Chitins from Seafood Waste as Sustainable Porous Carbon Precursors for the Development of Eco-Friendly Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biocarbon Derived from Fish Waste (Prawn and Squid Chitins) Preparation
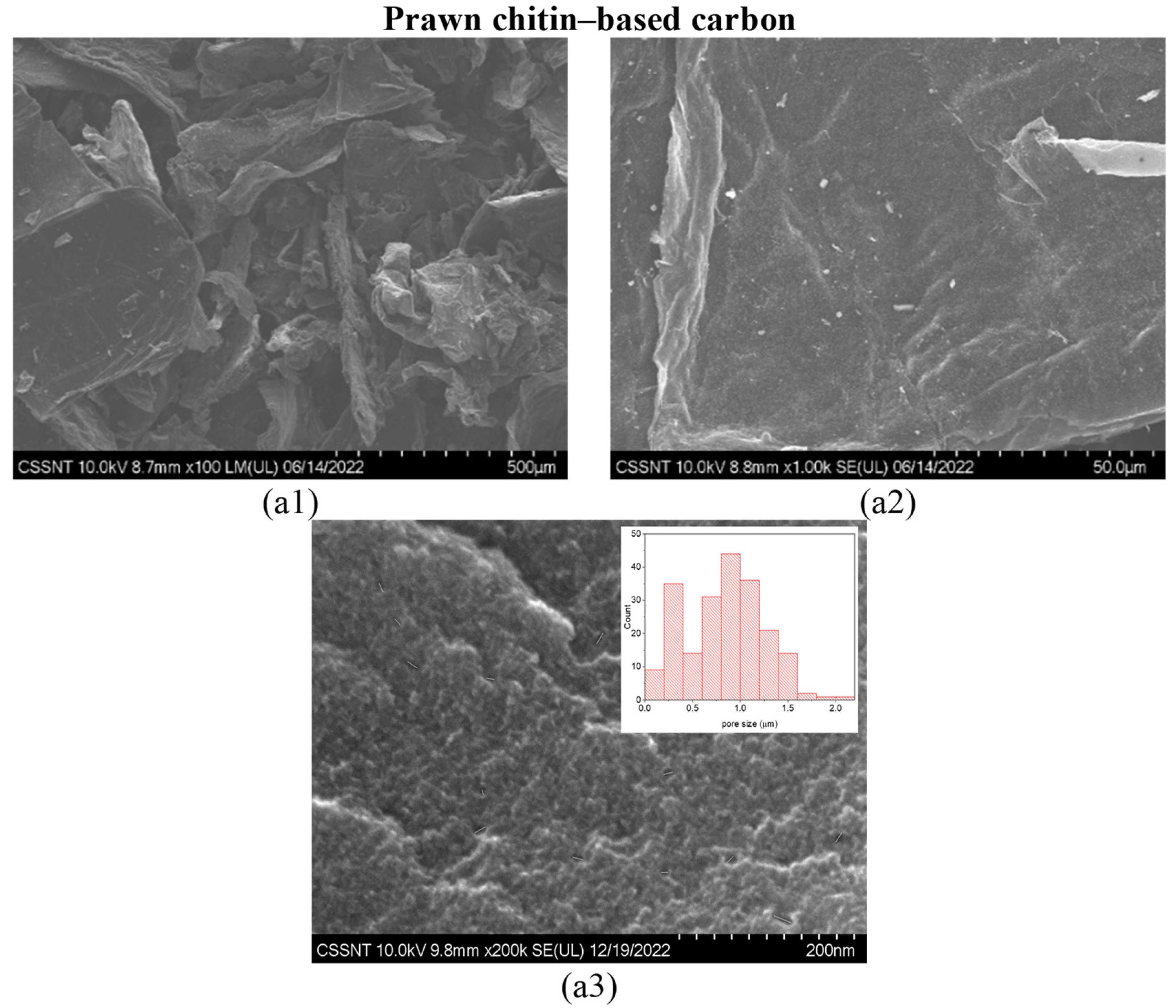
2.2. Morphological Characterization of the Carbon Materials
2.3. Chemical Activation of Chitin-Based Carbons with NaOH
2.4. Electrochemical Studies
3. Results
3.1. Structural Characteristics
3.2. XPS (X-ray Photoelectron Spectroscopy)
3.3. TEM (Transmission Electron Microscopy)
3.4. ATR-FTIR and Raman Spectra Analysis
3.5. XRD Analysis
3.6. BET Analysis
3.7. Electrochemical Characterization
3.7.1. Cyclic Voltammetry and Galvanostatic Charge–Discharge (GCD) Analysis
3.7.2. EIS Analysis
3.8. Preliminary Studies: Carbon Activation and Aqueous Electrolytes Effect on the Capacitance
3.8.1. Chitin-Based Carbon Activation with NaOH
3.8.2. Aqueous Electrolytes’ Effect on the Capacitance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Harindintwali, J.D.; Yuan, Z. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Stevic, Z.; Radovanovic, I. Supercapacitors: The Innovation of Energy Storage. In Updates on Supercapacitors; Stevic, D.Z.M., Ed.; IntechOpen: Rijeka, Croatia, 2022; p. 5. ISBN 978-1-83962-642-5. [Google Scholar]
- Zhou, Q.; Yao, H. Recent development of carbon electrode materials for electrochemical supercapacitors. Energy Rep. 2022, 8, 656–661. [Google Scholar] [CrossRef]
- Al-Shetwi, A.Q. Sustainable development of renewable energy integrated power sector: Trends, environmental impacts, and recent challenges. Sci. Total Environ. 2022, 822, 153645. [Google Scholar] [CrossRef] [PubMed]
- Akadiri, P.O.; Chinyio, E.A.; Olomolaiye, P.O. Design of A Sustainable Building: A Conceptual Framework for Implementing Sustainability in the Building Sector. Buildings 2012, 2, 126–152. [Google Scholar] [CrossRef] [Green Version]
- Gaddam, R.R.; Yang, D.; Narayan, R.; Raju, K.; Kumar, N.A.; Zhao, X.S. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy 2016, 26, 346–352. [Google Scholar] [CrossRef] [Green Version]
- De, S.; Acharya, S.; Sahoo, S.; Chandra Nayak, G. Chapter 12—Present status of biomass-derived carbon-based composites for supercapacitor application. In Functional, and Flexible Materials for Energy Conversion and Storage Systems; Pandikumar, A., Rameshkumar, P.B.T.-N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–415. ISBN 978-0-12-819552-9. [Google Scholar]
- Parameswaran, I.; Sampath, V.; Kumar, V.; Sathapathi, S. Extraction of Chitin and Chitosan from Prawn Shell Waste. Bull. Environ. Pharmacol. Life Sci. 2022, 1, 557–562. [Google Scholar]
- Majid, S.; Ali, A.S.G.; Cao, W.Q.; Reza, R.; Ge, Q. Biomass-derived porous carbons as supercapacitor electrodes—A review. Xinxing Tan Cailiao/New Carbon Mater. 2021, 36, 546–572. [Google Scholar]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal carbonization as a valuable tool for energy and environmental applications: A review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Shanmuga Priya, M.; Divya, P.; Rajalakshmi, R. A review status on characterization and electrochemical behaviour of biomass derived carbon materials for energy storage supercapacitors. Sustain. Chem. Pharm. 2020, 16, 100243. [Google Scholar] [CrossRef]
- Berktas, I.; Hezarkhani, M.; Haghighi Poudeh, L.; Saner Okan, B. Recent developments in the synthesis of graphene and graphene-like structures from waste sources by recycling and upcycling technologies: A review. Graphene Technol. 2020, 5, 59–73. [Google Scholar] [CrossRef]
- Kamal, A.S.; Othman, R.; Jabarullah, N.H. Preparation and synthesis of synthetic graphite from biomass waste: A review. Syst. Rev. Pharm. 2020, 11, 881–894. [Google Scholar]
- Reis, G.S.; Larsson, S.H.; de Oliveira, H.P.; Thyrel, M.; Claudio Lima, E. Sustainable Biomass Activated Carbons as Electrodes for Battery and Supercapacitors—A Mini-Review. Nanomaterials 2020, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Elisadiki, J.; Kibona, T.E.; Machunda, R.L.; Saleem, M.W.; Kim, W.; Jande, Y.A.C.; Kim, W. Biomass-based carbon electrode materials for capacitive deionization: A review. Biomass Convers. Biorefinery 2020, 10, 1327–1356. [Google Scholar] [CrossRef]
- Lionetto, F.; Bagheri, S.; Mele, C. Sustainable materials from fish industry waste for electrochemical energy systems. Energies 2021, 14, 7928. [Google Scholar] [CrossRef]
- Liu, M.; Niu, J.; Zhang, Z.; Dou, M.; Wang, F. Potassium compound-assistant synthesis of multi-heteroatom doped ultrathin porous carbon nanosheets for high performance supercapacitors. Nano Energy 2018, 51, 366–372. [Google Scholar] [CrossRef]
- Raj, C.J.; Rajesh, M.; Manikandan, R.; Yu, K.H.; Anusha, J.R.; Ahn, J.H.; Kim, D.-W.; Park, S.Y.; Kim, B.C. High electrochemical capacitor performance of oxygen and nitrogen enriched activated carbon derived from the pyrolysis and activation of squid gladius chitin. J. Power Sources 2018, 386, 66–76. [Google Scholar] [CrossRef]
- Shan, B.; Cui, Y.; Liu, W.; Zhang, Y.; Liu, S.; Wang, H.; Sun, L.; Wang, Z.; Wu, R. Fibrous Bio-Carbon Foams: A New Material for Lithium-Ion Hybrid Supercapacitors with Ultrahigh Integrated Energy/Power Density and Ultralong Cycle Life. ACS Sustain. Chem. Eng. 2018, 6, 14989–15000. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Li, Z. Unusual interconnected graphitized carbon nanosheets as the electrode of high-rate ionic liquid-based supercapacitor. Carbon N. Y. 2017, 119, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Qu, J.; Zhao, Z.; Wang, Z.; Qiu, J. Nitrogen-doped activated carbon derived from prawn shells for high-performance supercapacitors. Electrochim. Acta 2016, 190, 1134–1141. [Google Scholar] [CrossRef]
- Wang, J.; Shen, L.; Xu, Y.; Dou, H.; Zhang, X. Lamellar-structured biomass-derived phosphorus- and nitrogen-co-doped porous carbon for high-performance supercapacitors. New J. Chem. 2015, 39, 9497–9503. [Google Scholar] [CrossRef]
- Liu, H.-J.; Wang, X.-M.; Cui, W.-J.; Dou, Y.-Q.; Zhao, D.-Y.; Xia, Y.-Y. Highly ordered mesoporous carbon nanofiber arrays from a crab shell biological template and its application in supercapacitors and fuel cells. J. Mater. Chem. 2010, 20, 4223–4230. [Google Scholar] [CrossRef]
- Vinodh, R.; Sasikumar, Y.; Kim, H.J.; Atchudan, R.; Yi, M. Chitin and chitosan based biopolymer derived electrode materials for supercapacitor applications: A critical review. J. Ind. Eng. Chem. 2021, 104, 155–171. [Google Scholar] [CrossRef]
- Ikram, R.; Jan, B.M.; Qadir, M.A.; Sidek, A.; Stylianakis, M.M.; Kenanakis, G. Recent advances in chitin and chitosan/graphene-based bio-nanocomposites for energetic applications. Polymers 2021, 13, 3226. [Google Scholar] [CrossRef]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and Chitosan Based Composites for Energy and Environmental Applications: A Review. Waste Biomass Valorization 2021, 12, 4777–4804. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Tian, Y.; Sun, Z.; Huang, Z.; Wu, X.; Li, B. Heteroatoms-doped hierarchical porous carbon derived from chitin for flexible all-solid-state symmetric supercapacitors. Chem. Eng. J. 2020, 384, 123263. [Google Scholar] [CrossRef]
- Pandey, G.P.; Hashmi, S.A. Studies on electrical double layer capacitor with a low-viscosity ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate as electrolyte. Bull. Mater. Sci. 2013, 36, 729–733. [Google Scholar] [CrossRef] [Green Version]
- Laheäär, A.; Arenillas, A.; Béguin, F. Change of self-discharge mechanism as a fast tool for estimating long-term stability of ionic liquid based supercapacitors. J. Power Sources 2018, 396, 220–229. [Google Scholar] [CrossRef]
- Timperman, L.; Vigeant, A.; Anouti, M. Eutectic mixture of Protic Ionic Liquids as an Electrolyte for Activated Carbon-Based Supercapacitors. Electrochim. Acta 2015, 155, 164–173. [Google Scholar] [CrossRef]
- Tuhania, P.; Singh, P.K.; Bhattacharya, B.; Dhapola, P.S. PVDF-HFP and 1-ethyl-3-methylimidazolium thiocyanate—Doped polymer electrolyte for efficient supercapacitors. High Perform. Polym. 2018, 30, 911–917. [Google Scholar] [CrossRef]
- David, M. Applications of Ionic Liquids in Polymer Science and Technology, 1st ed.; Springer Berlin: Berlin/Heidelberg, Germany, 2015; ISBN 9783662449035. [Google Scholar]
- Abbott, A.P.; Ballantyne, A.; Harris, R.C.; Juma, J.A.; Ryder, K.S.; Forrest, G. A Comparative Study of Nickel Electrodeposition Using Deep Eutectic Solvents and Aqueous Solutions. Electrochim. Acta 2015, 176, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.; Aldous, L.; Borisenko, N.; Coles, S.; Fontaine, O.; Gamarra Garcia, J.D.; Gardas, R.; Hammond, O.; Hardwick, L.J.; Haumesser, P.H.; et al. Electrochemistry: General discussion. Faraday Discuss. 2018, 206, 405–426. [Google Scholar] [CrossRef]
- Brandão, A.T.S.C.; Costa, R.; Silva, A.F.; Pereira, C.M. Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte. Nanomaterials 2021, 11, 3258. [Google Scholar] [CrossRef] [PubMed]
- Brandão, A.T.S.C.; Rosoiu, S.; Costa, R.; Silva, A.F.; Anicai, L.; Enachescu, M.; Pereira, C.M. Characterization of Carbon Nanomaterials Dispersions: Can Metal Decoration of MWCNTs Improve Their Physicochemical Properties? Nanomaterials 2022, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Brandão, A.T.S.C.; Rosoiu, S.; Costa, R.; Lazar, O.A.; Silva, A.F.; Anicai, L.; Pereira, C.M.; Enachescu, M. Characterization and electrochemical studies of MWCNTs decorated with Ag nanoparticles through pulse reversed current electrodeposition using a deep eutectic solvent for energy storage applications. J. Mater. Res. Technol. 2021, 15, 342–359. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Ramos, P.; Mirón, J.; Valcarcel, J.; Sotelo, C.G.; Pérez-Martín, R.I. Production of Chitin from Penaeus vannamei By-Products to Pilot Plant Scale Using a Combination of Enzymatic and Chemical Processes and Subsequent Optimization of the Chemical Production of Chitosan by Response Surface Methodology. Mar. Drugs 2017, 15, 180. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Noriega, D.; Ramos, P.; Valcarcel, J.; Novoa-Carballal, R.; Pastrana, L.; Reis, R.L.; Pérez-Martín, R.I. Optimization of high purity chitin and chitosan production from Illex argentinus pens by a combination of enzymatic and chemical processes. Carbohydr. Polym. 2017, 174, 262–272. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Salomé, S.; Pereira, N.M.; Ferreira, E.S.; Pereira, C.M.; Silva, A.F. Tin electrodeposition from choline chloride based solvent: Influence of the hydrogen bond donors. J. Electroanal. Chem. 2013, 703, 80–87. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, J.; Wang, L. Magnetic N-doped carbon aerogel from sodium carboxymethyl cellulose/collagen composite aerogel for dye adsorption and electrochemical supercapacitor. Int. J. Biol. Macromol. 2018, 115, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Preparation of Activated Carbon-SnO2, TiO2, and WO3 Catalysts. Study by FT-IR Spectroscopy. Ind. Eng. Chem. Res. 2016, 55, 5200–5206. [Google Scholar] [CrossRef]
- Al Bahri, M.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J. Activated carbon from grape seeds upon chemical activation with phosphoric acid: Application to the adsorption of diuron from water. Chem. Eng. J. 2012, 203, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Luo, Y.; Li, D.; Chen, Y.; Sun, X.; Cao, Q.; Liu, X. The performance of phosphoric acid in the preparation of activated carbon-containing phosphorus species from rice husk residue. J. Mater. Sci. 2019, 54, 5008–5021. [Google Scholar] [CrossRef]
- Zhang, S.; Su, Y.; Zhu, S.; Zhang, H.; Zhang, Q. Effects of pretreatment and FeCl3 preload of rice husk on synthesis of magnetic carbon composites by pyrolysis for supercapacitor application. J. Anal. Appl. Pyrolysis 2018, 135, 22–31. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ruiz, B.; Martínez-Blanco, D.; Sánchez-Arenillas, M.; Diez, M.A.; Marco, J.F.; Gorria, P.; Fuente, E. Towards advanced industrial waste-based magnetic activated carbons with tunable chemical, textural and magnetic properties. Appl. Surf. Sci. 2021, 551, 149407. [Google Scholar] [CrossRef]
- Hu, S.C.; Cheng, J.; Wang, W.P.; Sun, G.T.; Hu, L.L.; Zhu, M.Q.; Huang, X.H. Structural changes and electrochemical properties of lacquer wood activated carbon prepared by phosphoric acid-chemical activation for supercapacitor applications. Renew. Energy 2021, 177, 82–94. [Google Scholar] [CrossRef]
- Flygare, M.; Svensson, K. Quantifying crystallinity in carbon nanotubes and its influence on mechanical behaviour. Mater. Today Commun. 2019, 18, 39–45. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.; Kasuga, T.; Nogi, M.; Koga, H. Chitin-derived-carbon nanofibrous aerogel with anisotropic porous channels and defective carbon structures for strong microwave absorption. Chem. Eng. J. 2022, 450, 137943. [Google Scholar] [CrossRef]
- Wang, L.; Mu, G.; Tian, C.; Sun, L.; Zhou, W.; Yu, P.; Yin, J.; Fu, H. Porous graphitic carbon nanosheets derived from cornstalk biomass for advanced supercapacitors. ChemSusChem 2013, 6, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Stephan, A.M.; Kumar, T.P.; Ramesh, R.; Thomas, S.; Jeong, S.K.; Nahm, K.S. Pyrolitic carbon from biomass precursors as anode materials for lithium batteries. Mater. Sci. Eng. A 2006, 430, 132–137. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Yang, K.; Zhu, L.; Yang, C.; Zhang, H. Bio-inspired beehive-like hierarchical nanoporous carbon derived from bamboo-based industrial by-product as a high performance supercapacitor electrode material. J. Mater. Chem. A 2015, 3, 5656–5664. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379–386. [Google Scholar] [CrossRef]
- Kubicka, M.; Bakierska, M.; Chudzik, K.; Rutkowska, M.; Pacek, J.; Molenda, M. Electrochemical Properties and Structure Evolution of Starch-Based Carbon Nanomaterials as Li-Ion Anodes with Regard to Thermal Treatment. Polymers 2019, 11, 1527. [Google Scholar] [CrossRef] [Green Version]
- Örkün, Y.; Karatepe, N.; Yavuz, R. Influence of temperature and impregnation ratio of H3PO4 on the production of activated carbon from hazelnut shell. Acta Phys. Pol. A 2012, 121, 277–280. [Google Scholar] [CrossRef]
- Wang, A.; Sun, K.; Xu, R.; Sun, Y.; Jiang, J. Cleanly synthesizing rotten potato-based activated carbon for supercapacitor by self-catalytic activation. J. Clean. Prod. 2021, 283, 125385. [Google Scholar] [CrossRef]
- Yakaboylu, G.A.; Jiang, C.; Yumak, T.; Zondlo, J.W.; Wang, J.; Sabolsky, E.M. Engineered hierarchical porous carbons for supercapacitor applications through chemical pretreatment and activation of biomass precursors. Renew. Energy 2021, 163, 276–287. [Google Scholar] [CrossRef]
- Heimböckel, R.; Hoffmann, F.; Fröba, M. Insights into the influence of the pore size and surface area of activated carbons on the energy storage of electric double layer capacitors with a new potentially universally applicable capacitor model. Phys. Chem. Chem. Phys. 2019, 21, 3122–3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taer, E.; Agustino, A.; Farma, R.; Taslim, R.; Awitdrus; Paiszal, M.; Ira, A.; Yardi, S.D.; Sari, Y.P.; Yusra, H.; et al. The relationship of surface area to cell capacitance for monolith carbon electrode from biomass materials for supercapacitor aplication. J. Phys. Conf. Ser. 2018, 1116, 032040. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, L.; Abbas, M.A.; Bang, J.H. The influence of surface area, porous structure, and surface state on the supercapacitor performance of titanium oxynitride: Implications for a nanostructuring strategy. Phys. Chem. Chem. Phys. 2017, 19, 21140–21151. [Google Scholar] [CrossRef]
- Lockett, V.; Sedev, R.; Ralston, J.; Horne, M.; Rodopoulos, T. Differential Capacitance of the Electrical Double Layer in Imidazolium-Based Ionic Liquids: Influence of Potential, Cation Size, and Temperature. J. Phys. Chem. C 2008, 112, 7486–7495. [Google Scholar] [CrossRef]
- Figueiredo, M.; Gomes, C.; Costa, R.; Martins, A.; Pereira, C.M.; Silva, F. Differential capacity of a deep eutectic solvent based on choline chloride and glycerol on solid electrodes. Electrochim. Acta 2009, 54, 2630–2634. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon N. Y. 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Kasprzak, D.; Galiński, M. Chitin as a universal and sustainable electrode binder for electrochemical capacitors. J. Power Sources 2023, 553, 232300. [Google Scholar] [CrossRef]
- Brandão, A.T.S.C.; Costa, R.; Silva, A.F.; Pereira, C.M. Hydrogen Bond Donors Influence on the Electrochemical Performance of Composite Graphene Electrodes/Deep Eutectic Solvents Interface. Electrochem 2022, 3, 129–142. [Google Scholar] [CrossRef]
- Perez-Salcedo, K.Y.; Ruan, S.; Su, J.; Shi, X.; Kannan, A.M.; Escobar, B. Seaweed-derived KOH activated biocarbon for electrocatalytic oxygen reduction and supercapacitor applications. J. Porous Mater. 2020, 27, 959–969. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Ong, W.K.; Lu, X. Ultrafast-Freezing-Assisted Mild Preparation of Biomass-Derived, Hierarchically Porous, Activated Carbon Aerogels for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 403–411. [Google Scholar] [CrossRef]
- Usha Rani, M.; Nanaji, K.; Rao, T.N.; Deshpande, A.S. Corn husk derived activated carbon with enhanced electrochemical performance for high-voltage supercapacitors. J. Power Sour. 2020, 471, 228387. [Google Scholar] [CrossRef]










| At% | ||||
|---|---|---|---|---|
| Chitin-Based Carbons | Raw Material | |||
| Element | Squid Chitin | Prawn Chitin | Squid Chitin | Prawn Chitin |
| C1s | 87.1 | 78.4 | 59.8 | 51.4 |
| N1s | 2.2 | 2.1 | 0.91 | 1.1 |
| O1s | 9.0 | 14.3 | 37.5 | 43.4 |
| Na1s | 0.3 | 0.7 | - | - |
| P2p | 0.3 | 0.8 | 0.52 | 0.5 |
| S2p | 0.06 | 0.05 | - | - |
| K2p | 0.5 | 2.3 | 0.62 | 2.4 |
| Ca2p | 0.6 | 1.4 | 0.65 | 1.2 |
| Carbon Source | R2 | ID/IG | La/nm |
|---|---|---|---|
| Prawn chitin | 0.99 | 1.68 ± 0.01 | 12 |
| Squid chitin | 0.98 | 1.59 ± 0.02 | 11 |
| No. | 2θ (Deg) | d (Å) | FWHM (Deg) | Int. I (Counts Deg) | |
|---|---|---|---|---|---|
| Squid chitin-based carbon | 1 | 8.2 | 10.6 | 9.6 | 4579.1 |
| 2 | 24.1 | 3.7 | 9.6 | 563.5 | |
| 3 | 44.7 | 2.0 | 9.6 | 462.3 | |
| Prawn chitin-based carbon | 1 | 9.0 | 9.8 | 0.1 | 66.5 |
| 2 | 24.4 | 3.6 | 0.1 | 43.1 | |
| 3 | 43.7 | 2.1 | 0.1 | 7.1 |
| BET Isotherms Analysis | |||||
|---|---|---|---|---|---|
| Carbon Source | SBET (m2 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Vtotal (cm3 g−1) | Dp (Å) |
| Prawn chitin | 85.0 | 0.029 | 0.009 | 0.038 | 8.47 |
| Squid chitin | 149.3 | 0.053 | 0.059 | 0.112 | 7.12 |
| Carbonization | Electrochemistry 30 °C Ethaline | ||||
|---|---|---|---|---|---|
| Carbon Precursor | Temperature/°C | Time/h | C/F g−1 1st Cycle | % Retention after 1000th Cycle | % Retention after 5000th Cycle |
| Squid Chitin | 1000 | 1 | 20 ± 1 | 95.7 | 93.3 |
| Prawn Chitin | 15 ± 2 | 92.1 | 84.1 | ||
| BET Isotherms Analysis | |||||
|---|---|---|---|---|---|
| Carbon Source | SBET (m2 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Vtotal (cm3 g−1) | Dp (Å) |
| Prawn Chitin_NaOH activation | 86.2 | 0.021 | 0.011 | 0.032 | 1.110 |
| Squid Chitin_NaOH activation | 154.2 | 0.066 | 0.071 | 0.137 | 0.981 |
| Carbonization | Electrochemistry 30 °C Ethaline | ||||
|---|---|---|---|---|---|
| Carbon Precursor | Temperature/°C | Time/h | C (F g−1) 1st cycle | % Retention after 1000th Cycle | % Retention after 5000th Cycle |
| Squid Chitin_NaOH activation | 1000 | 1 | 32 ± 4 | 93.9 | 88.1 |
| Prawn Chitin_NaOH activation | 29 ± 3 | 90.2 | 79.4 | ||
| Marine Waste Source | Material | Configuration | Surface Area m2 g−1 | Electrolyte | Current Density A g−1 | Capacitance F g−1 | Capacitance Retention % | Reference |
|---|---|---|---|---|---|---|---|---|
| Squid chitin (Illex argentinus) byproducts from the industrial processing | Porous carbon | 3 electrode- cell (Active mass: 4 mg) (electrode diameter: 4 mm) | 149 | Liquid DES | 1 | 20 ± 1 | 96 @ 1000 cycles/93.3 @ 5000 cycles | This work |
| Porous carbon NaOH activation | 154 | 32 ± 4 | 93 @ 1000 cycles/88.1 @ 5000 cycles | |||||
| Porous carbon | 149 | 1 mol L−1 H2SO4 | 23 ± 2 | 86 @ 1000 cycles/45 @ 5000 cycles | ||||
| 149 | 1 mol L−1 KOH | 12 ± 3 | 80 @ 1000 cycles/33 @ 5000 cycles | |||||
| Prawn chitin (Penaeus vannamei) byproducts from the industrial processing | Porous carbon | 85 | Liquid DES | 15 ± 2 | 92 @ 1000 cycles/84.1@ 5000 cycles | |||
| Porous carbon NaOH activation | 86 | 29 ± 3 | 94 @ 1000 cycles/79.4 @ 5000 cycles | |||||
| Prawn shells “Bohai prawn” | N- activated carbon | 2 electrode “sandwich” cell (Active mass: 3–4 mg) | 1918 | 1 mol L−1 H2SO4 6 mol L−1 KOH | 0.05 | 695 357 | 95 @ 5000 cycles | [22] |
| Shrimp shells chitin (α-Chitin from BioLog) | Porous carbon | Swagelok®-type cell (3-electrode–cell) (electrode diameter: 11.8 mm) | 1298 | 1 mol L−1 Li2SO4 | 15 | 142 | 92 | [71] |
| Gladius of Squid fish (Todarodes pacificus) | N- and O- activated carbon | Stainless-steel split test cell (EQ-STC) (2 electrode–cell) (Active mass: 2.5 mg) | 1129 | 1 mol L−1 H2SO4 | 1 | 204 | 100 @ 20,000 cycles | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, A.T.S.C.; Costa, R.; State, S.; Potorac, P.; Dias, C.; Vázquez, J.A.; Valcarcel, J.; Silva, A.F.; Enachescu, M.; Pereira, C.M. Chitins from Seafood Waste as Sustainable Porous Carbon Precursors for the Development of Eco-Friendly Supercapacitors. Materials 2023, 16, 2332. https://doi.org/10.3390/ma16062332
Brandão ATSC, Costa R, State S, Potorac P, Dias C, Vázquez JA, Valcarcel J, Silva AF, Enachescu M, Pereira CM. Chitins from Seafood Waste as Sustainable Porous Carbon Precursors for the Development of Eco-Friendly Supercapacitors. Materials. 2023; 16(6):2332. https://doi.org/10.3390/ma16062332
Chicago/Turabian StyleBrandão, Ana T. S. C., Renata Costa, Sabrina State, Pavel Potorac, Catarina Dias, José A. Vázquez, Jesus Valcarcel, A. Fernando Silva, Marius Enachescu, and Carlos M. Pereira. 2023. "Chitins from Seafood Waste as Sustainable Porous Carbon Precursors for the Development of Eco-Friendly Supercapacitors" Materials 16, no. 6: 2332. https://doi.org/10.3390/ma16062332










