Wet Blue Enzymatic Treatment and Its Effect on Leather Properties and Post-Tanning Processes
Abstract
:1. Introduction
- (1)
- Wastewater treatment and cleaner solid waste processing;
- (2)
- Cleaner technologies that reduce the pollution load or do not use hazardous chemicals in the processing of leather [2].
2. Materials and Methods
2.1. Materials
2.2. Technological Processes
2.3. Analysis Methods
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivaram, N.M.; Barik, D. Toxic waste in leather industry. In Energy from Toxic Organic Waste for Heat and Power Generation, 1st ed.; Barik, D., Ed.; Woodhead Publishing: Sawston, UK, 2019; Volume 1, pp. 55–67. [Google Scholar]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Khambhaty, Y. Applications of enzymes in leather processing. Environ. Chem. Lett. 2020, 18, 747–769. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Jana, A.K.; Jha, M.K. Enzyme technology applications in leather processing. IJCT 2004, 11, 659–671. [Google Scholar]
- Schropfer, M.; Klurer, E.; Meyer, M. Influence of elastin degradation on the mechanical properties of leather. J. Am. Leather Chem. Assoc. 2014, 109, 306–313. [Google Scholar]
- Thanikaivelan, P.; Rao, J.R.; Nair, B.U.; Ramasami, T. Progress and recent trends in biotechnological methods for Leather Processing. Trends Biotechnol. 2004, 22, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, M.; Chattha, S.A.; Zhu, Y.; Peng, B.; Ye, Y. Application of acidic protease in the pickling to simplify the pelt bating process. J. Leather Sci. Eng. 2021, 3, 27. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Zeng, Y.; Shi, B. Effect of the surface charge of the acid protease on leather bating performance. Process Biochem. 2022, 121, 330–338. [Google Scholar] [CrossRef]
- Yongquan, L. Sheep-pelt bating with acid protease. J. Am. Leather Chem. Assoc. 2001, 96, 398–400. [Google Scholar]
- Sirvaityte, J.; Valeika, V.; Beleska, K.; Valeikiene, V. Bating of pelts after deliming with peracetic acid. Proc. Estonian Acad. Sci. Chem 2006, 55, 93–100. [Google Scholar]
- Griyanitasari, G.; Rahmawati, D.; Sugihartono; Erwanto, Y. Cleaner leather tanning process using gambir: The influence of rebating on the properties of leather. Rev. Piel. Incaltaminte 2019, 19, 217–226. [Google Scholar] [CrossRef]
- Nugraha, A.W.; Suparno, O.; Indrasti, N.S.; Hoerudin, H. The Properties of Wet Blue Added Crude Enzyme from Rhizopus oligosporus in the Acid Bating Process. Trop. Anim. Sci. J. 2022, 45, 104–111. [Google Scholar] [CrossRef]
- Song, Y.; Wu, S.; Yang, Q.; Liu, H.; Zeng, Y.; Shi, B. Factors affecting mass transfer of protease in pelt during enzymatic bating process. J. Leather Sci. Eng. 2019, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Biškauskaitė, R.; Valeikienė, V.; Valeika, V. Enzymes for leather processing: Effect on pickling and chroming. Materials 2021, 14, 1480. [Google Scholar] [CrossRef]
- Zhang, X.; Chattha, S.A.; Song, J.; Zhang, C.; Peng, B. An integrated pickling-bating technology for reducing ammonia-nitrogen and chloride pollution in leather manufacturing. J. Clean. Prod. 2022, 375, 134070. [Google Scholar] [CrossRef]
- Li, H.; Zhu, D.; Li, Y.; Cao, S.; Xiao, J. Analyzing the Mechanism and Effect of Acid Protease in Wet Blue Bating Process for Leather Production. J. Am. Leather Chem. Assoc. 2020, 115, 10–15. [Google Scholar] [CrossRef]
- Jayakumar, G.C.; Karthik, V.; Fathima, A.A.; Selvi, A.T.; Muralidharan, C.; Kanth, S.V. High Exhaustion System (HES) for Leather Process-235; FILK Freiberg Institute gGmbH: Freiberg, Germany, 2019. [Google Scholar]
- Zhang, X.; Wan, X.; Xian, J.; Peng, B. Enzymatic Baiting Technology for Wet Blue: I. Characterization of Protease Activities Towards Chrome-tanned Elastin and Collagen Fibers. J. Am. Leather Chem. Assoc. 2018, 113, 217–224. [Google Scholar]
- Standard USSR: GOST 20264-88; Enzyme Preparations. Methods of Determination of Proteolytic Activity. Izdatelstvo Standartov: Moscow, Russia, 1988. (In Russian)
- Xian, J.; Bu, D.Y.; Tian, Y.X.; Zhang, C.X.; Peng, B.Y.; Long, Z.Z. Properties and bating effects of trypsins from different sources. J. Leather Sci. Eng. 2020, 30, 40–46. [Google Scholar]
- Zaides, A.; Mikhailov, A.; Pushenko, O. Modiphitsirovanyi method opredeleniya oksiprolina. Biokhimiya 1964, 1, 5–6. (In Russian) [Google Scholar]
- Golovteeva, A.A.; Kutsidi, D.A.; Sankin, L.B. Laboratornyj Praktikum po Khimiyi i Tekhnologiyi Kozhy i Mekha; Legkaiya i Pischevaiya Prom: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- Pat. USSR 767649; Method of Determination of Leather Quality. VNIIPI: Moscow, Russia, 1980. (In Russian)
- Standard ISO 3376:2011; Leather Physical and Mechanical Tests Determination of Tensile Strength and Percentage Extension. ISO: Geneva, Switzerland, 2011.
- Standard ISO 17229:2016; IULTCS/IUP 42 Leather—Physical and mechanical tests—Determination of water vapour absorption. ISO: Geneva, Switzerland, 2016.
- Standard ISO 5398-2:2009; Leather Chemical Determination of Chromic Oxide Content Part 2: Quantification by Colorimetric Determination. ISO: Geneva, Switzerland, 2009.
- Standard ISO 4048:2008; Leather Chemical Tests Determination of Matter Soluble in Dichloromethane and Free Fatty Acid Content. ISO: Geneva, Switzerland, 2008.
- Lyu, B.; Cheng, K.; Ma, J.; Hou, X.; Gao, D.; Gao, H.; Zhang, J.; Qi, Y. A Cleaning and Efficient Approach to Improve Wet-Blue Sheepleather Quality by Enzymatic Degreasing. J. Clean. Prod. 2017, 148, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, E.; High, K.E. The Use of Infrared Spectroscopy and Chemometrics to Investigate Deterioration in Vegetable Tanned Leather: Potential Applications in Heritage Science. Herit. Sci. 2022, 10, 65. [Google Scholar] [CrossRef]
- Mehta, M.; Naffa, R.; Maidment, C.; Holmes, G.; Waterland, M. Raman and ATR-FTIR Spectroscopy towards Classification of Wet Blue Bovine Leather Using Ratiometric and Chemometric Analysis. J. Leather Sci. Eng. 2020, 2, 3. [Google Scholar] [CrossRef]
- Mohamed, O.A.; El Sayed, N.H.; Abdelhakim, A.A. Preparation and Characterization of Polyamide-Leather Wastes Polymer Composites. J. Appl. Polym. Sci. 2010, 118, 446–451. [Google Scholar] [CrossRef]
- Kohler, A.; Bertrand, D.; Martens, H.; Hannesson, K.; Kirschner, C.; Ofstad, R. Multivariate image analysis of a set of FTIR microspectroscopy images of aged bovine muscle tissue combining image and design information. Anal. Bioanal. Chem. 2007, 389, 1143–1153. [Google Scholar] [CrossRef]
- Sanden, K.W.; Kohler, A.; Afseth, N.K.; Böcker, U.; Rønning, S.B.; Liland, K.H.; Pedersen, M.E. The use of Fourier-transform infrared spectroscopy to characterize connective tissue components in skeletal muscle of Atlantic cod (Gadus morhua L.). J. Biophotonics 2019, 12, e201800436. [Google Scholar] [CrossRef] [Green Version]
- China, C.R.; Maguta, M.M.; Nyandoro, S.S.; Hilonga, A.; Kanth, S.V.; Njau, K.N. Alternative tanning technologies and their suitability in curbing environmental pollution from the leather industry: A comprehensive review. Chemosphere 2020, 254, 126804. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Kim, S.M.; Kim, H.R. Improvement of dye affinity in natural dyeing usingterminalia chebula retzius (T chebula) applied to leather. Int. J. Cloth. Sci. Technol. 2017, 29, 610–626. [Google Scholar] [CrossRef]
- Kanth, S.V.; Venba, R.; Jayakumar, G.C.; Chandrababu, N.K. Kinetics of leather dyeing pretreated with enzymes: Role of acid protease. Bioresour. Technol. 2009, 100, 2430–2435. [Google Scholar] [CrossRef]
- Kanth, S.V.; Venba, R.; Madhan, B.; Chandrababu, N.K.; Sadulla, S. Studies on the influence of bacterial collagenase in leather dyeing. Dye. Pigment. 2008, 76, 338–347. [Google Scholar] [CrossRef]
- Haroun, A. Evaluation of modified leather dyeing technique using black dyestuffs from the Economical View. Dye. Pigment. 2005, 67, 215–221. [Google Scholar] [CrossRef]
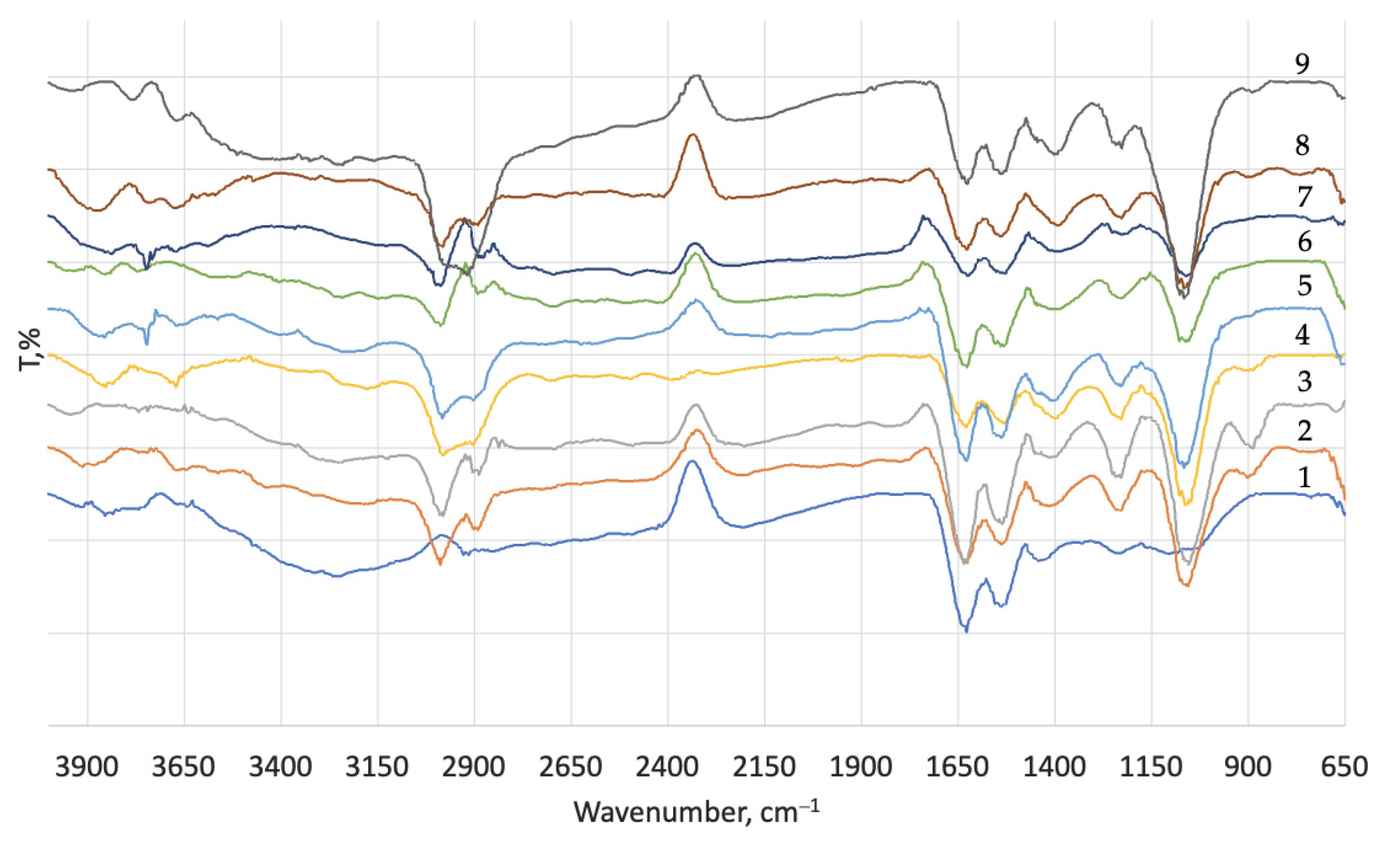

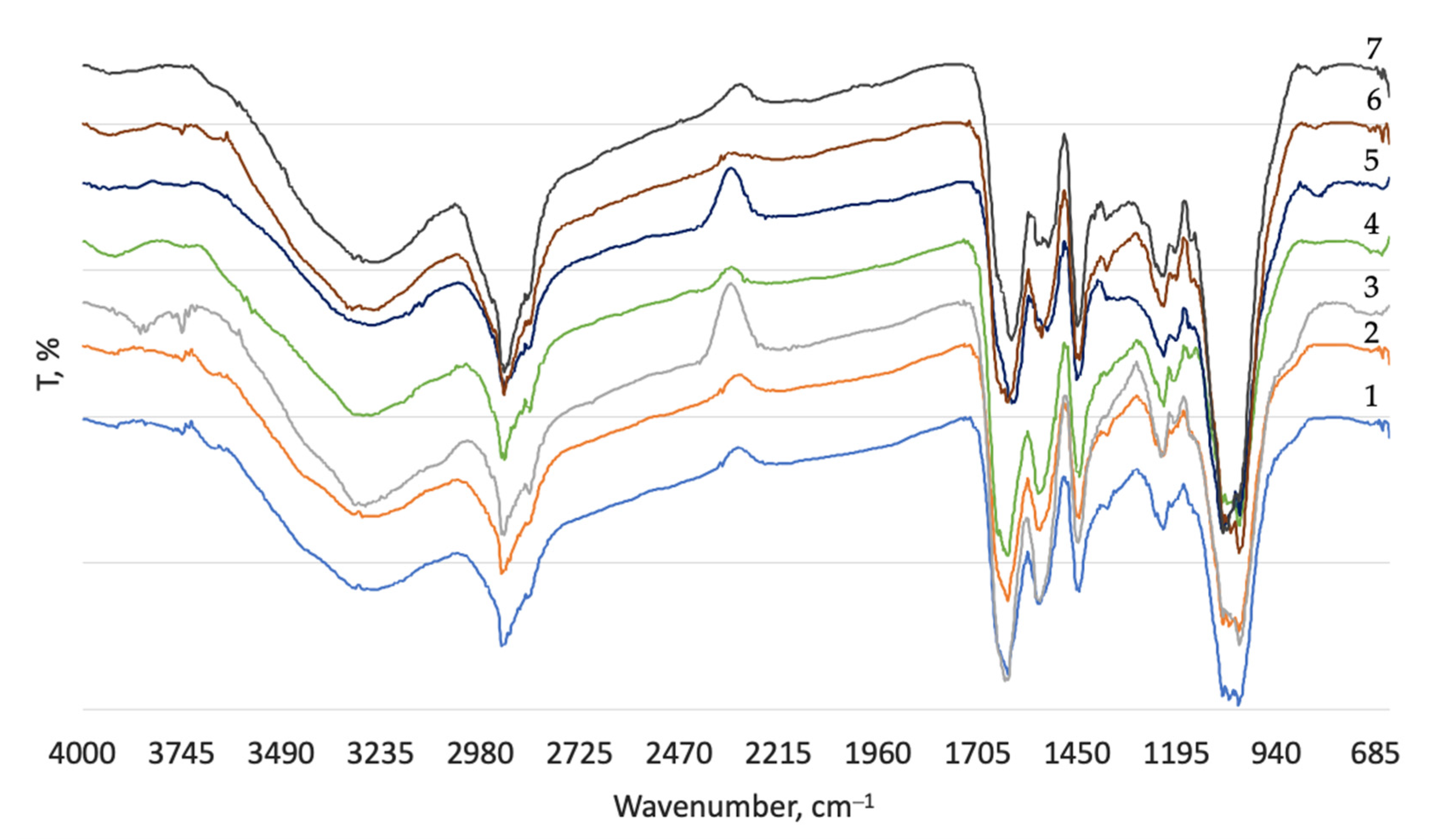
| Process | Materials 1, % | Process Duration, min | Process Temperature, °C |
|---|---|---|---|
| Washing | H2O–300 | 30 | 40 |
| Re-bating 2 | H2O–200; EP–1 or 5 | 60 or 210 | |
| Washing | H2O–100 | 15 | |
| Re-chroming | H2O–150 Chromeco 33 Extra–4 | 30 | |
| Neutragene MG-120–0.15 | 10 | ||
| Neutragene MG-120–0.15 | 50 | ||
| Washing | H2O–150 | 30 | |
| Neutralisation | H2O–150; NaHCO3–1.5 | 30 | |
| NaHCOO–2 | 90 | ||
| Washing | H2O–100 | 30 | |
| Washing | H2O–200 | 15 | 60 |
| Dyeing | Sellaset red H–4.5 | 60 | |
| Fat-liquoring | Oleal 146–2; Oleal 1946–4; Fospholiker 661–3; Fospholiker 6146–4 | 90 | |
| HCOOH–1 | 30 | ||
| Washing | H2O–200 | 15 | 30 |
| Re-tanning | H2O–100; Mimosa tannins–2; Quebracho tannins–2 | 60 | |
| Washing | H2O–100 | 15 |
| Enzyme Preparation | Indexes | ||
|---|---|---|---|
| Caseinolytic Activity, U/g | Collagenolytic Activity, U/g | Ratio of Caseinolytic and Collagenolytic Activities | |
| Zime SB | 161.73 ± 7.52 | 3.5 ± 0.16 | 46.2 |
| NovoBate WB | 700.70 ± 27.35 | 27.3 ± 1.17 | 25.7 |
| Oropon DVP | 8.54 ± 0.41 | 0.7 ± 0.02 | 12.2 |
| Oropon WB | 4.20 ± 0.17 | 2.8 ± 0.11 | 1.5 |
| Enzymatic Treatment | Indexes | ||||
|---|---|---|---|---|---|
| Variant Number | Enzyme Preparation | Amount of EP, % Based on Wet Blue Mass | Duration, Hours | Amount of Removed Collagen, g/kg Wet Blue | Shrinkage Temperature, °C |
| 1 | Zime SB | 1 | 1 | 0.014 ± 0.0004 | 119.7 ± 0.4 |
| 2 | NovoBate WB | 1 | 1 | 0.039 ± 0.001 | 118.0 ± 0.1 |
| 3 | Oropon DVP | 1 | 1 | 0.019 ± 0.0001 | 119.8 ± 0.3 |
| 4 | Oropon WB | 1 | 1 | 0.001 ± 0.0001 | 115.8 ± 0.3 |
| 5 | Zime SB | 5 | 1 | 0.051 ± 0.002 | 119.4 ± 0.3 |
| 6 | NovoBate WB | 5 | 1 | 0.106 ± 0.006 | 114.0 ± 0.1 |
| 7 | Oropon DVP | 5 | 1 | 0.018 ± 0.0001 | 117.7 ± 0.3 |
| 8 | Oropon WB | 5 | 1 | 0.002 ± 0.0001 | 116.0 ± 0.1 |
| 9 | Zime SB | 1 | 3.5 | 0.014 ± 0.0005 | 119.9 ± 0.3 |
| 10 | NovoBate WB | 1 | 3.5 | 0.039 ± 0.0015 | 116.0 ± 0.1 |
| 11 | Oropon DVP | 1 | 3.5 | 0.018 ± 0.0009 | 118.9 ± 0.3 |
| 12 | Oropon WB | 1 | 3.5 | 0.001 ± 0.00004 | 114.0 ± 0.1 |
| 13 | Zime SB | 5 | 3.5 | 0.059 ± 0.002 | 119.3 ± 0.3 |
| 14 | NovoBate WB | 5 | 3.5 | 0.128 ± 0.005 | 114.7 ± 0.4 |
| 15 | Oropon DVP | 5 | 3.5 | 0.020 ± 0.001 | 117.7 ± 0.4 |
| 16 | Oropon WB | 5 | 3.5 | 0.002 ± 0.0001 | 117.8 ± 0.3 |
| Control | - | - | - | - | 113.3 ± 0.6 |
| Re-Bating Variant | Relative Elongation of Leather at the Strain 10 N/mm2, % | Relative Elongation of Leather at the Break, % | Tensile Strength of Leather, N/mm2 | Water Vapour Absorption, g/mm2 |
|---|---|---|---|---|
| 1 | 25.74 ± 0.78 | 51.73 ± 1.21 | 26.10 ± 0.85 | 20.52 ± 0.69 |
| 2 | 26.14 ± 0.75 | 51.94 ± 2.07 | 25.86 ± 0.62 | 24.20 ± 1.18 |
| 3 | 27.21 ± 0.85 | 48.51 ± 2.11 | 23.00 ± 0.60 | 22.50 ± 0.58 |
| 4 | 26.57 ± 0.43 | 49.59 ± 0.70 | 25.32 ± 0.56 | 25.86 ± 1.21 |
| 5 | 26.15 ± 0.51 | 52.05 ± 2.11 | 26.04 ± 0.74 | 24.80 ± 1.12 |
| 6 | 26.06 ± 0.81 | 50.39 ± 2.24 | 25.53 ± 0.82 | 24.28 ± 0.64 |
| 7 | 26.71 ± 0.64 | 49.16 ± 2.05 | 24.93 ± 0.38 | 22.06 ± 0.37 |
| 8 | 26.53 ± 0.51 | 48.70 ± 2.34 | 24.96 ± 0.88 | 25.48 ± 1.00 |
| Control (without re-bating) | 27.08 ± 0.91 | 51.89 ± 1.01 | 25.47 ± 0.67 | 20.32 ± 0.37 |
| Re-Bating Variant | Indexes | ||
|---|---|---|---|
| Shrinkage Temperature, °C | Exhaustion of Chromium Compounds, % | Cr2O3 in Leather, % | |
| 1 | 123.8 ± 0.3 | 68.8 ± 0.4 | 6.78 ± 0.31 |
| 2 | 122.7 ± 0.4 | 66.4 ± 0.8 | 6.80 ± 0.24 |
| 3 | 123.1 ± 0.4 | 66.5 ± 0.3 | 6.66 ± 0.23 |
| 4 | 123.5 ± 0.5 | 67.1 ± 0.6 | 6.69 ± 0.31 |
| 5 | 124.1 ± 0.4 | 78.9 ± 0.3 | 6.78 ± 0.29 |
| 6 | 122.3 ± 0.6 | 67.6 ± 0.4 | 6.46 ± 0.13 |
| 7 | 123.0 ± 0.1 | 62.4 ± 0.8 | 6.39 ± 0.21 |
| 8 | 121.3 ± 0.3 | 63.5 ± 0.5 | 6.59 ± 0.32 |
| 9 | 123.2 ± 0.4 | 71.6 ± 0.2 | 6.89 ± 0.24 |
| 10 | 122.2 ± 0.6 | 70.5 ± 0.7 | 6.49 ± 0.18 |
| 11 | 118.4 ± 0.3 | 72.7 ± 0.3 | 6.54 ± 0.14 |
| 12 | 123.0 ± 0.1 | 66.9 ± 0.6 | 6.61 ± 0.21 |
| 13 | 123.1 ± 0.4 | 80.3 ± 0.7 | 6.77 ± 0.29 |
| 14 | 123.1 ± 0.4 | 68.9 ± 0.7 | 6.46 ± 0.18 |
| 15 | 123.2 ± 0.6 | 66.9 ± 0.9 | 6.68 ± 0.27 |
| 16 | 122.0 ± 0.1 | 62.7 ± 0.8 | 6.51 ± 0.21 |
| Control (without re-bating) | 123.0 ± 0.1 | 58.4 ± 0.6 | 6.34 ± 0.24 |
| Enzymatic Treatment Parameters | Indexes of Finished Leather | |||
|---|---|---|---|---|
| Variant Number | Enzyme Preparation | Amount of EP, % Based on Wet Blue Mass | Dye Consumption, % | Amount of Matter Soluble in Dichloromethane, % |
| 1 | Zime SB | 1 | 86.22 ± 3.73 | 9.98 ± 0.52 |
| 3 | Oropon DVP | 1 | 84.92 ± 4.14 | 9.13 ± 0.45 |
| 4 | Oropon WB | 1 | 83.42 ± 3.79 | 9.52 ± 0.47 |
| 5 | Zime SB | 5 | 82.02 ± 3.92 | 8.44 ± 0.42 |
| 7 | Oropon DVP | 5 | 84.92 ± 4.24 | 9.28 ± 0.46 |
| 8 | Oropon WB | 5 | 87.82 ± 2.33 | 8.67 ± 0.35 |
| Control (without re-bating) | - | - | 88.19 ± 4.09 | 9.40 ± 0.46 |
| Re-Bating Variant | Relative Elongation of Leather at the Strain 10 N/mm2, % | Relative Elongation of Leather at the Break, % | Tensile Strength of Leather, N/mm2 | Strain When Grain Layer Breaks, N/mm2 |
|---|---|---|---|---|
| 1 | 27.09 ± 1.21 | 61.34 ± 1.84 | 29.04 ± 1.47 | 25.92 ± 1.21 |
| 3 | 26.70 ± 1.14 | 56.53 ± 2.05 | 31.14 ± 1.43 | 23.62 ± 1.18 |
| 4 | 29.90 ± 1.30 | 69.59 ± 3.08 | 33.73 ± 1.54 | 24.75 ± 1.20 |
| 5 | 27.07 ± 1.33 | 64.44 ± 2.64 | 30.68 ± 1.49 | 22.08 ± 1.00 |
| 7 | 26.84 ± 1.28 | 64.18 ± 1.79 | 33.89 ± 1.63 | 20.28 ± 1.01 |
| 8 | 27.62 ± 1.36 | 61.72 ± 1.49 | 32.68 ± 1.61 | 25.56 ± 1.27 |
| Control (without re-bating) | 31.24 ± 1.56 | 65.96 ± 2.53 | 32.14 ± 1.59 | 21.85 ± 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biškauskaitė, R.; Valeika, V. Wet Blue Enzymatic Treatment and Its Effect on Leather Properties and Post-Tanning Processes. Materials 2023, 16, 2301. https://doi.org/10.3390/ma16062301
Biškauskaitė R, Valeika V. Wet Blue Enzymatic Treatment and Its Effect on Leather Properties and Post-Tanning Processes. Materials. 2023; 16(6):2301. https://doi.org/10.3390/ma16062301
Chicago/Turabian StyleBiškauskaitė, Renata, and Virgilijus Valeika. 2023. "Wet Blue Enzymatic Treatment and Its Effect on Leather Properties and Post-Tanning Processes" Materials 16, no. 6: 2301. https://doi.org/10.3390/ma16062301





