Kinetics of Carbon Enrichment in Austenite during Partitioning Stage Studied via In-Situ Synchrotron XRD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Thermal Treatments
2.2. High Energy X-ray Diffraction (HEXRD)
2.3. Dilatometry
2.4. Rietveld Refinement Analysis
2.4.1. Debye–Scherrer Ring Integration
2.4.2. Instrument Calibration
3. Results
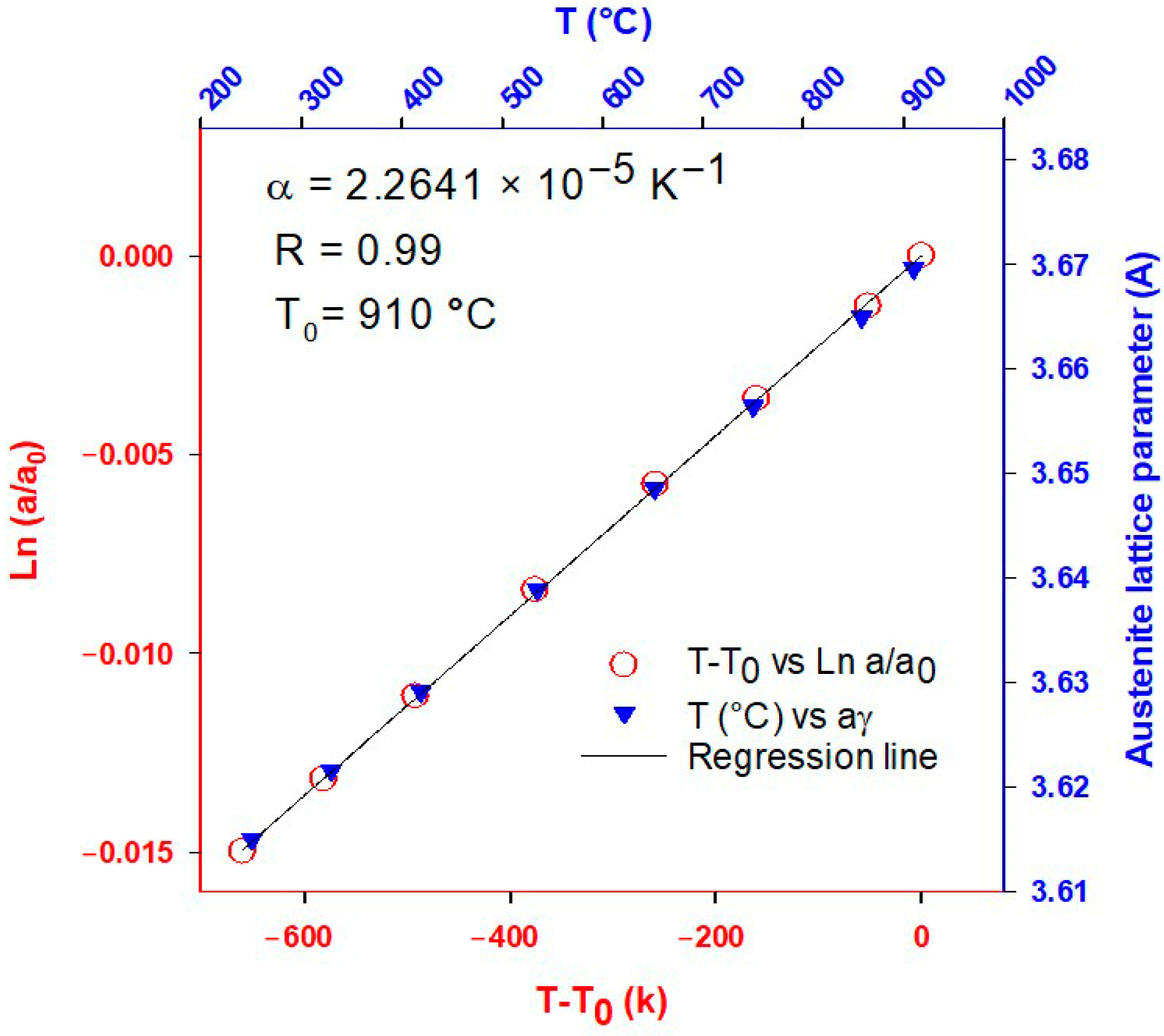
3.1. Coefficient of Thermal Expansion (CTE)
3.2. Austenite Carbon Content Estimation
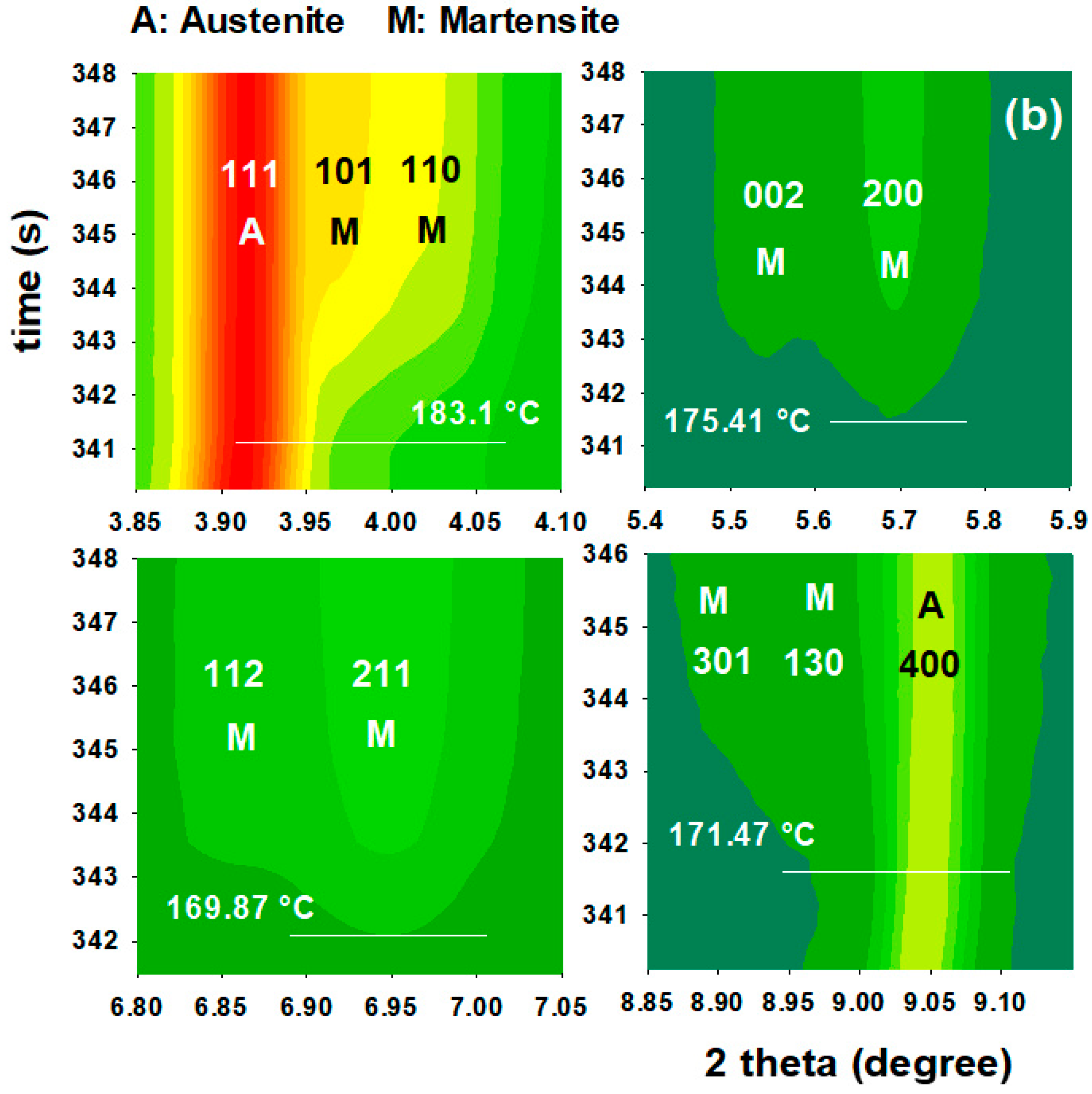
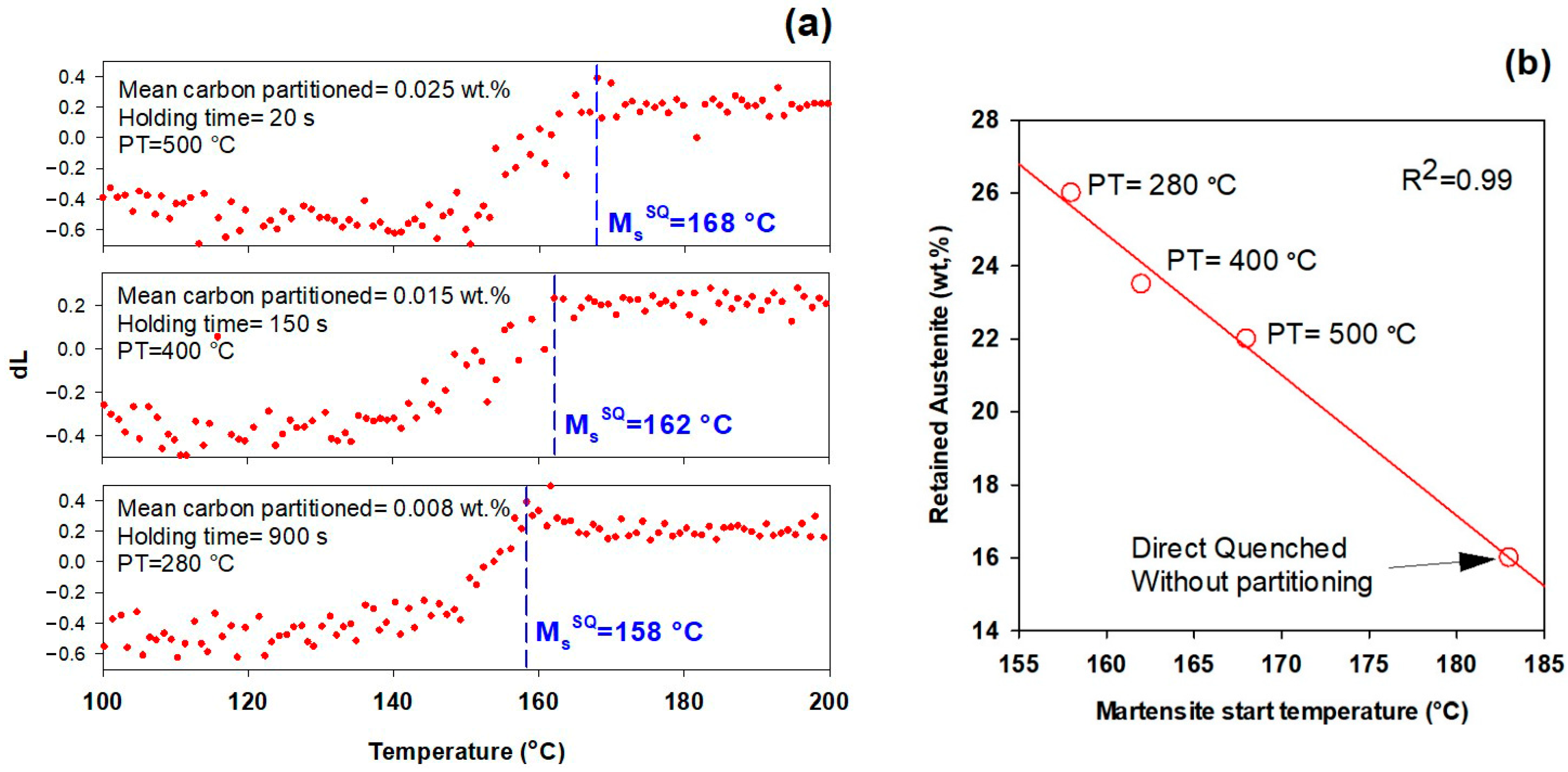
3.3. First Quench (Initial Martensite State)
3.4. Reheating Stage to the Partitioning Temperature
3.4.1. FCC Phase Evolution
3.4.2. Tetragonal BCT Phase
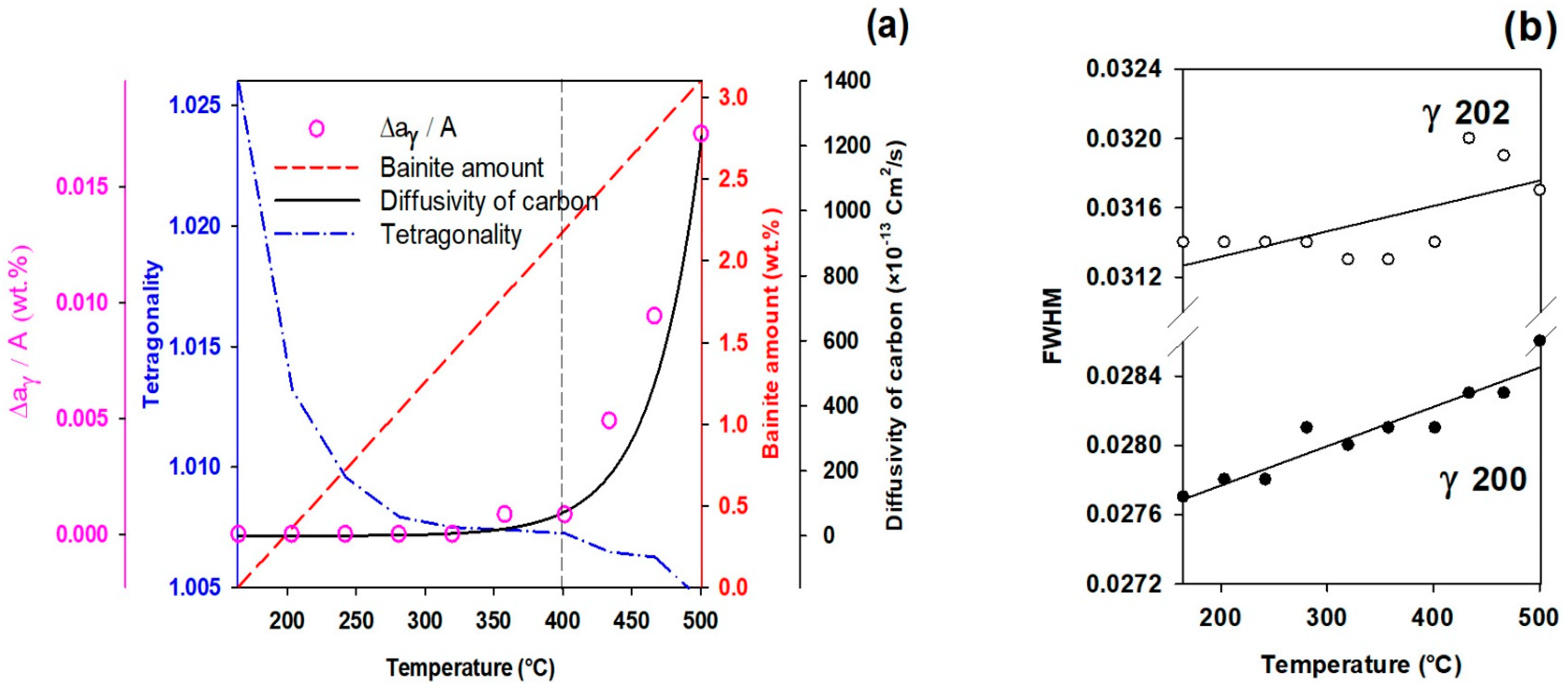
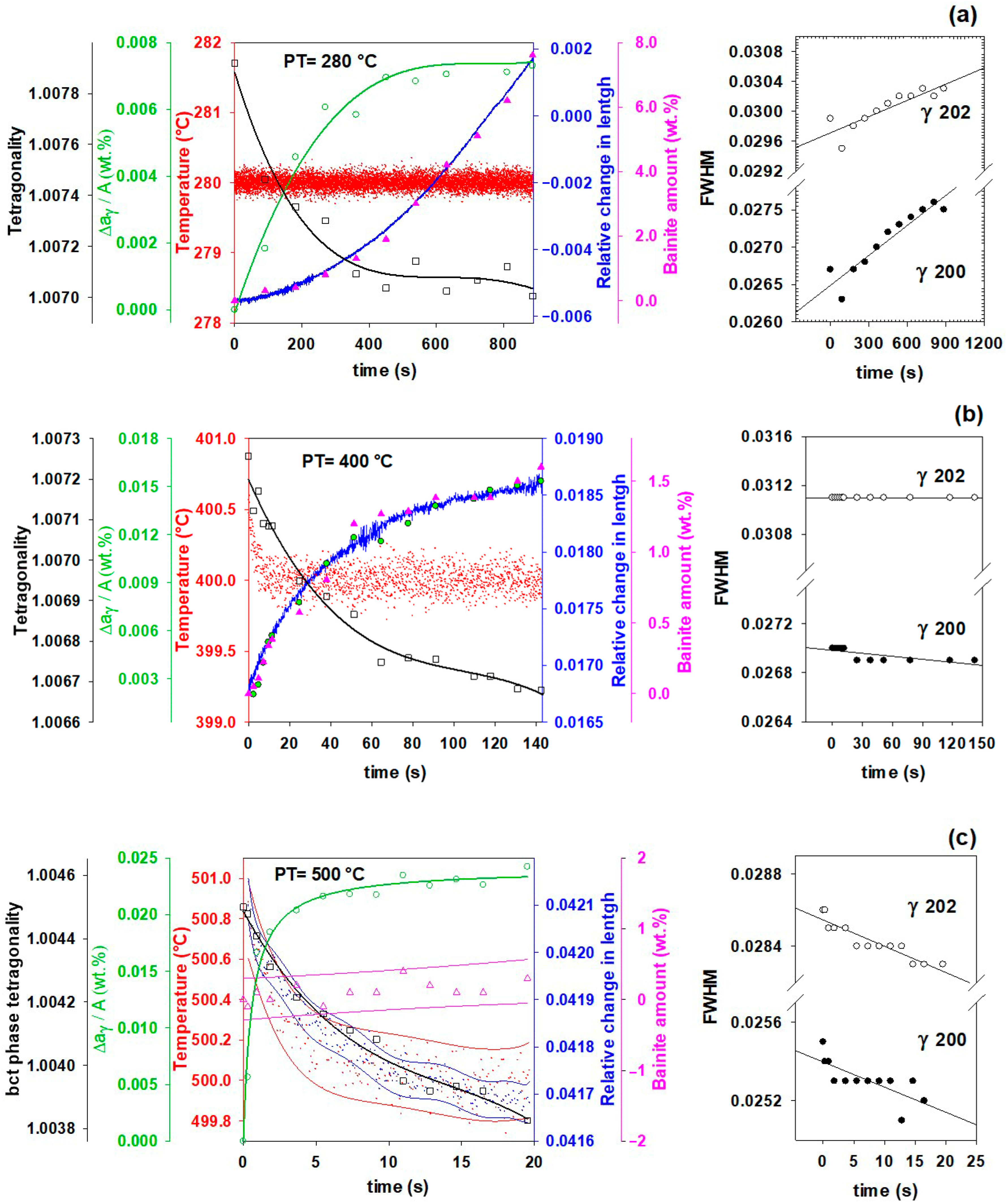
3.5. Effect of Partitioning Temperature on Partitioning Mechanisms
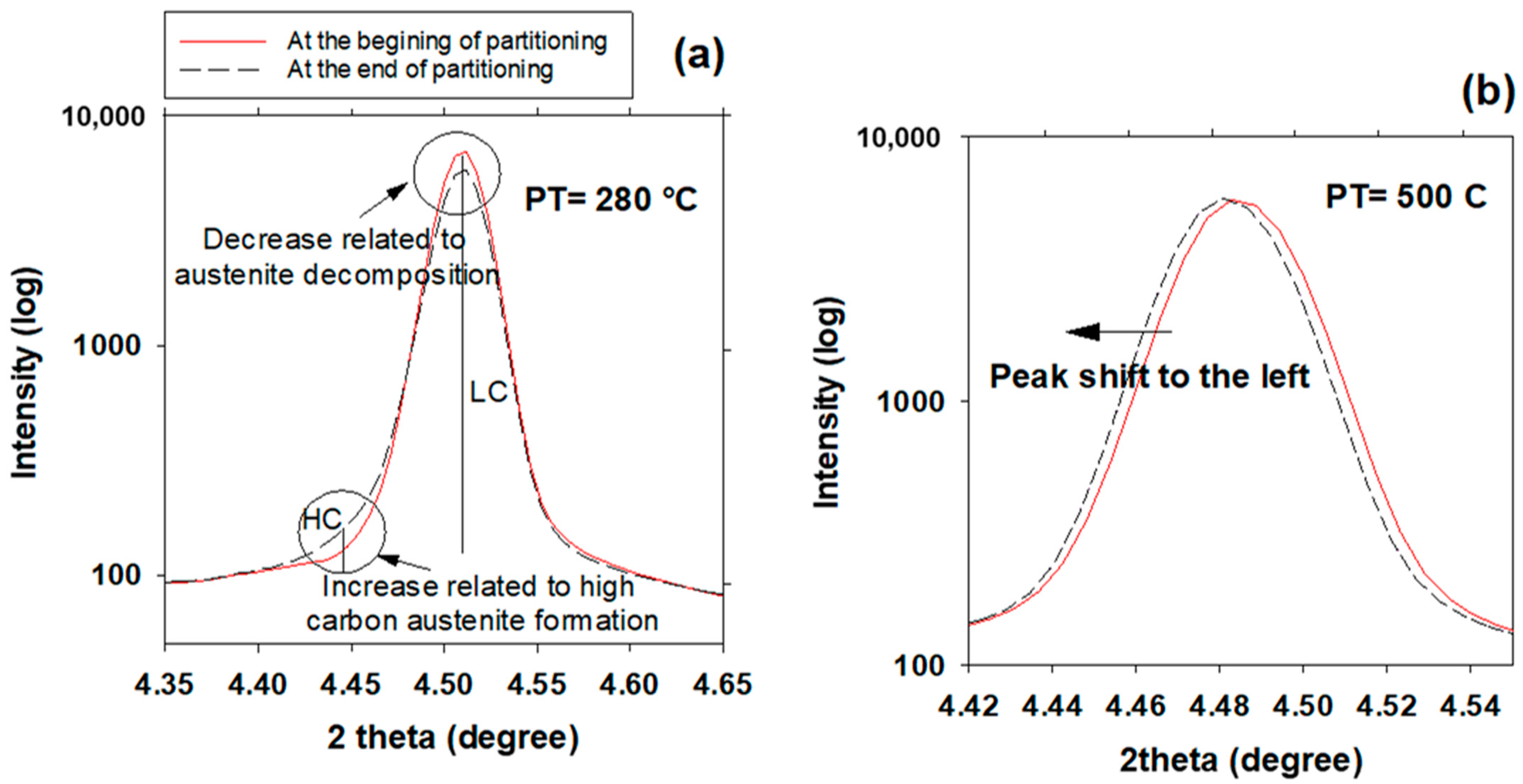
3.6. High- and Low-Carbon Austenite
3.7. Effect of Partitioning Treatment on the Final Microstructure
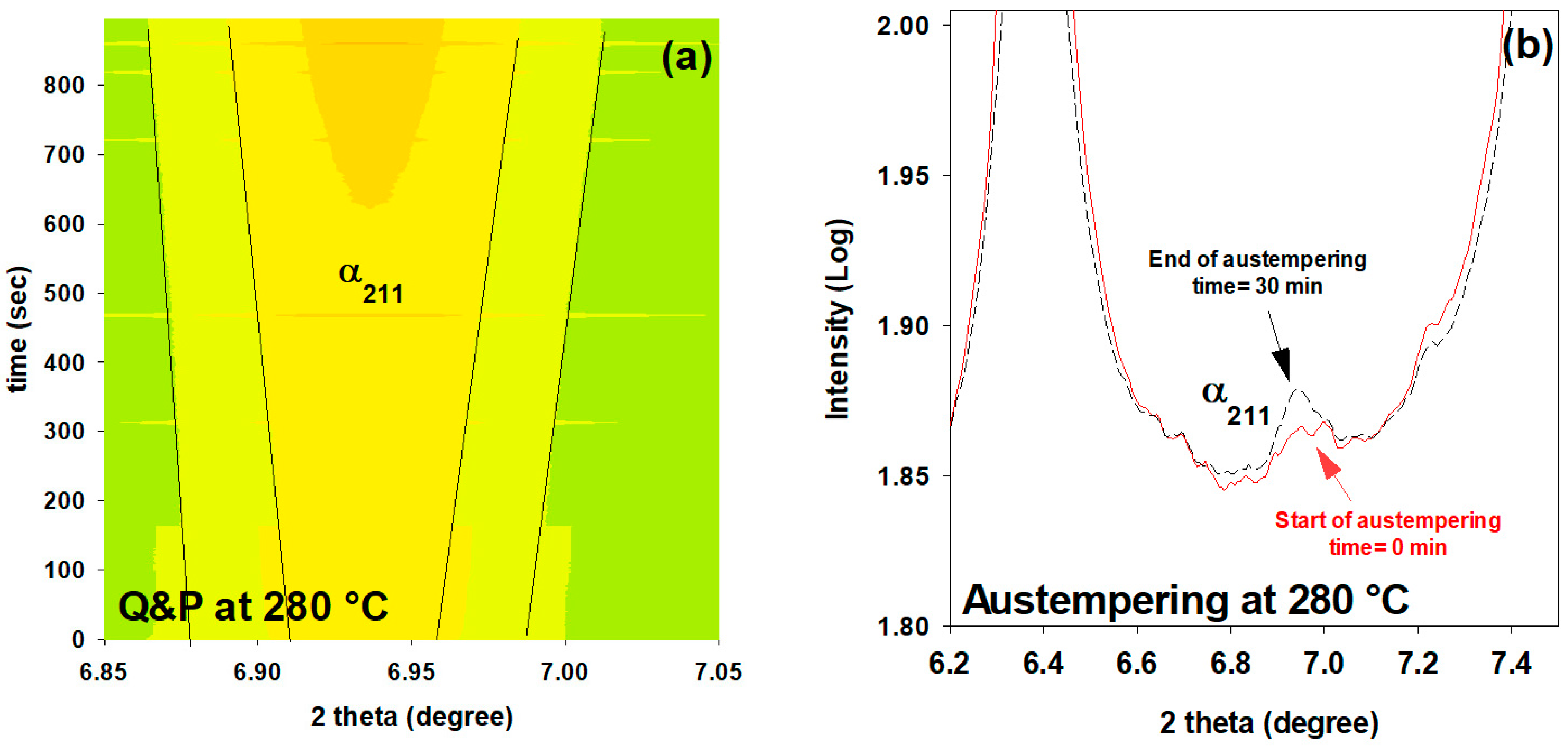
3.8. Influence of Prior Martensite on Bainite Transformation
4. Conclusions
- In addition to carbon partitioning from martensite to austenite, the bainite formation during the partitioning stage was another active mechanism for austenite carbon enrichment. When the partitioning temperature was increased, the dominant carbon enrichment mechanism in the austenite changed from bainitic ferrite transformation to carbon partitioning from supersaturated martensite. It has been shown that partitioning at 280 °C for 900 s produces the highest percentage of retained austenite (almost 26 wt.%).
- In comparison with austempering heat treatment with no prior martensite, the presence of initial martensite in the Q&P microstructure accelerates the subsequent bainitic transformation during the partitioning stage.
- The FWHM results measured from and peaks during partitioning at 280 °C showed a positive slope, indicating heterogeneity in the austenite carbon content when bainitic ferrite is formed in the microstructure (this occurs especially at lower partitioning temperatures). The lattice parameters in the austenite varied from 3.618 to 3.660 nm, showing that the carbon concentration in the austenite was between 0.6 and 1.9 wt.%.
- At temperatures below 400 °C, a new bct phase was detected. Tetragonality of this phase was much higher than for ferrite (bcc structure), indicating that this phase could be tetragonal ferrite, which would be consistent with the symmetry of ferrite in equilibrium with austenite not being cubic when first formed, since it is supersaturated with carbon.
- The FWHM results for the austenite peaks at 500 °C indicate a more homogeneous distribution of carbon. This phenomenon is related to carbon diffusion in the austenite and an equalization of the carbon concentration in the austenitic grains.
- The dilation behavior of the samples shows a significant correlation with bainitic ferrite fraction as a function of increasing temperature. However, at 500 °C, when the bainitic ferrite fraction is minor and carbon partitioning from supersaturated martensite into austenite is the dominant mechanism, no volume expansion occurs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Cooman, B.C.; Speer, J.G. Quench and Partitioning Steel: A New AHSS Concept for Automotive Anti-Intrusion Applications. Steel Res. Int. 2006, 77, 634–640. [Google Scholar] [CrossRef]
- Santofimia, M.; Zhao, L.; Petrov, R.; Kwakernaak, C.; Sloof, W.; Sietsma, J. Microstructural development during the quenching and partitioning process in a newly designed low-carbon steel. Acta Mater. 2011, 59, 6059–6068. [Google Scholar] [CrossRef]
- Clarke, A.J.; Speer, J.G.; Miller, M.K.; Hackenberg, R.E.; Edmonds, D.V.; Matlock, D.K.; Rizzo, F.C.; Clarke, K.D.; De Moor, E. Carbon partitioning to austenite from martensite or bainite during the quench and partition (Q&P) process: A critical assessment. Acta Mater. 2008, 56, 16–22. [Google Scholar] [CrossRef]
- Mateo, C.G.; Caballero, F.; Miller, M.K.; Jiménez, J.A. On measurement of carbon content in retained austenite in a nanostructured bainitic steel. J. Mater. Sci. 2012, 47, 1004–1010. [Google Scholar] [CrossRef]
- Toji, Y.; Matsuda, H.; Herbig, M.; Choi, P.-P.; Raabe, D. Atomic-scale analysis of carbon partitioning between martensite and austenite by atom probe tomography and correlative transmission electron microscopy. Acta Mater. 2014, 65, 215–228. [Google Scholar] [CrossRef]
- Guo, L.; Bhadeshia, H.K.D.H.; Roelofs, H.; Lembke, M.I. In situ synchrotron X-ray study of bainite transformation kinetics in a low-carbon Si-containing steel. Mater. Sci. Technol. 2017, 33, 2147–2156. [Google Scholar] [CrossRef]
- Huyghe, P.; Caruso, M.; Collet, J.L.; Dépinoy, S.; Godet, S. Into the quenching & partitioning of a 0.2 C steel: An in-situ synchrotron study. Mater. Sci. Eng. A 2019, 743, 175–184. [Google Scholar]
- Maheswari, N.; Amirthalingam, M.; Schwedt, A.; Brokmeier, H.G.; Schell, N.; Mayer, J.; Kumar, K.H.; Sankaran, S. Temperature dependent partitioning mechanisms and its associated microstructural evolution in a CMnSiAl quenching and partitioning (Q&P) steel. Mater. Today Commun. 2021, 29, 102918. [Google Scholar] [CrossRef]
- De Moor, E.; Lacroix, S.; Clarke, A.; Penning, J.; Speer, J. Effect of Retained Austenite Stabilized via Quench and Partitioning on the Strain Hardening of Martensitic Steels. Met. Mater. Trans. A 2008, 39, 2586–2595. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Sietsma, J. Overview of Mechanisms Involved During the Quenching and Partitioning Process in Steels. Met. Mater. Trans. A 2011, 42, 3620–3626. [Google Scholar] [CrossRef]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Edmonds, D.; He, K.; Miller, M.K.; Rizzo, F.; Clarke, A.; Matlock, D.K.; Speer, J.G. Microstructural Features of ‘Quenching and Partitioning’: A New Martensitic Steel Heat Treatment. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2007. [Google Scholar]
- Pierce, D.; Coughlin, D.; Williamson, D.; Clarke, K.; Clarke, A.; Speer, J.; De Moor, E. Characterization of transition carbides in quench and partitioned steel microstructures by Mössbauer spectroscopy and complementary techniques. Acta Mater. 2015, 90, 417–430. [Google Scholar] [CrossRef]
- HajyAkbary, F.; Sietsma, J.; Miyamoto, G.; Furuhara, T.; Santofimia, M.J. Interaction of carbon partitioning, carbide precipitation and bainite formation during the Q&P process in a low C steel. Acta Mater. 2016, 104, 72–83. [Google Scholar] [CrossRef]
- Li, H.; Lu, X.; Wu, X.; Min, Y.; Jin, X. Bainitic transformation during the two-step quenching and partitioning process in a medium carbon steel containing silicon. Mater. Sci. Eng. A 2010, 527, 6255–6259. [Google Scholar] [CrossRef]
- Speer, J.G.; Edmonds, D.V.; Rizzo, F.C.; Matlock, D.K. Partitioning of carbon from supersaturated plates of ferrite, with application to steel processing and fundamentals of the bainite transformation. Curr. Opin. Solid State Mater. Sci. 2004, 8, 219–237. [Google Scholar] [CrossRef]
- Van Dijk, N.H.; Butt, A.M.; Zhao, L.; Sietsma, J.; Offerman, S.E.; Wright, J.P.; Van Der Zwaag, S. Thermal stability of retained austenite in TRIP steels studied by synchrotron X-ray diffraction during cooling. Acta Mater. 2005, 53, 5439–5447. [Google Scholar] [CrossRef]
- Martinez-Perez, M.; Mompean, F.; Ruiz-Hervias, J.; Borlado, C.; Atienza, J.; Garcia-Hernandez, M.; Elices, M.; Gil-Sevillano, J.; Peng, R.L.; Buslaps, T. Residual stress profiling in the ferrite and cementite phases of cold-drawn steel rods by synchrotron X-ray and neutron diffraction. Acta Mater. 2004, 52, 5303–5313. [Google Scholar] [CrossRef]
- Elmer, J.W.; Palmer, T.A.; Specht, E.D. Direct Observations of Sigma Phase Formation in Duplex Stainless Steels Using In-Situ Synchrotron X-Ray Diffraction. Metall. Mater. Trans. A 2007, 38, 464–475. [Google Scholar] [CrossRef]
- Militzer, M. A Synchrotron Look at Steel. Science 2002, 298, 975–976. [Google Scholar] [CrossRef]
- Elmer, J.; Palmer, T.; Specht, E. In situ observations of sigma phase dissolution in 2205 duplex stainless steel using synchrotron X-ray diffraction. Mater. Sci. Eng. A 2007, 459, 151–155. [Google Scholar] [CrossRef]
- Young, M.; Almer, J.; Daymond, M.; Haeffner, D.; Dunand, D. Load partitioning between ferrite and cementite during elasto-plastic deformation of an ultrahigh-carbon steel. Acta Mater. 2007, 55, 1999–2011. [Google Scholar] [CrossRef]
- Allain, S.; Geandier, G.; Hell, J.; Soler, M.; Danoix, F.; Gouné, M. In-situ investigation of quenching and partitioning by High Energy X-Ray Diffraction experiments. Scr. Mater. 2017, 131, 15–18. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Geandier, G.; Hell, J.-C.; Soler, M.; Danoix, F.; Gouné, M. Effects of Q&P Processing Conditions on Austenite Carbon Enrichment Studied by In Situ High-Energy X-ray Diffraction Experiments. Metals 2017, 7, 232. [Google Scholar] [CrossRef]
- Allain, S.Y.; Gaudez, S.; Geandier, G.; Danoix, F.; Soler, M.; Goune, M. Carbon heterogeneities in austenite during Quenching & Partitioning (Q&P) process revealed by in situ High Energy X-Ray Diffraction (HEXRD) experiments. Scr. Mater. 2020, 181, 108–114. [Google Scholar] [CrossRef]
- Ebner, S.; Suppan, C.; Stark, A.; Schnitzer, R.; Hofer, C. Austenite decomposition and carbon partitioning during quenching and partitioning heat treatments studied via in-situ X-ray diffraction. Mater. Des. 2019, 178, 107862. [Google Scholar] [CrossRef]
- Wang, S.; Kistanov, A.A.; King, G.; Ghosh, S.; Singh, H.; Pallaspuro, S.; Rahemtulla, A.; Somani, M.; Kömi, J.; Cao, W.; et al. In-situ quantification and density functional theory elucidation of phase transformation in carbon steel during quenching and partitioning. Acta Mater. 2021, 221, 117361. [Google Scholar] [CrossRef]
- Ebner, S.; Schnitzer, R.; Suppan, C.; Stark, A.; Liu, H.; Hofer, C. Characterization of carbides in Q&P steels using a combination of high-resolution methods. Mater. Charact. 2020, 163, 110242. [Google Scholar] [CrossRef]
- Gaudez, S.; Teixeira, J.; Denis, S.; Geandier, G.; Allain, S.Y. Martensite and nanobainite transformations in a low alloyed steel studied by in situ high energy synchrotron diffraction. Mater. Charact. 2022, 185, 111740. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Gaudez, S.; Geandier, G.; Hell, J.-C.; Gouné, M.; Danoix, F.; Soler, M.; Aoued, S.; Poulon-Quintin, A. Internal stresses and carbon enrichment in austenite of Quenching and Partitioning steels from high energy X-ray diffraction experiments. Mater. Sci. Eng. A 2018, 710, 245–250. [Google Scholar] [CrossRef]
- Hu, Y. Phase Transformations in Steels during Quenching and Partitioning Heat Treatment. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2016. Available online: http://resolver.tudelft.nl/uuid:b6327e6f-abe3-492d-973a-a6f22aaac24a (accessed on 29 January 2023).
- Rieger, T.; Herrmann, K.; Carmele, D.; Meyer, S.; Lippmann, T.; Stark, A.; Bleck, W.; Klemradt, U. ‘Quenching and Partitioning’—An In Situ Approach to Characterize the Process Kinetics and the Final Microstructure of TRIP-Assisted Steel. Adv. Mater. Res. 2012, 409, 713–718. [Google Scholar] [CrossRef]
- Guo, H.; Feng, X.; Zhao, A.; Li, Q.; Ma, J. Influence of Prior Martensite on Bainite Transformation, Microstructures, and Mechanical Properties in Ultra-Fine Bainitic Steel. Materials 2019, 12, 527. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Tomota, Y.; Harjo, S.; Su, Y.H.; Aizawa, K. Effect of prior martensite on bainite transformation in nanobainite steel. Acta Mater. 2015, 85, 243–249. [Google Scholar] [CrossRef]
- Lu, X.; Yang, Z.; Qian, D.; Lan, J.; Hua, L. Effect of martensite pre-quenching on bainite transformation kinetics, martensite/bainite duplex microstructures, mechanical properties and retained austenite stability of GCr15 bearing steel. J. Mater. Res. Technol. 2021, 15, 2429–2438. [Google Scholar] [CrossRef]
- Zhou, W.; Hou, T.; Tao, L.; Wu, K. In Situ Observation on the Effects of Prior Martensite Formation on Nanostructured Low–Temperature Bainite Transformation. Metals 2018, 8, 818. [Google Scholar] [CrossRef]
- Hosseini, N.; Forouzan, F.; Vuorinen, E. In-situ microstructural evolution during quenching and partitioning of a high-carbon steel by high-temperature X-Ray Diffraction. Mater. Today Commun. 2022, 31, 103503. [Google Scholar] [CrossRef]
- Lutterotti, L.; Vasin, R.; Wenk, H.-R. Rietveld texture analysis from synchrotron diffraction images. I. Calibration and basic analysis. Powder Diffr. 2014, 29, 76–84. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Lu, X.-G.; Selleby, M.; Sundman, B. Assessments of molar volume and thermal expansion for selected bcc, fcc and hcp metallic elements. Calphad 2005, 29, 68–89. [Google Scholar] [CrossRef]
- Denand, B.; Esin, V.; Dehmas, M.; Geandier, G.; Denis, S.; Sourmail, T.; Aeby-Gautier, E. Carbon content evolution in austenite during austenitization studied by in situ synchrotron X-ray diffraction of a hypoeutectoid steel. Materialia 2020, 10, 100664. [Google Scholar] [CrossRef]
- van Bohemen, S. Austenite in multiphase microstructures quantified by analysis of thermal expansion. Scr. Mater. 2014, 75, 22–25. [Google Scholar] [CrossRef]
- Mohapatra, G.; Sommer, F.; Mittemeijer, E. Calibration of a quenching and deformation differential dilatometer upon heating and cooling: Thermal expansion of Fe and Fe–Ni alloys. Thermochim. Acta 2007, 453, 31–41. [Google Scholar] [CrossRef]
- Yan, S.; Liu, X.; Liu, W.J.; Lan, H.; Wu, H. Comparison on mechanical properties and microstructure of a C–Mn–Si steel treated by quenching and partitioning (Q&P) and quenching and tempering (Q&T) processes. Mater. Sci. Eng. A 2015, 620, 58–66. [Google Scholar] [CrossRef]
- Sugimoto, K.-I.; Usui, N.; Kobayashi, M.; Hashimoto, S.-I. Effects of Volume Fraction and Stability of Retained Austenite on Ductility of TRIP-aided Dual-phase Steels. ISIJ Int. 1992, 32, 1311–1318. [Google Scholar] [CrossRef]
- Hummelshøj, T.S.; Christiansen, T.L.; Somers, M.A. Lattice expansion of carbon-stabilized expanded austenite. Scr. Mater. 2010, 63, 761–763. [Google Scholar] [CrossRef]
- Epp, J.; Surm, H.; Kessler, O.; Hirsch, T. In situ X-ray phase analysis and computer simulation of carbide dissolution of ball bearing steel at different austenitizing temperatures. Acta Mater. 2007, 55, 5959–5967. [Google Scholar] [CrossRef]
- Chen, X.; Vuorinen, E.; Grahn, J. In-situ SEM observation on fracture behavior of austempered silicon alloyed steel. China Foundry 2009, 6, 185–190. [Google Scholar]
- Forouzan, F.; Borasi, L.; Vuorinen, E.; Mücklich, F. Optimization of Quenching Temperature to Minimize the Micro Segregation Induced Banding Phenomena in Quenching and Partitioning (Q&P) Steels. Steel Res. Int. 2019, 90, 1800281. [Google Scholar] [CrossRef]
- Forouzan, F.; Guitar, M.A.; Vuorinen, E.; Mücklich, F. Effect of Carbon Partitioning, Carbide Precipitation, and Grain Size on Brittle Fracture of Ultra-High-Strength, Low-Carbon Steel after Welding by a Quenching and Partitioning Process. Metals 2018, 8, 747. [Google Scholar] [CrossRef]
- Jang, J.H.; Bhadeshia, H.; Suh, D.-W. Solubility of carbon in tetragonal ferrite in equilibrium with austenite. Scr. Mater. 2013, 68, 195–198. [Google Scholar] [CrossRef]
- Chen, X.; Vuorinen, E. In-Situ High Temperature X-ray Studies of Austempering Transformation in High Silicon Cast Steel. ISIJ Int. 2009, 49, 1220–1224. [Google Scholar] [CrossRef]
- Hackenberg, R.E. The historical development of phase transformations understanding in ferrous alloys. Phase Transform. Steels 2012, 1, 3–55. [Google Scholar]
- Lu, Y.; Yu, H.; Sisson, R.D. The effect of carbon content on the c/a ratio of as-quenched martensite in Fe-C alloys. Mater. Sci. Eng. A 2017, 700, 592–597. [Google Scholar] [CrossRef]
- Caballero, F.; Miller, M.; Clarke, A.; Garcia-Mateo, C. Examination of carbon partitioning into austenite during tempering of bainite. Scr. Mater. 2010, 63, 442–445. [Google Scholar] [CrossRef] [Green Version]
- Rementeria, R.; Poplawsky, J.D.; Urones-Garrote, E.; Domínguez-Reyes, R.; Garcia-Mateo, C.; Caballero, F.G. Carbon supersaturation and clustering in bainitic ferrite at low temperature. In Proceedings of the 5th International Symposium on Steel Science, Kyoto, Japan, 13–16 November 2017. [Google Scholar]
- Wang, J.; Van Der Wolk, P.J.; Van Der Zwaag, S. Effects of Carbon Concentration and Cooling Rate on Continuous Cooling Transformations Predicted by Artificial Neural Network. ISIJ Int. 1999, 39, 1038–1046. [Google Scholar] [CrossRef]
- Lepera, F.S. Improved etching technique for the determination of percent martensite in high-strength dual-phase steels. Metallography 1979, 12, 263–268. [Google Scholar] [CrossRef]
- Forouzan, F.; Borasi, L.; Vuorinen, E.; Mücklich, F. Process control maps to design an ultra-high strength-ductile steel. Mater. Sci. Technol. 2019, 35, 1173–1184. [Google Scholar] [CrossRef]
- Girault, E.; Jacques, P.; Harlet, P.; Mols, K.; Van Humbeeck, J.; Aernoudt, E.; Delannay, F. Metallographic Methods for Revealing the Multiphase Microstructure of TRIP-Assisted Steels. Mater. Charact. 1998, 40, 111–118. [Google Scholar] [CrossRef]
- Ko, T.; Cottrell, S.A. The formation of bainite. J. Iron Steel Inst. 1952, 172, 307. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forouzan, F.; Surki Aliabad, R.; Hedayati, A.; Hosseini, N.; Maawad, E.; Blasco, N.; Vuorinen, E. Kinetics of Carbon Enrichment in Austenite during Partitioning Stage Studied via In-Situ Synchrotron XRD. Materials 2023, 16, 1557. https://doi.org/10.3390/ma16041557
Forouzan F, Surki Aliabad R, Hedayati A, Hosseini N, Maawad E, Blasco N, Vuorinen E. Kinetics of Carbon Enrichment in Austenite during Partitioning Stage Studied via In-Situ Synchrotron XRD. Materials. 2023; 16(4):1557. https://doi.org/10.3390/ma16041557
Chicago/Turabian StyleForouzan, Farnoosh, Roohallah Surki Aliabad, Ali Hedayati, Nazanin Hosseini, Emad Maawad, Núria Blasco, and Esa Vuorinen. 2023. "Kinetics of Carbon Enrichment in Austenite during Partitioning Stage Studied via In-Situ Synchrotron XRD" Materials 16, no. 4: 1557. https://doi.org/10.3390/ma16041557
APA StyleForouzan, F., Surki Aliabad, R., Hedayati, A., Hosseini, N., Maawad, E., Blasco, N., & Vuorinen, E. (2023). Kinetics of Carbon Enrichment in Austenite during Partitioning Stage Studied via In-Situ Synchrotron XRD. Materials, 16(4), 1557. https://doi.org/10.3390/ma16041557







