Corrosion Behavior of Reinforcing Steel in the Immersed Tube Tunnel (ITT) under Submarine Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Mix Ratio
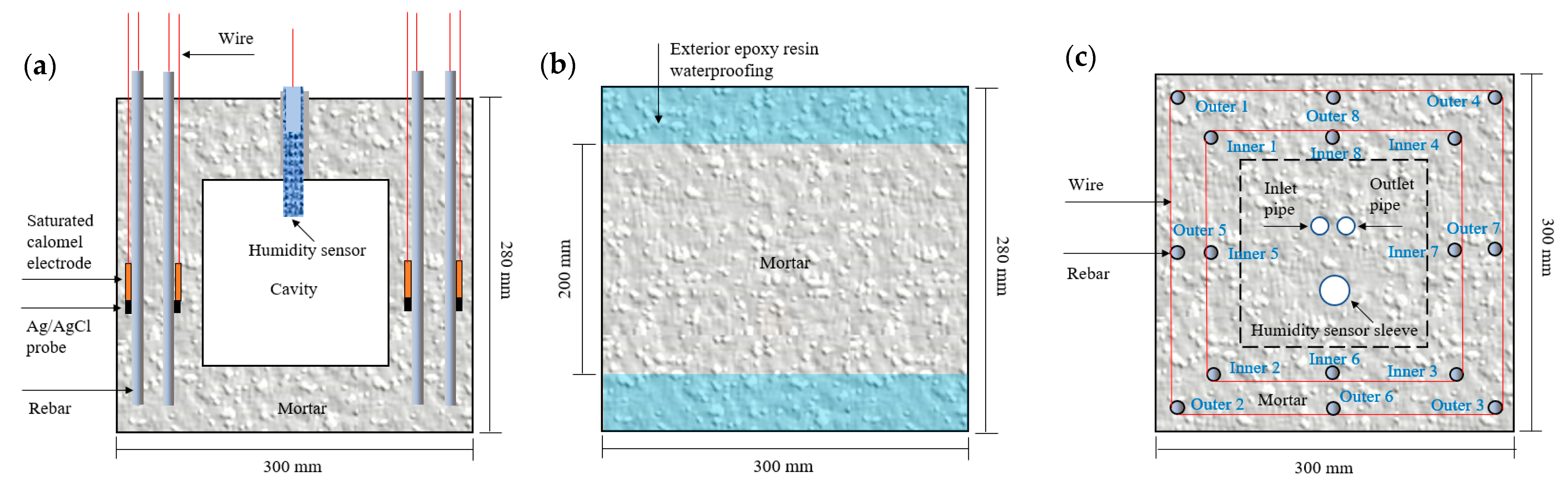
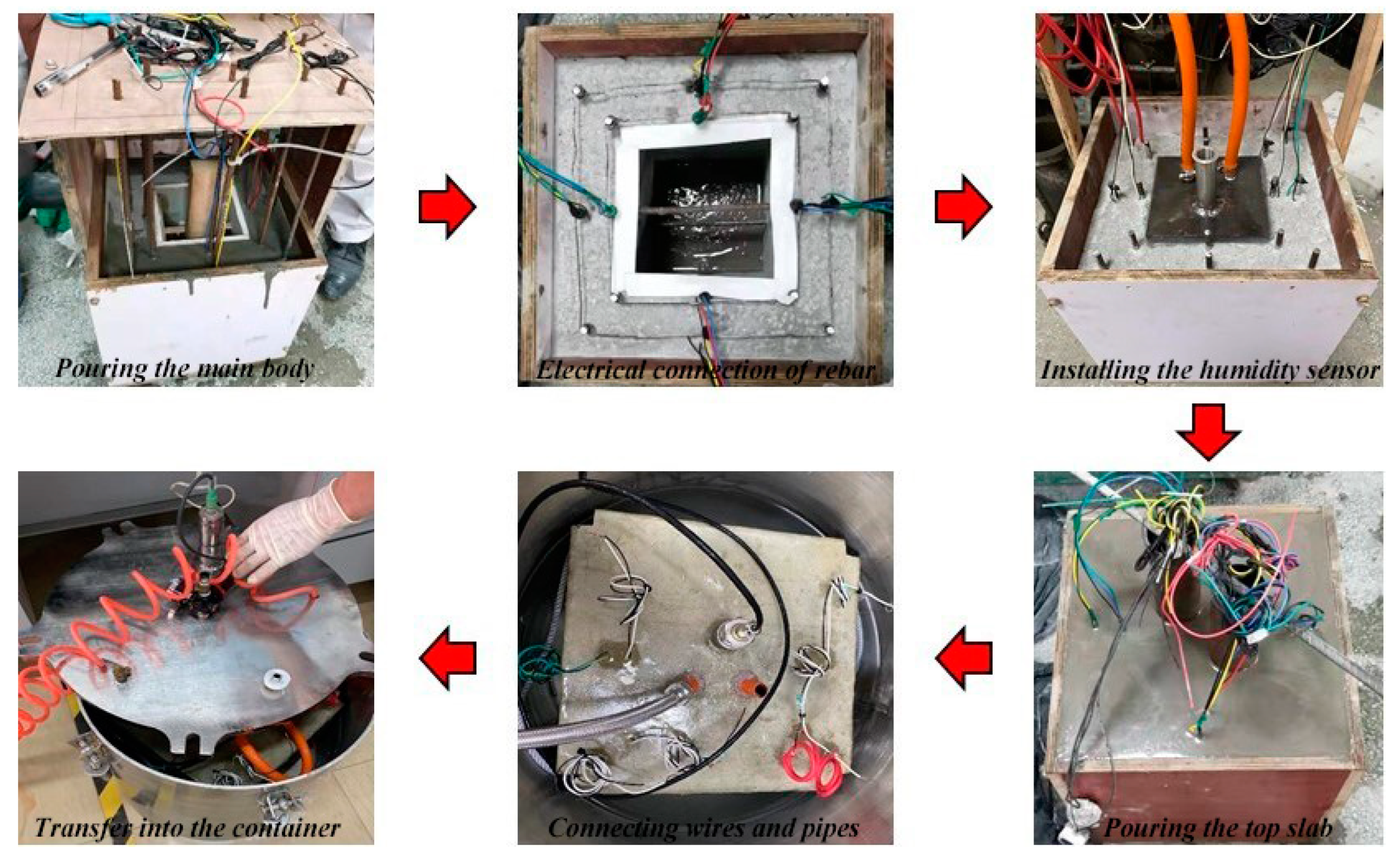
2.2. Design and Fabrication of ITT Scaled-Down Models
2.3. Pressurized Seawater Corrosion
2.4. Electrochemical Test Methods
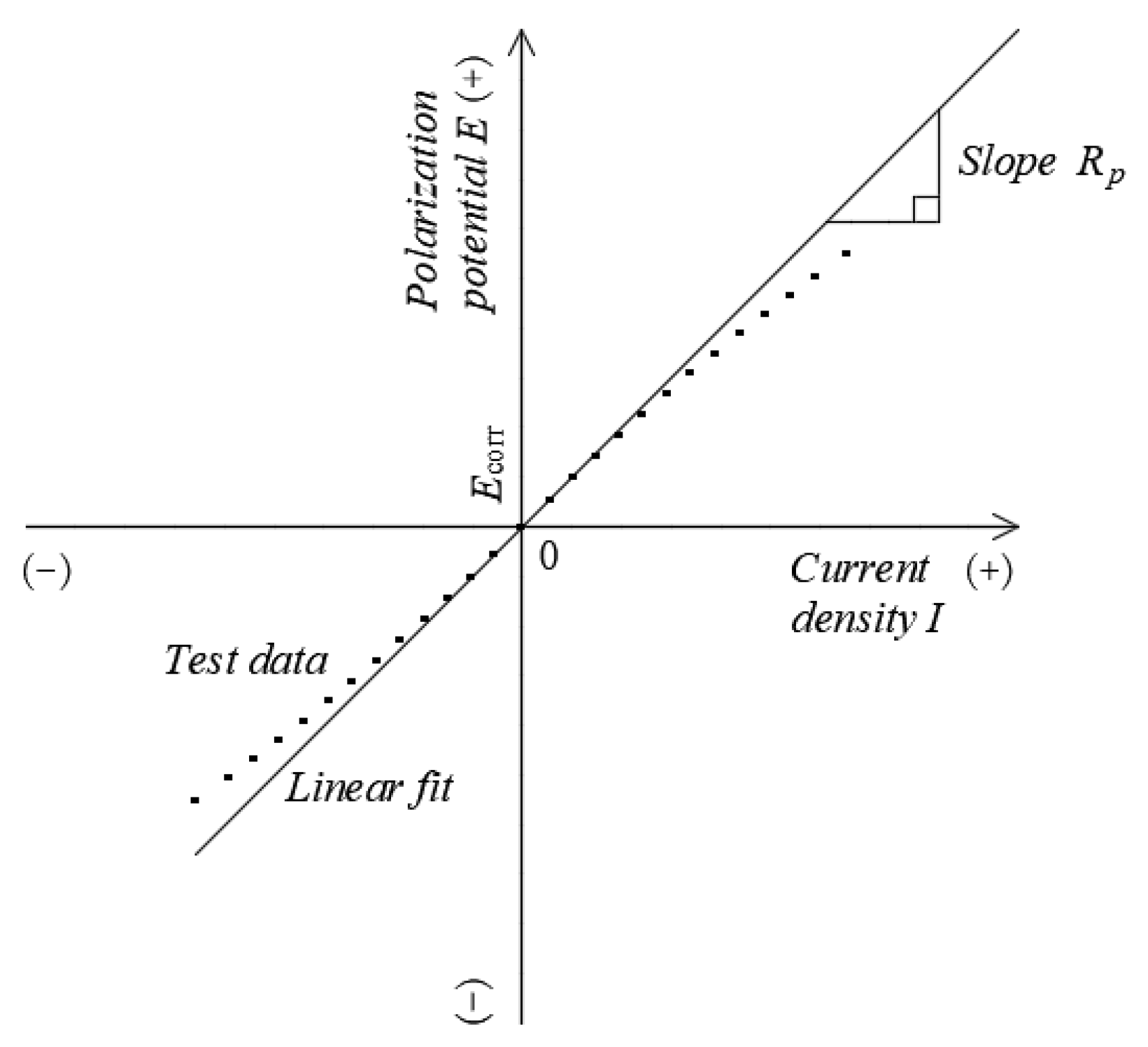
2.4.1. Polarization Curves
2.4.2. AC Impedance Spectra
3. Results
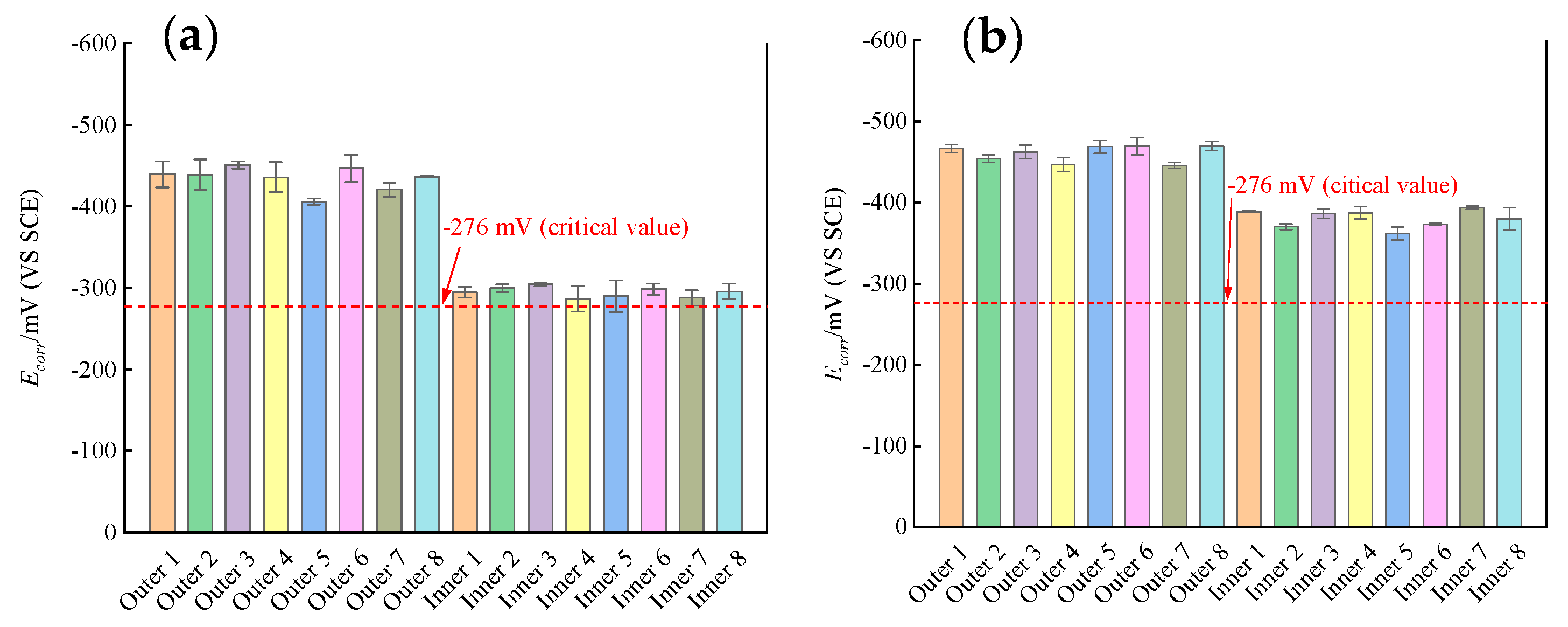
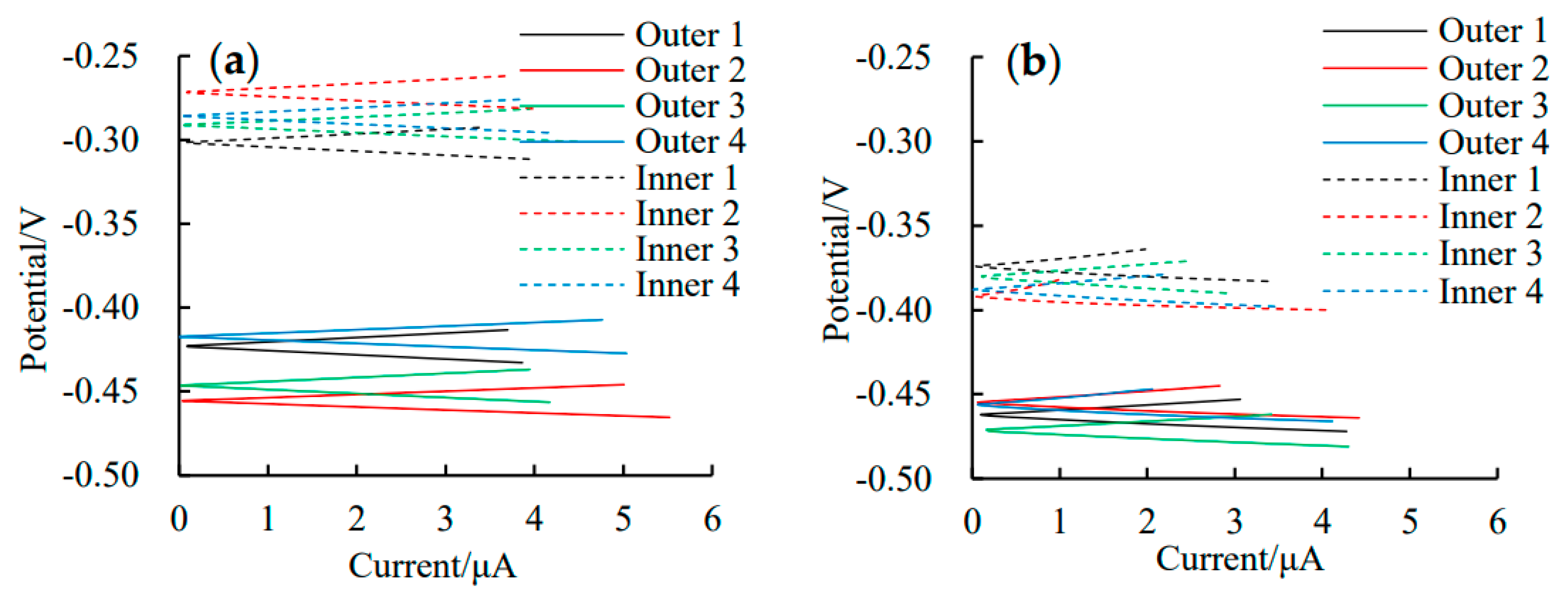
3.1. Self-Corrosion Potential Ecorr
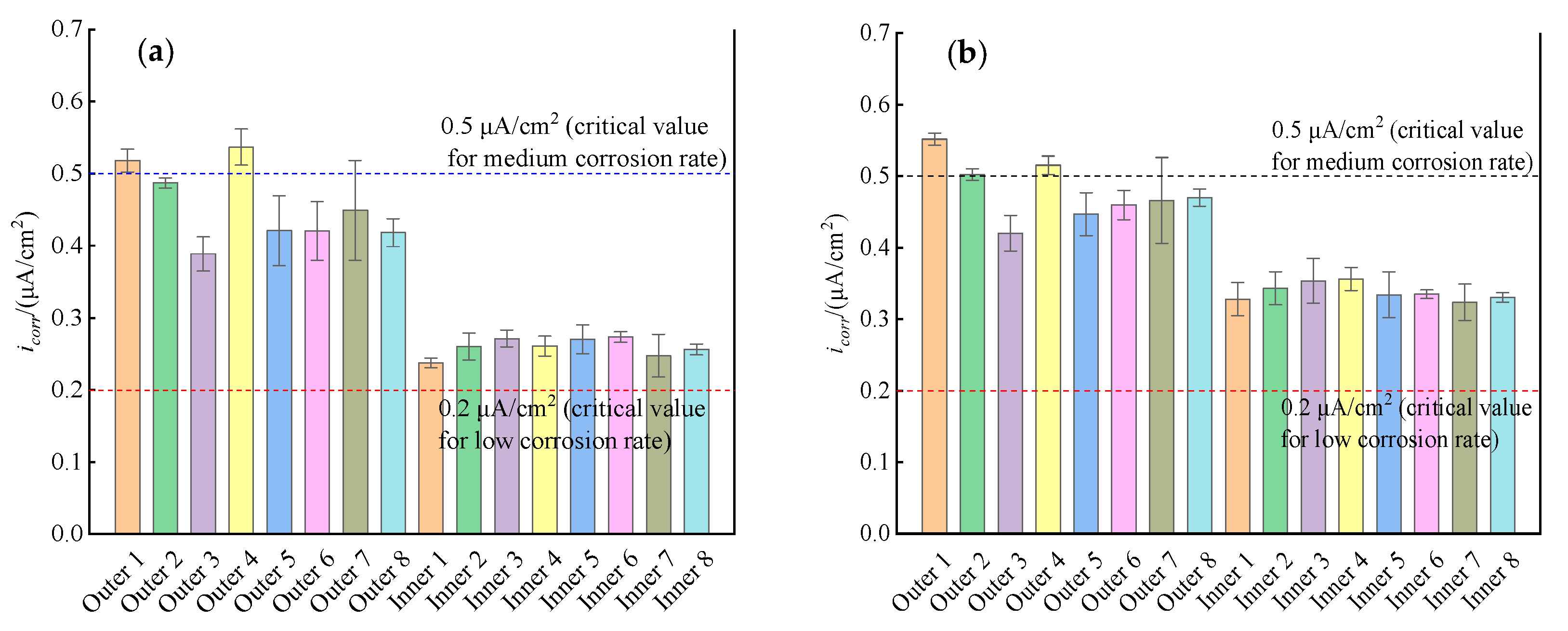
3.2. Linear Polarization Curves
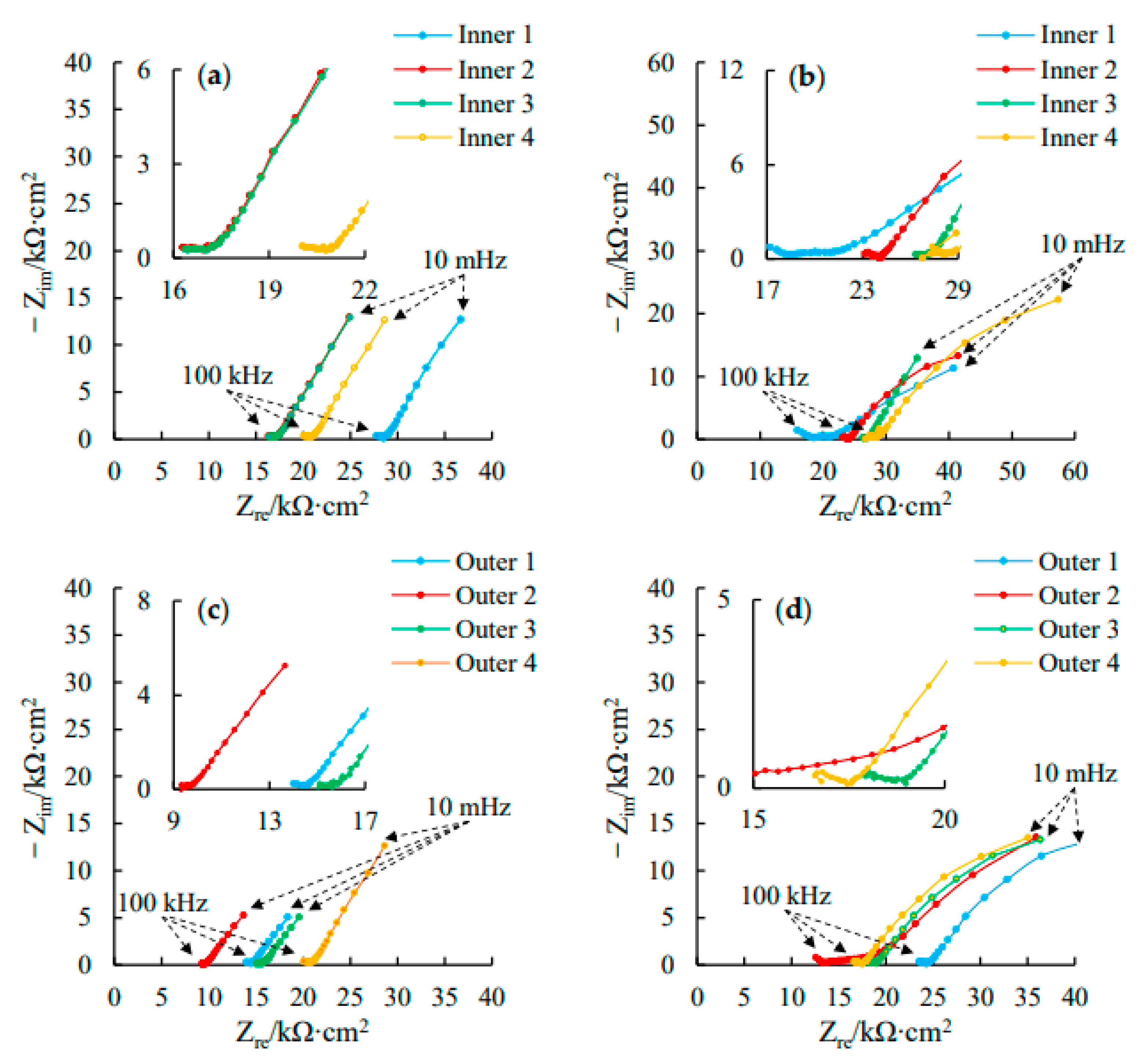
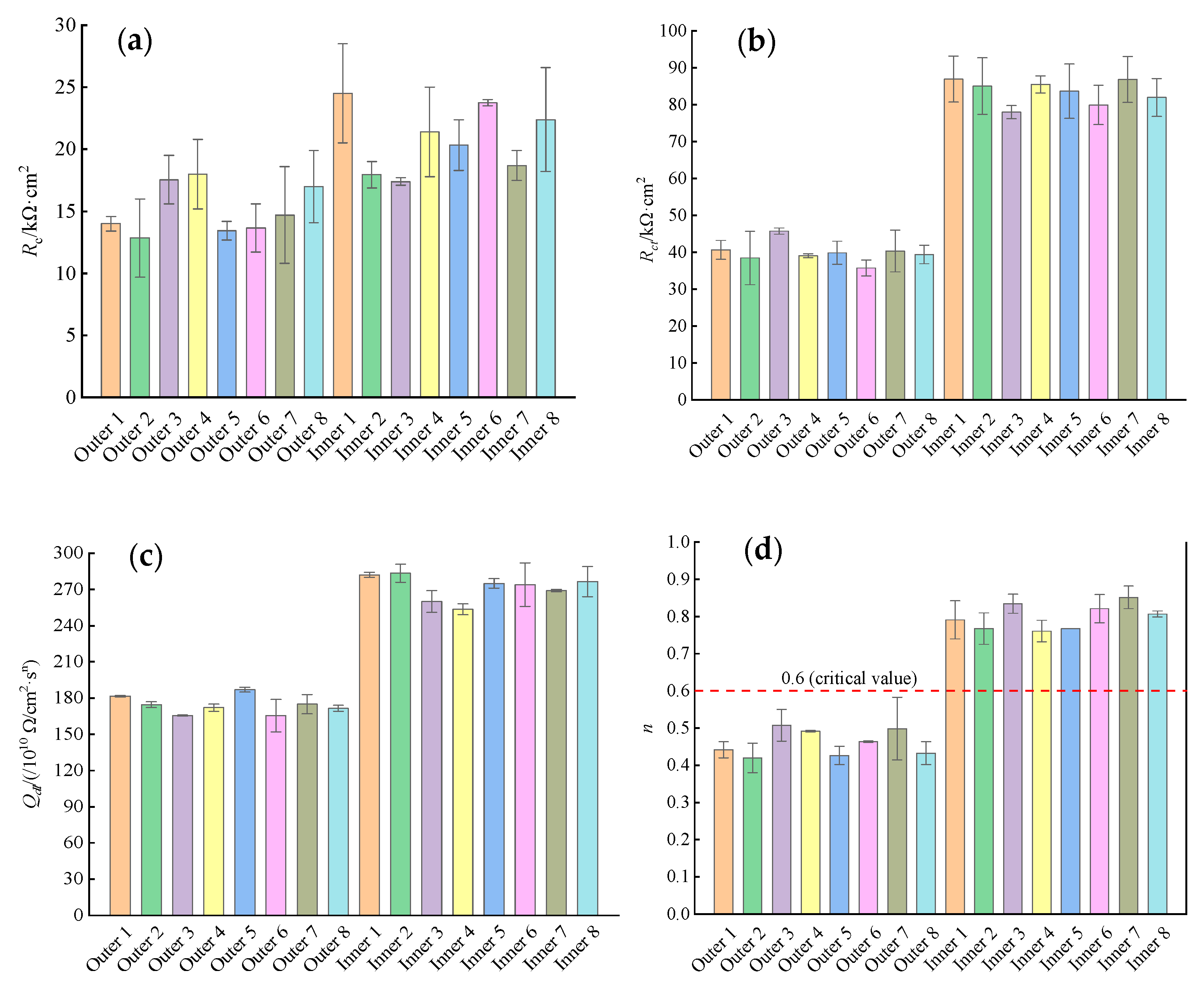
3.3. AC Impedance Spectra
4. Conclusions
- In the ITT models, the corrosion of the rebars on the seawater side happened earlier than the rebars on the cavity side, and the corrosion rate of the rebars on the seawater side was significantly higher than that of the rebars on the cavity side during the 365 d of erosion. The corrosion rate of the seawater side of the rebars maintained a high corrosion rate, while the corrosion rate of the cavity side of the rebars gradually increased with the growth of corrosion time.
- The electrochemical corrosion rates of the rebars on both the seawater side and the cavity side of the ITT models were controlled by anodic polarization, which indicated that chloride ion transport played a key role in the corrosion of the rebars, while oxygen transport had a small effect on the corrosion of the rebars.
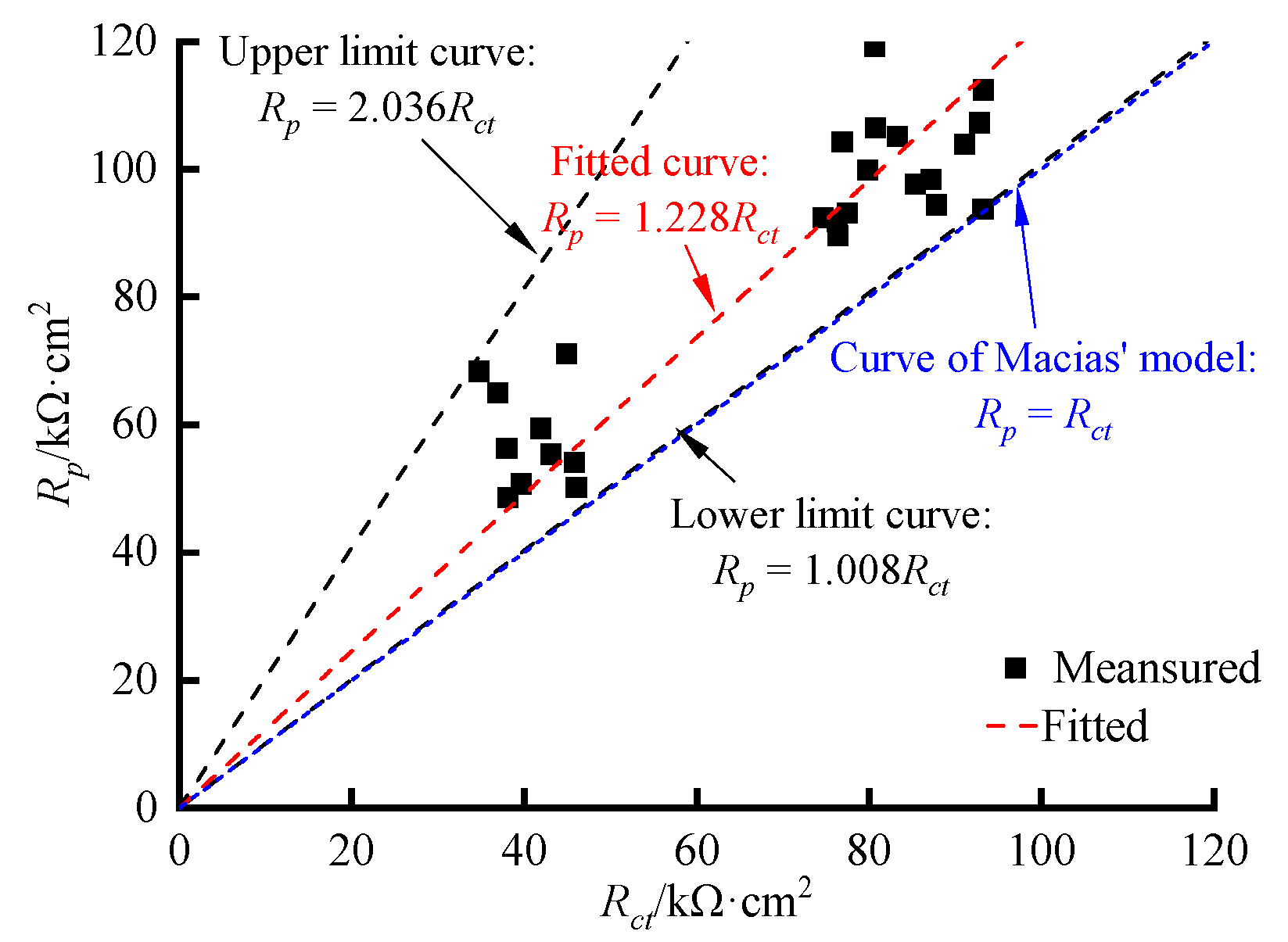
- There was an obvious linear relationship between Rp obtained by the linear polarization curves and Rct obtained by the AC impedance spectra in the reinforced concrete ITT models under the submarine environment, and the linear scale factor of 1.228 was obtained from the regression equation for both.
- Considering the reinforcing steel on the seawater side in the ITT is weaker in durability than that on the cavity side, it is recommended in the durability design to give priority to anti-corrosion methods for the reinforcing steel on the seawater side in order to prolong the service life of the ITT, such as applying polyurethane to the concrete surface on the seawater side to prevent seawater ingress, and using cathodic protection or surface epoxy for the reinforcing steel on the seawater side. Given the large size and high construction cost of the ITT, the results of this work show that it is feasible and more reasonable and economical to adopt durability enhancement methods for parts rather than for the entirety of the structure, while the corresponding service life assessment methods and life cycle cost analysis deserve further study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rasmussen, N.; Grantz, W. Catalogue of immersed tunnels. Tunn. Undergr. Space Technol. 1997, 12, 163–316. [Google Scholar] [CrossRef]
- Li, K.; Pang, X.; Dangla, P. Thermo–hydro–ionic transport in sea immersed tube tunnel. Tunn. Undergr. Space Technol. Inc. Trenchless Technol. Res. 2016, 58, 147–158. [Google Scholar] [CrossRef]
- Chen, W.; Huang, L.; Wang, D.; Liu, C.; Xu, L.; Ding, Z. Effects of siltation and desiltation on the wave–induced stability of foundation trench of immersed tunnel. Soil Dyn. Earthq. Eng. 2022, 160, 107360. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, G.; Bao, C.; Xu, J.; Sun, F. Spatial and economic effects of the Bohai Strait Cross–Sea Channel on the transportation accessibility in China. Appl. Geogr. 2017, 83, 86–99. [Google Scholar] [CrossRef]
- Guo, Y. Key construction technology research of Qingdao Jiaozhou Bay Subsea Tunnel. Constr. Technol. 2022, 51, 2022. [Google Scholar]
- Rasmussen, N. Concrete immersed tunnels—Forty years of experience. Tunn. Undergr. Space Technol. 1997, 12, 33–46. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Jing, G. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Marques, P.; Costa, A. Service life of RC structures: Carbonation induced corrosion. Prescriptive vs. performance–based methodologies. Constr. Build. Mater. 2010, 24, 258–265. [Google Scholar] [CrossRef]
- Hussain, R.; Ishida, T.; Wasim, M. Oxygen transport and corrosion of steel in concrete under varying concrete cover, w/c, and moisture. ACI Mater. J. 2012, 109, 3–10. [Google Scholar] [CrossRef]
- Diamond, S. Two Danish flyashes on alkali contents of pore solutions of cement–flyash pastes. Cem. Concr. Res. 1981, 11, 383–394. [Google Scholar] [CrossRef]
- Warkus, J.; Raupach, M. Modelling of reinforcement corrosion–geometrical effects on macrocell corrosion. Mater. Corros. 2009, 61, 494–504. [Google Scholar] [CrossRef]
- Hansson, C.; Poursaee, A.; Laurent, A. Macrocell and microcell corrosion of steel in ordinary Portland cement and high–performance concretes. Cem. Concr. Res. 2006, 36, 2098–2102. [Google Scholar] [CrossRef]
- Chalhoub, C.; Francois, R.; Garcia, D.; Laurens, S.; Carcasses, M. Macrocell corrosion of steel in concrete: Characterization of anodic behavior in relation to the chloride content. Mater. Corros. 2020, 71, 1424–1441. [Google Scholar] [CrossRef]
- Chen, L.; Kai, R.; Su, L. On the corrosion rate measurement of reinforcing steel in chloride–induced macrocell corrosion. Cem. Concr. Compos. 2022, 134, 104775. [Google Scholar] [CrossRef]
- Li, J.; Xiong, J.; Fan, Z.; Chen, M.; Sun, L.; Zhu, C.; Liu, W.; Zheng, H.; Li, W. Mechanistic study of effect on corrosion initiation and propagation of reinforcement in submarine immersed tunnel microcell. Cem. Concr. Compos. 2023, 136, 104890. [Google Scholar] [CrossRef]
- Lliso–Ferrando, J.; Gasch, I.; Martinez–Ibernon, A.; Valcuende, M. Effect of macrocell currents on rebar corrosion in reinforced concrete structures exposed to a marine environment. Ocean. Eng. 2022, 257, 111680. [Google Scholar] [CrossRef]
- Chen, D.; Mei, G.; Xiao, L. Analysis of chloride invasion process in undersea RC circular lined tunnel based on nonlinear ADE theory: Analytical solution, numerical verification and experimental prediction. Constr. Build. Mater. 2022, 343, 128140. [Google Scholar] [CrossRef]
- Li, C.; Chen, Q.; Wang, R.; Wu, M.; Jiang, Z. Corrosion assessment of reinforced concrete structures exposed to chloride environments in underground tunnels: Theoretical insights and practical data interpretations. Cem. Concr. Compos. 2020, 112, 103652. [Google Scholar] [CrossRef]
- Gao, W.; Chen, X.; Chen, D. Genetic programming approach for predicting service life of tunnel structures subject to chloride–induced corrosion. J. Adv. Res. 2019, 20, 141–152. [Google Scholar] [CrossRef]
- Hu, X.; He, C.; Feng, K.; Liu, S.; Walton, G. Effects of polypyrrole coated rebar on corrosion behavior of tunnel lining with the combination effect of sustained loading and pre-existing cracks when exposed to chlorides. Constr. Build. Mater. 2019, 221, 318–331. [Google Scholar] [CrossRef]
- Liu, H.; Song, K.; Ye, Z.; Wang, C.; Liu, H. Seismic fragility analysis of in-service shield tunnels considering surface building and joint-bolt corrosion. Soil Dyn. Earthq. Eng. 2022, 162, 107455. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, Y.; Li, C.; Chen, H.; Chen, Z. Seismic responses of long segmental immersed tunnel under unfavorable loads combination. Transp. Geotech. 2021, 30, 100621. [Google Scholar] [CrossRef]
- Xu, L.; Chen, L.; Fang, Q.; Dong, Y. Blast resistance of a folded arch cross–section immersed tunnel subjected to internal explosion. Tunn. Undergr. Space Technol. 2022, 125, 104521. [Google Scholar] [CrossRef]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Redaelli, E.; Polde, R. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair, 2nd ed.; Wiley–VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Sanchez, L.; Fricker, T.; Cong, H. AC induced pitting and pit–to–crack transition of low carbon steels under cathodic protection. Corros. Sci. 2022, 203, 110335. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Dehghanian, C.; Kosari, A. Corrosion protection of the reinforcing steels in chloride–laden concrete environment through epoxy/polyaniline-camphorsulfonate nanocomposite coating. Corros. Sci. 2015, 90, 239–247. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, H.; Ma, H.; Da, B.; Mei, Q. Effect of rust inhibitors and surface strengthening materials on service life of marine RC structures. Anti-Corros. Methods Mater. 2021, 68, 255–268. [Google Scholar] [CrossRef]
- Bastidas, D.; Martin, U.; Bastidas, J.; Ress, J. Corrosion inhibition mechanism of steel reinforcements in mortar using soluble phosphates: A critical review. Materials 2021, 14, 6168. [Google Scholar] [CrossRef]
- Liu, B.; Shi, J.; Sun, M.; He, Z.; Xu, H.; Tan, J. Mechanical and permeability properties of polymer-modified concrete using hydrophobic agent. J. Build. Eng. 2020, 31, 101337. [Google Scholar] [CrossRef]
- Gou, J.; Wang, G.; Ning, Y.; Guan, L.; Zhang, Y.; Liao, J.; Wang, Y. Preparation and corrosion resistance of chromium-free Zn–Al coatings with two different silane coupling agents. Surf. Coat. Technol. 2019, 366, 1–6. [Google Scholar] [CrossRef]
- Lafhaj, Z.; Goueygou, M.; Djerbi, A.; Kaczmarek, M. Correlation between porosity, permeability and ultrasonic parameters of mortar with variable water/cement ratio and water content. Cem. Concr. Res. 2006, 36, 625–633. [Google Scholar] [CrossRef]
- Ramli, M.; Tabassi, A.; Hoe, K. Porosity, pore structure and water absorption of polymer-modified mortars: An experimental study under different curing conditions. Compos. Part B Eng. 2013, 55, 221–233. [Google Scholar] [CrossRef]
- ASTM C192/C192M–18; Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory. ASTM International: West Conshohocken, PA, USA, 2018.
- Tian, Y.; Wen, C.; Wang, G.; Hu, J.; Mai, Z. Marine field test for steel reinforcement embedded in mortar: Coupled influence of the environmental conditions on corrosion. Mar. Struct. 2020, 73, 102788. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, L. Influence of hydrostatic pressure and cationic type on the diffusion behavior of chloride in concrete. Materials 2021, 14, 2851. [Google Scholar] [CrossRef] [PubMed]
- ASTM G3–2014; Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing. ASTM International: West Conshohocken, PA, USA, 2014.
- Andrade, C.; Keddam, M.; Novoa, X.; Perez, M.; Takenouti, H. Electrochemical behaviour of steel rebars in concrete: Influence of environmental factors and cement chemistry. Electrochimica Acta 2001, 46, 3905–3912. [Google Scholar] [CrossRef]
- Jamil, H.; Shriri, A.; Boulif, R.; Montemor, M.; Ferreira, M. Corrosion behaviour of reinforcing steel exposed to an amino alcohol–based corrosion inhibitor. Cem. Concr. Compos. 2005, 27, 671–678. [Google Scholar] [CrossRef]
- ASTM C876–2015; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM International: West Conshohocken, PA, USA, 2015.
- Poursaee, A. Corrosion of Steel in Concrete Structures; Woodhead Publishing: Duxford, UK, 2016; pp. 1–10. [Google Scholar]
- Li, G.; Yang, B.; Guo, C.; Du, J.; Wu, X. Time dependence and service life prediction of chloride resistance of concrete coatings. Constr. Build. Mater. 2015, 83, 19–25. [Google Scholar] [CrossRef]
- Bastidas, D.; Cobo, A.; Otero, E.; González, J. Electrochemical rehabilitation methods for reinforced concrete structures: Advantages and pitfalls. Corros. Eng. Sci. Technol. 2008, 43, 248–255. [Google Scholar] [CrossRef]
- Chen, C.; Cai, J.; Liu, J.; Liu, J. Use of aminoalcohol as in inhibitor in chloride–contaminated steel reinforced concrete. J. Chin. Ceram. Soc. 2015, 43, 393–399. [Google Scholar] [CrossRef]
- Macias, A. Comparison of different electrochemical techniques for corrosion-rate determination of zinc-coated reinforcements in simulated concrete pore solutions. Mater. Struct. 1991, 24, 456–465. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, H.; Ma, H. Electrochemical study on effect of rust inhibitors on corrosion of reinforcing bar in concrete in marine environment. J. Southeast Univ. (Nat. Sci. Ed.) 2020, 50, 109–119. [Google Scholar] [CrossRef]

| LOI | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | MnO | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.37 | 19.26 | 4.70 | 3.28 | 64.91 | 3.46 | 2.05 | 0.83 | 0.14 | - | - |
| Initial Setting Time/min | Final Setting Time/min | Specific Surface Area/(m2/kg) | Standard Consistency Water Consumption% | Stability | Compressive Strength/MPa | Flexural Strength/MPa | ||
|---|---|---|---|---|---|---|---|---|
| 3 d | 28 d | 3 d | 28 d | |||||
| 113 | 180 | 445.9 | 26.1 | qualified | 28.5 | 50.6 | 4.7 | 8.0 |
| Materials/(kg/m3) | w/c | Fluidity /mm | 28 d Flexural Strength/MPa | 28 d Compressive Strength/MPa | 28 d DRCM /(m2/s) | ||
|---|---|---|---|---|---|---|---|
| Cement | Sand | Water | |||||
| 486 | 751 | 243 | 0.5 | 163 | 6.2 | 34.6 | 15.626/1012 |
| Fe | C | Mn | Si | P | S |
|---|---|---|---|---|---|
| 98.197 | 0.168 | 1.425 | 0.160 | 0.023 | 0.027 |
| Number of Rebars | Rc | Rct | Qdl | n | ||||
|---|---|---|---|---|---|---|---|---|
| Fitting Result/ kΩ∙cm2 | Standard Error | Fitting Result/ kΩ∙cm2 | Standard Error | Fitting Result/(/1010 Ω/cm2∙sn) | Standard Error | Fitting Result | Standard Error | |
| Model 1−Outer 1 | 14.6 | 4.3% | 43.2 | 2.0% | 181 | 10.9% | 0.42 | 13.4% |
| Model 1−Outer 2 | 9.7 | 4.0% | 31.2 | 2.2% | 172 | 8.9% | 0.38 | 10.3% |
| Model 1−Outer 3 | 15.6 | 3.8% | 46.6 | 5.9% | 166 | 21.0% | 0.55 | 30.3% |
| Model 1−Outer 4 | 20.8 | 3.8% | 38.5 | 5.6% | 175 | 21.4% | 0.49 | 30.9% |
| Model 1−Outer 5 | 14.2 | 4.6% | 36.7 | 1.8% | 189 | 5.8% | 0.45 | 7.0% |
| Model 1−Outer 6 | 15.6 | 4.2% | 33.6 | 1.7% | 152 | 6.0% | 0.46 | 9.0% |
| Model 1−Outer 7 | 18.6 | 8.4% | 46.0 | 3.6% | 167 | 17.3% | 0.41 | 26.1% |
| Model 1−Outer 8 | 14.1 | 3.9% | 36.9 | 8.7% | 174 | 11.3% | 0.46 | 3.2% |
| Model 1−Inner 1 | 28.5 | 11.4% | 93.2 | 3.7% | 284 | 20.1% | 0.74 | 12.2% |
| Model 1−Inner 2 | 16.9 | 8.0% | 77.4 | 1.7% | 276 | 5.3% | 0.81 | 6.4% |
| Model 1−Inner 3 | 17.1 | 8.2% | 76.2 | 1.7% | 269 | 11.4% | 0.86 | 14.5% |
| Model 1−Inner 4 | 17.8 | 7.3% | 83.2 | 4.1% | 258 | 7.2% | 0.79 | 8.8% |
| Model 1−Inner 5 | 22.4 | 7.8% | 91.0 | 5.0% | 279 | 5.1% | 0.77 | 6.8% |
| Model 1−Inner 6 | 24.0 | 6.6% | 85.3 | 3.7% | 256 | 5.9% | 0.86 | 9.2% |
| Model 1−Inner 7 | 17.5 | 6.1% | 80.6 | 1.6% | 268 | 5.1% | 0.82 | 6.2% |
| Model 1−Inner 8 | 26.6 | 7.1% | 87.1 | 1.8% | 289 | 4.9% | 0.80 | 5.1% |
| Model 2−Outer 1 | 13.4 | 15.9% | 38.1 | 1.3% | 182 | 13.4% | 0.46 | 14.8% |
| Model 2−Outer 2 | 16.0 | 10.9% | 45.7 | 11.3% | 177 | 7.3% | 0.46 | 5.6% |
| Model 2−Outer 3 | 19.5 | 7.0% | 44.9 | 12.4% | 165 | 5.6% | 0.47 | 16.7% |
| Model 2−Outer 4 | 15.2 | 6.7% | 39.6 | 11.5% | 169 | 7.5% | 0.49 | 3.7% |
| Model 2−Outer 5 | 12.7 | 3.8% | 43.0 | 7.9% | 185 | 14.0% | 0.40 | 21.4% |
| Model 2−Outer 6 | 11.7 | 10.4% | 37.9 | 14.9% | 179 | 7.2% | 0.47 | 8.1% |
| Model 2−Outer 7 | 10.8 | 4.8% | 34.7 | 3.5% | 183 | 11.4% | 0.58 | 16.2% |
| Model 2−Outer 8 | 19.9 | 6.6% | 41.9 | 9.5% | 169 | 4.2% | 0.40 | 9.7% |
| Model 2−Inner 1 | 20.5 | 4.0% | 80.7 | 6.0% | 280 | 17.9% | 0.84 | 5.4% |
| Model 2−Inner 2 | 19.0 | 13.3% | 92.7 | 13.6% | 291 | 7.6% | 0.73 | 15.6% |
| Model 2−Inner 3 | 17.7 | 6.1% | 79.8 | 9.5% | 251 | 7.2% | 0.81 | 10.9% |
| Model 2−Inner 4 | 25.0 | 10.4% | 87.8 | 9.8% | 249 | 5.4% | 0.73 | 9.2% |
| Model 2−Inner 5 | 18.3 | 9.4% | 76.3 | 6.3% | 271 | 4.6% | 0.77 | 12.8% |
| Model 2−Inner 6 | 23.5 | 8.6% | 74.6 | 6.7% | 292 | 7.2% | 0.78 | 6.2% |
| Model 2−Inner 7 | 19.9 | 8.4% | 93.1 | 4.0% | 270 | 14.0% | 0.88 | 7.1% |
| Model 2−Inner 8 | 18.2 | 5.5% | 76.8 | 10.3% | 264 | 4.3% | 0.82 | 9.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhu, H.; Fan, Z.; Zhao, J.; Jiang, S. Corrosion Behavior of Reinforcing Steel in the Immersed Tube Tunnel (ITT) under Submarine Environment. Materials 2023, 16, 3300. https://doi.org/10.3390/ma16093300
Yan Y, Zhu H, Fan Z, Zhao J, Jiang S. Corrosion Behavior of Reinforcing Steel in the Immersed Tube Tunnel (ITT) under Submarine Environment. Materials. 2023; 16(9):3300. https://doi.org/10.3390/ma16093300
Chicago/Turabian StyleYan, Yu, Haiwei Zhu, Zhihong Fan, Jiaqi Zhao, and Shuping Jiang. 2023. "Corrosion Behavior of Reinforcing Steel in the Immersed Tube Tunnel (ITT) under Submarine Environment" Materials 16, no. 9: 3300. https://doi.org/10.3390/ma16093300
APA StyleYan, Y., Zhu, H., Fan, Z., Zhao, J., & Jiang, S. (2023). Corrosion Behavior of Reinforcing Steel in the Immersed Tube Tunnel (ITT) under Submarine Environment. Materials, 16(9), 3300. https://doi.org/10.3390/ma16093300







