Repairing Annulus Fibrosus Fissures Using Methacrylated Gellan Gum Combined with Novel Silk
Abstract
1. Introduction
2. Materials and Methods
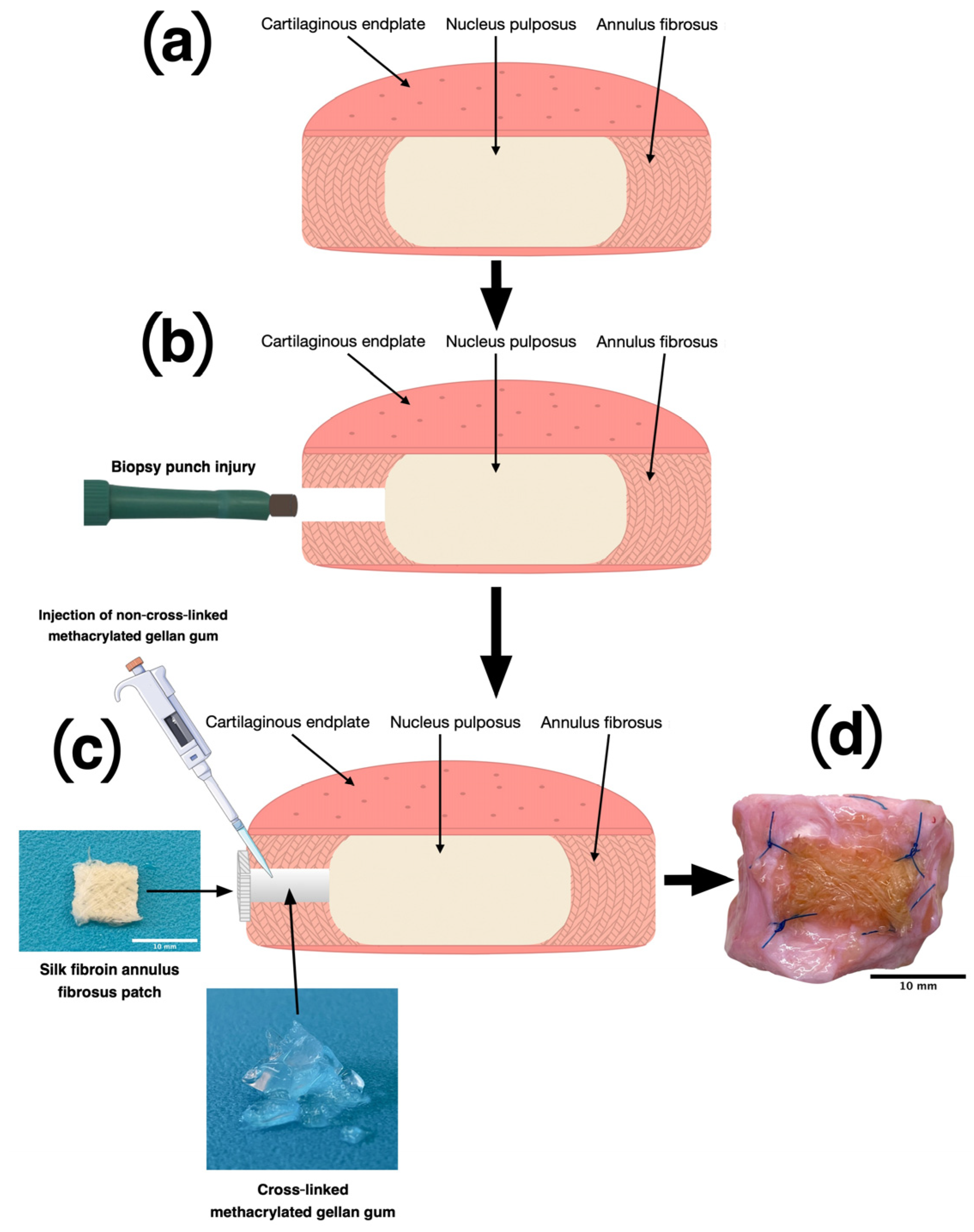
2.1. Dissection of Bovine IVDs
2.2. Assembly of the Embroidered Silk Yarn Patches
2.3. Production of Methacrylated Gellan Gum
2.4. AF Damage and Repair
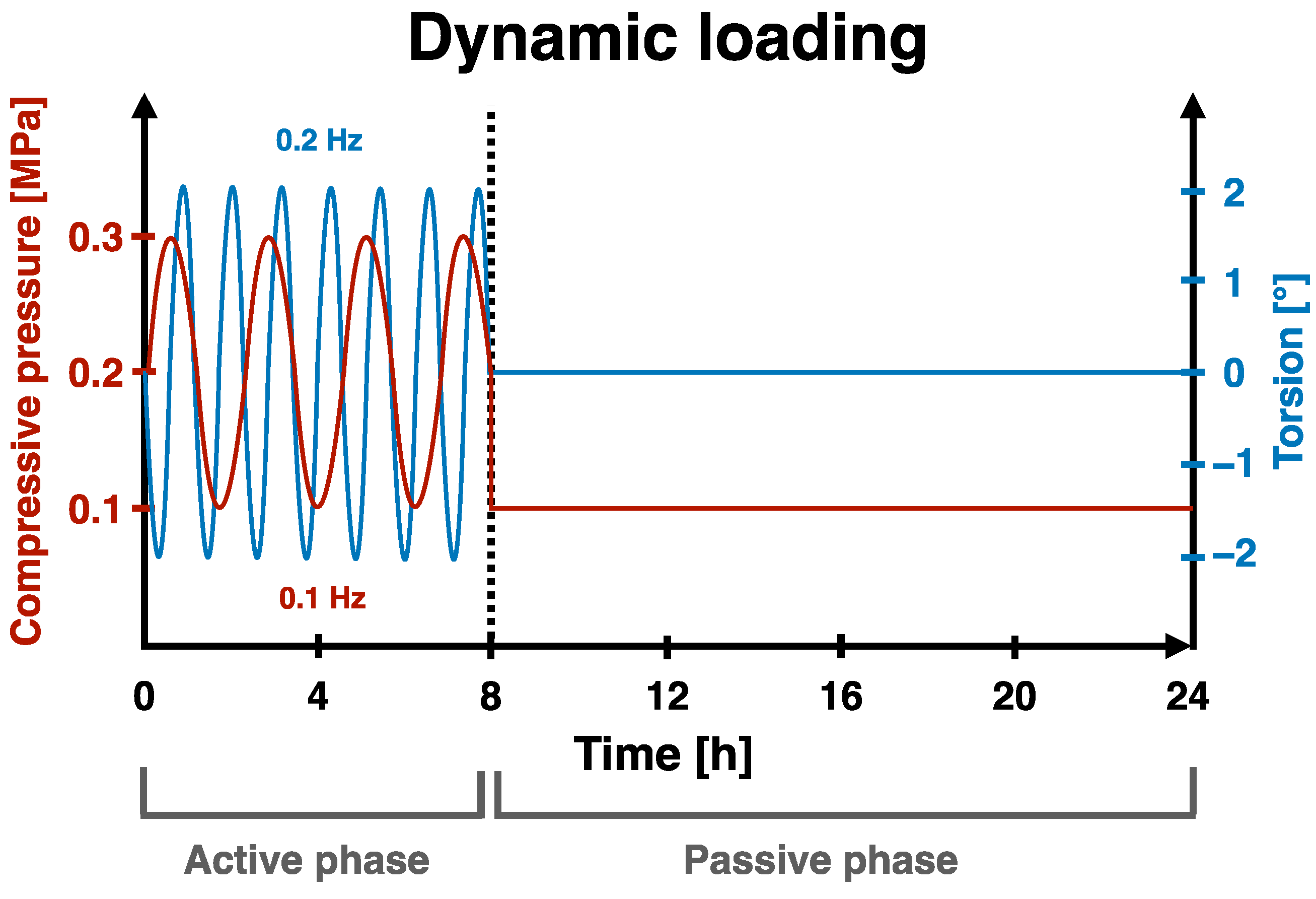
2.5. Organ Culture
2.6. Tissue Activity and Digestion
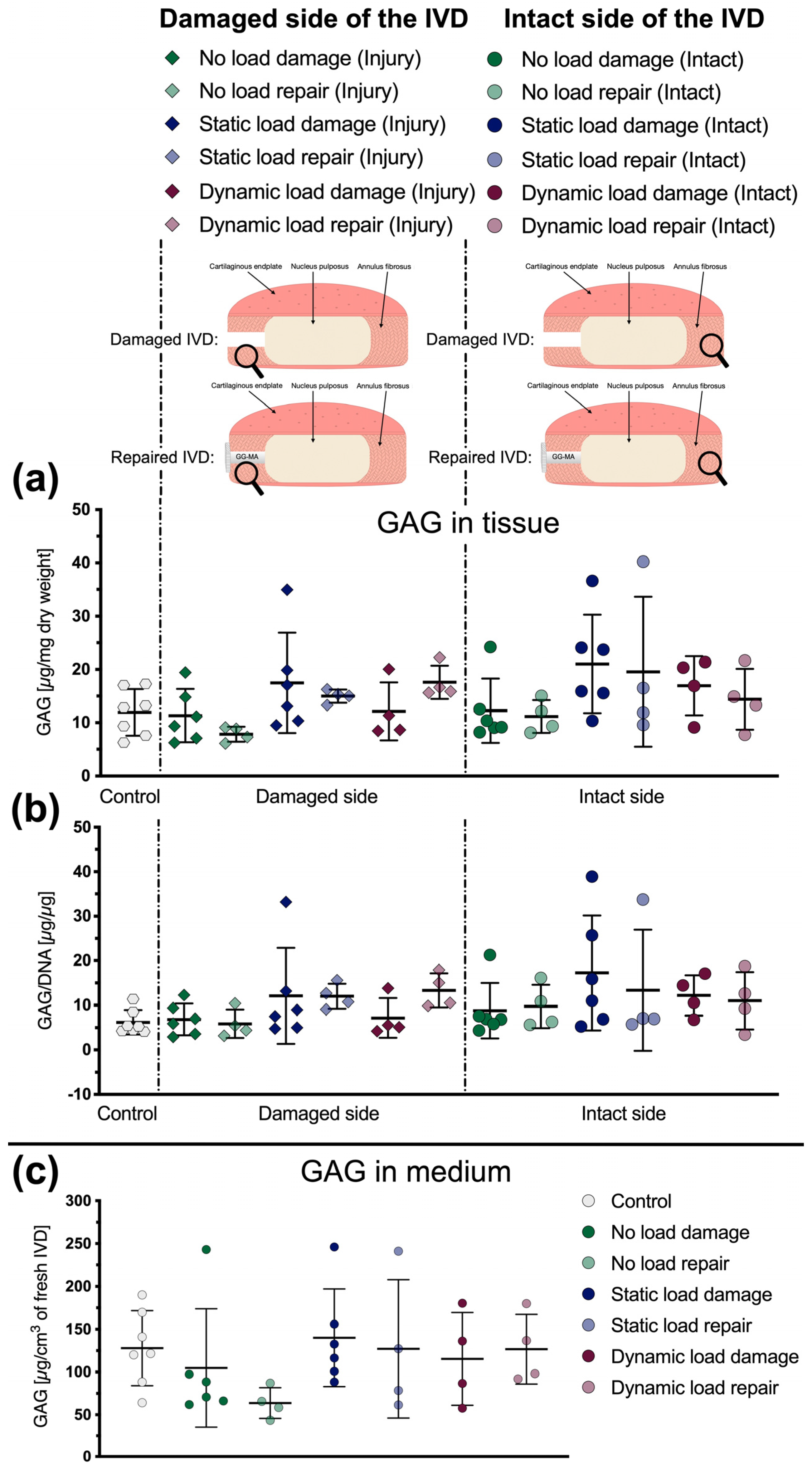
2.7. Glycosaminglycan (GAG) Content
2.8. DNA Content
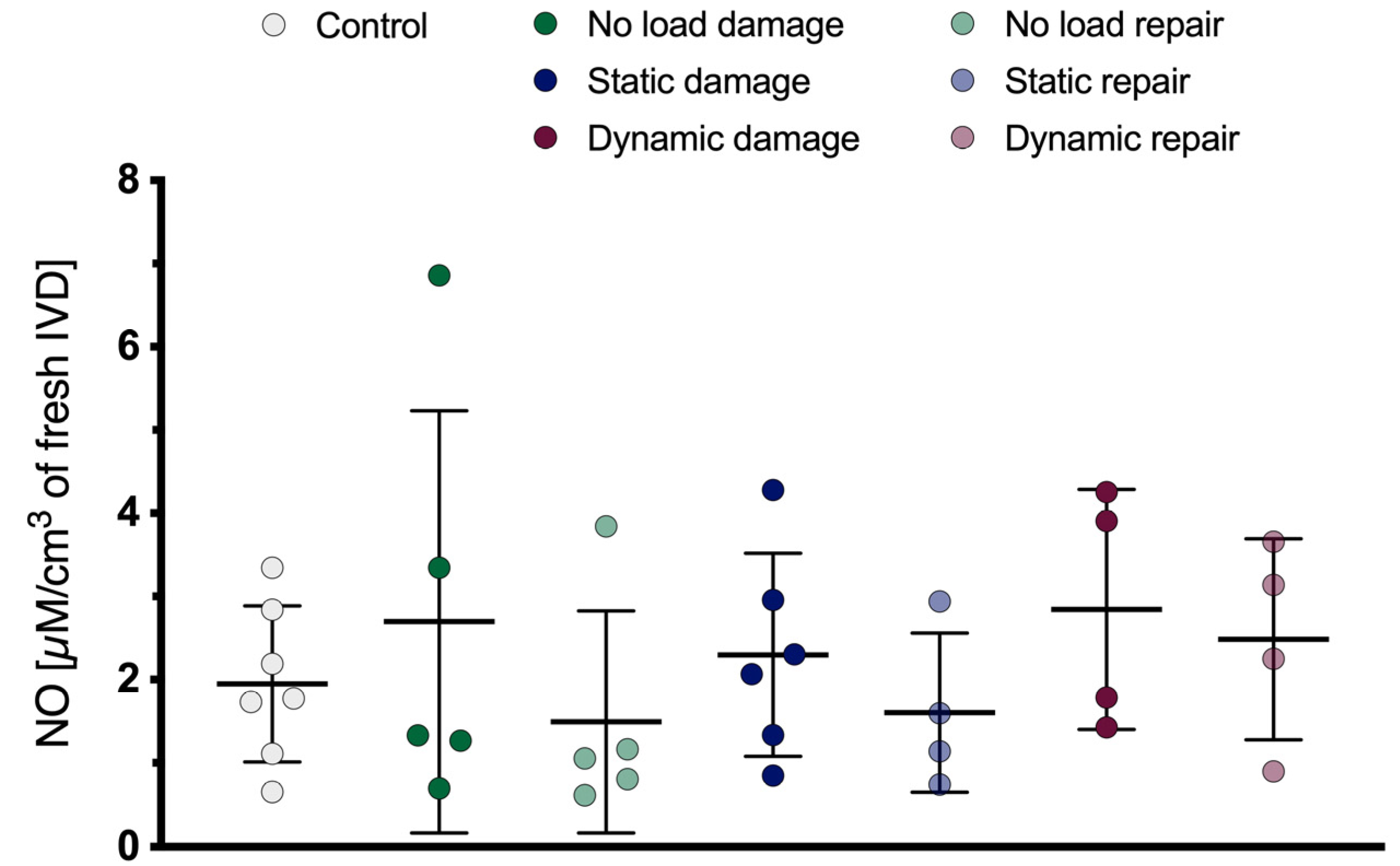
2.9. Nictric Oxide Content
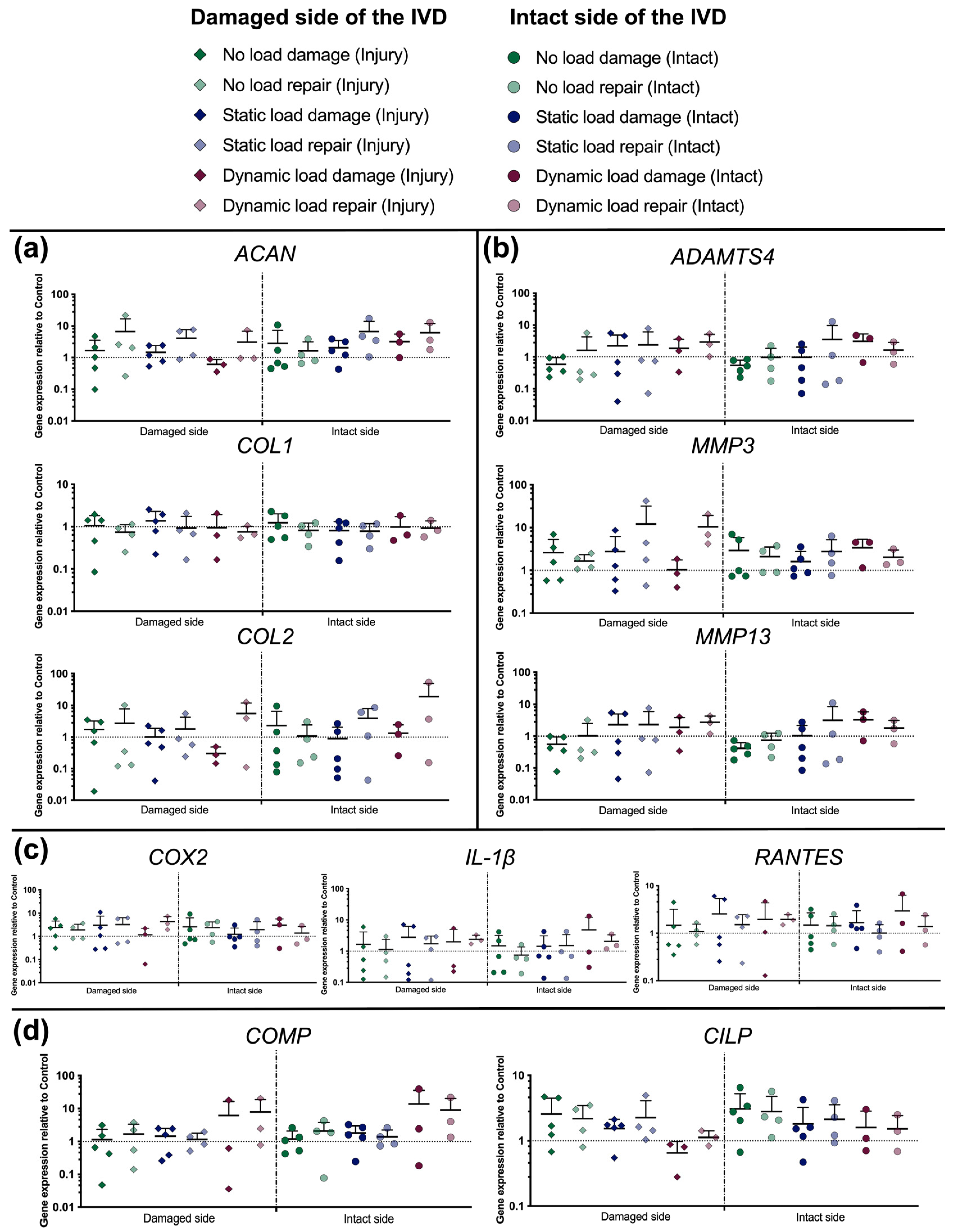
2.10. RNA Extraction and qPCR
2.11. Statistics
3. Results
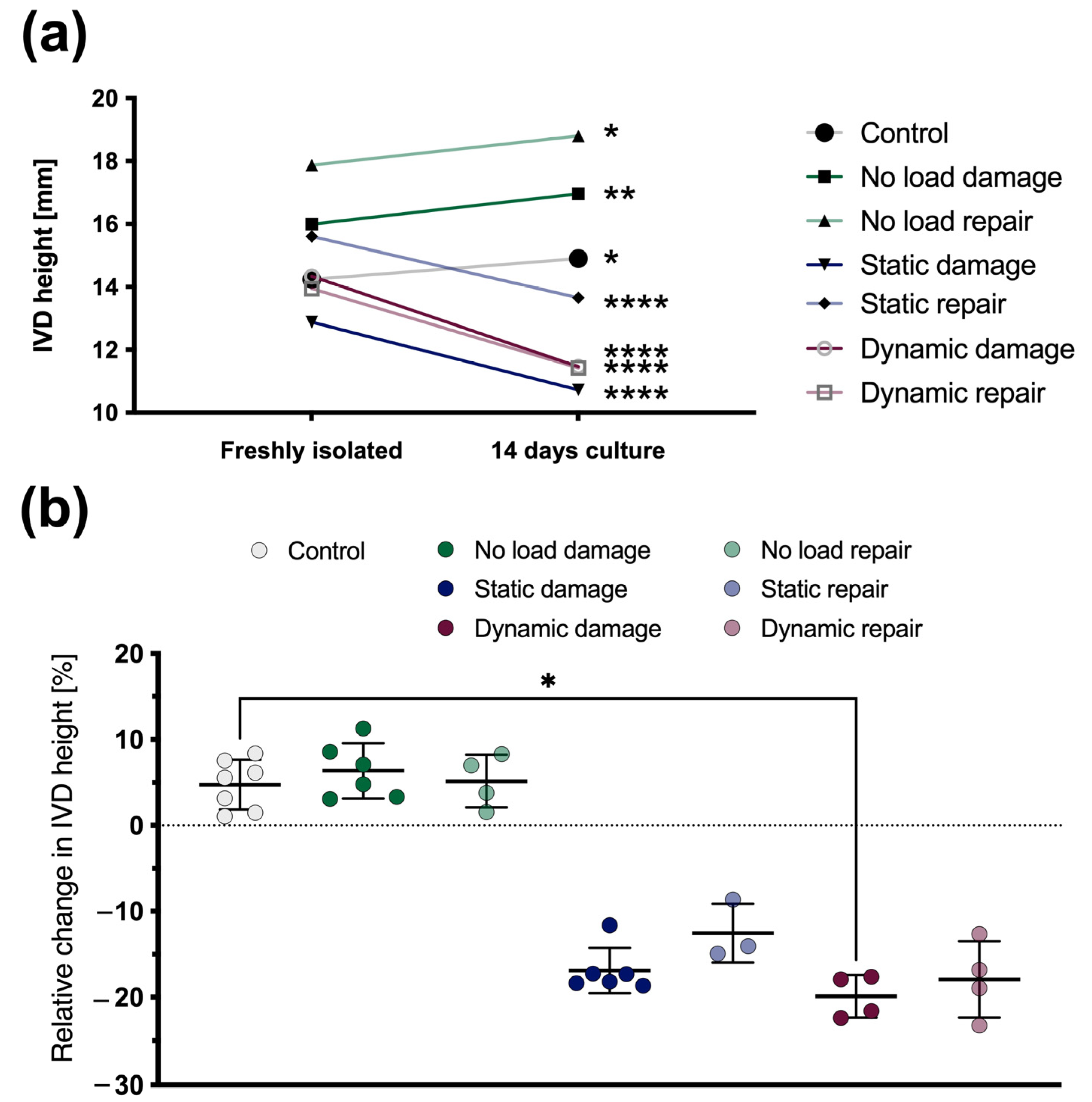
3.1. Changes in the IVD Height
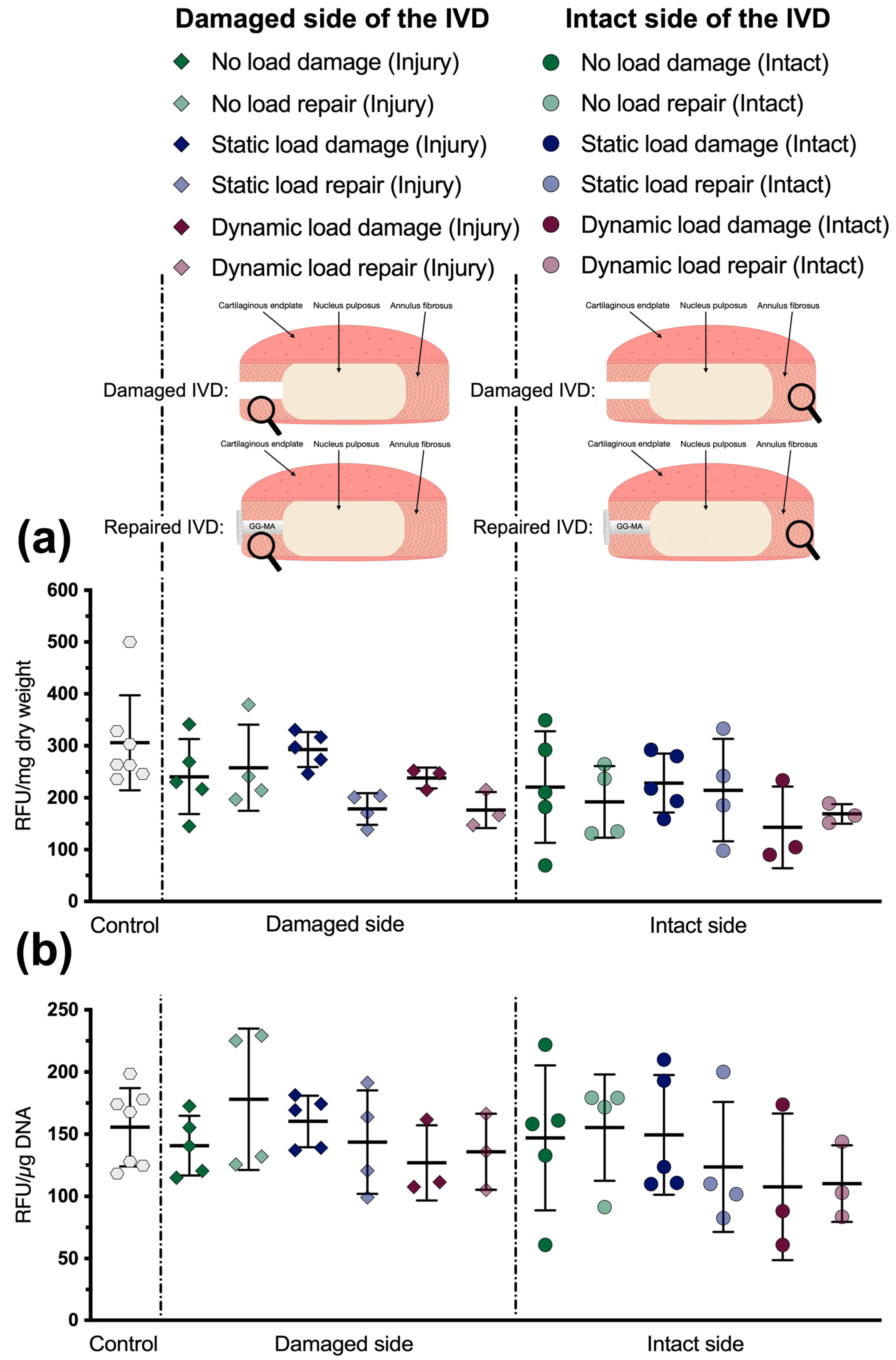
3.2. Metabolic Activity
3.3. Glycosaminoglycan Content
3.4. Nitric Oxide Content
3.5. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray-Offor, O.D.; Wachukwu, C.M.; Onubiyi, C.C.B. Intervertebral disc herniation: Prevalence and association with clinical diagnosis. Niger. J. Med. 2016, 25, 107–112. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Dydyk, A.M.; Ngnitewe Massa, R.; Mesfin, F.B. Disc Herniation; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Jordan, J.; Konstantinou, K.; O’Dowd, J. Herniated lumbar disc. BMJ Clin. Evid. 2011, 2011, 1118. [Google Scholar] [PubMed]
- Kreiner, D.S.; Hwang, S.W.; Easa, J.E.; Resnick, D.K.; Baisden, J.L.; Bess, S.; Cho, C.H.; DePalma, M.J.; Dougherty, P., 2nd; Fernand, R.; et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014, 14, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; McNally, D.S.; Dolan, P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J. Bone Jt. Surg. Br. 1996, 78, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 2020, 3, e1076. [Google Scholar] [CrossRef]
- Adams, M.A.; Freeman, B.J.; Morrison, H.P.; Nelson, I.W.; Dolan, P. Mechanical initiation of intervertebral disc degeneration. Spine (Phila Pa 1976) 2000, 25, 1625–1636. [Google Scholar] [CrossRef]
- Adams, M.A.; Dolan, P.; McNally, D.S. The internal mechanical functioning of intervertebral discs and articular cartilage, and its relevance to matrix biology. Matrix Biol. 2009, 28, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Kepler, C.K.; Ponnappan, R.K.; Tannoury, C.A.; Risbud, M.V.; Anderson, D.G. The molecular basis of intervertebral disc degeneration. Spine J. 2013, 13, 318–330. [Google Scholar] [CrossRef]
- Boos, N.; Weissbach, S.; Rohrbach, H.; Weiler, C.; Spratt, K.F.; Nerlich, A.G. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002, 27, 2631–2644. [Google Scholar] [CrossRef]
- Cosamalon-Gan, I.; Cosamalon-Gan, T.; Mattos-Piaggio, G.; Villar-Suarez, V.; Garcia-Cosamalon, J.; Vega-Alvarez, J.A. Inflammation in the intervertebral disc herniation. Neurocirugia (Astur. Engl. Ed.) 2021, 32, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Vroomen, P.C.; de Krom, M.C.; Wilmink, J.T.; Kester, A.D.; Knottnerus, J.A. Diagnostic value of history and physical examination in patients suspected of lumbosacral nerve root compression. J. Neurol. Neurosurg. Psychiatry 2002, 72, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Meng, Z.; Cao, Y.; Yu, J.; Wang, S.; Luo, C.; Yu, L.; Xu, Y.; Sun, Y.; Jiang, L. Nonsurgical medical treatment in the management of pain due to lumbar disc prolapse: A network meta-analysis. Semin. Arthritis. Rheum. 2019, 49, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Benzakour, T.; Igoumenou, V.; Mavrogenis, A.F.; Benzakour, A. Current concepts for lumbar disc herniation. Int. Orthop. 2019, 43, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Loupasis, G.A.; Stamos, K.; Katonis, P.G.; Sapkas, G.; Korres, D.S.; Hartofilakidis, G. Seven- to 20-year outcome of lumbar discectomy. Spine (Phila Pa 1976) 1999, 24, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dou, Q.; Kong, Q. Repair and Regenerative Therapies of the Annulus Fibrosus of the Intervertebral Disc. J. Coll. Physicians Surg. Pak. 2016, 26, 138–144. [Google Scholar]
- Costa, L.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Gellan Gum-Based Hydrogels for Osteochondral Repair. Adv. Exp. Med. Biol. 2018, 1058, 281–304. [Google Scholar] [CrossRef]
- Croft, A.S.; Spessot, E.; Bhattacharjee, P.; Yang, Y.; Motta, A.; Woltje, M.; Gantenbein, B. Biomedical applications of silk and its role for intervertebral disc repair. JOR Spine 2022, 5, e1225. [Google Scholar] [CrossRef]
- Yang, Y.; Greco, G.; Maniglio, D.; Mazzolai, B.; Migliaresi, C.; Pugno, N.; Motta, A. Spider (Linothele megatheloides) and silkworm (Bombyx mori) silks: Comparative physical and biological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110197. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Fialho, A.M.; Martins, L.O.; Donval, M.L.; Leitao, J.H.; Ridout, M.J.; Jay, A.J.; Morris, V.J.; Sa-Correia, I.I. Structures and properties of gellan polymers produced by sphingomonas paucimobilis ATCC 31461 from lactose compared with those produced from glucose and from cheese whey. Appl. Environ. Microbiol. 1999, 65, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; In Het Panhuis, M. Tissue engineering with gellan gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regen. Med. 2011, 5, e97–e107. [Google Scholar] [CrossRef]
- Tsaryk, R.; Silva-Correia, J.; Oliveira, J.M.; Unger, R.E.; Landes, C.; Brochhausen, C.; Ghanaati, S.; Reis, R.L.; Kirkpatrick, C.J. Biological performance of cell-encapsulated methacrylated gellan gum-based hydrogels for nucleus pulposus regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.; Gantenbein-Ritter, B. Preparation of intact bovine tail intervertebral discs for organ culture. J. Vis. Exp. 2012. [Google Scholar] [CrossRef]
- Frost, B.A.; Camarero-Espinosa, S.; Foster, E.J. Materials for the Spine: Anatomy, Problems, and Solutions. Materials 2019, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Frauchiger, D.A.; Chan, S.C.W.; Benneker, L.M.; Gantenbein, B. Intervertebral disc damage models in organ culture: A comparison of annulus fibrosus cross-incision versus punch model under complex loading. Eur. Spine J. 2018, 27, 1785–1797. [Google Scholar] [CrossRef]
- Li, Z.; Lezuo, P.; Pattappa, G.; Collin, E.; Alini, M.; Grad, S.; Peroglio, M. Development of an ex vivo cavity model to study repair strategies in loaded intervertebral discs. Eur. Spine J. 2016, 25, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Croft, A.S.; Illien-Junger, S.; Grad, S.; Guerrero, J.; Wangler, S.; Gantenbein, B. The Application of Mesenchymal Stromal Cells and Their Homing Capabilities to Regenerate the Intervertebral Disc. Int. J. Mol. Sci. 2021, 22, 3519. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, Y.; Wang, J.; Yu, W.; Wang, W.; Ma, X. Monitoring of cell viability and proliferation in hydrogel-encapsulated system by resazurin assay. Appl. Biochem. Biotechnol. 2010, 162, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Sayers, C.A.; Barrett, A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect. Tissue Res. 1982, 9, 247–248. [Google Scholar] [CrossRef]
- Frauchiger, D.A.; May, R.D.; Bakirci, E.; Tekari, A.; Chan, S.C.W.; Woltje, M.; Benneker, L.M.; Gantenbein, B. Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model. J. Funct. Biomater. 2018, 9, 40. [Google Scholar] [CrossRef]
- Verdon, C.P.; Burton, B.A.; Prior, R.L. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal. Biochem. 1995, 224, 502–508. [Google Scholar] [CrossRef]
- Reno, C.; Marchuk, L.; Sciore, P.; Frank, C.B.; Hart, D.A. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques 1997, 22, 1082–1086. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Botsford, D.J.; Esses, S.I.; Ogilvie-Harris, D.J. In vivo diurnal variation in intervertebral disc volume and morphology. Spine (Phila Pa 1976) 1994, 19, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Neef, P.; Caimi, M.; Hoogland, T.; Claes, L.E. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine (Phila Pa 1976) 1999, 24, 755–762. [Google Scholar] [CrossRef]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J 2008, 17, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Long, R.G.; Ferguson, S.J.; Benneker, L.M.; Sakai, D.; Li, Z.; Pandit, A.; Grijpma, D.W.; Eglin, D.; Zeiter, S.; Schmid, T.; et al. Morphological and biomechanical effects of annulus fibrosus injury and repair in an ovine cervical model. JOR Spine 2020, 3, e1074. [Google Scholar] [CrossRef]
- Saghari Fard, M.R.; Krueger, J.P.; Stich, S.; Berger, P.; Kuhl, A.A.; Sittinger, M.; Hartwig, T.; Endres, M. A Biodegradable Polymeric Matrix for the Repair of Annulus Fibrosus Defects in Intervertebral Discs. Tissue Eng. Regen. Med. 2022, 19, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.R.; Silva-Correia, J.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Salgado, A.J.; Oliveira, J.M.; Mano, J.F.; Sousa, N.; Reis, R.L. Development of gellan gum-based microparticles/hydrogel matrices for application in the intervertebral disc regeneration. Tissue Eng. Part C Methods 2011, 17, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Martins, L.; Picciochi, R.; Malafaya, P.B.; Sousa, R.A.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum: A new biomaterial for cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010, 93, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Gardel, L.S.; Rada, T.; Martins, L.; Gomes, M.E.; Reis, R.L. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J. Orthop. Res. 2010, 28, 1193–1199. [Google Scholar] [CrossRef]
| Gene Type | Full Name | Symbol | NCBI Gene ID | Forward and Reverse Primer Sequences |
|---|---|---|---|---|
| Reference gene | 18S ribosomal RNA | 18S | 493779 | f—ACG GAC AGG ATT GAC AGA TTG r—CCA GAG TCT CGT TCG TTA TCG |
| Anabolic markers | Aggrecan | ACAN | 280985 | f—GGC ATC GTG TTC CAT TAC AG r—ACT CGT CCT TGT CTC CAT AG |
| Collagen Type 2, Alpha 1 Chain | COL2 | 407142 | f—CGG GTG AAC GTG GAG AGA CA r—GTC CAG GGT TGC CAT TGG AG | |
| Collagen Type 1, Alpha 2 Chain | COL1 | 282188 | f—GCC TCG CTC ACC AAC TTC r—AGT AAC CAC TGC TCC ATT CTG | |
| Catabolic markers | ADAM Metallopeptidase with Thrombospondin Type 1 Motif 4 | ADAMTS4 | 286806 | f—AGA TTT GTG GAG ACT CTG r—ATA ACT GTC AGC AGG TAG |
| Matrix Metallopeptidase 13 | MMP13 | 281914 | f—TCC TGG CTG GCT TCC TCT TC r—CCT CGG ACA AGT CTT CAG AAT CTC | |
| Matrix Metallopeptidase 3 | MMP3 | 281309 | f—CTT CCG ATT CTG CTG TTG CTA TG r—ATG GTG TCT TCC TTG TCC CTT G | |
| Mechanosensitive markers | Cartilage Oligomeric Matrix Protein | COMP | 281088 | f—TGC GAC GAC GAC ATA CAC r—ATC TCC TAC ACC ATC ACC ATC |
| Cartilage Intermediate Layer Protein | CILP | 100336614 | f—AGG ACT TCG TGC TGT ATG r—CTT GCT CAG GAG GTA GAC | |
| Inflammatory markers | Cyclooxygenase 2 | COX2 | 3283880 | f—GGT AAT CCT ATA TGC TCT C r—GTA TCT TGA ACA CTG AAT G |
| Regulated Upon Activation, Normally T-Expressed, And Presumably Secreted | RANTES | 327712 | f—GTG CGA GAG TAC ATC AAC r—TTA GGA CAA GAG CGA GAA | |
| Interleukin 1 Beta | IL-1β | 281251 | f—AGT GCC ATC CTT CTG TCA r—CAT TGC CTT CTC CGC TAT T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croft, A.S.; Ćorluka, S.; Fuhrer, J.; Wöltje, M.; Silva-Correia, J.; Oliveira, J.M.; Erbach, G.F.; Reis, R.L.; Gantenbein, B. Repairing Annulus Fibrosus Fissures Using Methacrylated Gellan Gum Combined with Novel Silk. Materials 2023, 16, 3173. https://doi.org/10.3390/ma16083173
Croft AS, Ćorluka S, Fuhrer J, Wöltje M, Silva-Correia J, Oliveira JM, Erbach GF, Reis RL, Gantenbein B. Repairing Annulus Fibrosus Fissures Using Methacrylated Gellan Gum Combined with Novel Silk. Materials. 2023; 16(8):3173. https://doi.org/10.3390/ma16083173
Chicago/Turabian StyleCroft, Andreas S., Slavko Ćorluka, Janine Fuhrer, Michael Wöltje, Joana Silva-Correia, Joaquim M. Oliveira, Georg F. Erbach, Rui L. Reis, and Benjamin Gantenbein. 2023. "Repairing Annulus Fibrosus Fissures Using Methacrylated Gellan Gum Combined with Novel Silk" Materials 16, no. 8: 3173. https://doi.org/10.3390/ma16083173
APA StyleCroft, A. S., Ćorluka, S., Fuhrer, J., Wöltje, M., Silva-Correia, J., Oliveira, J. M., Erbach, G. F., Reis, R. L., & Gantenbein, B. (2023). Repairing Annulus Fibrosus Fissures Using Methacrylated Gellan Gum Combined with Novel Silk. Materials, 16(8), 3173. https://doi.org/10.3390/ma16083173










