Effects of Cu and Ag Elements on Corrosion Resistance of Dual-Phase Fe-Based Medium-Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Thermodynamic Parameters of Fe-Based Medium Entropy Alloys
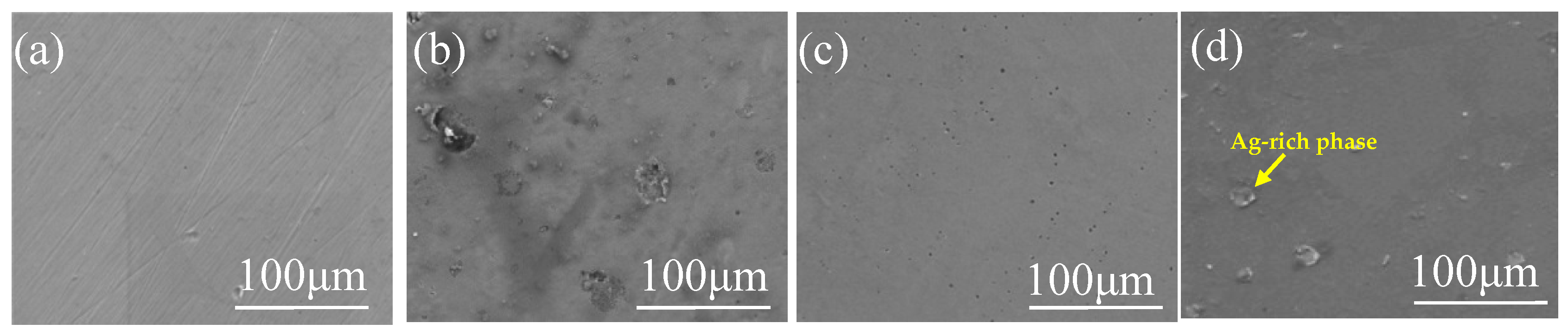
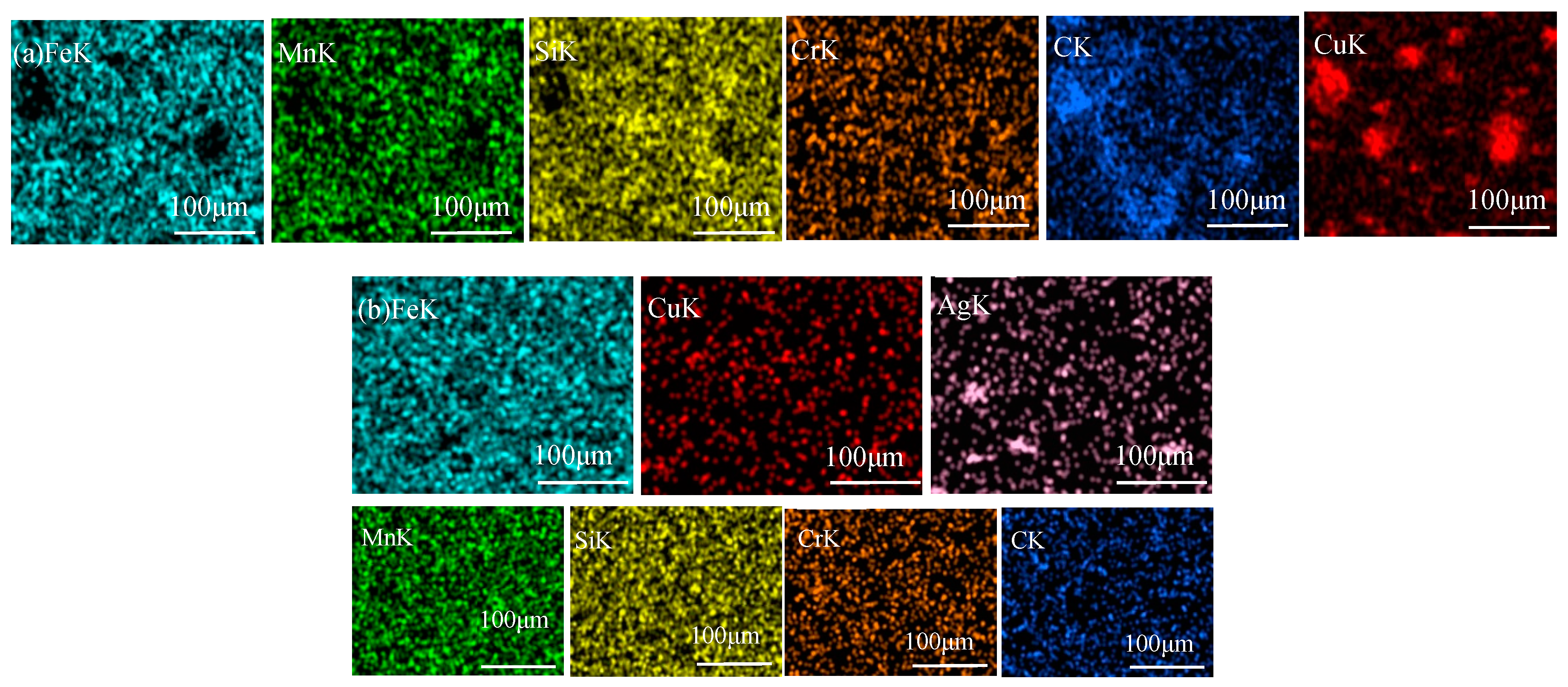
3.2. Microstructure of Fe-Based Medium-Entropy Alloys
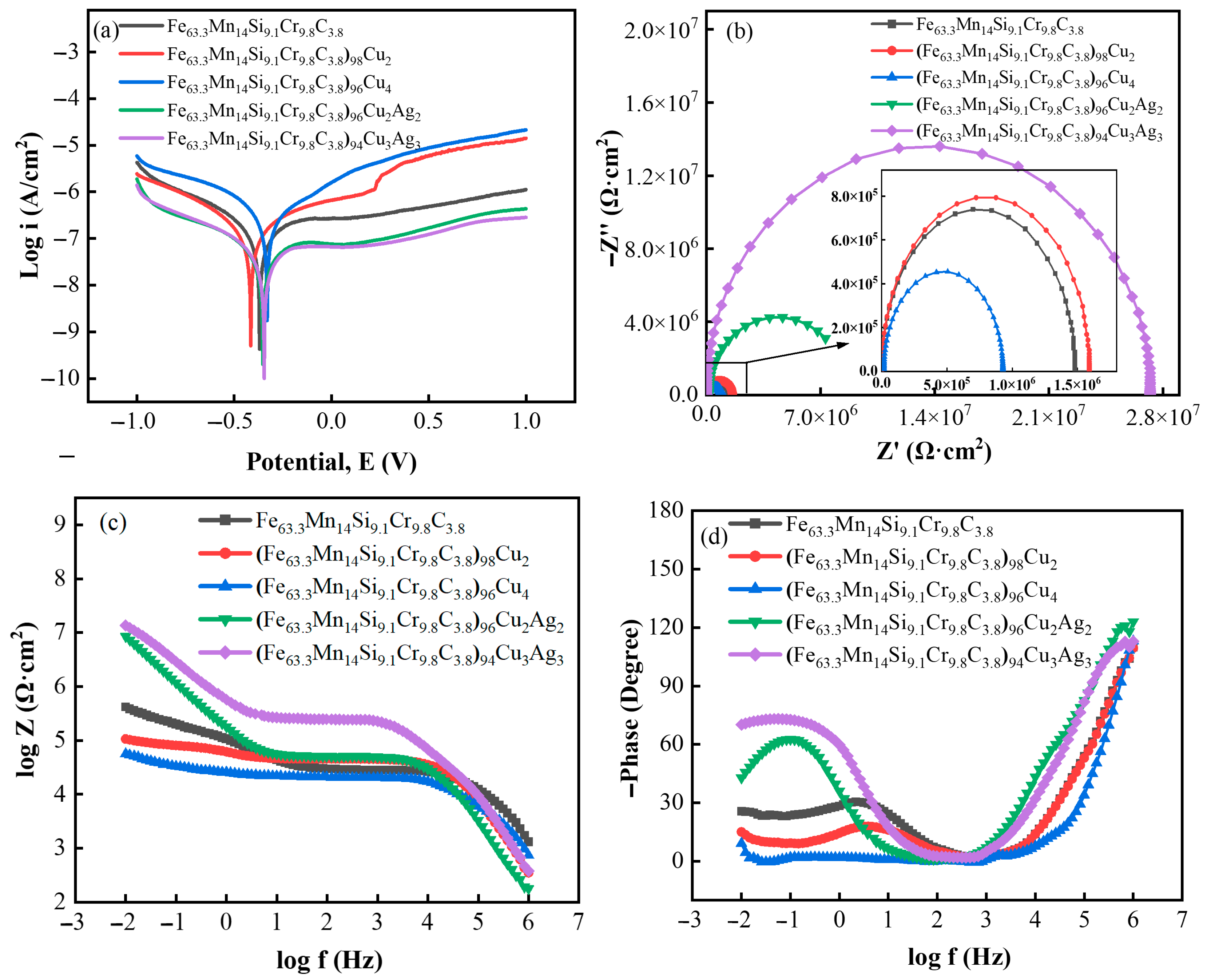
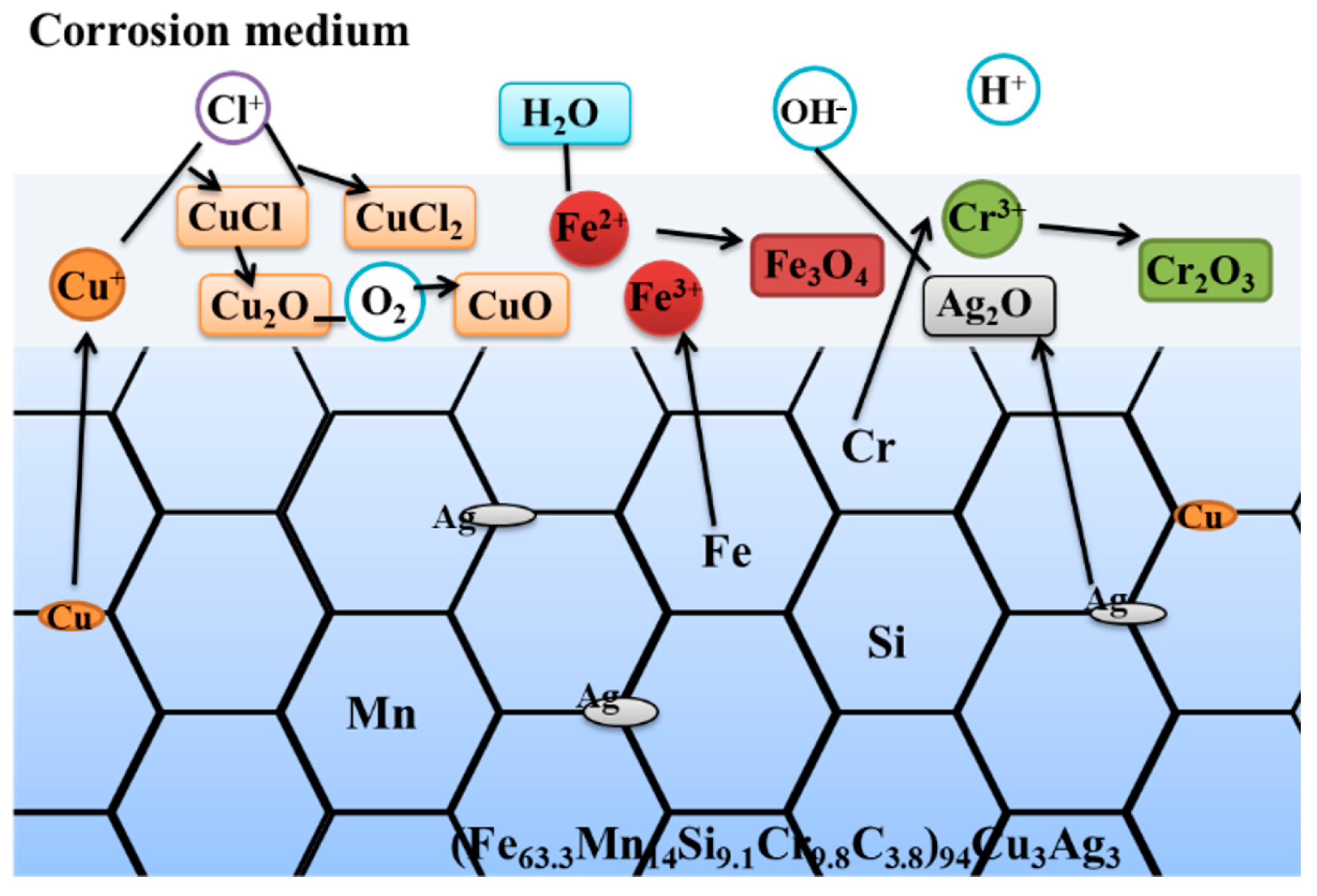
3.3. Corrosion Resistance
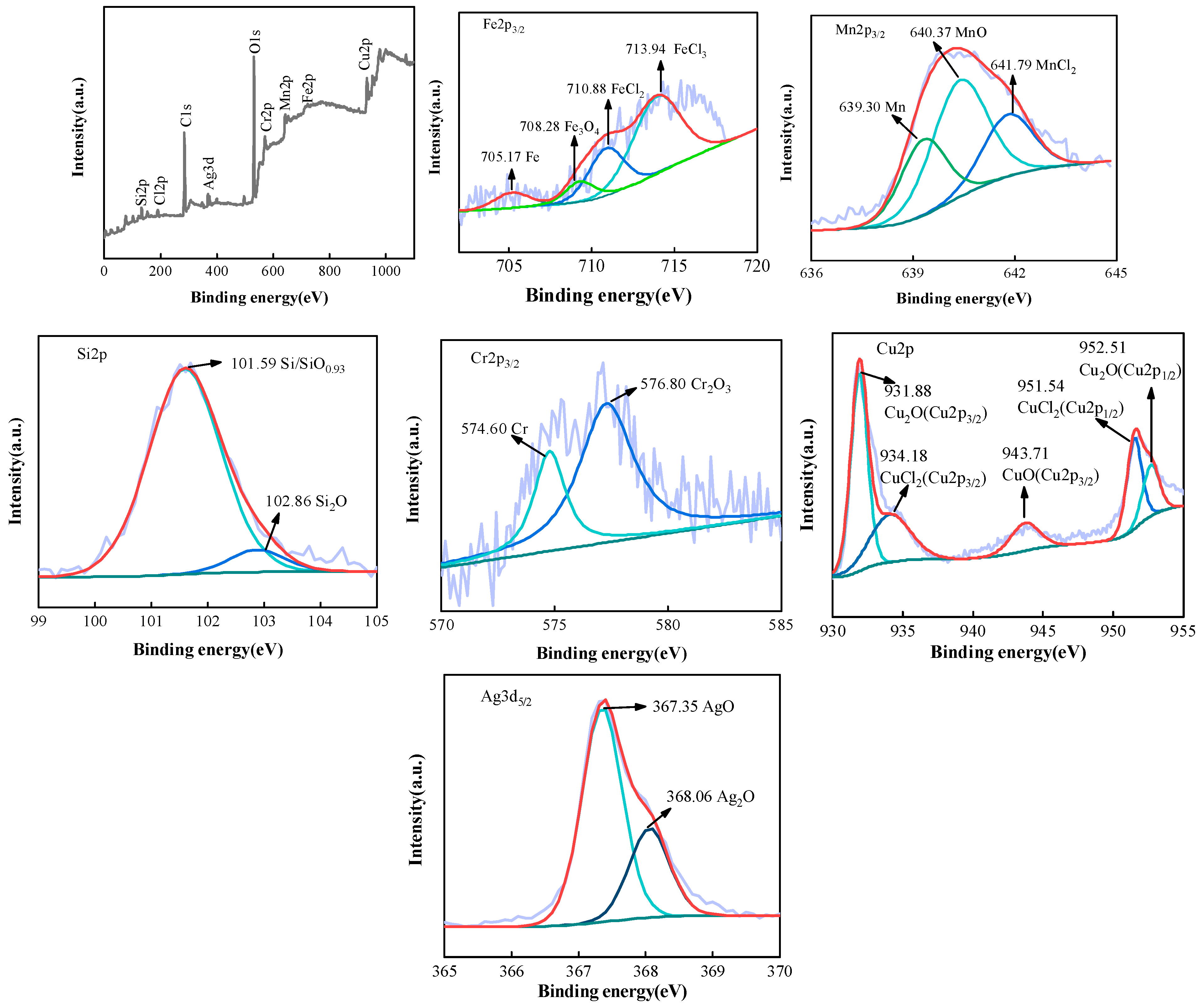
4. Discussion
5. Conclusions
- (1)
- After adding different proportions of Cu and Ag elements, the entropy value of the alloy was still in the range of entropy alloys (from 1R to 1.5R); the mixing enthalpy of the alloy was all negative. Regarding the alloy’s morphology, it was observed that Cu and Ag elements were enriched in the intergranular area, as the binary mixing enthalpies of Cu and Ag and the main elements Fe, Mn, Si, and Cr of the medium-entropy alloy were all positive;
- (2)
- With an increase in Cu and Ag content, the alloy’s corrosion potential increased, the corrosion current density decreased, the radius of the capacitive arc gradually increased, and the alloy’s corrosion resistance was enhanced. The corrosion current density of (Fe63.3Mn14Si9.1Cr9.8C3.8)94Cu3Ag3 in PBS solution reached 1.357 × 10−8 A·cm−2;
- (3)
- The synergistic effect of Cu and Ag elements made the alloy exhibit excellent corrosion resistance. Si, Cr, Ag, and other elements in the corrosion product film were combined with electron holes to fill in the corrosion in the product lattice; the passivation film’s conductivity was reduced, the corrosion rate slowed, and the alloy’s corrosion resistance was guaranteed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Yang, B.; Liaw, P. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Yao, Q.; Sebastian, T.; Mark, A.G.; Hamish, L.F.; Nick, B. Corrosion of high entropy alloys. NPJ Mater. Degrad. 2017, 1, 15. [Google Scholar]
- Salishchev, G.A.; Tikhonovsky, M.A.; Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Kolodiy, I.V.; Tortika, A.S.; Senkov, O.N. Effect of Mn and V on structure and mechanical properties of high entropy alloys based on CoCrFeNi system. J. Alloys Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef]
- Han, J.S.; Su, B.; Meng, J.H.; Zhang, A.J.; Wu, Y.Z. Microstructure and composition evolution of a fused slurry silicide coating on MoNbTaTiW refractory high-entropy alloy in high temperature oxidation environment. Materials 2020, 13, 3592. [Google Scholar] [CrossRef]
- Laplanche, G.; Kostka, A.; Reinhart, C.; Hunfeld, J.; Eggeler, G.; Georgeet, E.P. Reasons for the superior mechanical properties of medium entropy CrCoNi compared to high entropy CrMnFeCoNi. Acta Mater. 2017, 128, 292–303. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Ma, H.W.; Zhang, L.H.; Zhang, D.; Kou, S.Z.; Wang BLi, W.S.; Liaw, P.K. Mn content optimum on microstructures and mechanical behavior of Fe-based medium entropy alloys. Mater. Des. 2022, 223, 111241. [Google Scholar] [CrossRef]
- Shi, P.; Li, R.; Li, Y.; Wen, Y.; Zhong, Y.; Ren, W.; Shen, Z.; Zheng, T.; Peng, J.; Liang, X.; et al. Hierarchical crack buffering triples ductility in eutectic herringbone high-entropy alloys. Science 2021, 373, 912–918. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Jiao, Z.M.; Bian, G.B.; Yang, H.J.; He, H.W.; Wang, Z.H.; Liaw, P.K.; Qiao, J.W. Dynamic tension and constitutive model in in Fe40Mn20Cr20Ni20 highentropy alloys with a heterogeneous structure. Mater. Sci. Eng. A 2022, 839, 142887–142898. [Google Scholar] [CrossRef]
- Lu, C.W.; Lu, Y.S.; Lai, Z.H.; Yen, H.W.; Lee, Y.L. Comparative corrosion behavior of Fe50Mn30Co10Cr10 dual-phase high-entropy alloy and CoCrFeMnNi high-entropy alloy in 3.5wt% NaCl solution. J. Alloys Comd. 2020, 842, 155824. [Google Scholar] [CrossRef]
- Guo, W.; Su, J.; Lu, W.; Liebscher, C.H.; Kirchlechner, C.; Ikeda, Y.; Körmann, F.; Liu, X.; Xue, Y.; Dehm, G. Dislocation-induced breakthrough of strength and ductility trade-off in a non-equiatomic high-entropy alloy. Acta Mater. 2020, 185, 45–54. [Google Scholar] [CrossRef]
- Gao, M.C.; Qiao, J.W. High-Entropy Alloys (HEAs). Metals 2018, 8, 108. [Google Scholar] [CrossRef]
- Liu, F.; Song, Q.; Chen, R.; Wang, C.; Sun, J. Effect of Co, Ni, Cu content on phase composition, microstructure and corrosion resistance of Co1−xCrFeNi1+xCuy series high-entropy alloys. Vacuum 2023, 210, 111830. [Google Scholar] [CrossRef]
- Li, J.C.; Liu, Z.X.; Chen, Y.D.; CAO, Y.J. Microstructure and Corrosion Behavior of CuxCr2Fe2Ni3Mn2Nb0.4Mo0.2 High Entropy Alloy. Spec. Cast. Nonferrous Alloys 2018, 38, 666–669. (In Chinese) [Google Scholar]
- Hsu, Y.J.; Chiang, W.C.; Wu, J.K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Gaskell, D.R. Introduction to the Thermodynamics of Materials, 3rd ed.; Taylor & Francis Ltd.: Washington, DC, USA, 2008; pp. 80–84. [Google Scholar]
- Swalin, R.A. Thermodynamics of Solids, 2nd ed.; Wiley: New York, NY, USA, 1972; pp. 35–41. [Google Scholar]
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multicomponent alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy design and properties optimization of high-entropy alloys. JOM 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.Y.; Cotton, J.D.; Zhang, Y. Phase stability of low-density, multiprincipal component alloys containing aluminum, magnesium, and lithium. JOM 2014, 10, 2009–2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Chen, R.; Qin, G.; Zheng, H.; Wang, L.; Su, Y.; Chiu, Y.; Ding, H.; Guo, J.; Fu, H. Composition design of high entropy alloys using the valence electron concentration to balance strength and ductility. Acta Mater. 2018, 144, 129–137. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, D.; Jin, X.; Zhang, L.; Du, X.; Li, B. Design of non-equiatomic medium-entropy alloys. Sci. Rep. 2018, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhang, Y.; Chen, X.; Fu, X.; Chen, D.; Chen, S.; Gu, L.; Wei, F.; Bei, H.; Gao, Y.; et al. Tuning element distribution, structure and properties by composition in high-entropy alloys. Nature 2019, 574, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Zhevnenko, S.N.; Chernyshikhin, S.V. Surface Phase Transitions in Cu-Based Solid Solutions. Appl. Surf. Sci. 2017, 421, 77–81. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Liaw, B.Y.; Zhang, J.B. Graphical analysis of electrochemical impedance spectroscopy data in Bode and Nyquist representations. J. Power Sources 2016, 309, 82–98. [Google Scholar] [CrossRef]
- Liang, C.H.; Liu, L.B.; Jia, Z.; Dai, C.S.; Xiong, Y.P. Synergy of Nyquist and Bode electrochemical impedance spectroscopy studies to particle size effect on the electrochemical properties of LiNi0.5Co0.2Mn0.3O2. Electrochim. Acta 2015, 186, 413–419. [Google Scholar] [CrossRef]
- Monaco, L.; Sodhi, R.N.; Palumbo, G.; Erb, U. XPS study on the passivity of coarse-grained polycrystalline and electrodeposited nanocrystalline nickel-iron (NiFe) alloys. Corros. Sci. 2020, 176, 108902. [Google Scholar] [CrossRef]
- Fajardo, S.; Llorente, I.; Jiménez, J.A.; Bastidas, J.M.; Bastidas, D.M. Effect of Mn additions on the corrosion behaviour of TWIP Fe-Mn-Al-Si austenitic steel in chloride solution. Corros. Sci. 2019, 154, 246–253. [Google Scholar] [CrossRef]
- Kim, Y.; Buchheit, R.G.; Kotula, P.G. Effect of alloyed Cu on localized corrosion susceptibility of Al–Cu solid solution alloys—Surface characterization by XPS and STEM. Electrochim. Acta 2010, 55, 7367–7375. [Google Scholar] [CrossRef]
- Stephan-Scherb, C.; Menneken, M.; Weber, K.; Jácome, L.A.; Nolze, G. Elucidation of orientation relations between Fe-Cr alloys and corrosion products after high temperature SO2 corrosion. Corros. Sci. 2020, 174, 108809. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.H.; Wang, S.B.; Liu, C.H.; Yang, S.S.; Wu, C.L. The effect of Si on precipitation in Al–Cu–Mg alloy with a high Cu/Mg ratio. Mater. Sci. Eng. A 2014, 606, 187–195. [Google Scholar] [CrossRef]
- Liu, G.; Song, H.; Feng, L.; Du, X.; Liu, J. Corrosion of Fe–B–Si alloys in liquid zinc. Mater. Chem. Phys. 2021, 273, 124933. [Google Scholar] [CrossRef]
- Yang, L.; Shi, Y.; Shen, L.; Zhang, E.; Qin, G.; Lu, X.; Zhou, X. Effect of Ag on cathodic activation and corrosion behaviour of Mg-Mn-Ag alloys. Corros. Sci. 2021, 185, 109408. [Google Scholar] [CrossRef]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of Electrochemical Corrosion; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Cao, Z.Q.; Yin, X.T.; Jia, Z.Q.; Tian, Q.Y.; Jie, L.U.; Zhang, K.; Yan, W.A. Corrosion behavior of bulk two-phase Ag−25Cu alloys with different microstructures in NaCl aqueous solution. Trans. Non. Metals Soc. China 2019, 29, 1495–1502. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, Z.; Tang, C.; Li, W.; You, C.; Chen, M. The mechanical and corrosion resistance of Mg-Zn-Ca-Ag alloys: The influence of Ag content. J. Mater. Res. Technol. 2020, 9, 10863–10875. [Google Scholar] [CrossRef]
- Deschamps, A.; Militzer, M.; Poole, W.J. Precipitation Kinetics and Strengthening of a Fe-0.8%Cu Alloy. ISIJ Int. 2001, 41, 196–205. [Google Scholar] [CrossRef]



| Alloys | ∆Smix (J·mol−1·K−1) | ∆Hmix (kJ/mol) | Ω | δ (%) | ∆χ | VEC |
|---|---|---|---|---|---|---|
| Fe63.3Mn14Si9.1Cr9.8C3.8 | 1.141R | −19.610 | 0.903 | 6.720 | 0.184 | 7.141 |
| (Fe63.3Mn14Si9.1Cr9.8C3.8)98Cu2 | 1.215R | −18.471 | 1.017 | 6.721 | 0.185 | 7.218 |
| (Fe63.3Mn14Si9.1Cr9.8C3.8)96Cu4 | 1.255R | −16.705 | 1.152 | 6.391 | 0.180 | 7.302 |
| (Fe63.3Mn14Si9.1Cr9.8C3.8)96Cu2Ag2 | 1.284R | −15.824 | 1.242 | 6.692 | 0.180 | 7.302 |
| (Fe63.3Mn14Si9.1Cr9.8C3.8)94Cu3Ag3 | 1.336R | −14.235 | 1.431 | 6.841 | 0.181 | 7.379 |
| Alloy | Ecorr (V) | Icorr (A·cm−2) | R (Ω·cm2) |
|---|---|---|---|
| Fe63.3Mn14Si9.1Cr9.8C3.8 | −0.370 | 8.089 × 10−8 | 5.7238 × 105 |
| (Fe63.3Mn14Si9.1Cr9.8C3.8)98Cu2 | −0.415 | 8.332 × 10−8 | 5.2500 × 105 |
| (Fe63.3Mn14Si9.1Cr9.8C3.8)96Cu4 | −0.331 | 1.960 × 10−7 | 2.3082 × 105 |
| (Fe63.3Mn14Si9.1Cr9.8C3.8)96Cu2Ag2 | −0.353 | 1.659 × 10−8 | 2.8842 × 106 |
| (Fe63.3Mn14Si9.1Cr9.8C3.8)94Cu3Ag3 | −0.346 | 1.357 × 10−8 | 3.4824 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhao, Y.; Hu, R.; Zhang, M.; Ding, Y. Effects of Cu and Ag Elements on Corrosion Resistance of Dual-Phase Fe-Based Medium-Entropy Alloys. Materials 2023, 16, 3243. https://doi.org/10.3390/ma16083243
Liu J, Zhao Y, Hu R, Zhang M, Ding Y. Effects of Cu and Ag Elements on Corrosion Resistance of Dual-Phase Fe-Based Medium-Entropy Alloys. Materials. 2023; 16(8):3243. https://doi.org/10.3390/ma16083243
Chicago/Turabian StyleLiu, Jianjun, Yanchun Zhao, Ruonan Hu, Minya Zhang, and Yutian Ding. 2023. "Effects of Cu and Ag Elements on Corrosion Resistance of Dual-Phase Fe-Based Medium-Entropy Alloys" Materials 16, no. 8: 3243. https://doi.org/10.3390/ma16083243
APA StyleLiu, J., Zhao, Y., Hu, R., Zhang, M., & Ding, Y. (2023). Effects of Cu and Ag Elements on Corrosion Resistance of Dual-Phase Fe-Based Medium-Entropy Alloys. Materials, 16(8), 3243. https://doi.org/10.3390/ma16083243






