Molecular Dynamics Simulation of Silane Inserted CSH Nanostructure
Abstract
:1. Introduction
2. Simulation Method
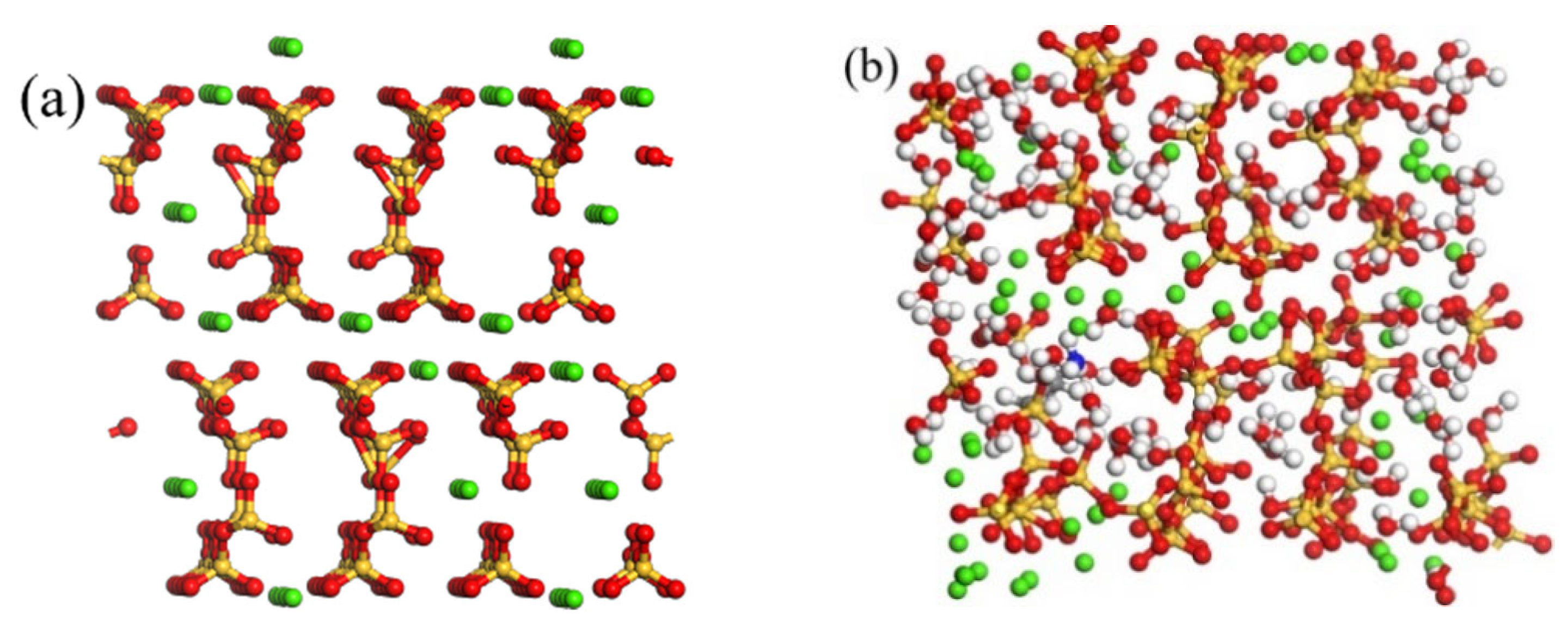
2.1. Models
2.2. Force Field
3. Molecular Dynamics Simulation Assessment
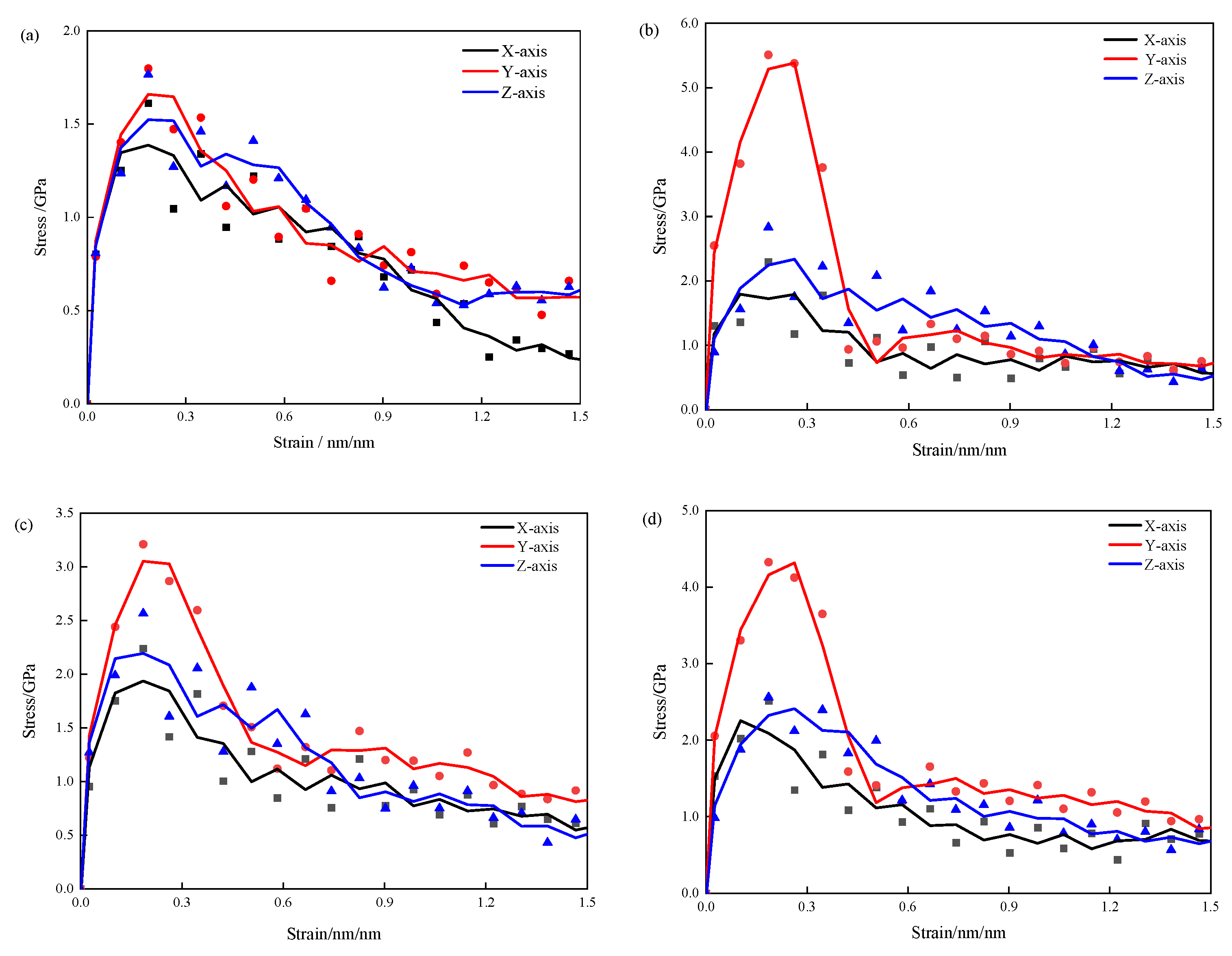
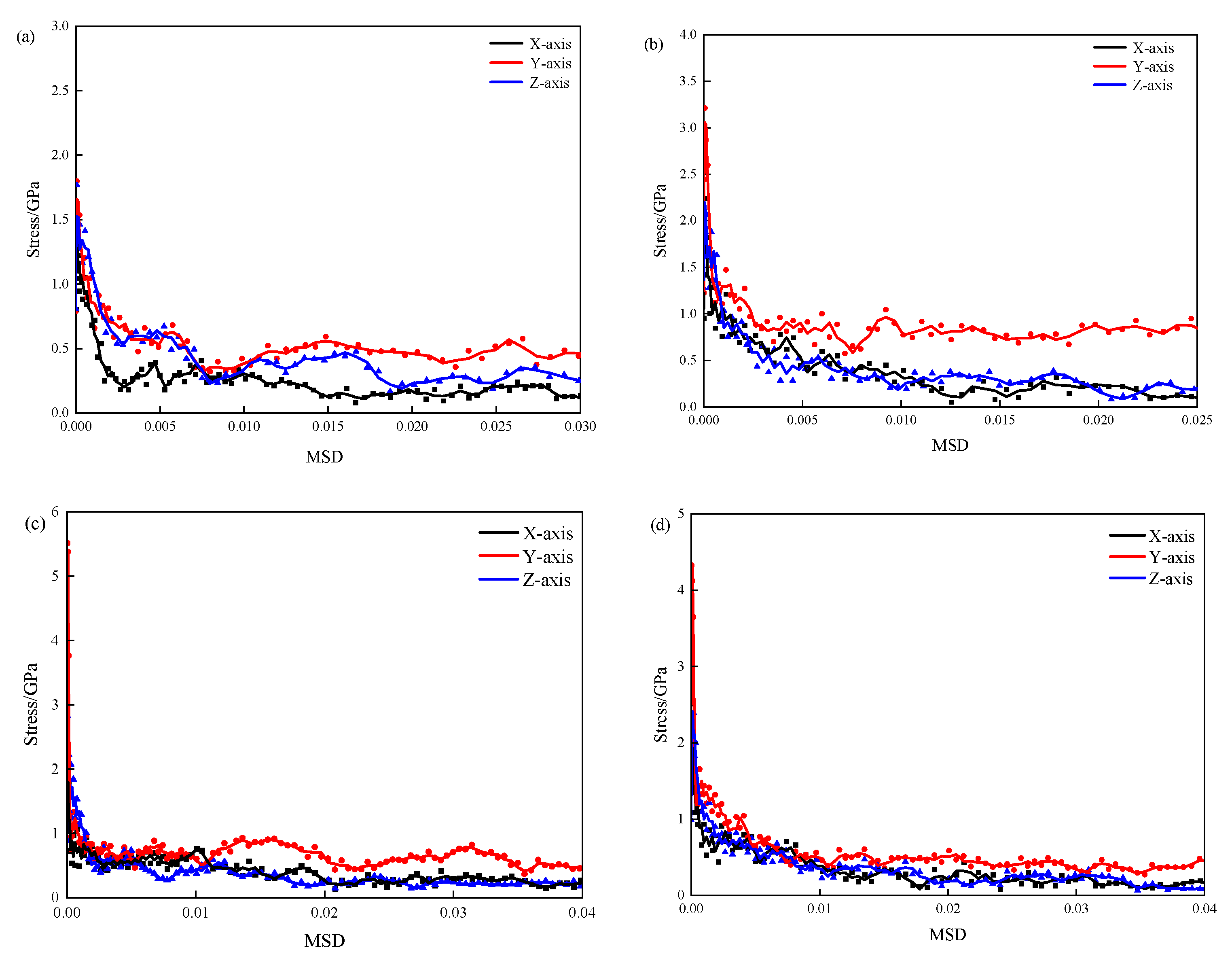
3.1. Tensile Mechanical Properties
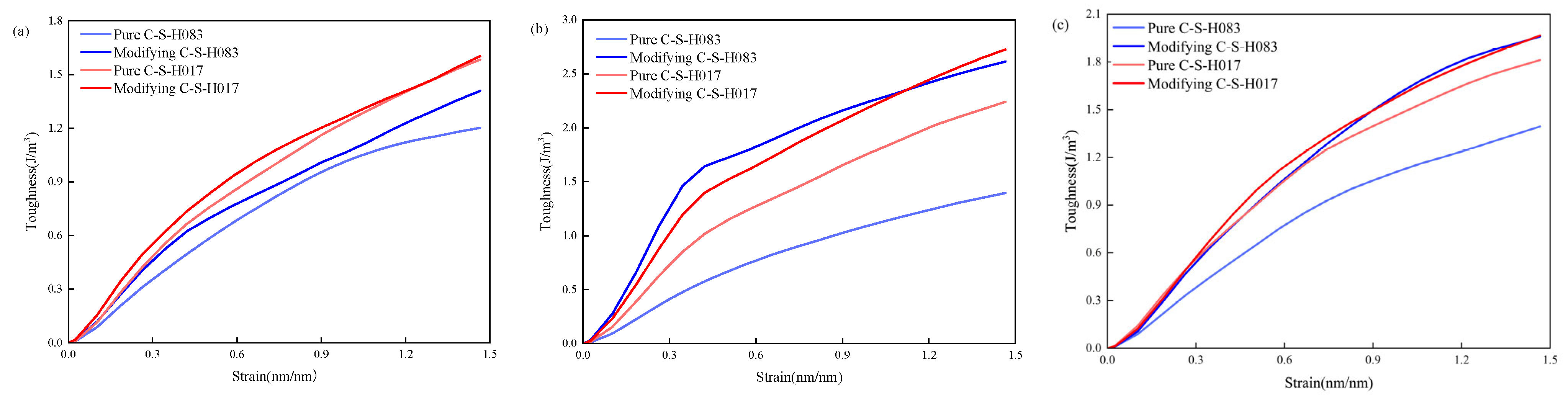
3.2. Toughness Performance
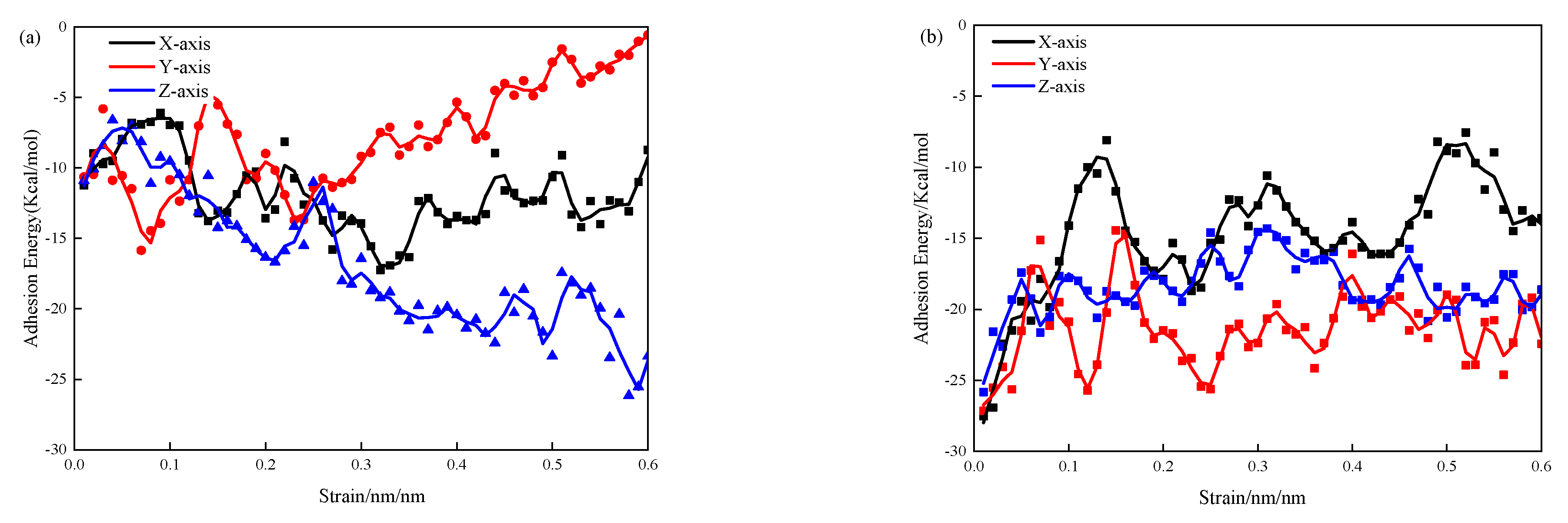
3.3. Adsorption Energy
3.4. Mean Square Displacement
3.5. Radial Distribution Function
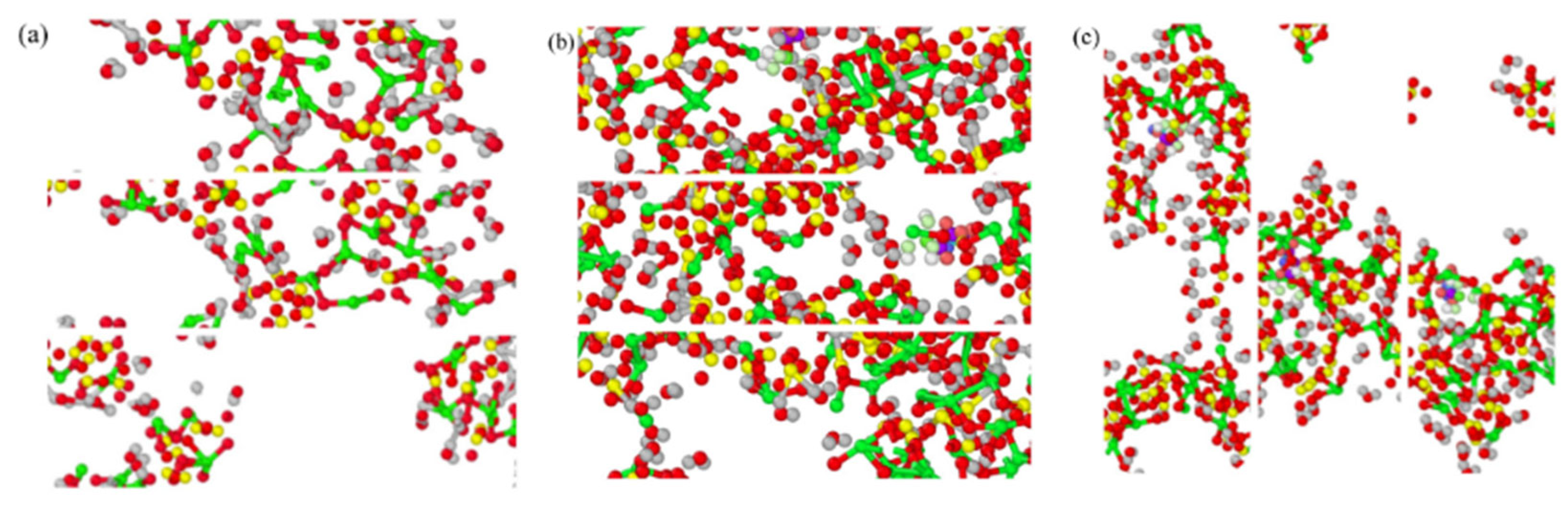
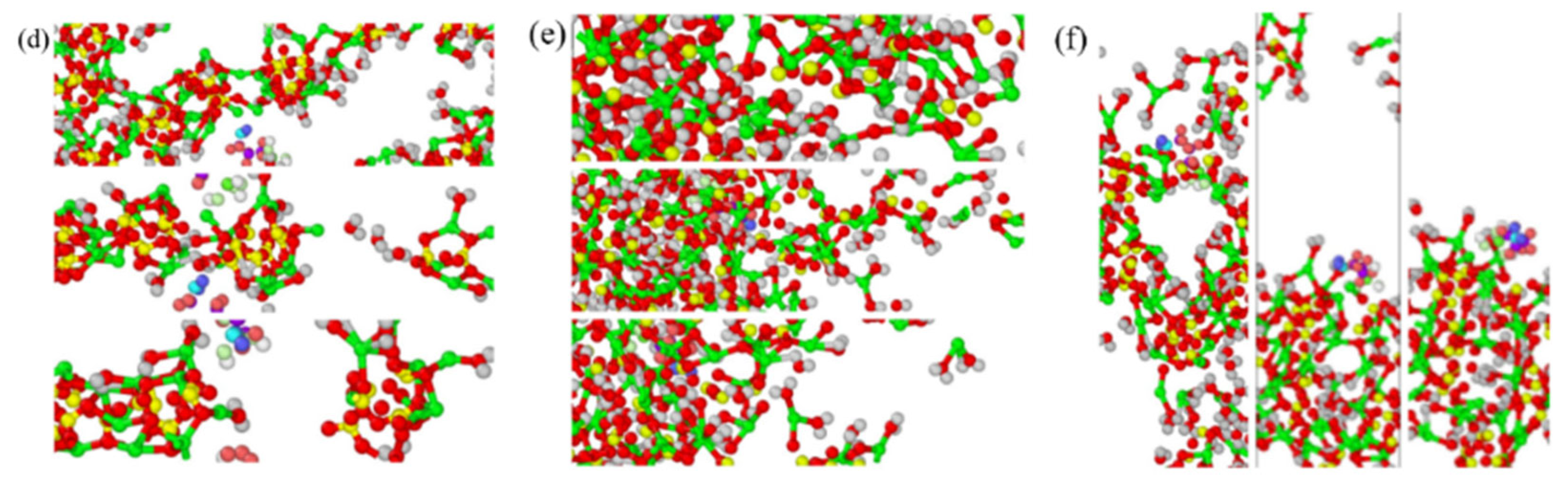
3.6. Tensile Dynamic Analysis
4. Conclusions
- The 3-APTES modified CSH structure exhibits better tensile properties than the pure CSH structure. Compared with the pure CSH structure, the maximum tensile stress of the 3-APTES modified CSH structure along the x-, y-, and z-axes increases by 45.4%, 78.6%, and 38.9%, respectively, at a 0.83 Ca/Si ratio. Moreover, at a 1.7 Ca/Si ratio, the maximum tensile stress along the x-, y-, and z-axes increases by 13.5%, 63.3%, and 16.6%, respectively.
- The toughness of the 3-APTES modified CSH structure is superior to that of the pure CSH structure. At a 0.83 Ca/Si ratio, the En values of the 3-APTES modified CSH structure along the x-, y-, and z-axes increase by 17.5%, 86.4%, and 41.0%, respectively, compared to the pure CSH structure. Moreover, at a 1.7 Ca/Si ratio, the En values along the x-, y-, and z-axes increase by 1.3%, 21.9%, and 8.8%, respectively.
- The incorporation of 3-APTES increases the adsorption energy of water molecules on the CSH structure model and silica tetrahedral skeleton. At a 0.83 Ca/Si ratio, the adsorption energy of the 3-APTES modified CSH structure along the x-, y-, and z-axes increases by 23.5%, 94.9%, and −97.0%, respectively, compared to the pure CSH structure. Moreover, at a 1.7 Ca/Si ratio, the adsorption energy along the x-, y-, and z-axes increases by 19.2%, 24.5%, and 25.4%, respectively. 3-APTES molecules can effectively increase the atom density within the CSH structure model. As the Ca/Si ratio increases, the Ca–O bond formation is enhanced, with noticeable aggregation occurring because of the 3-APTES-based CSH structural modification.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonaccorsi, E.; Merlino, S.; Taylor, H.F.W. The crystal structure of jennite, Ca9Si6O18(OH)6 8H2O. Cem. Concr. Res. 2003, 34, 1481–1488. [Google Scholar] [CrossRef]
- Akono, A.T. Nanostructure and Fracture Behavior of Carbon Nanofiber-Reinforced Cement Using Nanoscale Depth-Sensing Methods. Materials 2020, 13, 3837. [Google Scholar] [CrossRef] [PubMed]
- Zehtab, B.; Tarighat, A. Diffusion study for chloride ions and water molecules in C-S-H gel in nano-scale using molecular dynamics: Case study of tobermorite. Adv. Concr. Constr. 2016, 4, 305–317. [Google Scholar] [CrossRef]
- Plank, J.; Schönlein, M.; Kanchanason, V. Study on the early crystallization of calcium silicate hydrate (C-S-H) in the presence of polycarboxylate superplasticizers. J. Organomet. Chem. 2018, 869, 227–232. [Google Scholar] [CrossRef]
- Matsuyama, H.; Young, F.J. Synthesis of calcium silicate hydrate/polymer complexes: Part I. Anionic and nonionic polymers. J. Mater. Res. 1999, 14, 3379–3388. [Google Scholar] [CrossRef]
- Bahraq, A.A.; Al-Osta, M.A.; Obot, I.B.; Al-Amoudi, O.S.; Saleh, T.A.; Maslehuddin, M. Improving the adhesion properties of cement/epoxy interface using graphene-based nanomaterials: Insights from molecular dynamics simulation. Cem. Concr. Comp. 2022, 134, 104801. [Google Scholar] [CrossRef]
- Jennings, H.M. Refinements to colloid model of C-S-H in cement: CM-II. Cem. Concr. Res. 2007, 38, 275–289. [Google Scholar] [CrossRef]
- Kamali, M.; Ghahremaninezhad, A. Effect of Biomolecules on the Nanostructure and Nanomechanical Property of Calcium-Silicate-Hydrate. Sci. Rep. 2018, 8, 9491. [Google Scholar] [CrossRef]
- Liang, G.W.; Luo, L.; Wu, Y. Reusing waste red brick powder as partial mineral precursor in eco-friendly binders: Reaction kinetics, microstructure and life-cycle assessment. Resour. Conserv. Recycl. 2022, 185, 106523. [Google Scholar] [CrossRef]
- Dal Sasso, G.; Dalconi, M.C.; Ferrari, G.; Pedersen, J.S.; Tamburini, S.; Bertolotti, F.; Guagliardi, A.; Bruno, M.; Valentini, L.; Artioli, G. An Atomistic Model Describing the Structure and Morphology of Cu-Doped C-S-H Hardening Accelerator Nanoparticles. Nanomaterials 2022, 12, 342. [Google Scholar] [CrossRef]
- Murray, J.S.; Subramani, J.V.; Selvam, P.R.; Hall, K.D. Molecular Dynamics to Understand the Mechanical Behavior of Cement Paste. Transp. Res. Rec. 2010, 2142, 75–82. [Google Scholar] [CrossRef]
- Bahraq, A.A.; Obot, I.; Al-Osta, M.A.; Al-Amoudi, O.S.B.; Maslehuddin, M. Molecular-level investigation on the effect of surface moisture on the bonding behavior of cement-epoxy interface. J. Build. Eng. 2022, 61, 105299. [Google Scholar] [CrossRef]
- Sekkal, W.; Zaoui, A. Enhancing the interfacial bond strength of cement nanocomposite with carbonate nanostructure. Compos. Part B—Eng. 2017, 124, 111–119. [Google Scholar] [CrossRef]
- Cuesta, A.; Santacruz, I.; Angeles, G.; Dapiaggi, M.; Zea-Garcia, J.D.; Aranda, M.A. Local structure and Ca/Si ratio in C-S-H gels from hydration of blends of tricalcium silicate and silica fume. Cem. Concr. Res. 2021, 143, 106405. [Google Scholar] [CrossRef]
- Hajilar, S.; Shafei, B. Nano-scale investigation of elastic properties of hydrated cement paste constituents using molecular dynamics simulations. Comp. Mater. Sci. 2015, 101, 216–226. [Google Scholar] [CrossRef]
- Kakanakova-Georgieva, A.; Ivanov, I.G.; Suwannaharn, N.; Hsu, C.W.; Cora, I.; Pécz, B.; Giannazzo, F.; Sangiovanni, D.G.; Gueorguiev, G.K. MOCVD of AlN on epitaxial graphene at extreme temperatures. CrystEngComm 2021, 23, 385–390. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Ricardo, F.; Kostov, G.A.; Kakanakova-Georgieva, A. Discovering atomistic pathways for supply of metal atoms from methyl-based precursors to graphene surface. Phys. Chem. Chem. Phys. 2022, 25, 5887. [Google Scholar] [CrossRef]
- Skinner, L.B.; Chae, S.R.; Benmore, C.J.; Wenk, H.R.; Monteiro, P.J.M. Nanostructure of calcium silicate hydrates in cements. Phys. Rev. Lett. 2010, 104, 195502. [Google Scholar] [CrossRef]
- Sabet, A.B.; Hashemi, S.A.H.; Farokhzad, R.; Delnavaz, A. Synergic effect of defects on carbon nanoparticles under interaction with calcium silicate hydrate composites. Appl. Surf. Sci. 2023, 622, 156712. [Google Scholar] [CrossRef]
- Moradi, M.; Rezaei, M. Construction of highly anti-corrosion and super-hydrophobic polypropylene/graphene oxide nanocomposite coatings on carbon steel: Experimental, electrochemical and molecular dynamics studies. Constr. Build. Mater. 2022, 317, 126136. [Google Scholar] [CrossRef]
- Mazaheripour, H.; Faria, R.; Azenha, M.; Ye, G. Modelling Macroscopic Shrinkage of Hardened Cement Paste Considering C-S-H Densification. Adv. Cem. Res. 2021, 33, 257–284. [Google Scholar] [CrossRef]
- Duque-Redondo, E.; Masoero, E.; Manzano, H. Nanoscale shear cohesion between cement hydrates: The role of water diffusivity under structural and electrostatic confinement. Cem. Concr. Res. 2022, 154, 106716. [Google Scholar] [CrossRef]
- Bahraq, A.A.; Al-Osta, M.A.; Al-Amoudi, O.S.; Obot, I.B.; Adesina, A.Y.; Maslehuddin, M. A nanoscale adhesion mechanism of cement-epoxy interface under varying moisture conditions: A molecular dynamics study. Surf. Interfaces 2022, 35, 102446. [Google Scholar] [CrossRef]
- Geng, G.; Myers, R.J.; Li, J.; Maboudian, R.; Carraro, C.; Shapiro, D.A.; Monteiro, P.J. Aluminum-induced dreierketten chain cross-links increase the mechanical properties of nanocrystalline calcium aluminosilicate hydrate. Sci. Rep. 2017, 7, 44032. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.; Goyal, S.; Siddique, R.; Singh, M. Mechanical properties and microstructural analysis of cement mortar incorporating marble powder as partial replacement of cement. Constr. Build. Mater. 2015, 96, 615–621. [Google Scholar] [CrossRef]
- Liang, G.W.; Wu, Y.; She, A.M. New insights into the early-age reaction kinetics of metakaolin geopolymer by 1H low-field NMR and isothermal calorimetry. Cem. Concr. Comp. 2023, 137, 104932. [Google Scholar] [CrossRef]
- Eftekhari, M.; Mohammadi, S. Molecular dynamics simulation of the nonlinear behavior of the CNT-reinforced calcium silicate hydrate (C–S–H) composite. Compos. Part A Appl. Sci. Manuf. 2016, 82, 78–87. [Google Scholar] [CrossRef]
- Khoshnazar, R.; Beaudoin, J.J.; Raki, L.; Alizadeh, R. Volume Stability of C-S-H/Polyaniline Nanocomposites in Aqueous Salt Solutions. ACI Mater. J. 2014, 111, 623–632. [Google Scholar] [CrossRef]
- Fu, J.; Bernard, F.; Kamali-Bernard, S. Assessment of the elastic properties of amorphous calcium silicates hydrates (I) and (II) structures by molecular dynamics simulation. Mol. Simulat. 2018, 44, 285–299. [Google Scholar] [CrossRef]
- Liang, G.W.; Liu, T.J.; Li, H.R. Shrinkage mitigation, strength enhancement and microstructure improvement of alkali-activated slag/fly ash binders by ultrafine waste concrete powder. Compos. Part B—Eng. 2022, 231, 109570. [Google Scholar] [CrossRef]
- Abdolhosseini Qomi, M.J.; Ulm, F.J.; Pellenq, R.J. Evidence on the Dual Nature of Aluminum in the Calcium-Silicate-Hydrates Based on Atomistic Simulations. J. Am. Ceram. Soc. 2012, 95, 1128–1137. [Google Scholar] [CrossRef]
- Mohamed, A.K.; Parker, S.C.; Bowen, P.; Galmarini, S. An atomistic building block description of C-S-H—Towards a realistic C-S-H model. Cem. Concr. Res. 2018, 107, 221–235. [Google Scholar] [CrossRef]
- Pellenq, R.J.-M.; Kushima, A.; Shahsavari, R.; Van Vliet, K.J.; Buehler, M.J.; Yip, S.; Ulm, F.-J. A realistic molecular model of cement hydrates. Proc. Natl. Acad. Sci. USA 2009, 106, 16102–16107. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Rub, M.A.; Khan, F.; Al-Lohedan, H.A. Thermodynamic, interfacial and hydrodynamic aspects of interaction of cationic drug amitriptyline hydrochloride with anionic and nonionic polymers. J. Mol. Liq. 2013, 180, 200–206. [Google Scholar] [CrossRef]
- Paradiso, P.; Santos, R.; Horta, R.; Lopes, J.; Ferreira, P.; Colaço, R. Formation of nanocrystalline tobermorite in calcium silicate binders with low C/S ratio. Acta Mater. 2018, 152, 7–15. [Google Scholar] [CrossRef]
- Pellenq, R.M.; Lequeux, N.; Van Damme, H. Engineering the bonding scheme in C–S–H: The iono-covalent framework. Cem. Concr. Res. 2007, 38, 159–174. [Google Scholar] [CrossRef]
- Basquiroto de Souza, F.; Sagoe-Crentsil, K.; Duan, W. A century of research on calcium silicate hydrate (C–S–H): Leaping from structural characterization to nanoengineering. J. Am. Ceram. Soc. 2022, 105, 3081–3099. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Cui, Y.; She, A.; Hai, R.; Zhu, Z. Molecular Dynamics Simulation of Silane Inserted CSH Nanostructure. Materials 2024, 17, 149. https://doi.org/10.3390/ma17010149
Yang F, Cui Y, She A, Hai R, Zhu Z. Molecular Dynamics Simulation of Silane Inserted CSH Nanostructure. Materials. 2024; 17(1):149. https://doi.org/10.3390/ma17010149
Chicago/Turabian StyleYang, Fei, Yangyang Cui, Anming She, Ran Hai, and Zheyu Zhu. 2024. "Molecular Dynamics Simulation of Silane Inserted CSH Nanostructure" Materials 17, no. 1: 149. https://doi.org/10.3390/ma17010149
APA StyleYang, F., Cui, Y., She, A., Hai, R., & Zhu, Z. (2024). Molecular Dynamics Simulation of Silane Inserted CSH Nanostructure. Materials, 17(1), 149. https://doi.org/10.3390/ma17010149





