Enhancing the Performance of Hemihydrate Phosphogypsum by the Collaborative Effects of Calcium Hydroxide and Carbonation
Abstract
:1. Introduction
2. Materials and Experiments
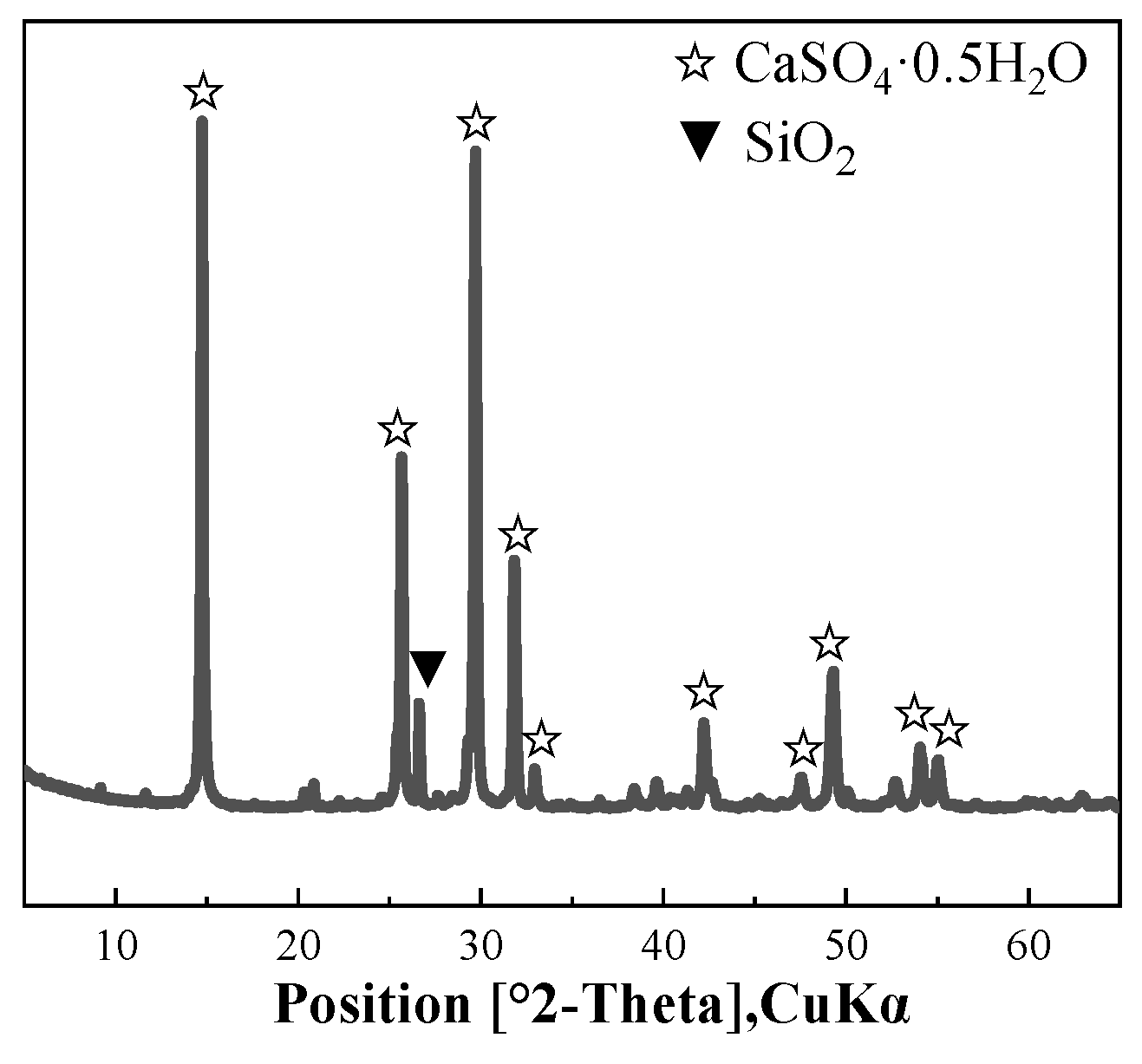
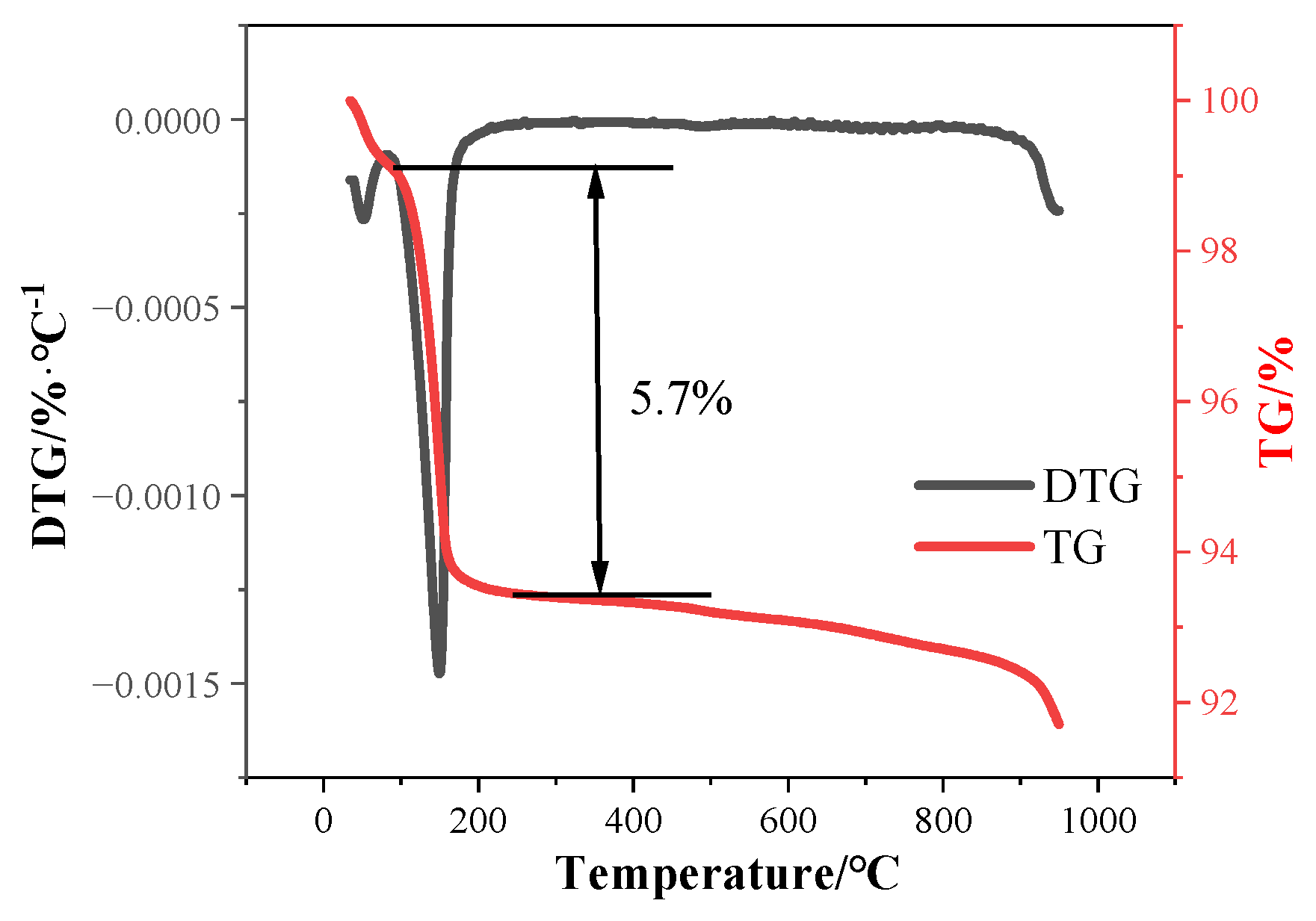
2.1. Raw Materials
2.2. Sample Preparation and Curing
2.3. Test Procedures
2.3.1. Water Requirement for the Normal Consistency, Fluidity and Setting Time
2.3.2. Mechanical Properties
2.3.3. Water Resistance
2.3.4. Carbonation Area
2.3.5. Chemical Composition Analysis
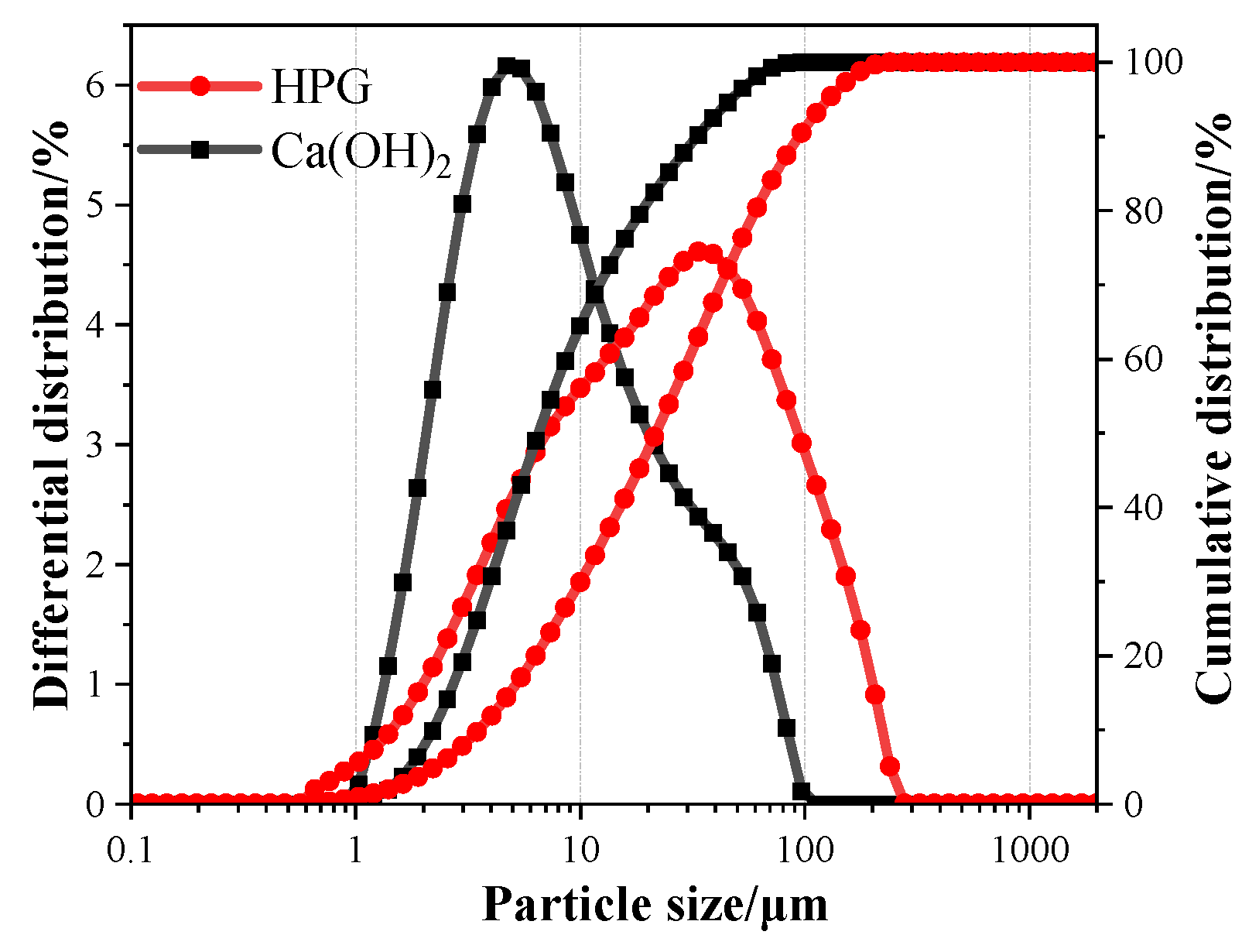
2.3.6. Particle Size Distribution Analysis
2.3.7. Hydration Products Analysis
2.3.8. Microstructure Analysis
2.3.9. Pore Structure Analysis
3. Results
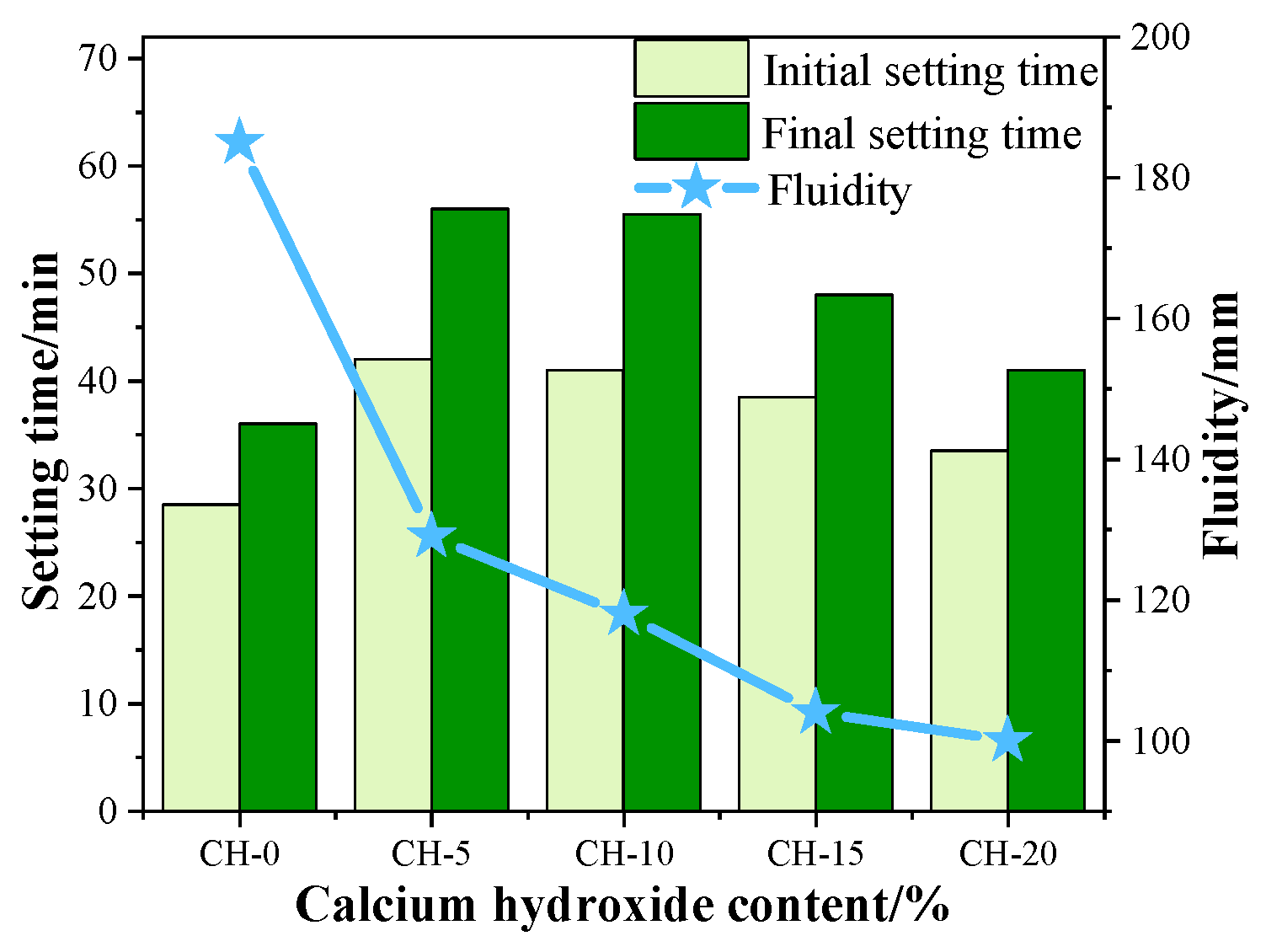
3.1. Effects of CH Addition on the Setting Time and Fluidity of HPG Pastes
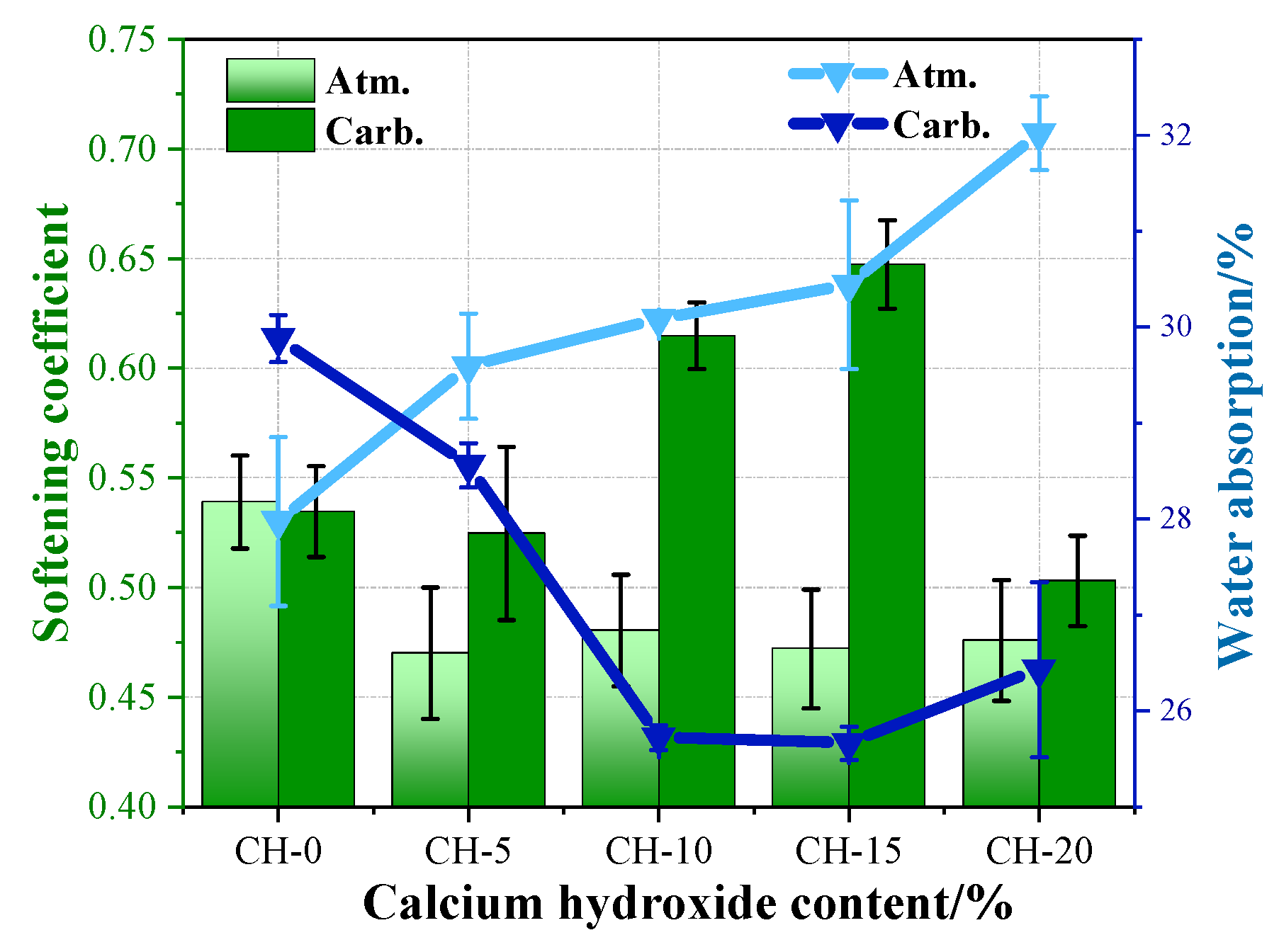
3.2. Effects of Carbonation on the Mechanical Properties and Water Resistance of HPG + CH Pastes
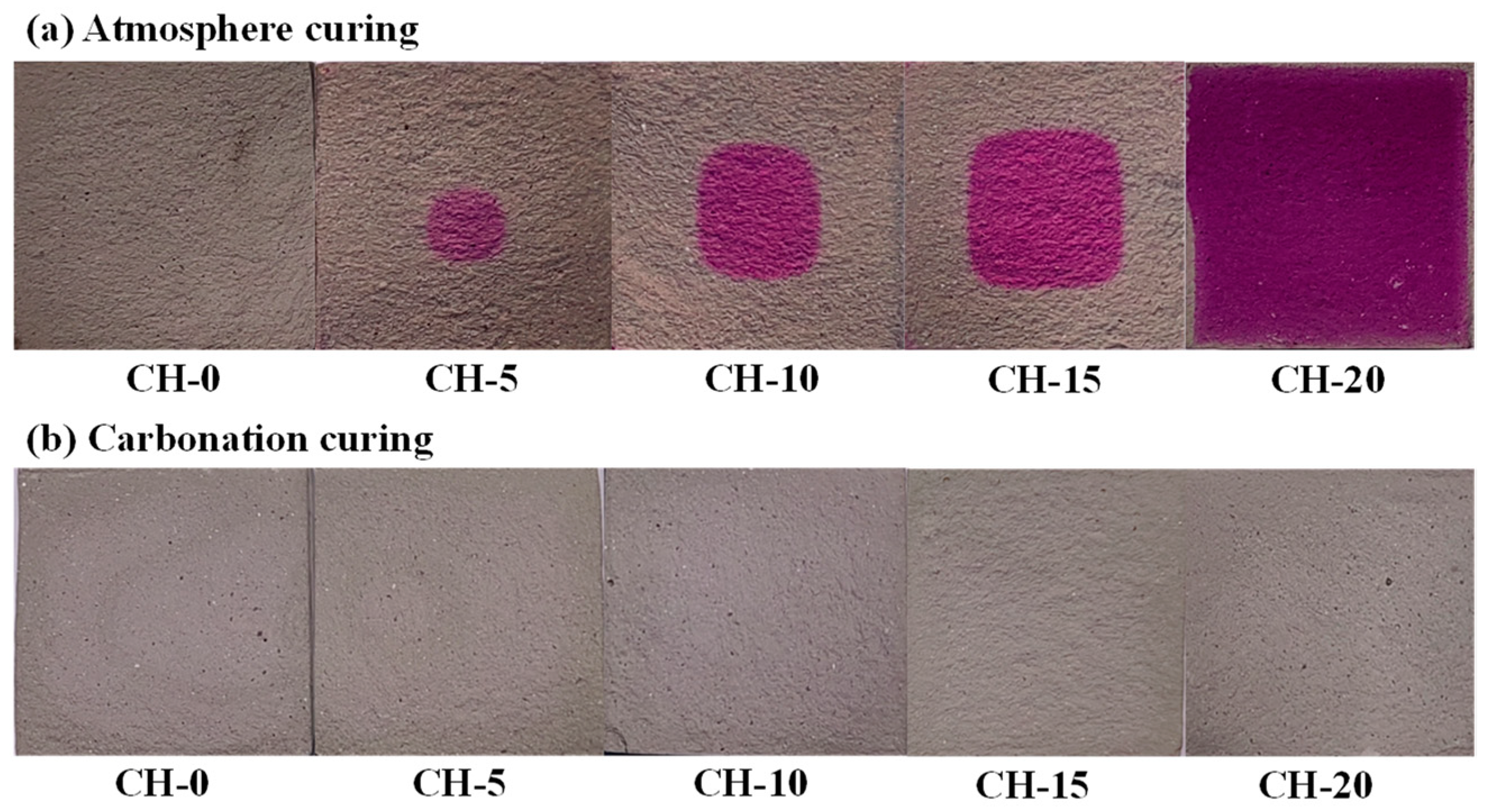
3.3. Carbonation Area of HPG + CH Pastes
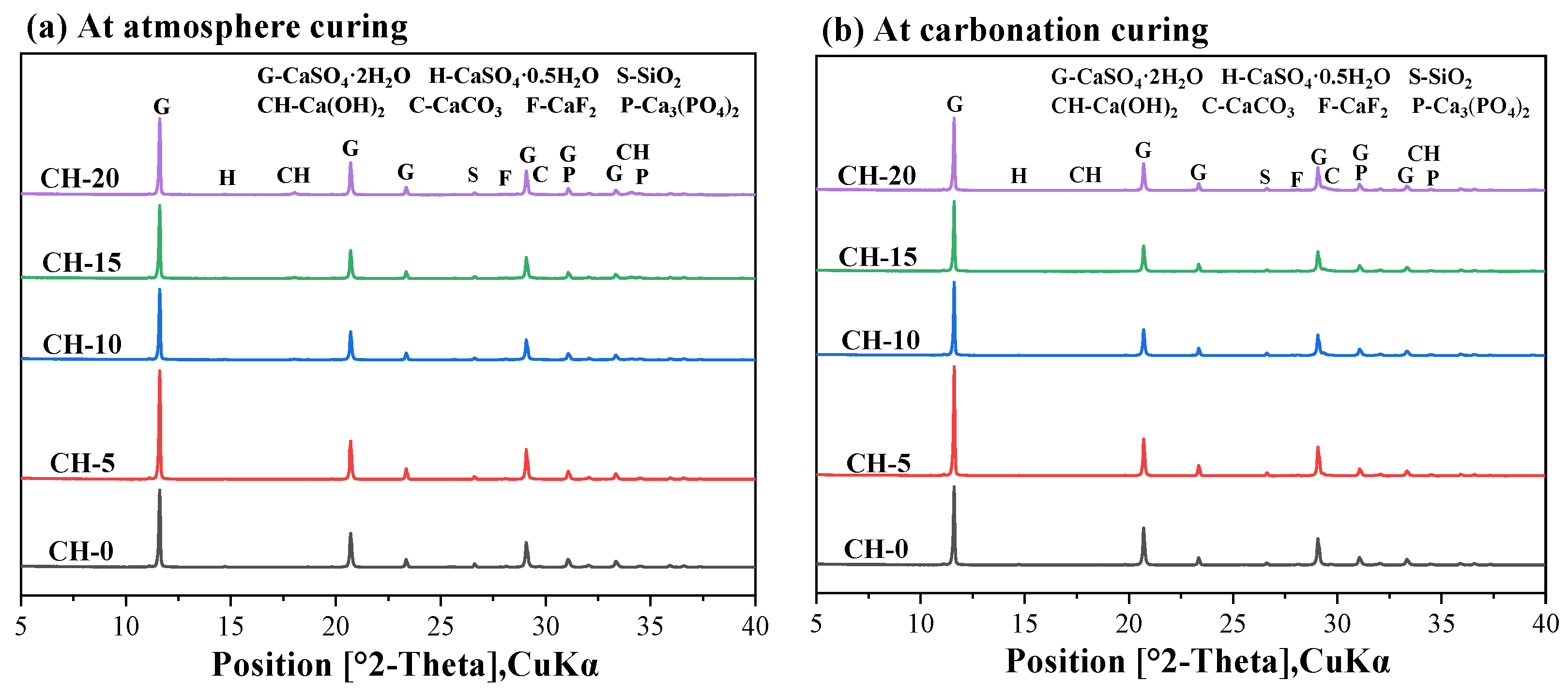
3.4. Products Identification of HPG + CH Pastes
3.4.1. XRD Analysis
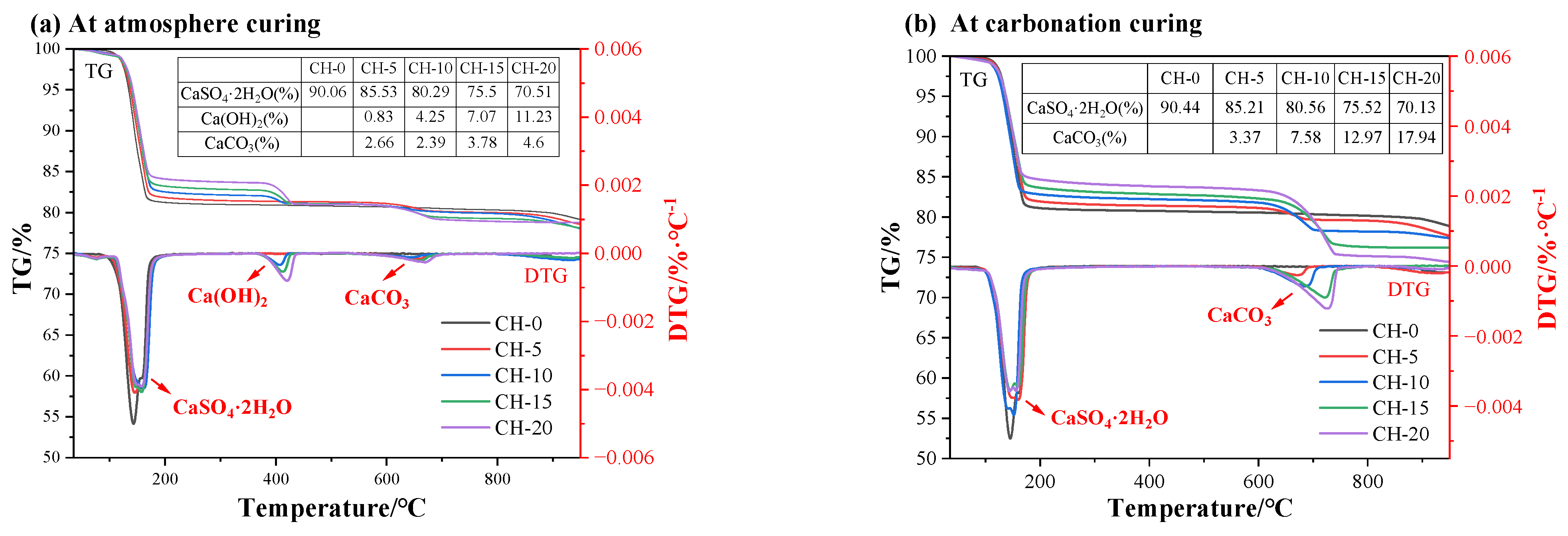
3.4.2. TG-DTG Analysis
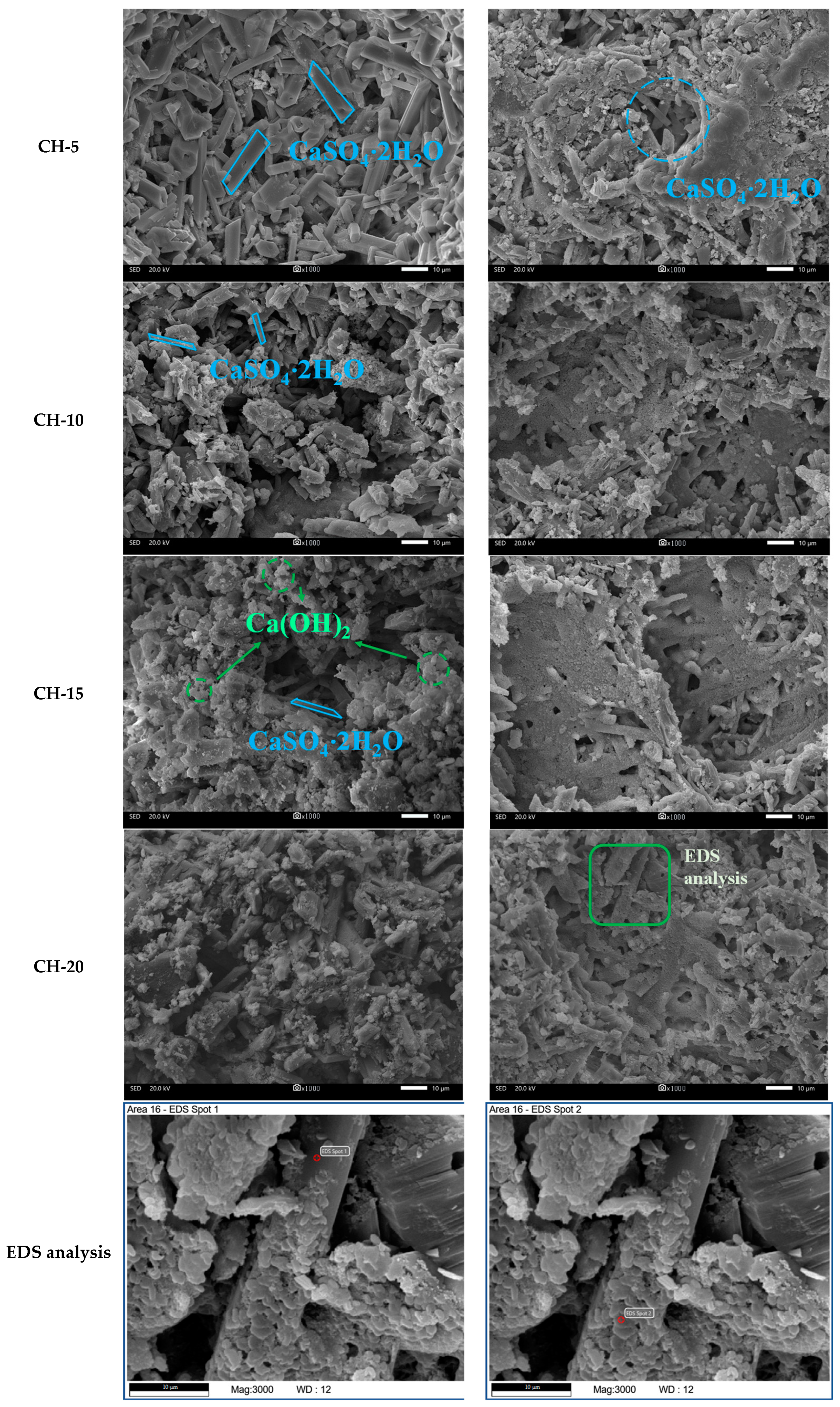
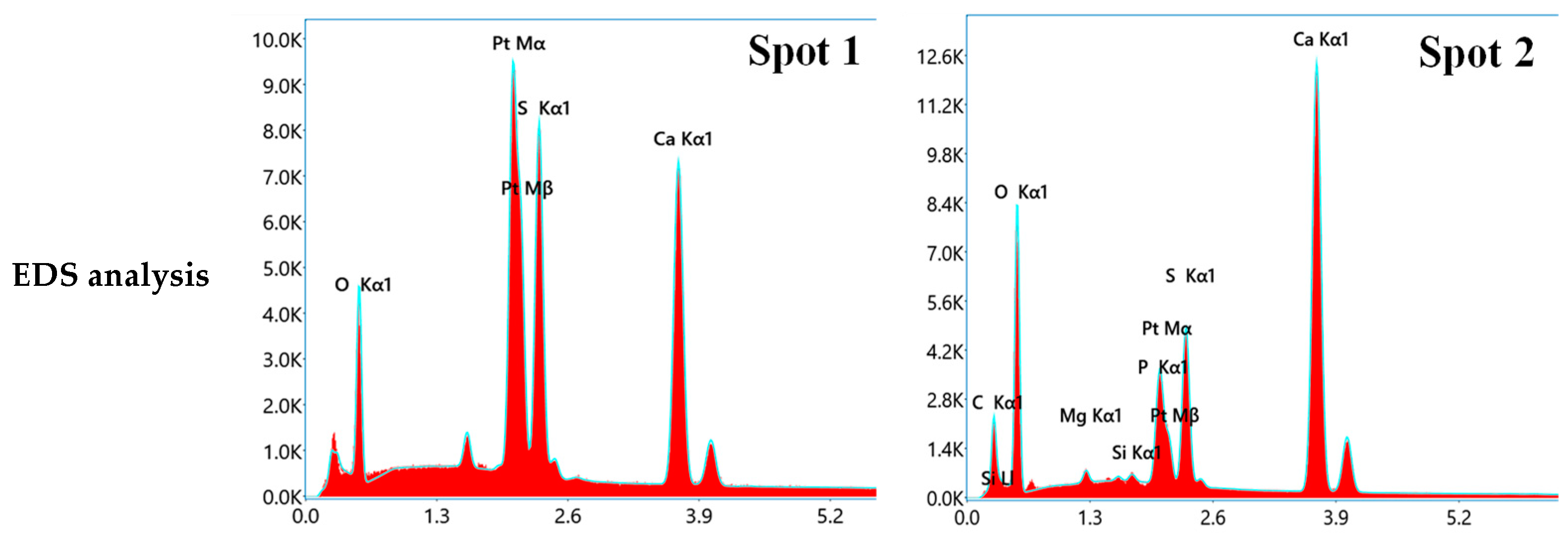
3.5. Microstructure Analysis
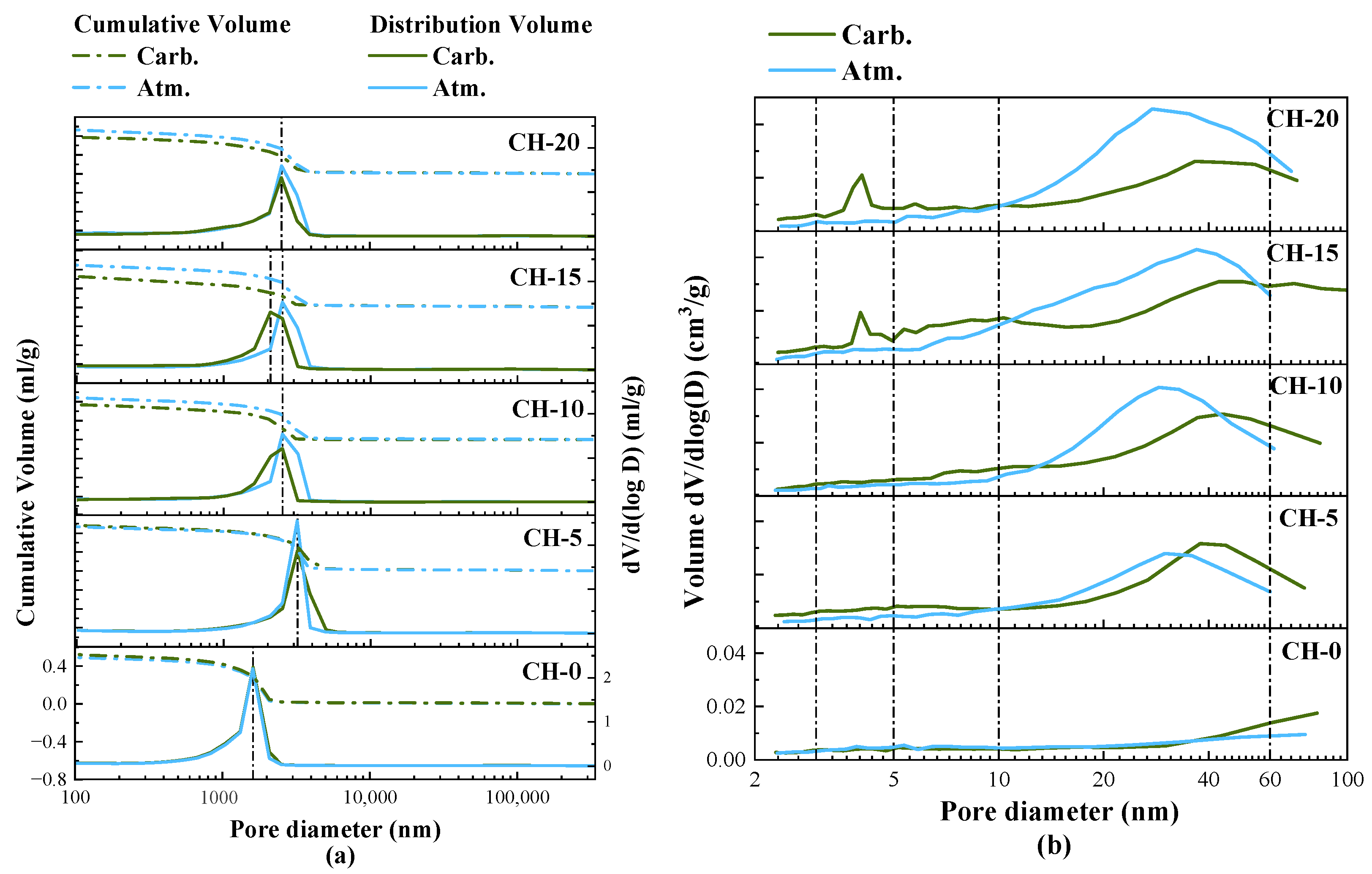
3.6. Pore Structure
4. Discussion
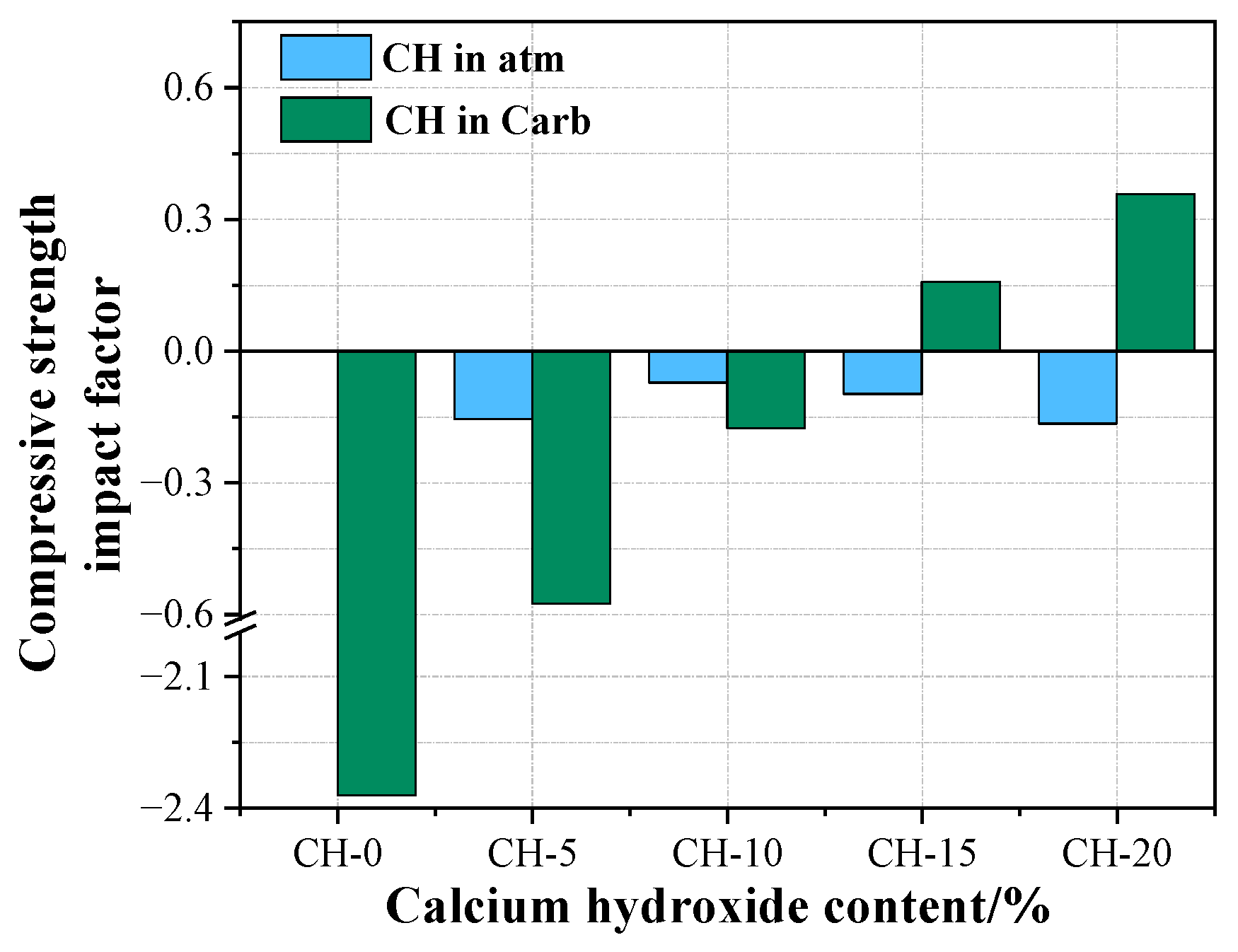
4.1. The Effects of CH on the Performance of HPG
4.2. The Effects of Carbonation Curing on the Performance of HPG + CH Pastes
4.3. Carbon Footprint and Energy Consumption of Carbonated HPG + CH Pastes
5. Conclusions
- The addition of 5 wt.% to 20 wt.% CH could prolong the setting times and decrease the fluidities of the fresh HPG pastes, and the compressive strength and the softening coefficient of hardened HPG pastes were decreased by about 9–37% and 13%, respectively;
- CaF2 and Ca3(PO4)2 could be identified in the HPG + CH pastes due to neutralization;
- Carbonation curing could significantly recover the compressive strength and water resistance of the hardened HPG + CH pastes. The carbonated HPG + 15 wt.% CH paste demonstrated the best performance—compared with the reference paste under atmosphere, the compressive strength and softening coefficient increased by 16.2% and 37.1%, respectively;
- A large number of nanoscale CaCO3 crystals were identified to fill the pores of the carbonated HPG + CH pastes and cover the surface of CaSO4·2H2O crystals. This refinement and coverage caused the improvement of compressive strength and water resistance;
- The carbonated HPG + 15 wt.% CH paste is also an environmentally friendly option, exhibiting the lowest normalized saturated conditions compressive strength carbon footprint and energy consumption, with 13.3% and 6.2% reductions compared to pure HPG paste.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Hwaiti, M.S. Influence of treated waste phosphogypsum materials on the properties of ordinary portland cement. Bangladesh J. Sci. Ind. Res. 2015, 50, 241–250. [Google Scholar] [CrossRef]
- Haneklaus, N.; Barbossa, S.; Basallote, M.D.; Bertau, M.; Bilal, E.; Chajduk, E.; Chernysh, Y.; Chubur, V.; Cruz, J.; Dziarczykowski, K.; et al. Closing the upcoming EU gypsum gap with phosphogypsum. Resour. Conserv. Recycl. 2022, 182, 106328. [Google Scholar] [CrossRef]
- Taher, M.A. Influence of thermally treated phosphogypsum on the properties of Portland slag cement. Resour. Conserv. Recycl. 2007, 52, 28–38. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- López, F.A.; Gázquez, M.; Alguacil, F.J.; Bolívar, J.P.; García-Díaz, I.; López-Coto, I. Microencapsulation of phosphogypsum into a sulfur polymer matrix: Physico-chemical and radiological characterization. J. Hazard. Mater. 2011, 192, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.; de Rezende, L.R.; Mascarenha, M.M.D.A.; de Oliveira, R.B. Phosphogypsum, tropical soil and cement mixtures for asphalt pavements under wet and dry environmental conditions. Resour. Conserv. Recycl. 2019, 144, 123–136. [Google Scholar] [CrossRef]
- Seraya, N.; Litvinov, V.; Daumova, G.; Zhusipov, N.; Idrisheva, Z.; Aubakirova, R. Production Waste Management: Qualitative and Quantitative Characteristics and the Calculation of the Hazard Class of Phosphogypsum. Processes 2023, 11, 3033. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, R.; Álvarez-Valero, A.M.; Nieto, J.M. Changes in mobility of toxic elements during the production of phosphoric acid in the fertilizer industry of Huelva (SW Spain) and environmental impact of phosphogypsum wastes. J. Hazard. Mater. 2007, 148, 745–750. [Google Scholar] [CrossRef]
- Altun, İ.A.; Sert, Y. Utilization of weathered phosphogypsum as set retarder in Portland cement. Cem. Concr. Res. 2004, 34, 677–680. [Google Scholar] [CrossRef]
- Murali, G.; Azab, M. Recent research in utilization of phosphogypsum as building materials: Review. J. Mater. Res. Technol. 2023, 25, 960–987. [Google Scholar] [CrossRef]
- Garbaya, H.; Jraba, A.; Khadimallah, M.A.; Elaloui, E. The Development of a New Phosphogypsum-Based Construction Material: A Study of the Physicochemical, Mechanical and Thermal Characteristics. Materials 2021, 14, 7369. [Google Scholar] [CrossRef]
- Bumanis, G.; Zorica, J.; Bajare, D.; Korjakins, A. Technological properties of phosphogypsum binder obtained from fertilizer production waste. Energy Procedia 2018, 147, 301–308. [Google Scholar] [CrossRef]
- Singh, M. Effect of phosphatic and fluoride impurities of phosphogypsum on the properties of selenite plaster. Cem. Concr. Res. 2003, 33, 1363–1369. [Google Scholar] [CrossRef]
- Singh, M. Role of phosphogypsum impurities on strength and microstructure of selenite plaster. Constr. Build. Mater. 2005, 19, 480–486. [Google Scholar] [CrossRef]
- Doleželová, M.; Krejsová, J.; Scheinherrová, L.; Keppert, M.; Vimmrová, A. Investigation of environmentally friendly gypsum based composites with improved water resistance. J. Clean Prod. 2022, 370, 133278. [Google Scholar] [CrossRef]
- Chen, X.M. Study on the Hydration-Hardening Mechanism and Microstructure Regulation of Hemihydrate Phosphgypsum under Alkaline Condition. Ph.D. Thesis, Southeast University, Nanjing, China, 25 January 2021. [Google Scholar]
- Neto, J.S.A.; Bersch, J.D.; Silva, T.S.M.; Rodriguez, E.D.; Suzuki, S.; Kirchheim, A.P. Influence of phosphogypsum purification with lime on the properties of cementitious matrices with and without plasticizer. Constr. Build. Mater. 2021, 299, 11. [Google Scholar] [CrossRef]
- Chen, X.M.; Gao, J.M.; Zhao, Y.S. Investigation on the hydration of hemihydrate phosphogypsum after post treatment. Constr. Build. Mater. 2019, 229, 9. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, H.; Wang, D.; Wang, Q. Effects of Ca(OH)2 on the early hydration, macro-performance and environmental risks of the calcined phosphogypsum. Constr. Build. Mater. 2022, 324, 126590. [Google Scholar] [CrossRef]
- Altiner, M. Use of Taguchi approach for synthesis of calcite particles from calcium carbide slag for CO2 fixation by accelerated mineral carbonation. Arab. J. Chem. 2019, 12, 531–540. [Google Scholar] [CrossRef]
- Hu, X.; He, P.; Shi, C. Carbonate binders: Historic developments and perspectives. Cem. Concr. Res. 2024, 175, 107352. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, W.; Sun, H.; Peng, T.; Ma, G. Utilization of Phosphogypsum to Prepare High-Purity CaCO3 in the NH4Cl–NH4OH–CO2 System. ACS Sustain. Chem. Eng. 2020, 8, 11649–11657. [Google Scholar] [CrossRef]
- GB/T 9776-2022; Calcined Gypsum. General Administration of Market Regulation of the People’s Republic of China. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- Campos, M.P.; Costa, L.J.P.; Nisti, M.B.; Mazzilli, B.P. Phosphogypsum recycling in the building materials industry: Assessment of the radon exhalation rate. J. Environ. Radioact. 2017, 172, 232–236. [Google Scholar] [CrossRef] [PubMed]
- GB/T 17669.3-1999; Gypsum Plasters-Determination of Mechanical Properties. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 1999.
- GB/T 17669.4-1999; Gypsum Plasters-Determination of Phycical Properties of Pure Paste. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 1999.
- Schofield, P.F.; Wilson, C.C.; Knight, K.S.; Stretton, I.C. Temperature related structural variation of the hydrous components in gypsum. Z. Krist.-Cryst. Mater. 2000, 215, 707–710. [Google Scholar] [CrossRef]
- Ikuta, D.; Kawame, N.; Banno, S.; Hirajima, T.; Ito, K.; Rakovan, J.F.; Downs, R.T.; Tamada, O. First in situ X-ray identification of coesite and retrograde quartz on a glass thin section of an ultrahigh-pressure metamorphic rock and their crystal structure details. Am. Miner. 2007, 92, 57–63. [Google Scholar] [CrossRef]
- Desgranges, L.; Grebille, D.; Calvarin, G.; Chevrier, G.; Floquet, N.; Niepce, J.-C. Hydrogen thermal motion in calcium hydroxide: Ca(OH)2. Acta Cryst. 1993, B49, 812–817. [Google Scholar] [CrossRef]
- Goergens, J.; Manninger, T.; Goetz-Neunhoeffer, F. In-situ XRD study of the temperature-dependent early hydration of calcium aluminate cement in a mix with calcite. Cem. Concr. Res. 2020, 136, 106160. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical Analysis by X-ray Diffraction: Classification and Use of X-ray Diffraction Patterns. Powder Diffr. 1986, 1, 2–14. [Google Scholar] [CrossRef]
- Batchelder, D.N.; Simmons, R.O. Lattice Constants and Thermal Expansivities of Silicon and of Calcium Fluoride between 6° and 322° K. J. Chem. Phys. 2004, 41, 2324–2329. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, H. Characterization and optimization of eco-friendly cementitious materials based on titanium gypsum, fly ash, and calcium carbide residue. Constr. Build. Mater. 2022, 349, 128635. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, J. Microstructure changes of waste hydrated cement paste induced by accelerated carbonation. Constr. Build. Mater. 2015, 76, 360–365. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.X.; Boyd, A.J.; He, Z. Microstructure of cement paste subject to early carbonation curing. Cem. Concr. Res. 2012, 42, 186–193. [Google Scholar] [CrossRef]
- Panesar, D.K.; Mo, L.W. Properties of binary and ternary reactive MgO mortar blends subjected to CO2 curing. Cem. Concr. Compos. 2013, 38, 40–49. [Google Scholar] [CrossRef]
- Chen, X.M.; Gao, J.M.; Liu, C.B.; Zhao, Y.S. Effect of neutralization on the setting and hardening characters of hemihydrate phosphogypsum plaster. Constr. Build. Mater. 2018, 190, 53–64. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, S.; Wang, K.; Qunaynah, S.A.; Wan, S.; Yuan, Z.; Xu, P.; Tang, S. A study on the hydration of calcium aluminate cement pastes containing silica fume using non-contact electrical resistivity measurement. J. Mater. Res. Technol. 2023, 24, 8135–8149. [Google Scholar] [CrossRef]
- Halamickova, P.; Detwiler, R.J.; Bentz, D.P.; Garboczi, E.J. Water permeability and chloride ion diffusion in portland cement mortars: Relationship to sand content and critical pore diameter. Cem. Concr. Res. 1995, 25, 790–802. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Bogner, A.; Schatz, J.; Dehn, F.; Muller, H.S. Influence of Drying on the Microstructure of Hardened Cement Paste: A Mercury Intrusion Porosimetry, Nitrogen Sorption and SAXS Study. J. Adv. Concr. Technol. 2020, 18, 83–94. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, J.; Deng, F.; Li, H.; Wang, K.; Tang, S. Hydration behavior and strength development of supersulfated cement prepared by calcined phosphogypsum and slaked lime. J. Build. Eng. 2023, 80, 108075. [Google Scholar] [CrossRef]
- Mo, L.W.; Panesar, D.K. Accelerated carbonation—A potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, H.; Hao, L.X.; Li, Q. Water resistant block from desulfurization gypsum. Constr. Build. Mater. 2012, 27, 531–533. [Google Scholar] [CrossRef]
- Garg, M.; Minocha, A.K.; Jain, N. Environment hazard mitigation of waste gypsum and chalk: Use in construction materials. Constr. Build. Mater. 2011, 25, 944–949. [Google Scholar] [CrossRef]
- Fornés, I.V.; Vaičiukynienė, D.; Nizevičienė, D.; Doroševas, V. The improvement of the water-resistance of the phosphogypsum by adding waste metallurgical sludge. J. Build. Eng. 2021, 43, 102861. [Google Scholar] [CrossRef]
- Jin, Z.; Ma, B.; Su, Y.; Lu, W.; Qi, H.; Hu, P. Effect of calcium sulphoaluminate cement on mechanical strength and waterproof properties of beta-hemihydrate phosphogypsum. Constr. Build. Mater. 2020, 242, 118198. [Google Scholar] [CrossRef]
- Zheng, X.X.; Bai, H.D.; Feng, S.S.; Cui, R.Z.; Wang, C.; Gao, Y.F. Carbon emission status of phosphate and compound fertilizer industry and low carbon green development path. Phosphate Compd. Fertil. 2022, 37, 1–7. [Google Scholar]
- Smith, P.; Martino, D.L.; Cai, Z.C.; Gwary, D.; Janzen, H.H.; Kumar, P.; McCarl, B.A.; Ogle, S.M.; O’Mara, F.P.; Rice, C.W.; et al. Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B-Biol. Sci. 2008, 363, 789–813. [Google Scholar] [CrossRef]
- Hammond, G.; Jones, C. Inventory of Carbon and Energy, 2nd ed.; University of Bath: Bath, UK, 2011. [Google Scholar]




| Radionuclide | Initial Setting Time (min) | Final Setting Time (min) | 2 h Flexural Strength (MPa) | 2 h Compressive Strength (MPa) | |
|---|---|---|---|---|---|
| Test results | IRa = 0.3, Ic = 0.3 | 7 | 16 | 3.3 | 8.7 |
| Composition | SO3 | CaO | SiO2 | F | P2O5 | Al2O3 | Fe2O3 | Na2O | TiO2 | K2O | SrO | BaO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPG | 46.69 | 35.96 | 5.598 | 1.8 | 1.03 | 0.755 | 0.36 | 0.085 | 0.0742 | 0.0729 | 0.0728 | 0.071 |
| CH | 0.292 | 82.56 | 0.98 | - | 0.01 | 0.408 | 0.239 | - | 0.022 | 0.0807 | 0.0211 | - |
| Mixtures Notation | HPG | Ca (OH)2 | Retarder XK (By Mass of HPG) | Water to Powder Ratio |
|---|---|---|---|---|
| CH-0 | 100 | 0 | 0.01 | 0.58 |
| CH-5 | 95 | 5 | ||
| CH-10 | 90 | 10 | ||
| CH-15 | 85 | 15 | ||
| CH-20 | 80 | 20 |
| Compound Name | Chemical Formula | PDF Code | ICSD Collection Code | Author [Ref.] |
|---|---|---|---|---|
| Gypsum | CaSO4·2H2O | 00-033-0311 | 92567 | Schofield [28] |
| Quartz | SiO2 | 156196 | Ikuta [29] | |
| Portlandite | Ca(OH)2 | 00-004-0733 | 73467 | Desgranges [30] |
| Calcite | CaCO3 | 00-005-0586 | 80869 | Goergens [31] |
| Calcium Phosphate | Ca3(PO4)2 | 10941 | Hanawalt [32] | |
| Calcium Fluoride | CaF2 | 01-077-2096 | 60371 | Batchelder [33] |
| Raw Materials | CO2 Emission (kgCO2/kg) | [Ref.] | Energy Consumption (MJ/kg) | [Ref.] | |
|---|---|---|---|---|---|
| HPG | Formation of PG | 0.18 | [50] | 1.0 | [51] |
| Calcination of PG | 0.13 | [52] | 1.8 | [52] | |
| CH | 0.78 | [52] | 5.3 | [52] | |
| HPG Pastes | CO2 Emission (kgCO2·m−3) | CO2 Capture (kgCO2·m−3) | (kgCO2·m −3·MPa−1) | Energy Consumption (MJ·m−3) | (MJ·m−3 ·MPa−1) | ||
|---|---|---|---|---|---|---|---|
| CH-0 | 1174.61 | 0.00 | 0.00 | 127.68 | 10,609.38 | 0.00 | 1153.19 |
| CH-5 | 1115.88 | 147.77 | 54.89 | 159.05 | 10,078.91 | 1004.10 | 1458.29 |
| CH-10 | 1057.15 | 295.55 | 124.41 | 136.48 | 9548.44 | 2008.20 | 1284.07 |
| CH-15 | 998.42 | 443.32 | 213.34 | 110.76 | 9017.97 | 3012.30 | 1083.81 |
| CH-20 | 939.69 | 591.09 | 296.47 | 128.57 | 8487.50 | 4016.41 | 1302.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Liu, Z.; Wei, X.; Ding, X.; Zhu, J.; Zhao, Y.; Iqbal, B.; Guo, S. Enhancing the Performance of Hemihydrate Phosphogypsum by the Collaborative Effects of Calcium Hydroxide and Carbonation. Materials 2024, 17, 2204. https://doi.org/10.3390/ma17102204
Huang J, Liu Z, Wei X, Ding X, Zhu J, Zhao Y, Iqbal B, Guo S. Enhancing the Performance of Hemihydrate Phosphogypsum by the Collaborative Effects of Calcium Hydroxide and Carbonation. Materials. 2024; 17(10):2204. https://doi.org/10.3390/ma17102204
Chicago/Turabian StyleHuang, Jiawen, Zanqun Liu, Xiangsong Wei, Xiaojiang Ding, Jiahui Zhu, Yilin Zhao, Babar Iqbal, and Shulai Guo. 2024. "Enhancing the Performance of Hemihydrate Phosphogypsum by the Collaborative Effects of Calcium Hydroxide and Carbonation" Materials 17, no. 10: 2204. https://doi.org/10.3390/ma17102204
APA StyleHuang, J., Liu, Z., Wei, X., Ding, X., Zhu, J., Zhao, Y., Iqbal, B., & Guo, S. (2024). Enhancing the Performance of Hemihydrate Phosphogypsum by the Collaborative Effects of Calcium Hydroxide and Carbonation. Materials, 17(10), 2204. https://doi.org/10.3390/ma17102204






