Nanoscale Surface Refinement of CoCrMo Alloy for Artificial Knee Joints via Chemical Mechanical Polishing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Slurry Preparation
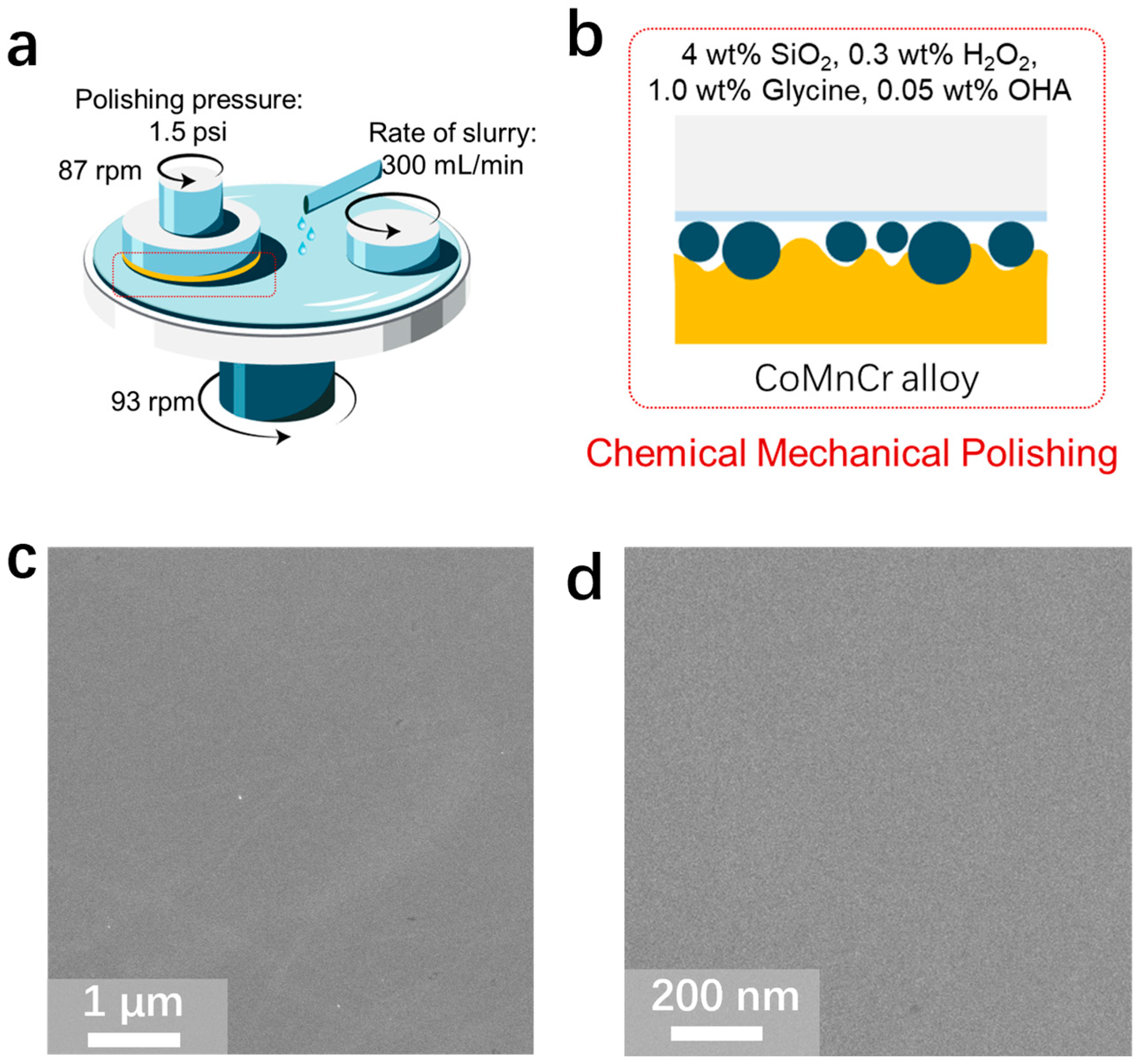
2.3. Polishing Experiments
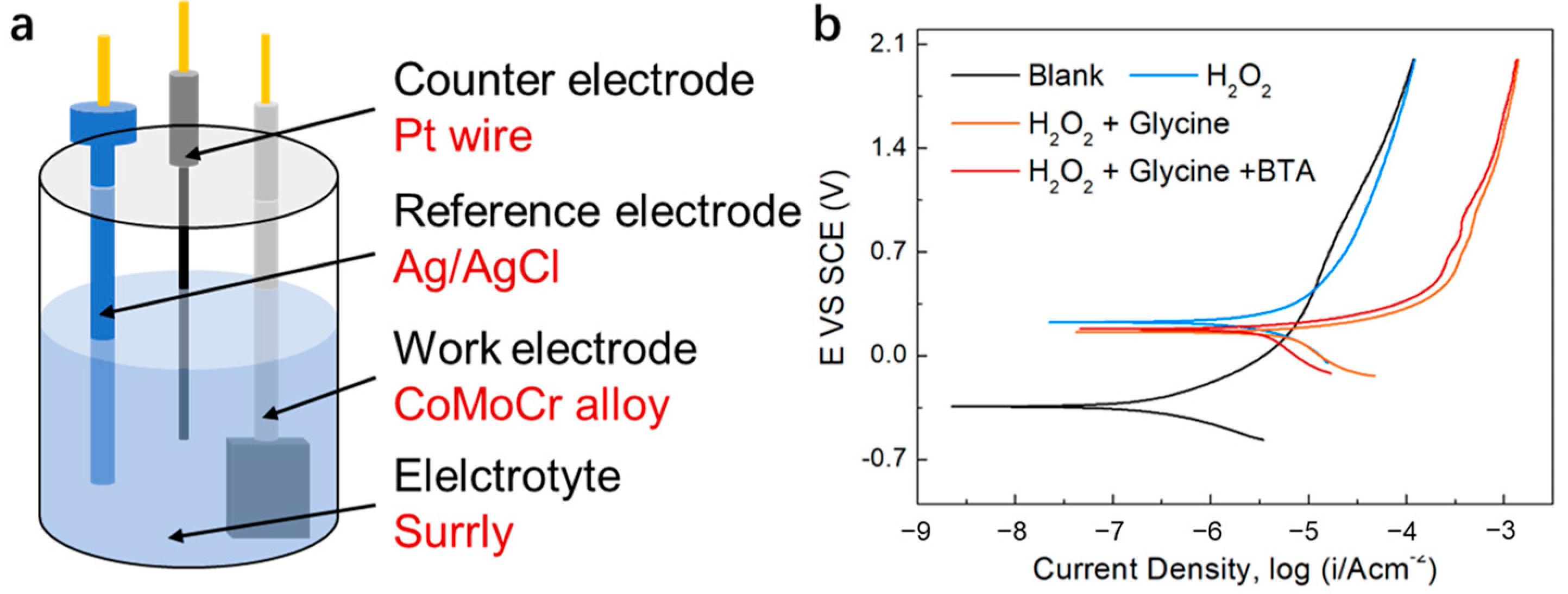
2.4. Electrochemical Measurements
2.5. Characterization Methods
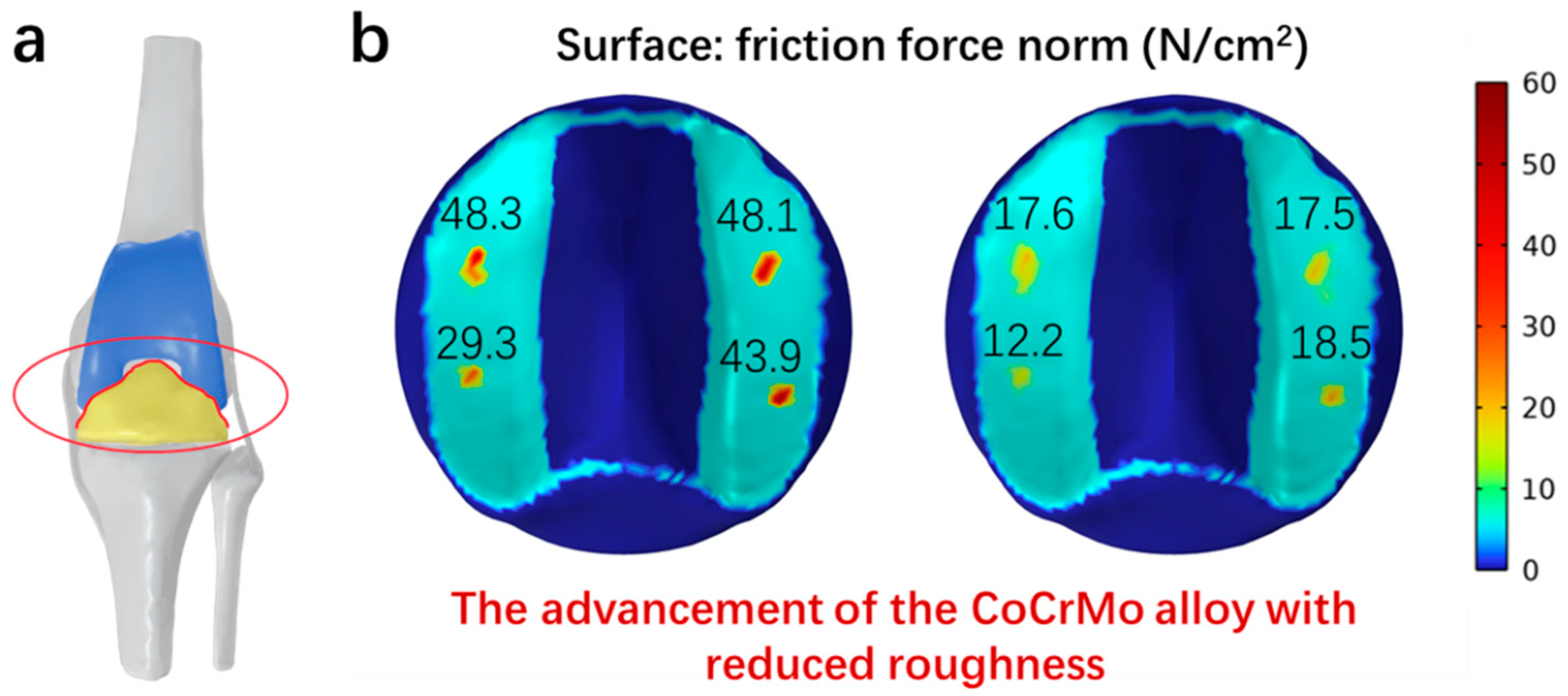
2.6. Finite Element Analysis
3. Results and Discussion
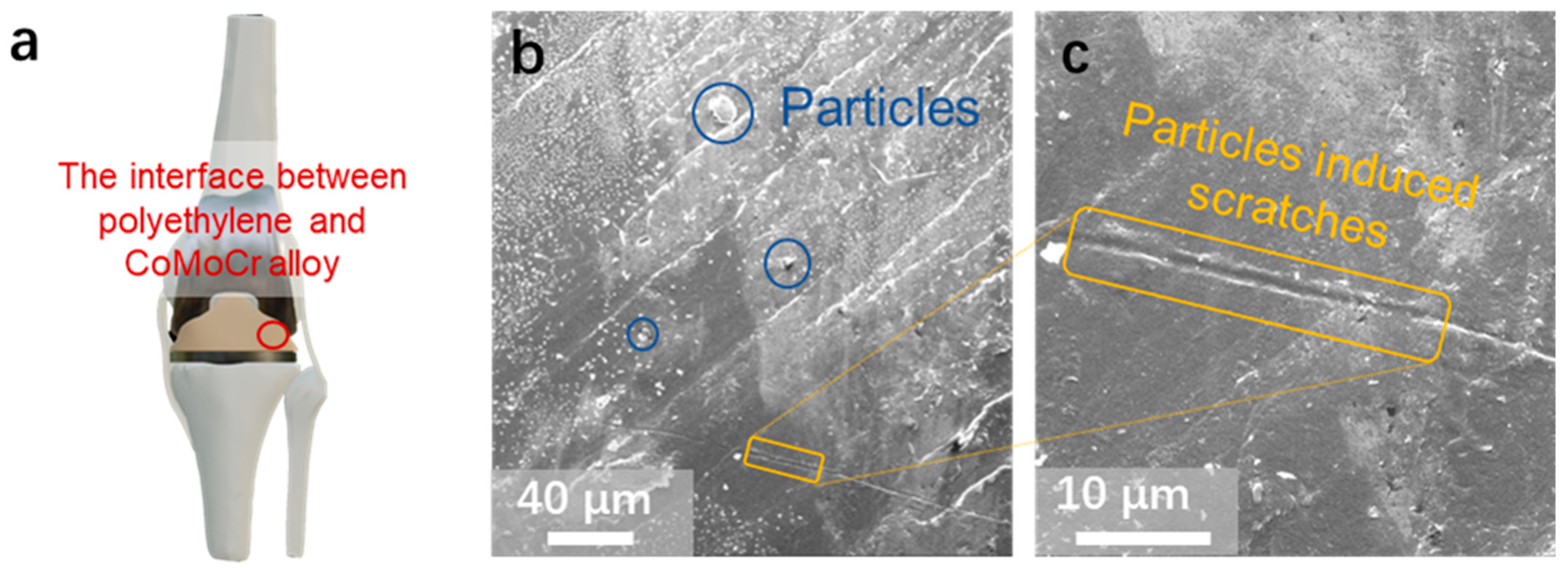
3.1. Surface Morphology of an Artificial Knee Joint
3.2. Chemical Mechanical Polishing of CoCrMo Alloys
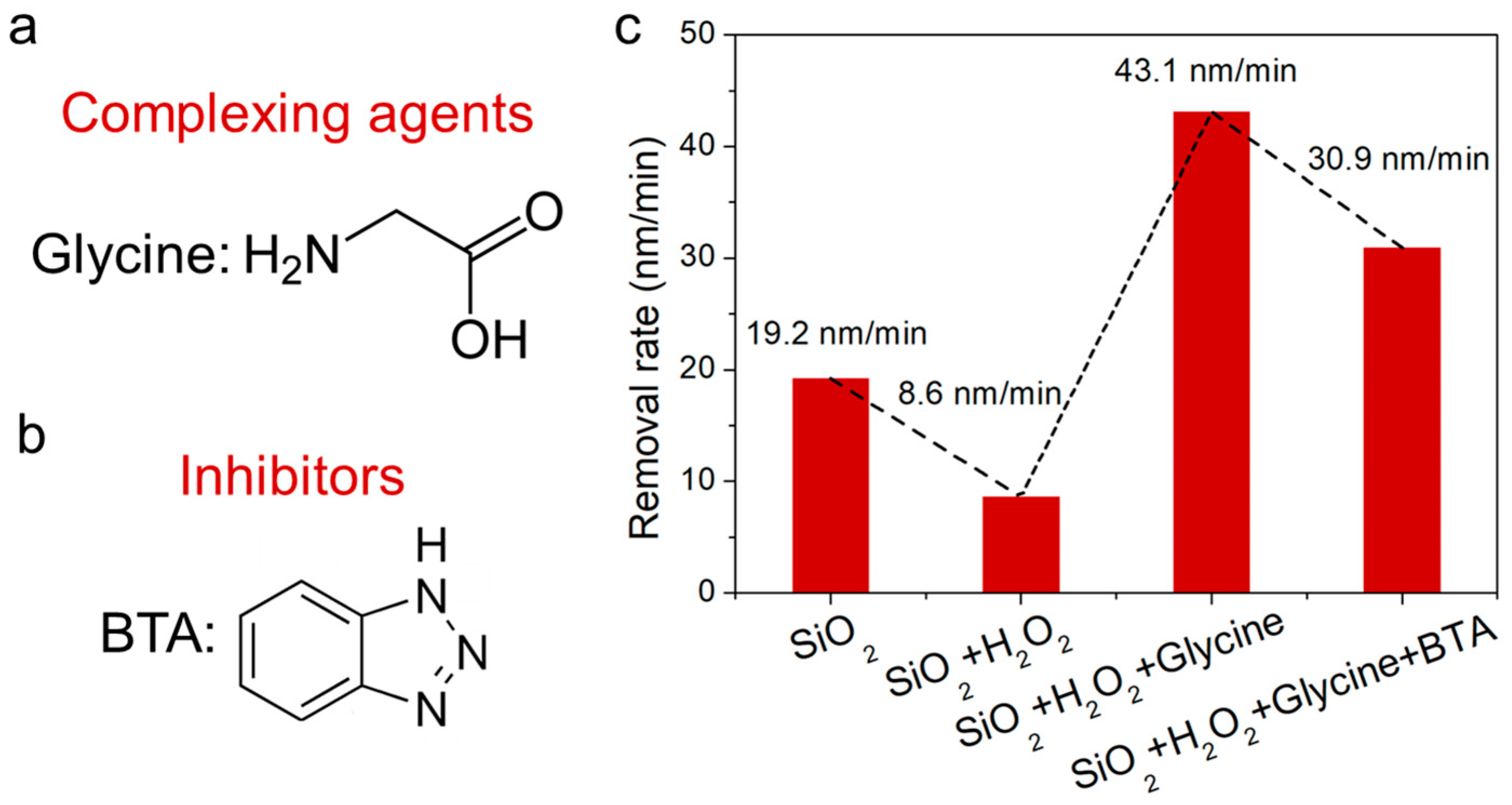
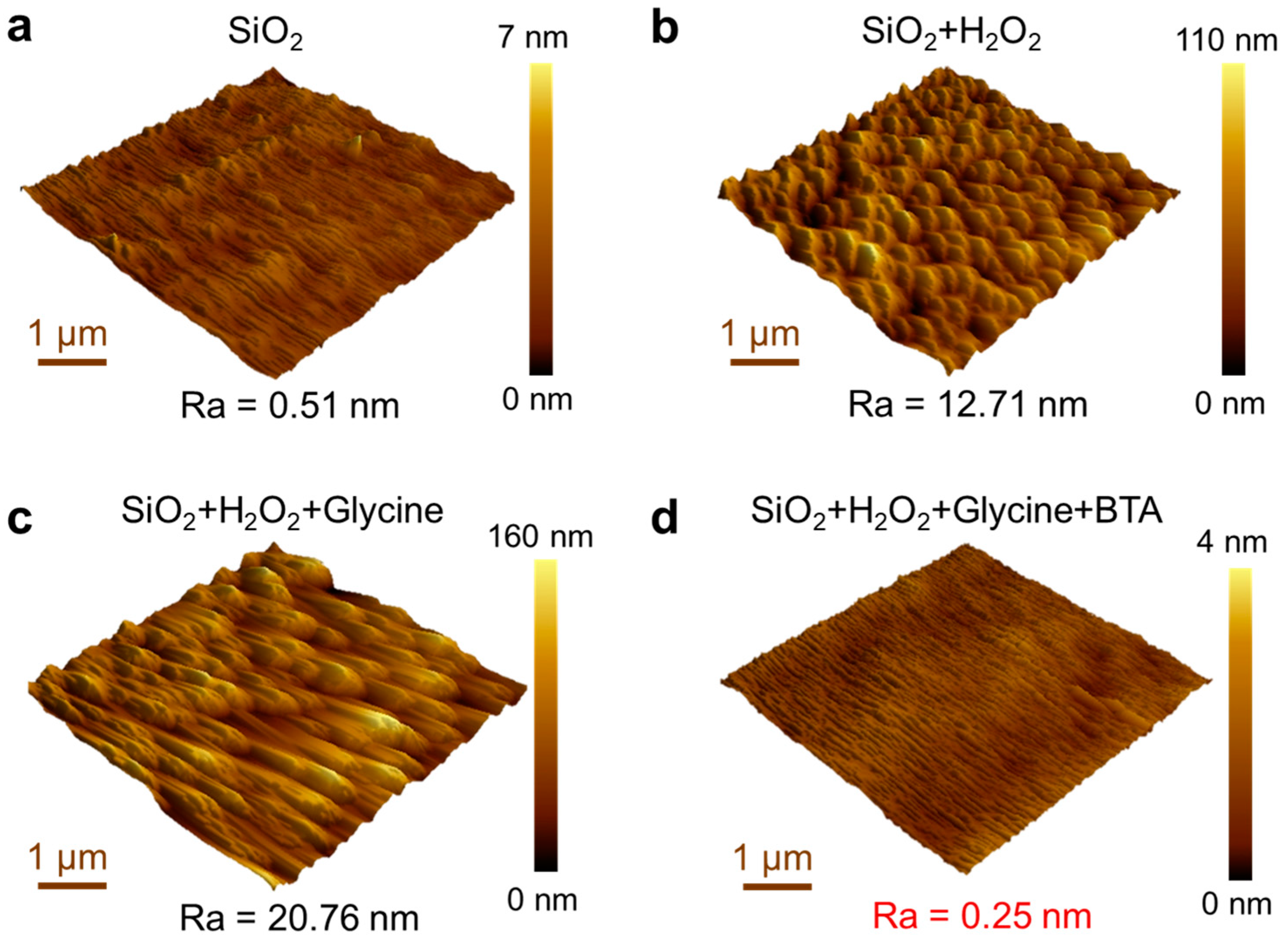
3.3. Investigating the Impact of Slurry Constituents on the Polishing Process
3.4. Investigating the Average Particle Size of Colloidal Silica
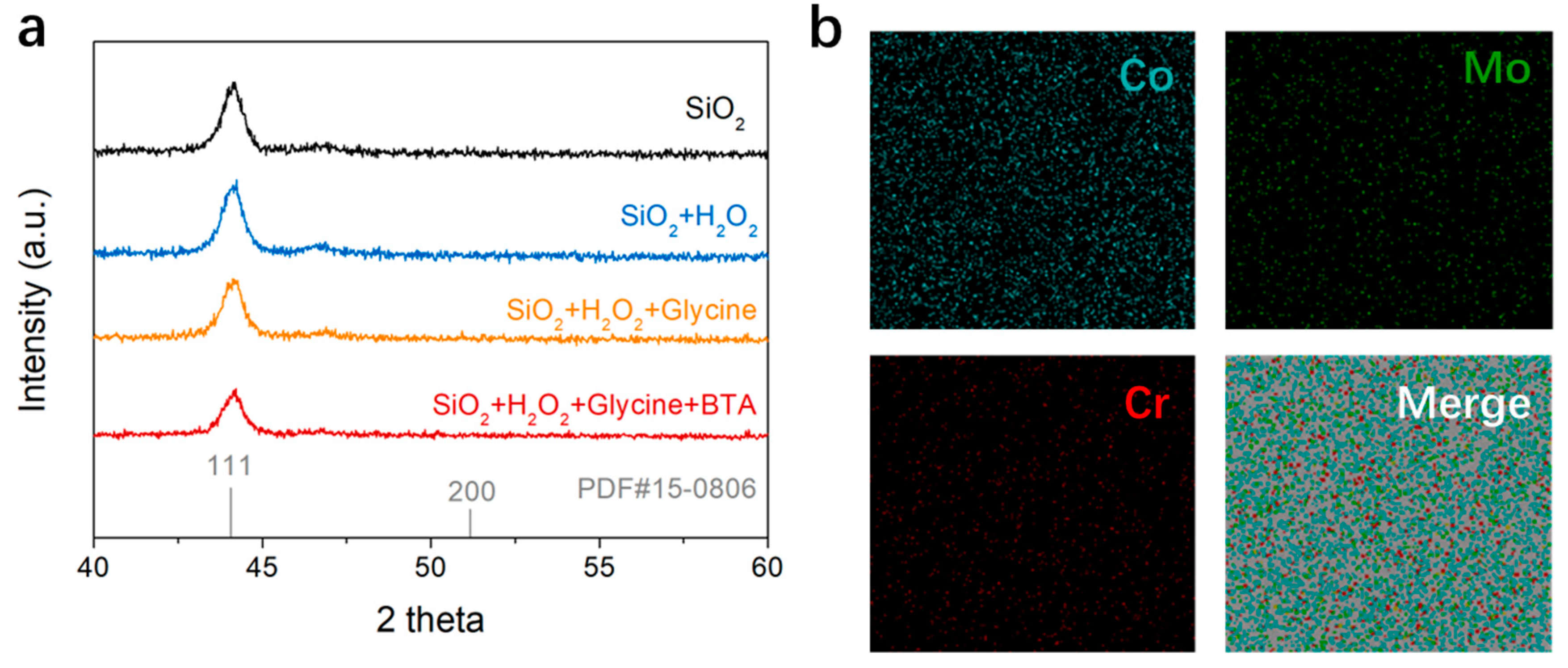
3.5. Investigating the Chemical States and Crystalline Structure of the CoCrMo Alloy
3.6. Investigating the Corrosion and Corrosion Inhibition Mechanisms of the CoCrMo Alloy
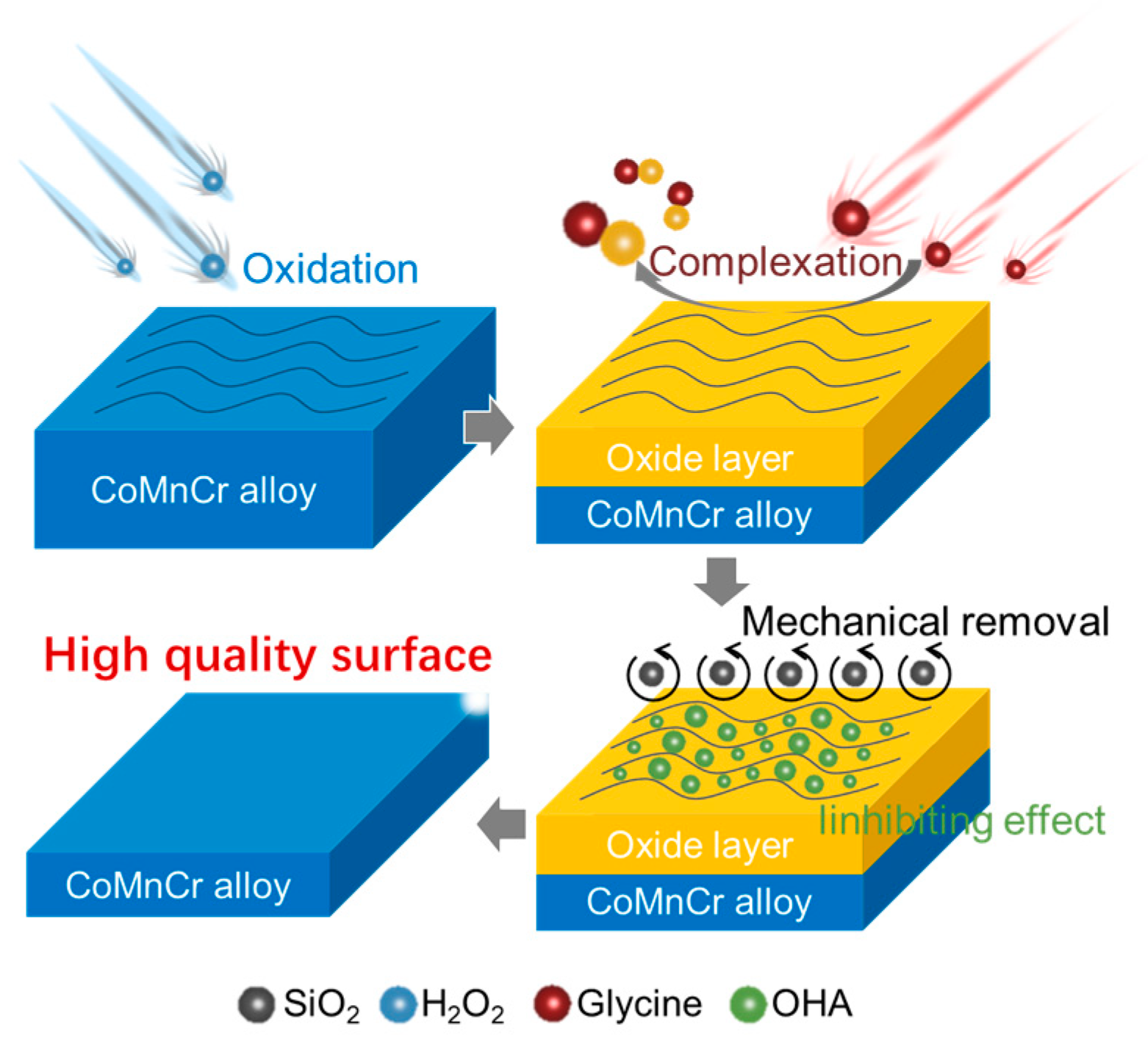
3.7. The Mechanisms of Chemical Mechanical Polishing in CoCrMo Alloys
3.8. Simulation of Frictional Forces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keener, J.D.; Callaghan, J.J.; Goetz, D.D.; Pederson, D.R.; Sullivan, P.M.; Johnston, R.C. Twenty-five-Year Results After Charnley Total Hip Arthroplasty in Patients Less than Fifty Years Old: A Concise Follow-up of a Previous Report. JBJS 2003, 85, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Tipper, J.L.; Firkins, P.J.; Besong, A.A.; Barbour, P.S.M.; Nevelos, J.; Stone, M.H.; Ingham, E.; Fisher, J. Characterisation of wear debris from UHMWPE on zirconia ceramic, metal-on-metal and alumina ceramic-on-ceramic hip prostheses generated in a physiological anatomical hip joint simulator. Wear 2001, 250, 120–128. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: Review over the past 30 years. Heliyon 2022, 8, 12050. [Google Scholar] [CrossRef]
- Moro, T.; Takatori, Y.; Ishihara, K.; Konno, T.; Takigawa, Y.; Matsushita, T.; Chung, U.-I.; Nakamura, K.; Kawaguchi, H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat. Mater. 2004, 3, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.; Hoving, D.; Aramburu, I.V.; Temkin, M.I.; De Vasconcelos, N.M.; Tsourouktsoglou, T.-D.; Wang, Q.; Boeing, S.; Goldstone, R.; Vernardis, S.; et al. Microbe capture by splenic macrophages triggers sepsis via T cell-death-dependent neutrophil lifespan shortening. Nat. Commun. 2022, 13, 4658. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, 17–71. [Google Scholar] [CrossRef]
- Rahman, M.D.M.; Biswas, M.A.S.; Hoque, K.N. Recent development on micro-texturing of UHMWPE surfaces for orthopedic bearings: A review. Biotribology 2022, 31, 100216. [Google Scholar] [CrossRef]
- Hashmi, A.W.; Mali, H.S.; Meena, A.; Saxena, K.K.; Ahmad, S.; Agrawal, M.K.; Sagbas, B.; Valerga Puerta, A.P.; Khan, M.I. A comprehensive review on surface post-treatments for freeform surfaces of bio-implants. J. Mater. Res. Technol. 2023, 23, 4866–4908. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Qiao, L. Protein adsorption on implant metals with various deformed surfaces. Colloids Surf. B 2017, 156, 62–70. [Google Scholar] [CrossRef]
- Seo, B.; Park, H.-K.; Park, K.B.; Kang, H.-S.; Park, K. Effect of hydrogen peroxide on Cr oxide formation of additive manufactured CoCr alloys during plasma electrolytic polishing. Mater. Lett. 2021, 294, 129736. [Google Scholar] [CrossRef]
- Barman, A.; Das, M. Nano-finishing of bio-titanium alloy to generate different surface morphologies by changing magnetorheological polishing fluid compositions. Precis. Eng. 2018, 51, 145–152. [Google Scholar] [CrossRef]
- Heng, L.; Song, J.H.; Mun, S.D. A novel methodology of MAF process via a multi-axis/multi-faceted polishing technique for enhancing surface quality of artificial hip joint component. J. Magn. Magn. Mater. 2023, 570, 170523. [Google Scholar] [CrossRef]
- Lu, P.; Wu, M.; Liu, X.; Ye, X.; Duan, W.; Miao, X. Surface modification and biotribological behavior of UHMWPE nanocomposites with GO infiltrated by ultrasonic induction. J. Biomed. Mater. Res. B 2021, 109, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Shiou, F.-J.; Ding, Z.-L.; Lin, S.-P. Reduction in the Volumetric Wear of a Ball Polishing Tool Using Ultrasonic-Vibration-Assisted Polishing Process. Lubricants 2022, 10, 339. [Google Scholar] [CrossRef]
- Sharif Azadeh, S.; Mak, J.C.C.; Chen, H.; Luo, X.; Chen, F.-D.; Chua, H.; Weiss, F.; Alexiev, C.; Stalmashonak, A.; Jung, Y.; et al. Microcantilever-integrated photonic circuits for broadband laser beam scanning. Nat. Commun. 2023, 14, 2641. [Google Scholar] [CrossRef]
- Han, S.-J.; Tang, J.; Kumar, B.; Falk, A.; Farmer, D.; Tulevski, G.; Jenkins, K.; Afzali, A.; Oida, S.; Ott, J.; et al. High-speed logic integrated circuits with solution-processed self-assembled carbon nanotubes. Nat. Nanotechnol. 2017, 12, 861–865. [Google Scholar] [CrossRef]
- Lee, J.; He, S.; Song, G.; Hogan, C.J. Size distribution monitoring for chemical mechanical polishing slurries: An intercomparison of electron microscopy, dynamic light scattering, and differential mobility analysis. Powder Technol. 2022, 396, 395–405. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Fang, T.-H. Revealing the mechanisms for inactive rolling and wear behaviour on chemical mechanical planarization. Appl. Surf. Sci. 2022, 595, 153524. [Google Scholar] [CrossRef]
- Srivastava, M.; Singh, J.; Mishra, D.K.; Singh, R.P. Review on the various strategies adopted for the polishing of silicon wafer—A chemical perspective. Mater. Today Proc. 2022, 63, 62–68. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Z.; Yu, S.; Chen, X.; Shi, C.; Zhou, H.; Meng, F.; Yu, J.; Wen, W. Unprecedented atomic surface of silicon induced by environmentally friendly chemical mechanical polishing. Nanoscale 2023, 15, 9304–9314. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Pan, G.; Yang, X.; Qi, Y.; Fang, Q.; She, L.; Di, Y. Electrochemical corrosion behavior and theoretical simulation of cobalt in chemical mechanical polishing process. Electrochim. Acta 2023, 468, 143184. [Google Scholar] [CrossRef]
- Rothammer, B.; Schwendner, M.; Bartz, M.; Wartzack, S.; Böhm, T.; Krauß, S.; Merle, B.; Schroeder, S.; Uhler, M.; Kretzer, J.P.; et al. Wear Mechanism of Superhard Tetrahedral Amorphous Carbon (ta-C) Coatings for Biomedical Applications. ADV Mater. Interfaces 2023, 10, 2202370. [Google Scholar] [CrossRef]
- Nayak, C.; Balani, K. Effects of reinforcements and gamma-irradiation on wear performance of ultra-high molecular weight polyethylene as acetabular cup liner in hip-joint arthroplasty: A review. J. Appl. Polym. Sci. 2021, 138, 51275. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Q.; Xia, R.; Pan, G.; Hu, L.; Qi, Y. Experimental and density functional theory study of benzohydroxamic acid as a corrosion inhibitor in chemical mechanical polishing of Co interconnects. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130848. [Google Scholar] [CrossRef]
- He, P.; Wu, B.; Shao, S.; Teng, T.; Wang, P.; Qu, X.-P. Characterization of 1, 2, 4-Triazole as Corrosion Inhibitor for Chemical Mechanical Polishing of Cobalt in H2O2 Based Acid Slurry. ECS J. Solid State Sci. Technol. 2019, 8, 3075. [Google Scholar] [CrossRef]
- Wen, J.; Ma, T.; Zhang, W.; van Duin, A.C.T.; van Duin, D.M.; Hu, Y.; Lu, X. Atomistic Insights into Cu Chemical Mechanical Polishing Mechanism in Aqueous Hydrogen Peroxide and Glycine: ReaxFF Reactive Molecular Dynamics Simulations. J. Phys. Chem. C 2019, 123, 26467–26474. [Google Scholar] [CrossRef]
- Kwon, O.; Bae, K.; Byun, J.; Lim, T.; Kim, J.J. Study on effect of complexing agents on Co oxidation/dissolution for chemical-mechanical polishing and cleaning process. Microelectron. Eng. 2020, 227, 111308. [Google Scholar] [CrossRef]
- Kim, E.; Choi, S.; Jeon, S.; Seok, H.; Cho, J.-i.; Shin, D.; Kim, T. Development of novel multi-selective slurry with mechanically driven etching for through silicon via chemical mechanical polishing. Mater. Sci. Semicond. Process. 2022, 152, 107025. [Google Scholar] [CrossRef]
- Liu, P.; Nam, Y.; Lee, S.; Kim, E.; Jeon, S.; Park, K.; Hong, S.; Kim, T. The mechanical effect of soft pad on copper chemical mechanical polishing. Mater. Sci. Semicond. Process. 2023, 155, 107256. [Google Scholar] [CrossRef]
- Bouvier, M.; Pacheco Bubi, I.; Wiegmann, T.; Qiu, C.; Allongue, P.; Magnussen, O.M.; Maroun, F. Unraveling the Cobalt Oxidation State at the Surface of Epitaxial Cobalt Oxide Films during the Oxygen Evolution Reaction by Operando X-ray Absorption Spectroscopy/Surface X-ray Diffraction. ACS Appl. Energ. Mater. 2023, 6, 7335–7345. [Google Scholar] [CrossRef]
- Wu, W.; Chen, R.; Chen, S.; Wang, Z.; Cheng, N. Optimizing d-Orbital Electronic Configuration via Metal–Metal Oxide Core–Shell Charge Donation for Boosting Reversible Oxygen Electrocatalysis. Small 2023, 19, 2300621. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Pei, L.; Yan, S.; Yu, Z.; Yu, T.; Zou, Z. In situ formed oxy/hydroxide antennas accelerating the water dissociation kinetics on a Co@N-doped carbon core–shell assembly for hydrogen production in alkaline solution. Dalton Trans. 2019, 48, 11927–11933. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Shaikh, S.; Chatzinoff, Y.; Munaweera, I.; Cheng, B.; Daly, S.M.; Xi, Y.; Bing, C.; Burns, D.; Greenberg, D.E. Employing high-frequency alternating magnetic fields for the non-invasive treatment of prosthetic joint infections. Sci. Rep. 2017, 7, 7520. [Google Scholar] [CrossRef]
- Peña, E.; Calvo, B.; Martínez, M.A.; Palanca, D.; Doblaré, M. Finite element analysis of the effect of meniscal tears and meniscectomies on human knee biomechanics. Clin. Biomech. 2005, 20, 498–507. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, J.; Lai, J.; Chen, Y.; Tian, M.; Pan, G.; Yang, X.; Qi, Y. Nanoscale Surface Refinement of CoCrMo Alloy for Artificial Knee Joints via Chemical Mechanical Polishing. Materials 2024, 17, 8. https://doi.org/10.3390/ma17010008
Zhang H, Zhang J, Lai J, Chen Y, Tian M, Pan G, Yang X, Qi Y. Nanoscale Surface Refinement of CoCrMo Alloy for Artificial Knee Joints via Chemical Mechanical Polishing. Materials. 2024; 17(1):8. https://doi.org/10.3390/ma17010008
Chicago/Turabian StyleZhang, Hanji, Jiangliang Zhang, Jinghui Lai, Yilin Chen, Mengqiang Tian, Guofeng Pan, Xueli Yang, and Yuhang Qi. 2024. "Nanoscale Surface Refinement of CoCrMo Alloy for Artificial Knee Joints via Chemical Mechanical Polishing" Materials 17, no. 1: 8. https://doi.org/10.3390/ma17010008
APA StyleZhang, H., Zhang, J., Lai, J., Chen, Y., Tian, M., Pan, G., Yang, X., & Qi, Y. (2024). Nanoscale Surface Refinement of CoCrMo Alloy for Artificial Knee Joints via Chemical Mechanical Polishing. Materials, 17(1), 8. https://doi.org/10.3390/ma17010008








