooking Oil. Polymer 2020, 190, 122164. [Google Scholar] [CrossRef]
- Coman, A.E.; Peyrton, J.; Hubca, G.; Sarbu, A.; Gabor, A.R.; Nicolae, C.A.; Iordache, T.V.; Averous, L. Synthesis and Characterization of Renewable Polyurethane Foams Using Different Biobased Polyols from Olive Oil. Eur. Polym. J. 2021, 149, 110363. [Google Scholar] [CrossRef]
- High Performance Enabled by Nature: First Bio-Based Crosslinker Desmodur Eco N 7300. Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://solutions.covestro.com/-/media/covestro/solution-center/brochures/packaging/desmodur-eco-n-7300.pdf%3Frev%3Df0ae701db2644c77aa60bf9a28397687%26hash%3DD3225E56EDCA4CA7527F02A9B64C6A56&ved=2ahUKEwj92LrFvbmFAxV1ia8BHeY6B08QFnoECBEQAQ&usg=AOvVaw3jgNcGAABcdzDbEHfJsFKE (accessed on 1 February 2024).
- Covestro. Desmodur CQ 44 V 20 L MB_TDS. Available online: https://solutions.covestro.com/en/products/desmodur/desmodur-cq-44-v-20-l-mb_86596164-23474960?SelectedCountry=AD (accessed on 1 February 2024).
- Çaylı, G.; Küsefoğlu, S. Biobased Polyisocyanates from Plant Oil Triglycerides: Synthesis, Polymerization, and Characterization. J. Appl. Polym. Sci. 2008, 109, 2948–2955. [Google Scholar] [CrossRef]
- Hojabri, L.; Kong, X.; Narine, S.S. Fatty Acid-Derived Diisocyanate and Biobased Polyurethane Produced from Vegetable Oil: Synthesis, Polymerization, and Characterization. Biomacromolecules 2009, 10, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Nanclares, J.; Petrović, Z.S.; Javni, I.; Ionescu, M.; Jaramillo, F. Segmented Polyurethane Elastomers by Nonisocyanate Route. J. Appl. Polym. Sci. 2015, 132, 42492. [Google Scholar] [CrossRef]
- Błażek, K.; Beneš, H.; Walterová, Z.; Abbrent, S.; Eceiza, A.; Calvo-Correas, T.; Datta, J. Synthesis and Structural Characterization of Bio-Based Bis(cyclic Carbonate)s for the Preparation of Non-Isocyanate Polyurethanes. Polym. Chem. 2021, 12, 1643–1652. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kahol, P.K. Polyurethane Chemistry: Renewable Polyols and Isocyanates; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, ISBN 9780841298408. [Google Scholar]
- Joshi, J.R.; Bhanderi, K.K.; Patel, J.V.; Karve, M. Chemical Modification of Waste Cooking Oil for the Biolubricant Production Through Transesterification Process. J. Indian Chem. Soc. 2023, 100, 100909. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel Fuel Production by Transesterification of Oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Shomchoam, B.; Yoosuk, B. Eco-Friendly Lubricant by Partial Hydrogenation of Palm Oil over Pd/-Al2O3 Catalyst. Ind. Crops Prod. 2014, 62, 395–399. [Google Scholar] [CrossRef]
- Fernández, M.B.; Sánchez, M.J.F.; Tonetto, G.M.; Damiani, D.E. Hydrogenation of Sunflower Oil over Different Palladium Supported Catalysts: Activity and Selectivity. Chem. Eng. J. 2009, 155, 941–949. [Google Scholar] [CrossRef]
- Hassan, A.A.; Abbas, A.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Wang, S. Development, Influencing Parameters and Interactions of Bioplasticizers: An Environmentally Friendlier Alternative to Petro Industry-Based Sources. Sci. Total Environ. 2019, 682, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Sovtić, N.; Predrag, K.S.; Bera, O.J.; Pavličević, J.M.; Govedarica, O.M.; Jovičić, M.C.; Govedarica, D.D. A Review of Environmentally Friendly Rubber Production Using Different Vegetable Oils. Polym. Eng. Sci. 2020, 60, 1097–1117. [Google Scholar] [CrossRef]
- Kirpluks, M.; Vanags, E.; Abolins, A.; Michalowski, S.; Fridrihsone, A.; Cabulis, U. High Functionality Bio-Polyols from Tall Oil and Rigid Polyurethane Foams Formulated Solely Using Bio-Polyols. Materials 2020, 13, 1985. [Google Scholar] [CrossRef] [PubMed]
- Babiak, M.; Błaszczyński, T. Zużyte Oleje Jako Wypełniacze Asfaltów Stosowanych Do Produkcji Materiałów Hydroizolacyjnych. Prz. Bud. 2017, 88, 121–124. [Google Scholar]
- Foo, W.H.; Chia, W.Y.; Tang, D.Y.Y.; Koay, S.S.N.; Lim, S.S.; Chew, K.W. The Conundrum of Waste Cooking Oil: Transforming Hazard into Energy. J. Hazard. Mater. 2021, 417, 126129. [Google Scholar] [CrossRef] [PubMed]
- Matušinec, J.; Hrabec, D.; Šomplák, R.; Nevrlý, V.; Pecha, J.; Smejkalová, V.; Redutskiy, Y. Cooking Oil and Fat Waste Management: A Review of the Current State. Chem. Eng. Trans. 2020, 81, 763–768. [Google Scholar]
- Awogbemi, O.; Kallon, D.V.V.; Aigbodion, V.S.; Panda, S. Advances in Biotechnological Applications of Waste Cooking Oil. Case Stud. Chem. Environ. Eng. 2021, 4, 100158. [Google Scholar] [CrossRef]
- De Feo, G.; Ferrara, C.; Giordano, L.; Ossèo, L.S. Assessment of Three Recycling Pathways for Waste Cooking Oil as Feedstock in the Production of Biodiesel, Biolubricant, and Biosurfactant: A Multi-Criteria Decision Analysis Approach. Recycling 2023, 8, 64. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Adamu, A.; Zhu, Z. Economic Evaluation and Production Process Simulation of Biodiesel Production from Waste Cooking Oil. Curr. Res. Green Sustain. Chem. 2021, 4, 100091. [Google Scholar] [CrossRef]
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J.; Tokgoz, S.; Hayes, D.; Yu, T.H. Use of U.S. Croplands for Biofuels Increases Greenhouse Gases Through Emissions from Land-Use Change. Science 2008, 319, 1238–1240. [Google Scholar] [CrossRef]
- Popp, J.; Lakner, Z.; Harangi-Rákos, M.; Fári, M. The Effect of Bioenergy Expansion: Food, Energy, and Environment. Renew. Sustain. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef]
- Hoekstra, A.Y.; Mekonnen, M.M. The Water Footprint of Humanity. Proc. Natl. Acad. Sci. USA 2012, 109, 3232–3237. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Aghbashlo, M.; Tabatabaei, M. Life Cycle Assessment of Bioenergy Product Systems: A Critical Review. e-Prime—Adv. Electr. Eng. Electron. Energy 2021, 1, 100015. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration to Mitigate Climate Change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Ozlu, E.; Arriaga, F.J.; Bilen, S.; Gozukara, G.; Babur, E. Carbon Footprint Management by Agricultural Practices. Biology 2022, 11, 1453. [Google Scholar] [CrossRef]
- Kajdas, C. Major Pathways for Used Oil Disposal and Recycling. Part 1. Tribotest 2000, 7, 61–74. [Google Scholar] [CrossRef]
- Parekh, K.; Gaur, R.; Shahabuddin, S. Recent Advances in Reclamation of Used Lubricant Oil. In Tailored Functional Materials; Springer Proceedings in Materials; Springer Nature: Singapore, 2022; Volume 15, pp. 273–282. [Google Scholar]
- Audibert, F. Waste Engine Oils: Rerefining and Energy Recovery; Elsevier: Amsterdam, The Netherlands, 2006; p. 323. ISBN 0444522026. [Google Scholar]
- Rupal, A.; Sharma, S.K.; Tyagi, G.D. Experimental Investigation on Mechanical Properties of Polyurethane Modified Bituminous Waterproofing Membrane. Mater. Today Proc. 2020, 27, 467–474. [Google Scholar] [CrossRef]
| Route Modification | Number of Production Steps | State of Art | References |
|---|---|---|---|
| epoxidation | 2 | commercial | [36,37,38,39,40] |
| transesterification | 1 | commercial | [41,42,43,44,45] |
| ozonolysis | 2 | investigation | [46] |
| thiol-ene coupling | 1 | investigation | [47] |
| hydroformylation, | 2 | investigation | [48,49] |
| photochemical oxidation | 2 | investigation | [50] |
| Plant Oil | Application | Investigated Polyurethane Properties | References |
|---|---|---|---|
| Castor oil | polyol/adhesive | cure characteristic, TGA, shore A hardness, density, tensile properties, DSC analysis, | [51] |
| Soybean oil | polyol/adhesive | DSC analysis, TGA, FTIR analysis, GPC analysis, mechanical properties | [52] |
| Rapeseed oil | polyol/coating | cure characteristic, rheology properties, density, tensile properties, FTIR analysis, hydrolytic stability tests, shore A hardness | [53] |
| Linseed oil | plasticizer/foam | FTIR analysis, DSC, TGA, morphological and optical properties, mechanical properties, density, dimensional stability, contact angle, water absorption, flammability | [54] |
| Jatropha oil | polyol/foam | FTIR analysis, TGA/DTG, SEM analysis, mechanical properties, content of closed–cell, average cell diameter, density, biodegradability | [55] |
| Palm oil, | polyol/adhesive | cure characteristic, chemical resistance, green strength, FTIR analysis, TGA, tensile properties | [56] |
| Coconut oil | polyol/foam | rheology properties, NMR analysis, FTIR analysis, GPC analysis, morphological and optical properties, DSC, TGA, thermal conductivity, cure characteristic, mechanical properties | [57] |
| Sunflower oil, | polyol/coating | NMR analysis, FTIR analysis, TGA, surface morphology, tensile properties, chemical resistance, biodegradability | [58] |
| Corn oil | polyol/foam | cure characteristic, density, and content of closed–cell mechanical properties, optical properties SEM, fire-retardant properties, TGA | [59] |
| Tall Oil | polyol/foam | density, content of closed–cell, thermal conductivity, mechanical properties, average cell size, | [76] |
| Rubber seed oil | polyol/adhesive | SEC analysis, FTIR analysis, NMR analysis, tensile properties, DSC analysis, TGA | [49] |
| Waste Cooking Oils | polyol/foam | foaming properties, morphology properties SEM, apparent density, thermal conductivity coefficients, compressive strength, TGA, dimensional stability | [61] |
| Olive oil | polyol/foam | FTIR analysis, NMR analysis, SEC analysis, rheology properties, DSC analysis, TGA, compression tests, the cell sizes, apparent foam density | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciastowicz, Ż.; Pamuła, R.; Białowiec, A. Utilization of Plant Oils for Sustainable Polyurethane Adhesives: A Review. Materials 2024, 17, 1738. https://doi.org/10.3390/ma17081738
Ciastowicz Ż, Pamuła R, Białowiec A. Utilization of Plant Oils for Sustainable Polyurethane Adhesives: A Review. Materials. 2024; 17(8):1738. https://doi.org/10.3390/ma17081738
Chicago/Turabian StyleCiastowicz, Żaneta, Renata Pamuła, and Andrzej Białowiec. 2024. "Utilization of Plant Oils for Sustainable Polyurethane Adhesives: A Review" Materials 17, no. 8: 1738. https://doi.org/10.3390/ma17081738
APA StyleCiastowicz, Ż., Pamuła, R., & Białowiec, A. (2024). Utilization of Plant Oils for Sustainable Polyurethane Adhesives: A Review. Materials, 17(8), 1738. https://doi.org/10.3390/ma17081738








