Effects of Ionic Liquid Content on the Electrical Properties of PVDF Films by Fused Deposition Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
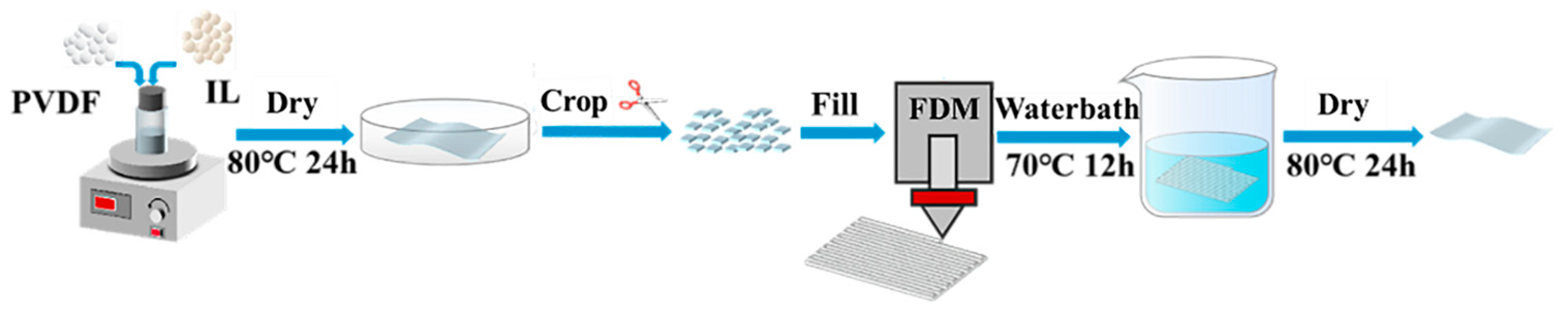
2.2. Preparation of PVDF Films
2.3. Polarization Process
2.4. Characterization
3. Results and Discussion
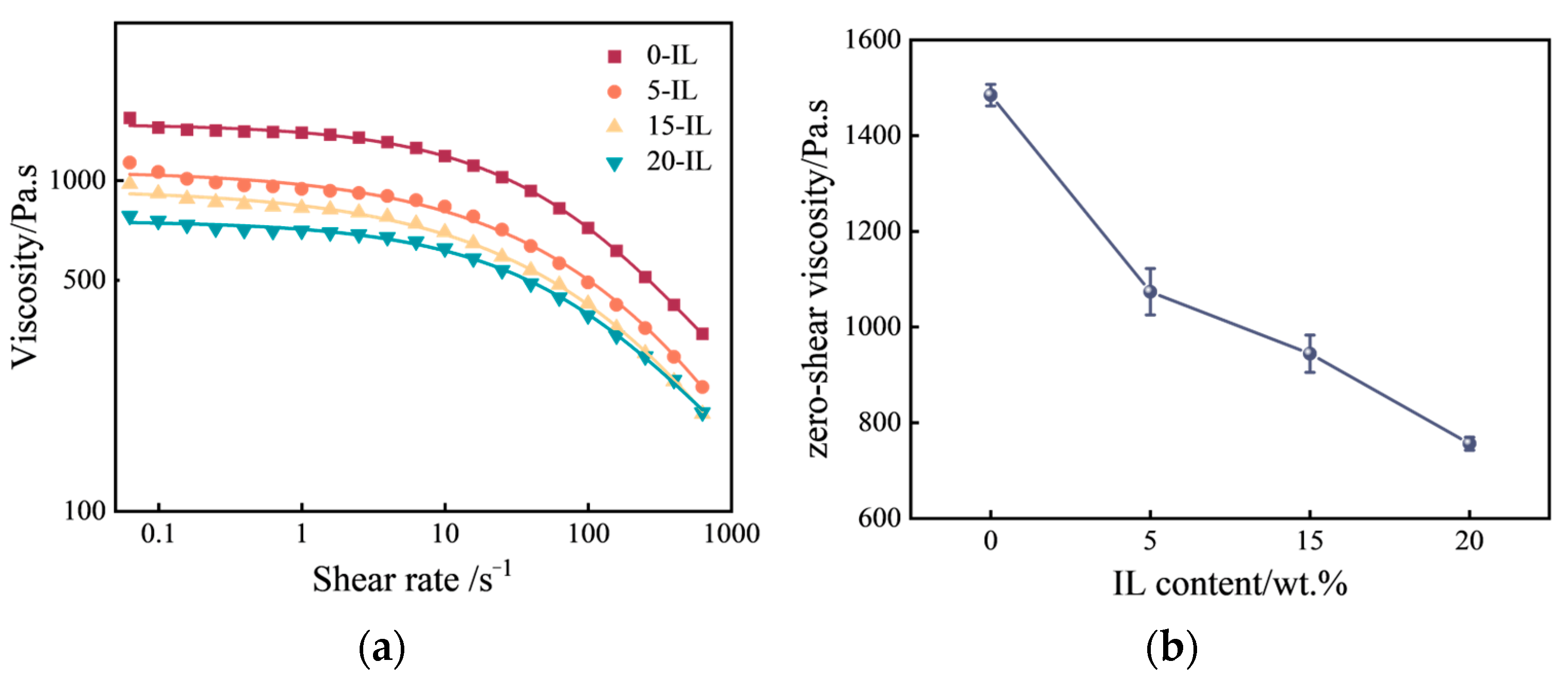
3.1. Melted PVDF Rheological Properties
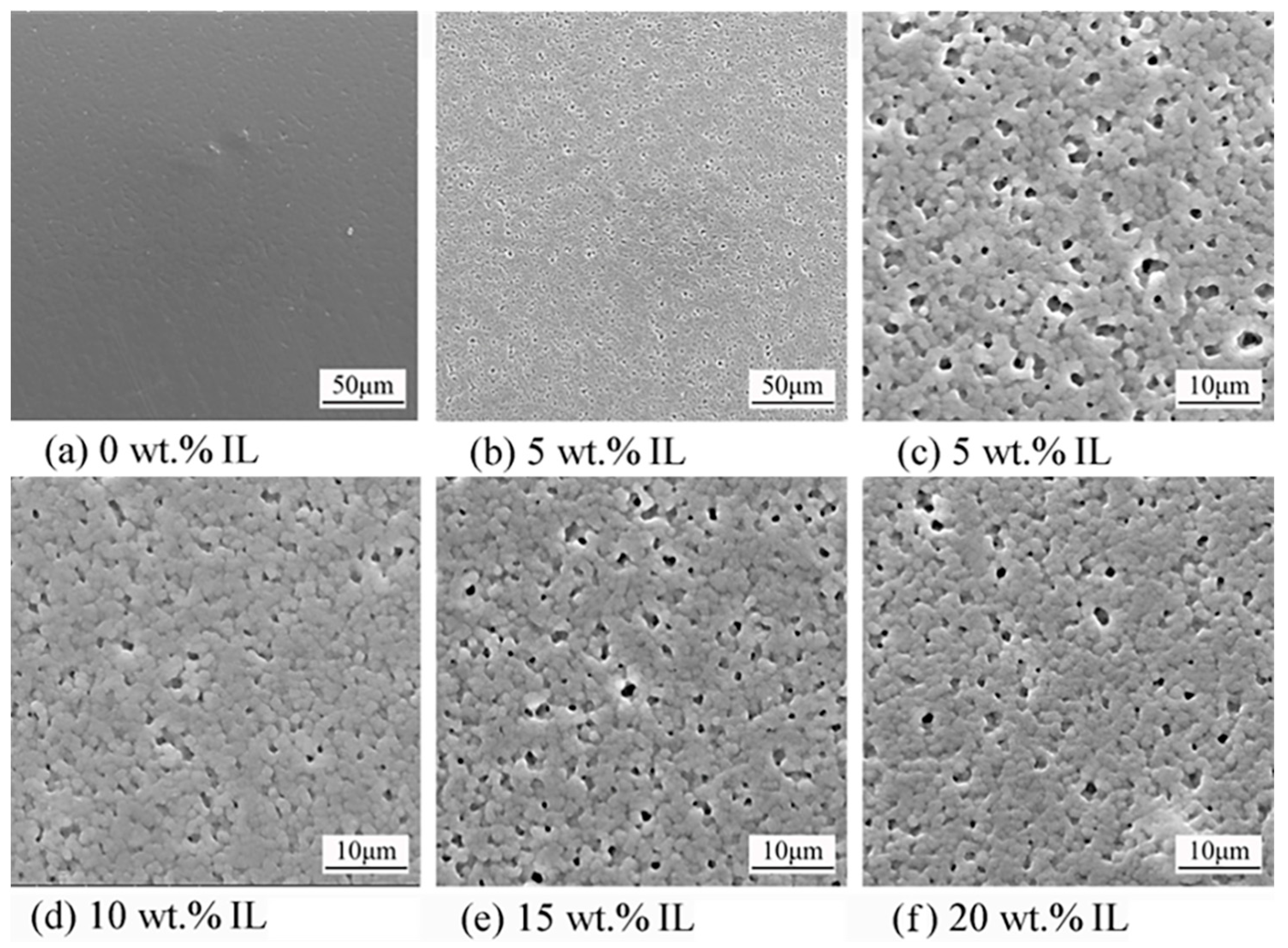
3.2. Topography and Structure
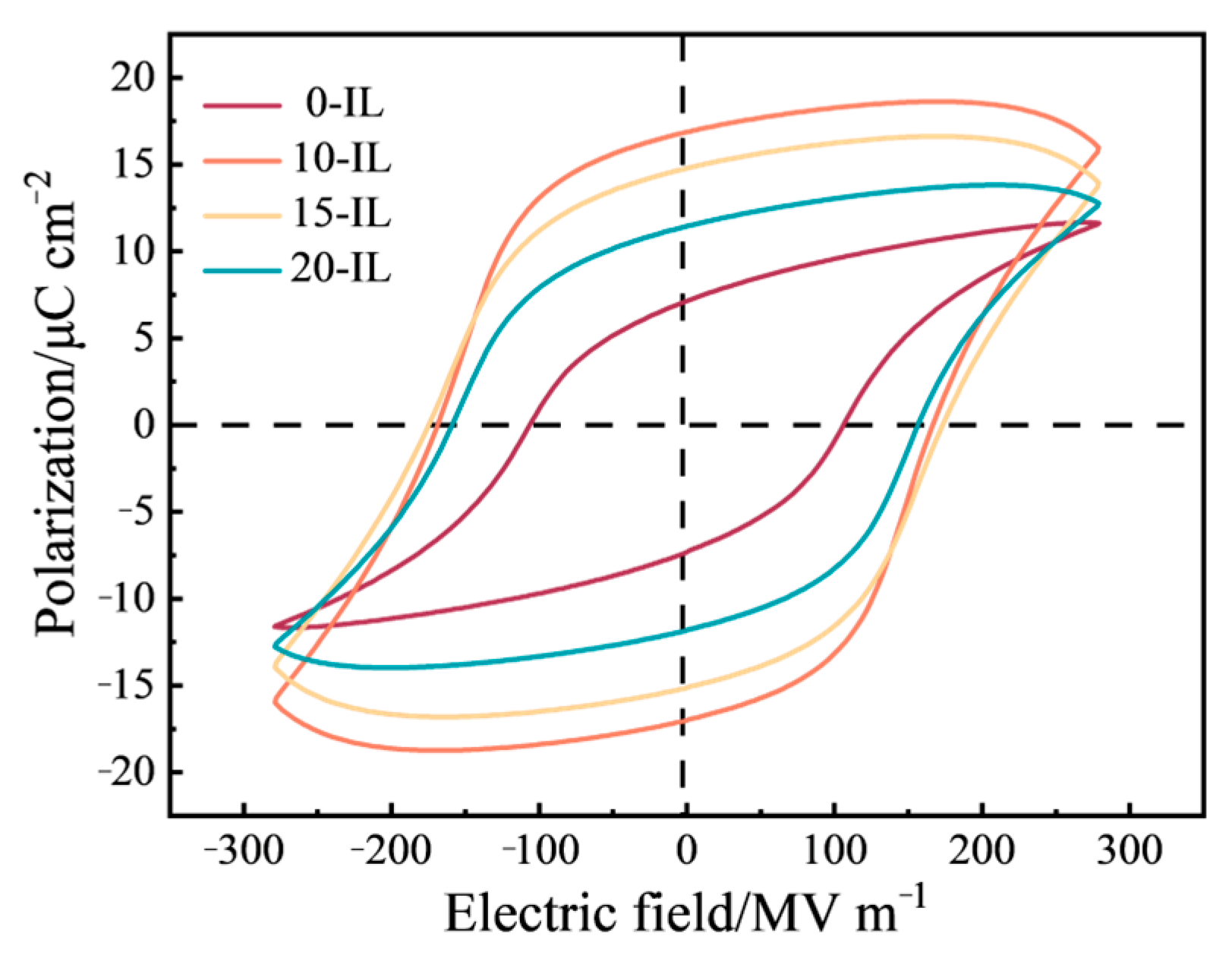
3.3. Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Fan, J.; Deneke, N.; Xu, S.; Newell, B.; Davis, C.; Wu, W.; Voyles, R.M.; Nawrocki, R.A. Electric poling-assisted additive manufacturing technique for piezoelectric active poly(vinylidene fluoride) films: Towards fully three-dimensional printed functional materials. Addit. Manuf. 2022, 60, 103248. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhou, J.; Wu, K.; Li, Y.; Jia, B.; Wang, Y.; Zhu, Q. Progress in the study of polycrystalline characteristics of polyvinylidene fluoride (PVDF). Insul. Mater. 2022, 55, 1–12. [Google Scholar]
- Rajeevan, S.; John, S.; George, S.C. Polyvinylidene fluoride: A multifunctional polymer in supercapacitor applications. J. Power Sources 2021, 504, 230037. [Google Scholar] [CrossRef]
- Porter, D.A.; Hoang, T.; Berfield, T.A. Effects of in-situ poling and process parameters on fused filament fabrication printed PVDF sheet mechanical and electrical properties. Addit. Manuf. 2017, 13, 81–92. [Google Scholar] [CrossRef]
- Momenzadeh, N.; Miyanaji, H.; Porter, D.A.; Berfield, T.A. Polyvinylidene fluoride (PVDF) as a feedstock for material extrusion additive manufacturing. Rapid Prototyp. J. 2020, 26, 156–163. [Google Scholar] [CrossRef]
- D1708; Standard Test Method for Tensile Properties of Plastics by Use of Microtensile Specimens. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- Liu, X.; Shang, Y.; Zhang, J.; Zhang, C. Ionic Liquid-Assisted 3D Printing of self-polarized beta-PVDF for flexible piezoelectric energy harvesting. ACS Appl. Mater. Interfaces 2021, 13, 14334–14341. [Google Scholar] [CrossRef]
- Kim, H.; Johnson, J.; Chavez, L.A.; Garcia Rosales, C.A.; Tseng, T.L.B.; Lin, Y. Enhanced dielectric properties of three phase dielectric MWCNTs/BaTiO3/PVDF nanocomposites for energy storage using fused deposition modeling 3D printing. Ceram. Int. 2018, 44, 9037–9044. [Google Scholar] [CrossRef]
- Zhang, H.; Ke, H.; Shao, J.; Wang, C.; Wang, H.; Chen, Y. One-step fabrication of highly sensitive pressure sensor by all FDM printing. Compos. Sci. Technol. 2022, 226, 109531. [Google Scholar] [CrossRef]
- Guo, R.; Luo, H.; Zhou, X.; Xiao, Z.; Xie, H.; Liu, Y.; Zhou, K.; Shen, Z.; Chen, L.; Zhang, D. Ultrahigh energy density of poly(vinylidene fluoride) from synergistically improved dielectric constant and withstand voltage by tuning the crystallization behavior. J. Mater. Chem. A 2021, 9, 27660–27671. [Google Scholar] [CrossRef]
- Lu, H.; Li, L. Crystalline structure, dielectric, and mechanical properties of simultaneously biaxially stretched polyvinylidene fluoride film. Polym. Adv. Technol. 2018, 29, 3056–3064. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, W. Piezoelectric properties of nano-SiO2/poly (vinylidene fluoride) composite film. J. Mater. Sci. Eng. 2019, 37, 599–603+618. [Google Scholar]
- Liu, R.; Liu, Q.; He, D.; Sun, G.; Li, Z.; Zhu, Y. Enhancement of the piezoelectric property of polyvinylidene fluoride through electroactive phase enrichment and the pplication in piezoelectric generators. ACS Appl. Electron. Mater. 2021, 3, 1804–1812. [Google Scholar] [CrossRef]
- Kim, H.; Torres, F.; Wu, Y.; Villagran, D.; Lin, Y.; Tseng, T.-L. Integrated 3D printing and corona poling process of PVDF piezoelectric films for pressure sensor application. Smart Mater. Struct. 2017, 26, 085027. [Google Scholar] [CrossRef]
- Zeyrek Ongun, M.; Paralı, L.; Oğuzlar, S.; Pechousek, J. Characterization of β-PVDF-based nanogenerators along with Fe2O3 NPs for piezoelectric energy harvesting. J. Mater. Sci. Mater. Electron. 2020, 31, 19146–19158. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Wang, L.; He, Y.; Wu, M. Fluorinated barium yitanate nanoparticles for wearable piezoelectric power generation. ACS Appl. Nano Mater. 2022, 5, 3352–3360. [Google Scholar] [CrossRef]
- Wang, A.; Chen, C.; Qian, J.; Yang, F.; Wang, L.; Zhang, M. Enhanced electrical properties of PVDF yhin film by addition of NaCl by near-electric-field 3D Printing. J. Electron. Mater. 2021, 50, 4781–4786. [Google Scholar] [CrossRef]
- Yi, H.; Huang, Y.; Jiang, J.; Chen, Z.; Yuan, S.; Deng, H.; Zhou, G.; Zhu, D.; Wu, X.; Hong, W. Characterization of PA6T/66 rheological properties. J. Chem. Eng. Chin. Univ. 2022, 36, 617–624. [Google Scholar]
- Liu, X.; Lin, J. Evaluation of asphalt and mixture high temperature performance with zero shear viscosity. Shandong Transp. Sci. Technol. 2017, 2, 41–44. [Google Scholar]
- Yasuda, K.R.; Armstrong, R.C.; Cohen, R.E. Shear flow properties of concentrated solutions of linear and star branched polystyrenes. Rheol. Acta 1981, 20, 163–178. [Google Scholar] [CrossRef]
- Zhu, J.; Li, C.; Xin, P. Analysis of viscosity-temperature characteristics and rheological properties of thick oil. J. Petrochem. Univ. 2011, 24, 66–68+72. [Google Scholar]
- Li, T.; Mei, P.; Yuan, H.; Li, F. Viscosity-temperature characteristics of asphalt pavement hot-applied sealant. J. Highw. Transp. Res. Dev. 2015, 32, 8–12. [Google Scholar]
- Xu, P.; Fu, W.; Luo, X.; Ding, Y. Enhanced dc conductivity and conductivity relaxation in PVDF/ionic liquid composites. Mater. Lett. 2017, 206, 60–63. [Google Scholar] [CrossRef]
- Xing, C. Modification of Polyvinylidene Fluoride by Ionic Liquids and Ionic Liquid-Modified Carbon Nanotubes. Master’s Thesis, Hangzhou Normal University, Hangzhou, China, 2013. [Google Scholar]
- Okkada, D.; Kaneko, H.; Kato, K.; Furumi, S.; Takeguchi, M.; Yamamoto, Y. Colloidal Crystallization and Ionic Liquid Induced Partial β-Phase Transformation of Poly(vinylidene fluoride) Nanoparticles. Macromolecules 2015, 48, 2570–2575. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, H.; Ke, F.; Wang, Z.; Xu, J.; Chen, Y.; Wang, H. Preparation and fused deposition modeling of multiwalled carbon nanotubes/ionic liquid/poly(vinylidene fluoride)dielectric materials with high β phase. Polym. Mater. Sci. Eng. 2021, 37, 116–123. [Google Scholar]
- Song, L.; Dai, R.; Li, Y.; Wang, Q.; Zhang, C. Polyvinylidene fluoride energy harvester with boosting piezoelectric performance through 3D printed biomimetic bone structures. ACS Sustain. Chem. Eng. 2021, 9, 7561–7568. [Google Scholar] [CrossRef]
- Rinaldo Gregorio, J.R.; Marcelo, C. Effect of crystallization temperature on the crystalline phase content and morphology of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Parangusan, H.; Ponnamma, D.; Almaadeed, M.A.A. Investigation on the effect of gamma-irradiation on the dielectric and piezoelectric properties of stretchable PVDF/Fe-ZnO nanocomposites for self-powering devices. Soft Matter 2018, 14, 8803–8813. [Google Scholar] [CrossRef]
- Benz, M.; Euler, W.B. Determination of the crystalline phases of poly(vinylidene fluoride) under different preparation conditions using differential scanning calorimetry and infrared spectroscop. J. Appl. Polym. Sci. 2003, 89, 1093–1100. [Google Scholar] [CrossRef]
- Luiso, S.; Henry, J.J.; Pourdeyhimi, B.; Fedkiw, P.S. Fabrication and Characterization of meltblown poly(vinylidene difluoride) membranes. ACS Appl. Polym. Mater. 2020, 2, 2849–2857. [Google Scholar] [CrossRef]
- Bencomo, J.A.; Lacono, S.T.; McCollum, J. 3D printing multifunctional fluorinated nanocomposites: Tuning electroactivity, rheology and chemical reactivity. J. Mater. Chem. A 2018, 6, 12308–12315. [Google Scholar] [CrossRef]
- Guo, H.; Yang, B.; Huang, G.; Xu, Q.; Li, S.; Wu, Z. Preparation and properties of polyvinylidene fluoride/graphene composites. CIESC J. 2020, 71, 1881–1888. [Google Scholar]
- Wang, Y.; Yang, W.; Lin, J.; Liu, X.; Zuo, Y.; Sun, H.; Yang, Y.; He, X. All organic polyimide-based composite films with high intrinsic polarization for superior energy density and low dielectric loss. J. Appl. Polym. Sci. 2023, 140, e54043. [Google Scholar] [CrossRef]
- Tu, R.; Sprague, E.; Sodano, H.A. Precipitation-printed high-β-phase poly(vinylidene fluoride) for energy harvesting. ACS Appl. Mater. Interfaces 2020, 12, 58072–58081. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, L.; Zhang, Y.; Luo, H.; Wang, R.; Zhang, D.; Wang, X. Electrical properties of PVDF films fabricated by direct ink writing. J. Cent. South Univ. 2023, 30, 1477–1489. [Google Scholar] [CrossRef]
- Yuan, X.; Gao, X.; Shen, X.; Yang, J.; Dong, S. A 3D-printed, alternatively tilt-polarized PVDF-TrFE polymer with enhanced piezoelectric effect for self-powered sensor application. Nano Energy 2021, 85, 105985. [Google Scholar] [CrossRef]
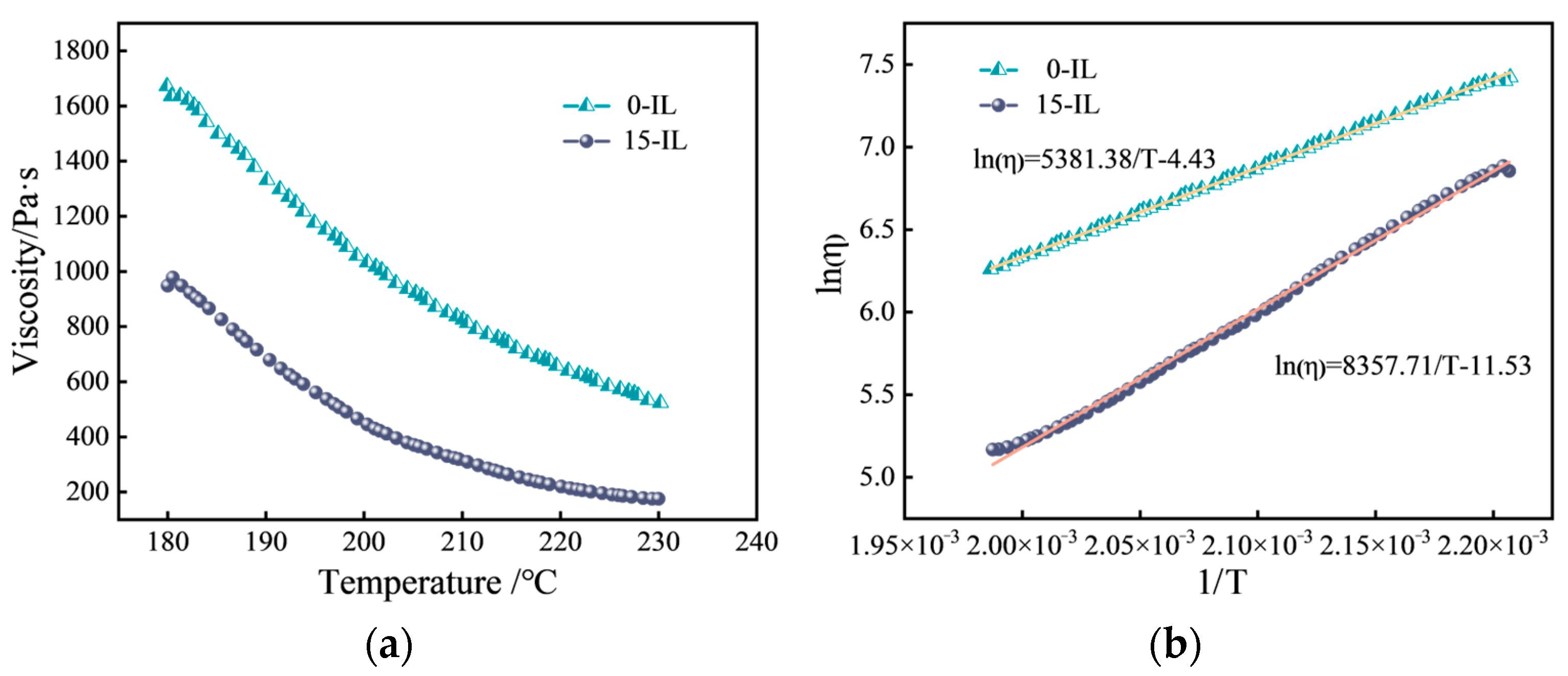
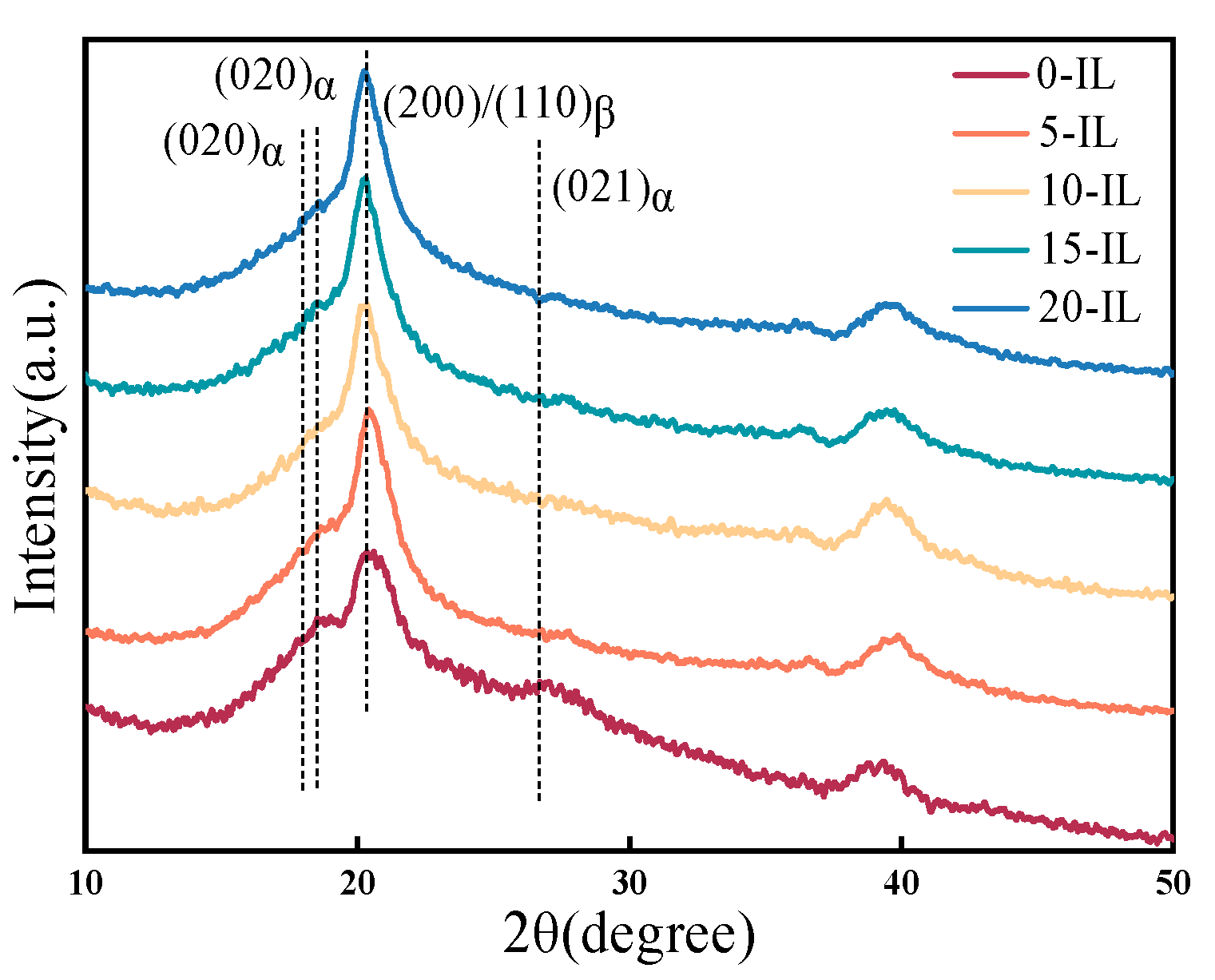
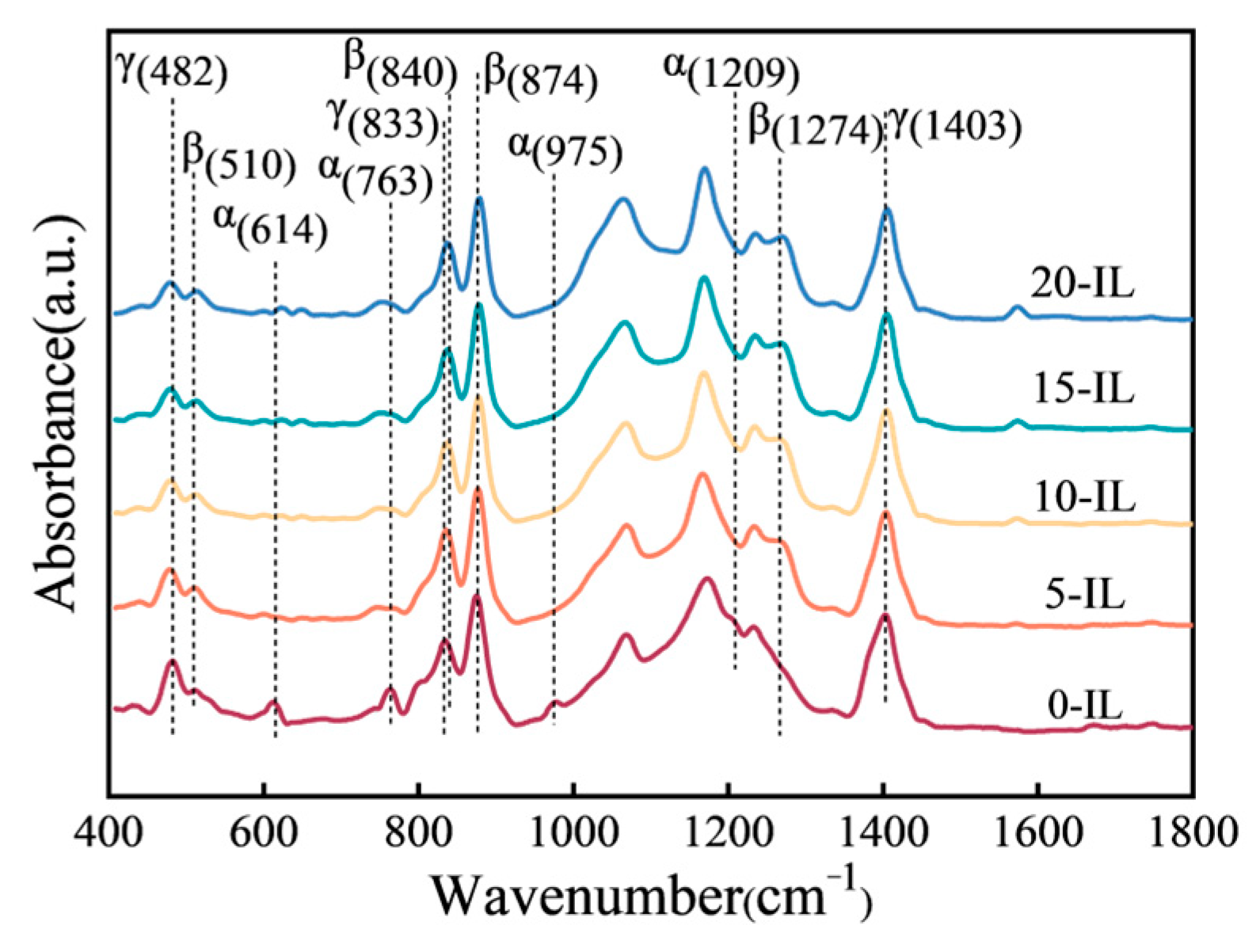

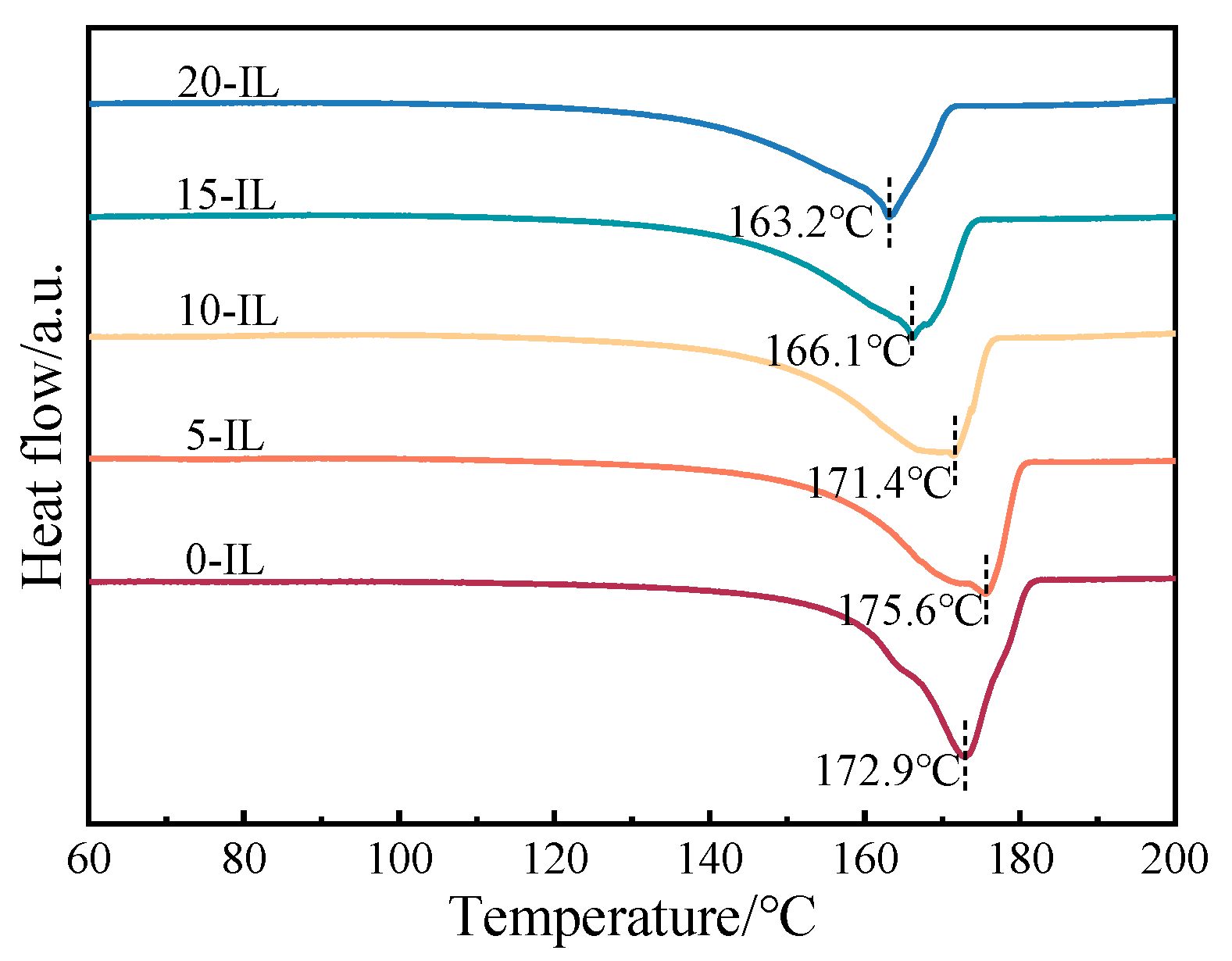
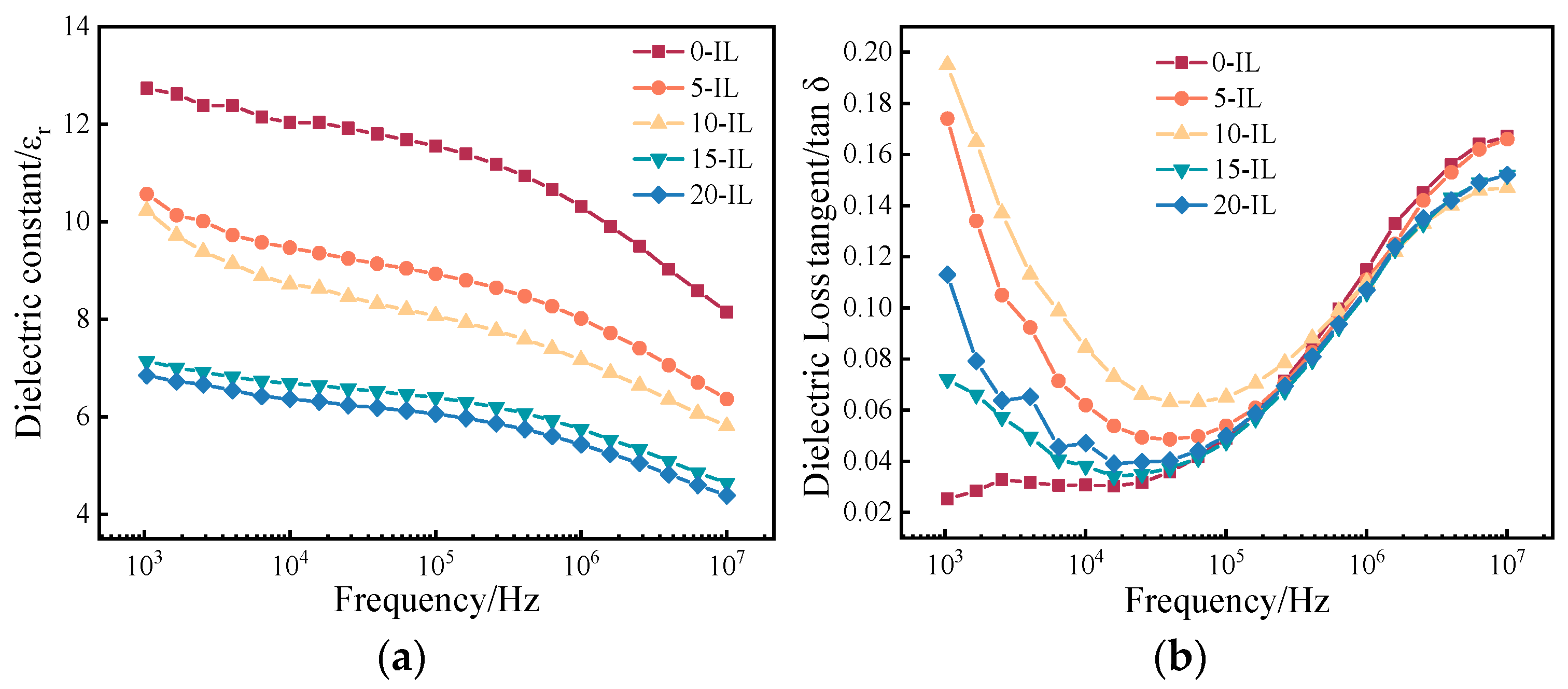
| IL Content (wt.%) | (J/mol) | (kJ/mol) | Linearity |
|---|---|---|---|
| 0 | 5381.38 | 44.74 | 0.99932 |
| 15 | 8357.71 | 69.46 | 0.99757 |
| Sample | 0-IL | 5-IL | 10-IL | 15-IL |
|---|---|---|---|---|
| F(β) (%) | 32.54 | 42.51 | 43.22 | 43.28 |
| (%) | 80.41 | 67.62 | 68.99 | 67.84 |
| (β) (%) | 26.17 | 28.74 | 29.81 | 29.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Yang, H.; Zhao, L.; Wang, C.; Peng, C.; Wang, R.; Zhang, D.; Wang, X. Effects of Ionic Liquid Content on the Electrical Properties of PVDF Films by Fused Deposition Modeling. Materials 2024, 17, 9. https://doi.org/10.3390/ma17010009
Zhou R, Yang H, Zhao L, Wang C, Peng C, Wang R, Zhang D, Wang X. Effects of Ionic Liquid Content on the Electrical Properties of PVDF Films by Fused Deposition Modeling. Materials. 2024; 17(1):9. https://doi.org/10.3390/ma17010009
Chicago/Turabian StyleZhou, Runkai, Hong Yang, Lianzhong Zhao, Chun Wang, Chaoqun Peng, Richu Wang, Dou Zhang, and Xiaofeng Wang. 2024. "Effects of Ionic Liquid Content on the Electrical Properties of PVDF Films by Fused Deposition Modeling" Materials 17, no. 1: 9. https://doi.org/10.3390/ma17010009
APA StyleZhou, R., Yang, H., Zhao, L., Wang, C., Peng, C., Wang, R., Zhang, D., & Wang, X. (2024). Effects of Ionic Liquid Content on the Electrical Properties of PVDF Films by Fused Deposition Modeling. Materials, 17(1), 9. https://doi.org/10.3390/ma17010009





