Carbon-Based Composites for Oxygen Evolution Reaction Electrocatalysts: Design, Fabrication, and Application
Abstract
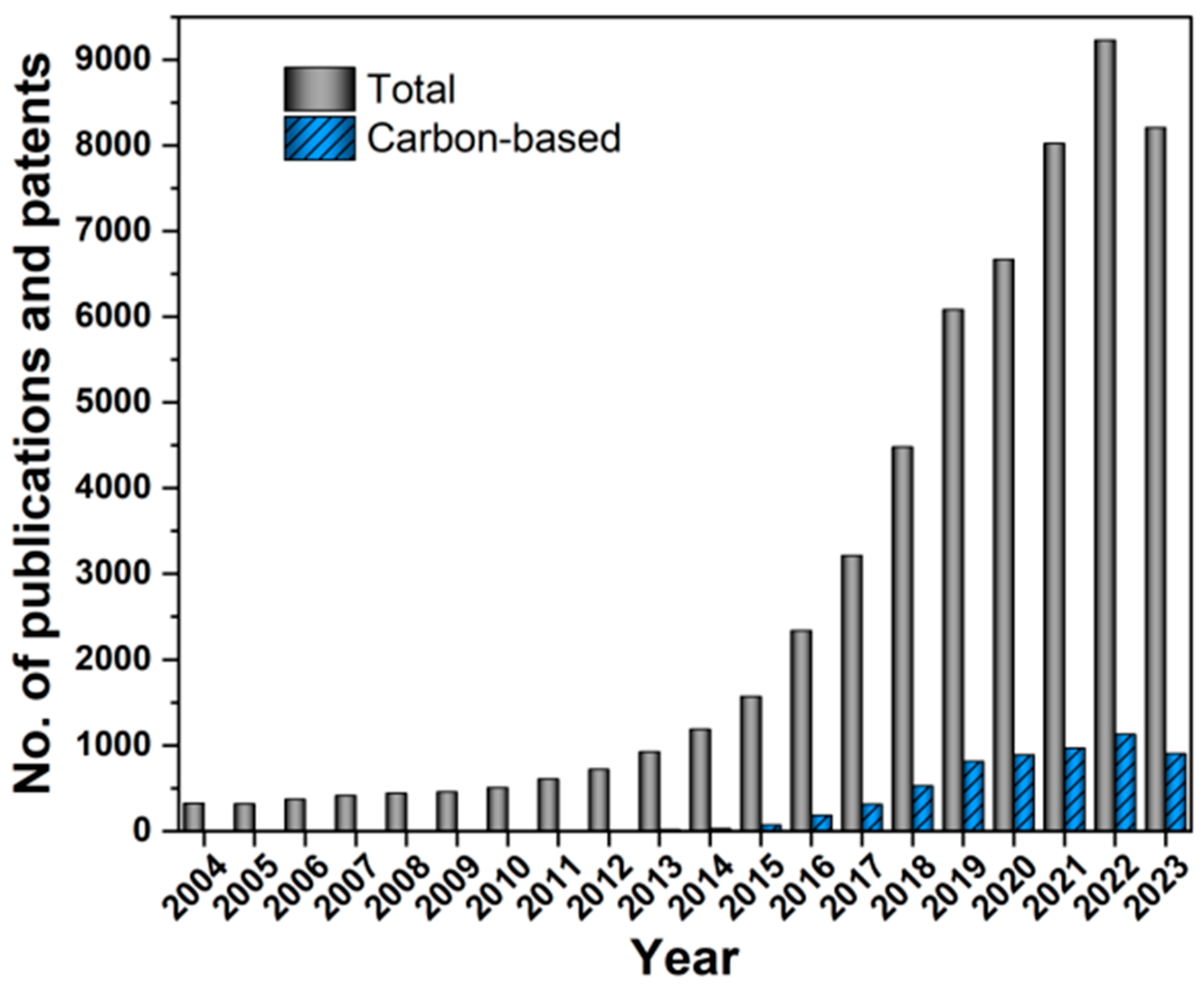
:1. Introduction
2. Design of Carbon-Based Composites
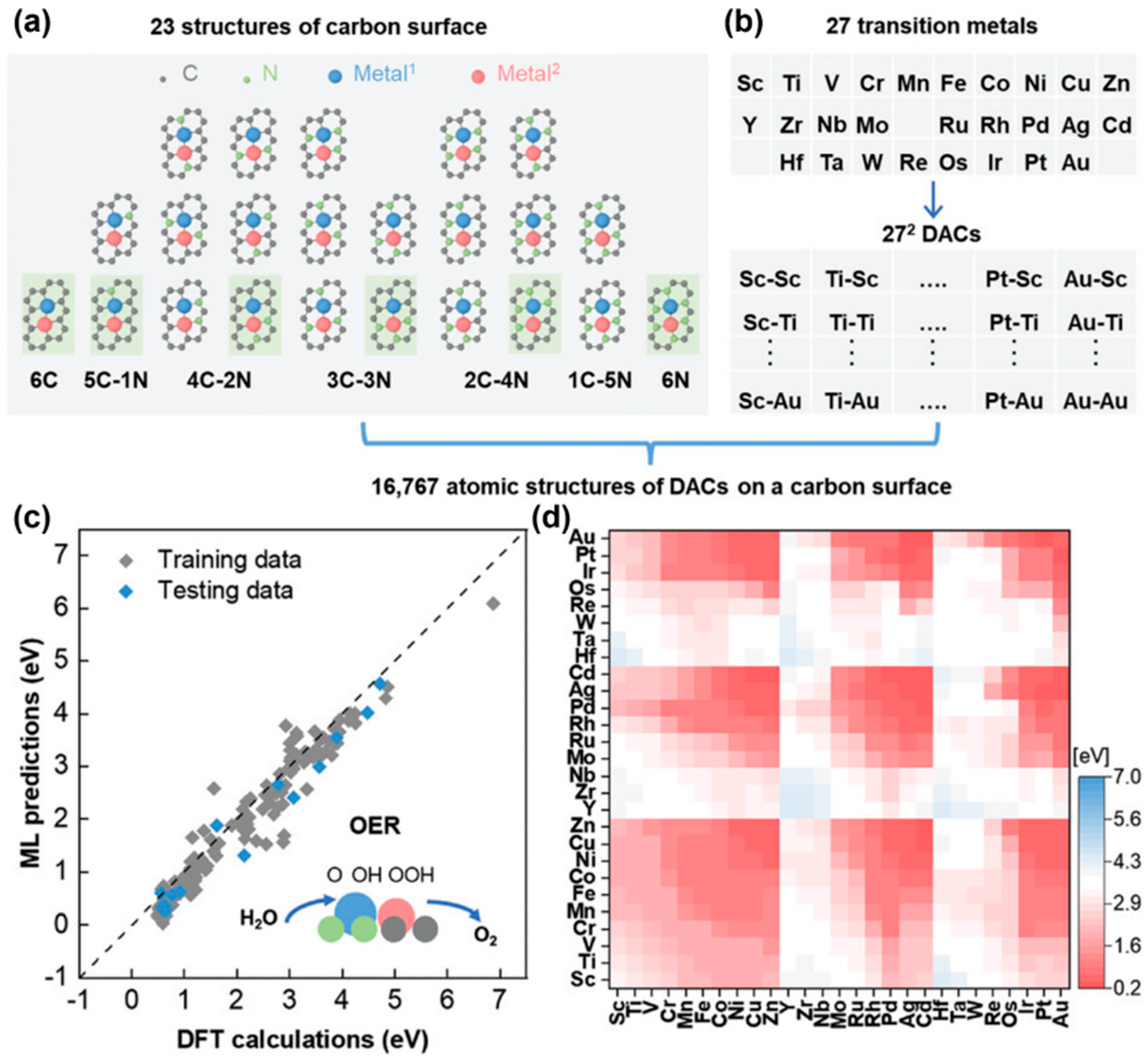
2.1. Theoretical Design
2.2. Combined Theoretical and Experimental Design
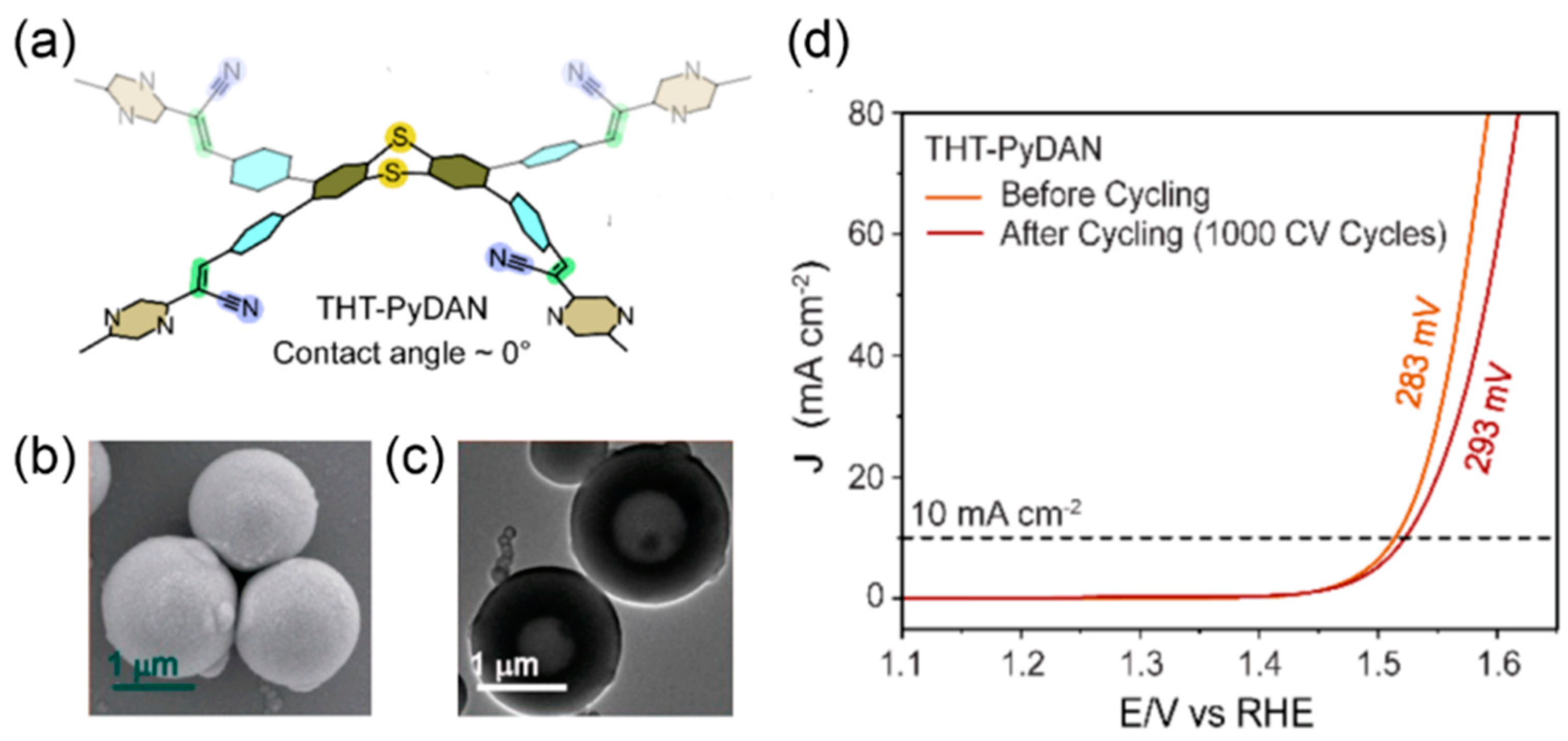
2.3. Experimental Design
3. Fabrication and Application
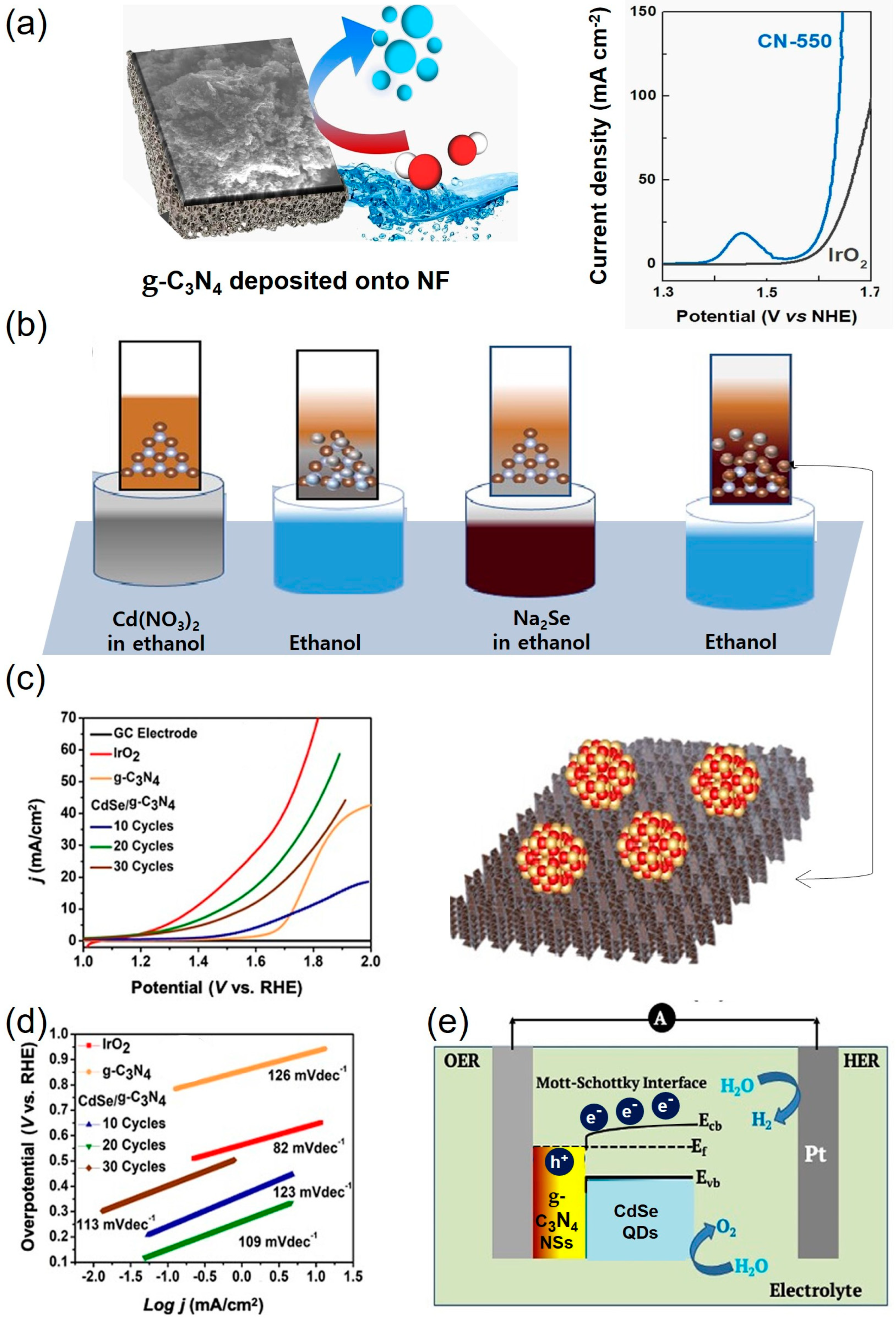
3.1. Metal-Free Carbon-Based Materials
| Materials | Morphology | OER | Water Splitting | Electrolyte | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| η at j | TS | Sta. | U at j | Stab. | ||||
| THT-PyDAN a | POPs, hollow sphere | 283@10 | 81 | 36@20 | 1.85@10 | 12@10 | 1 M KOH | [70] |
| TDA-Trz-POP | N,S-rich microporous organic polymer powder | 410@10 | 104.5 | 3@10 | 2.61@10 | N.A. | 1 M KOH | [27] |
| CCW-KOH-600 | Coffee waste-derived porous carbon, activated by KOH at 600 °C | 230@10 | 140 | 24@10 | N.A | N.A. | 6 M KOH | [80] |
| g-C3N4 | Layered composition | 355@10 | 46.8 | 45@10&50&100 | N.A. | N.A. | 1 M KOH | [81] |
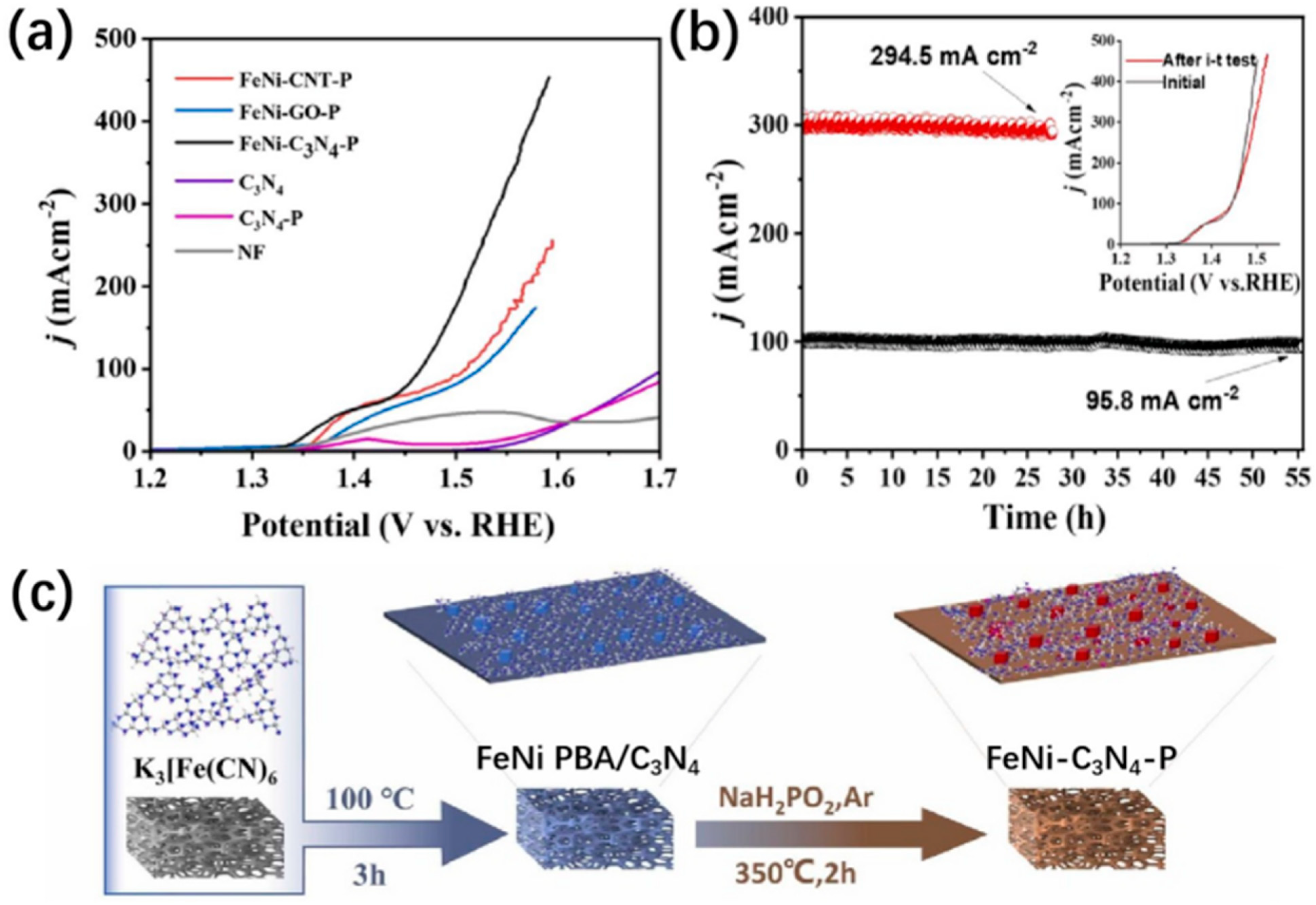
| FeNi-C3N4-P/NF | Interfacial hetero-structure of FeNi phosphide/C3N4 grown on NF | 235@100 | 40.4 | 55@100 | N.A. | N.A. | 1 M KOH | [82] |
| CoFe0.05P/CC | Nanorods grown on CC | 264@10 | 102 | 45@10 | 1.59@10 | 24@10 | 1 M KOH | [40] |
| Se-NiS2/CC | Se-doped NiS2 nanosheets grown on CC | 343@50 | 44.3 | 30@100 | N.A. | N.A. | 1 M KOH | [83] |
| W@Ni(OH)2/CC | Ni(OH)2 nanosheets and tungsten nanograins form a heterogeneous structure on CC | 290@10 | 67 | [email protected] V | N.A. | N.A. | 1 M KOH | [84] |
| d-NiFeP/CC | Array structure grown on CC | 185@10 | 24.56 | 100@10 | 1.48@10 | 50@10 | 1 M KOH | [85] |
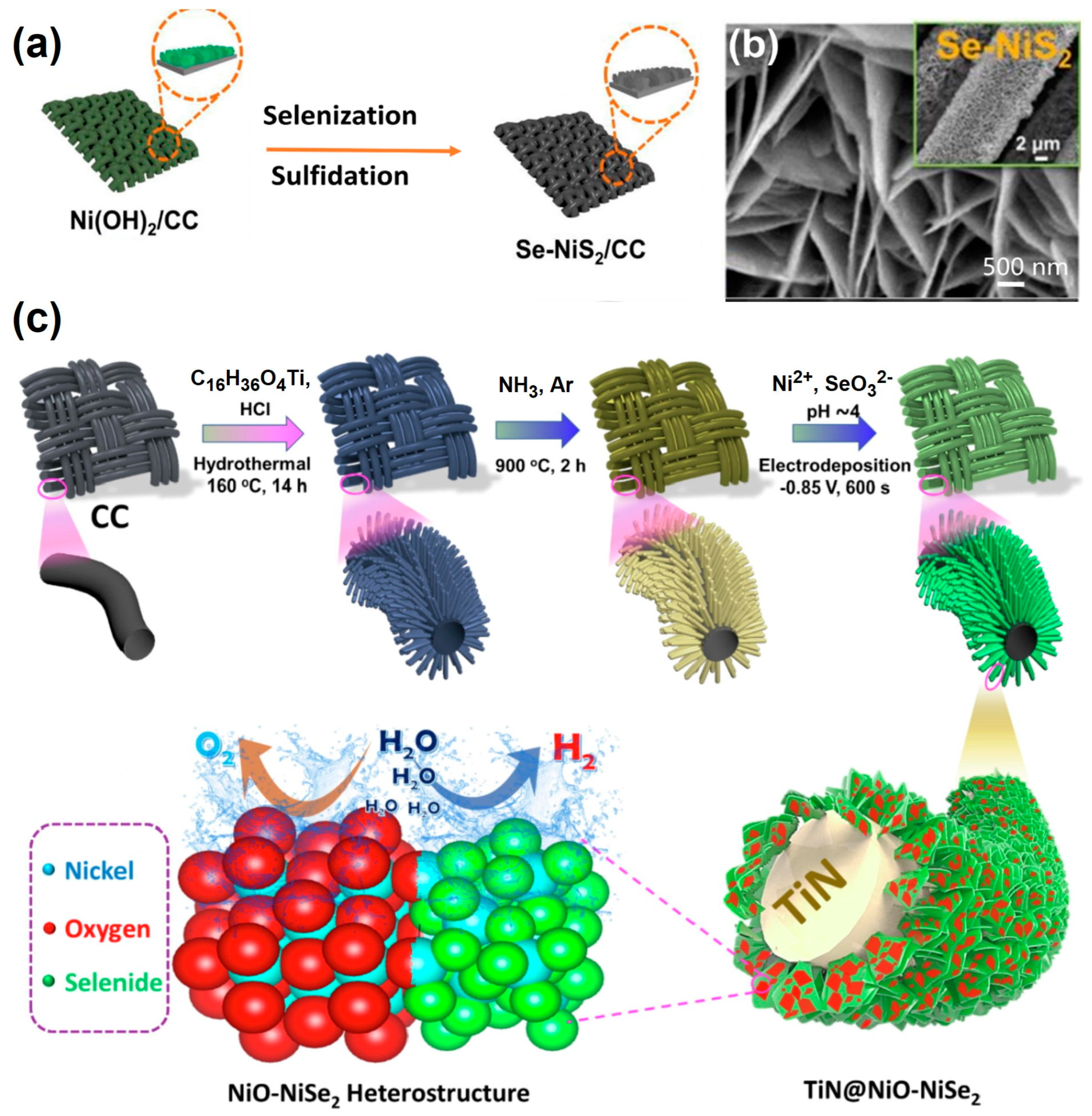
| TiN@NiO-NiSe2/CC | NiO-NiSe2 hetero-structured nanosheets shelling over 1D TiN nanoarrays supported by CC | 240@10 | 29 | 10@10 | 1.57@10 | 50 | 1 M KOH | [86] |
| FeCo/Co2P/Fe2P@NPC | FeCo/Co2P/Fe2P imbedded in N-doped porous carbon | 281@10 | 85 | 100@10 | 1.55@10 | [email protected] V | 1 M KOH | [87] |
| FeCoP2@NPPC | Phosphide embedded in a N and P co-doped porous carbon | 236@10 | 83 | [email protected] V | 1.6@10 | 16@10 | 1 M KOH | [88] |
| NiV2P/FeSe/CC | Ni-Fe-V trimetallic phosphorus–selenium composite grown on CC | 168@10 282@200 | 44.3 | 160@100 | 1.53@10 | N.A. | 1 M KOH | [89] |
| CuMn0.5Co2O4/CC | Nanoneedles of spinel oxides grown on CC | 189@10 327@200 | 76.2 | 24@10 | N.A. | N.A. | 1 M KOH | [90] |
| Fe-NiOOH/CQDs a | In situ activation of Fe-NiOOH nanoclusters on carbon quantum dots | 199@10 450@1000 | 35 | 80@10 | 1.5@10 | 12@500 | 1 M KOH | [91] |
3.2. Carbon-Based Supported Composites for OER
3.2.1. At Low Current Density (LCD)
3.2.2. At HCD
3.3. Carbon-Based Core–Shell Composite
3.3.1. At LCD
| Materials | Morphology | OER | Water Splitting a | Electrolyte | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| η at j | TS | Sta. | U at j | Stab. | ||||
| Fe-Co2P@Fe-N-C | Core–shell nanostructure; Fe-doped Co2P core and Fe-N-C shell | 300@10 | 79 | 24@50 | 1.58@10 | 10 | 1 M KOH | [120] |
| CFO@CSs | Carbon-coated spherical structure; core: CoFe2O4 | 390@10 | 57.75 | 6@10 | 1.87@10 | 20@10 | 1 M KOH | [123] |
| Ni@PMMA-BM | Carbon-coated honeycomb-like structure | 263@10 | 34 | 28@10 | N.A. | N.A. | 1 M KOH | [124] |
| Fe3C@C–N | Core–shell nanostructure; core: Fe3C; shell: nitrogen-doped graphene-like layers | 469@10 | 89 | [email protected] V | N.A. | N.A. | 0.1 M KOH | [121] |
| Fe/Fe3C@C/CNT | Carbon-coated Fe/Fe3C nanoparticles anchored to CNTs | 292@10 | 29 | 12@50 | N.A. | N.A. | 1 M KOH | [125] |
| Ni0.70Fe0.10V0.20S2-AH/CP | Core–shell trimetallic heterostructures grown on carbon paper | 204@10 | 39 | 24@10 | N.A. | N.A. | 1 M KOH | [100] |
| CoP/DCS | CoP nanoparticles encapsulated in a rich-defect carbon shell | 251@10 | 81.6 | 72 | 1.49@10 | 24@10 | 1 M KOH | [117] |
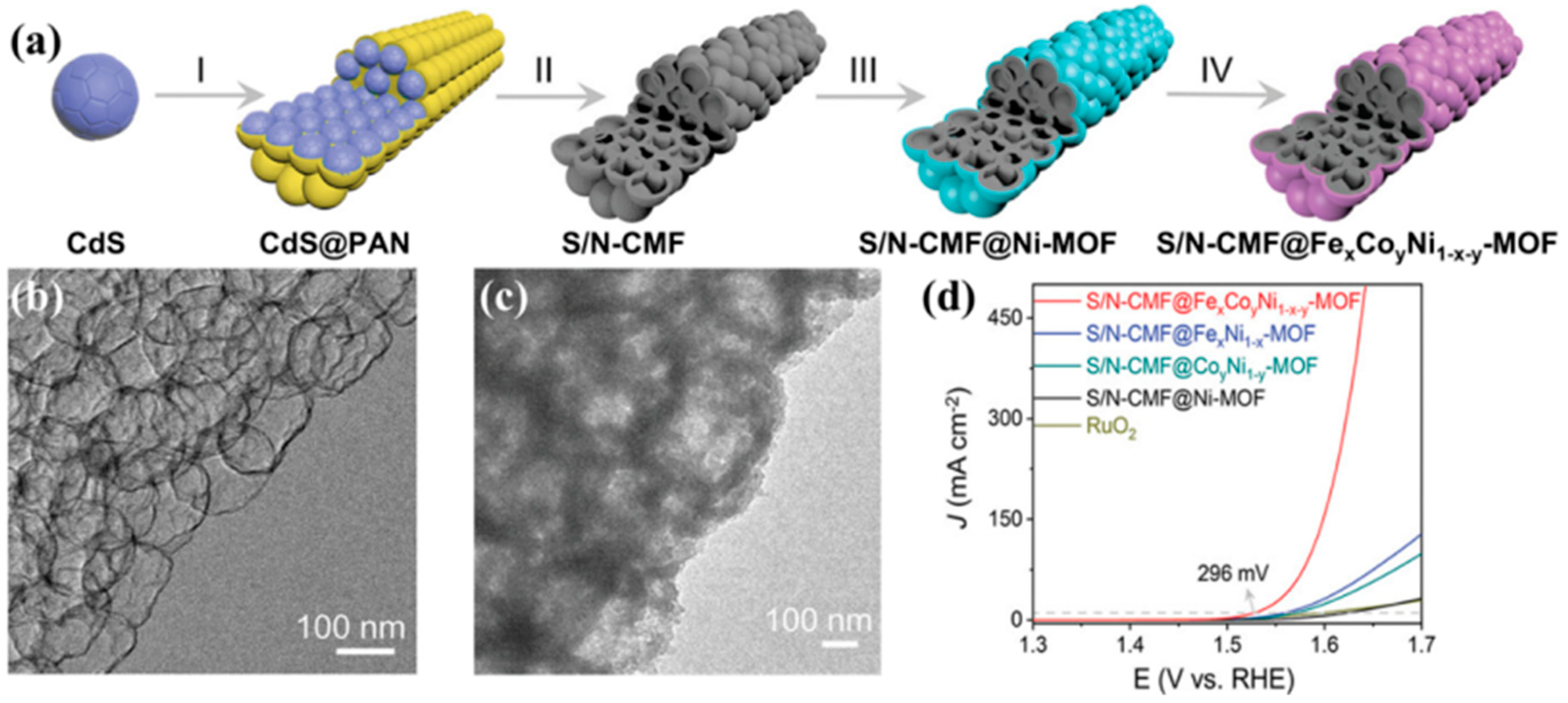
| S/N-CMF@FexCoyNi1−x−y-MOF | Core–shell nanostructure; core: S/N-doped carbon mesoporous fibers; shell: ternary metal centers MOFs | 296@10 377@200 | 53.5 | 100@10 | N.A. | N.A. | 1 M KOH | [68] |
| (V2O3−CoFe2O4)@C/NF a | V2O3-decorated spinel CoFe2O4 with carbon-encapsulated nanosheets grown on NF | 226@10 310@500 | 56 | 80@500 | 1.53@10 1.89@150 | 80@100 | 1 M KOH | [18] |
3.3.2. At HCD
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, R.; Liu, C.; Li, Z.; Huang, H.; Feng, J.; Li, Z.; Zou, Z. Ultrastable Electrocatalytic Seawater Splitting at Ampere-Level Current Density. Nat. Sustain. 2024, 7, 158–167. [Google Scholar] [CrossRef]
- Gu, J.; Li, L.; Xie, Y.; Chen, B.; Tian, F.; Wang, Y.; Zhong, J.; Shen, J.; Lu, J. Turing Structuring with Multiple Nanotwins to Engineer Efficient and Stable Catalysts for Hydrogen Evolution Reaction. Nat. Commun. 2023, 14, 5389. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, T.; Liu, J.; Chen, W.; Li, S.; Liang, J.; Liu, S.; Liu, X.; Cai, Z.; Wang, C.; et al. A Sodium-Ion-Conducted Asymmetric Electrolyzer to Lower the Operation Voltage for Direct Seawater Electrolysis. Nat. Commun. 2023, 14, 3934. [Google Scholar] [CrossRef]
- Magnier, L.; Cossard, G.; Martin, V.; Pascal, C.; Roche, V.; Sibert, E.; Shchedrina, I.; Bousquet, R.; Parry, V.; Chatenet, M. Fe-Ni-Based Alloys as Highly Active and Low-Cost Oxygen Evolution Reaction Catalyst in Alkaline Media. Nat. Mater. 2024, 23, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Y.; Duan, Y.; Feng, X.-Y.; Yu, X.; Gao, M.-R.; Yu, S.-H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; McGlynn, R.J.; Boies, A.; Maguire, P.; Mariotti, D.; Chakrabarti, S. Flexible Bifunctional Electrode for Alkaline Water Splitting with Long-Term Stability. ACS Appl. Mater. Interfaces 2024, 16, 12339–12352. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.X.; Zheng, Y.; Hu, Z.P.; Zheng, C.Y.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.Z.; Ling, T. Direct Seawater Electrolysis by Adjusting the Local Reaction Environment of a Catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Retuerto, M.; Pascual, L.; Torrero, J.; Salam, M.A.; Tolosana-Moranchel, Á.; Gianolio, D.; Ferrer, P.; Kayser, P.; Wilke, V.; Stiber, S.; et al. Highly Active and Stable OER Electrocatalysts Derived from Sr2MIrO6 for Proton Exchange Membrane Water Electrolyzers. Nat. Commun. 2022, 13, 7935. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Senthamaraikannan, T.G.; Baasanjav, E.; Bandyopadhyay, P.; Jin, B.; Park, Y.S.; Lim, D.-H.; Jeong, S.M. Surface Hydroxyl Group-Enriched Nickel Cobalt Molybdate Hydrate for Improved Oxygen Evolution Activity in an Anion Exchange Membrane Water Electrolyzer. Appl. Catal. B 2023, 328, 122504. [Google Scholar] [CrossRef]
- Shimada, H.; Yamaguchi, T.; Kishimoto, H.; Sumi, H.; Yamaguchi, Y.; Nomura, K.; Fujishiro, Y. Nanocomposite Electrodes for High Current Density over 3 A Cm−2 in Solid Oxide Electrolysis Cells. Nat. Commun. 2019, 10, 5432. [Google Scholar] [CrossRef]
- Dionigi, F.; Zeng, Z.; Sinev, I.; Merzdorf, T.; Deshpande, S.; Lopez, M.B.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-Situ Structure and Catalytic Mechanism of NiFe and CoFe Layered Double Hydroxides during Oxygen Evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Wang, C.; Zhao, Y.; Zhang, Y.; Gao, J.; Sun, L.; Hou, J. Regulating Electronic States of Nitride/Hydroxide to Accelerate Kinetics for Oxygen Evolution at Large Current Density. Nat. Commun. 2023, 14, 1873. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, J.; Gong, Z.; Lei, H.; Zhou, D.; Zhang, N.; Mai, W.; Zhao, S.; Chen, Y. Activating Lattice Oxygen in NiFe-Based (Oxy)Hydroxide for Water Electrolysis. Nat. Commun. 2022, 13, 2191. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Shi, X.-R.; He, X.; Zhang, X.; Cao, F.; Wang, P.; Sun, C.; Xu, S.; Zhang, M. Electronically Regulated FeOOH/c-NiMoO4 with Hierarchical Sandwich Structure as Efficient Electrode for Oxygen Evolution and Hybrid Supercapacitors. Electrochim. Acta 2022, 427, 140884. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.-Y.; Shi, X.; Zhu, Y.; Xia, C.; Zaman, S.; Hu, X.; Wang, X.; Xia, B.Y. A Zeolitic-Imidazole Framework-Derived Trifunctional Electrocatalyst for Hydrazine Fuel Cells. ACS Nano 2021, 15, 10286–10295. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jiang, H.; Liu, Y.; Xu, L.; Jiang, H.; Guo, Y.; Guo, Y.; Xuan, K.; Wang, L.; Ge, Q.; et al. Self-Supported Sheet-like Bi2O3 Electrodes for Co-Electrolysis of CO2 Conversion and Cl− Upgrading. Sep. Purif. Technol. 2024, 339, 126592. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, G.; Yu, T.; Chen, J.; Shen, F.; Luo, L.; Zou, Y.; Yin, S. Carbon Encapsulated FeWO4-Ni3S2 Nanosheets as a Highly Active Catalyst for Overall Water Splitting at Large Current Density. Chem. Eng. J. 2022, 434, 134669. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, G.; Chen, X.; Jiang, W.; Yu, T.; Wang, Y.; Luo, L.; Yin, S. V2O3-Decorated Spinel CoFe2O4 with Carbon-Encapsulated Mesoporous Nanosheets for Efficient Water Splitting. ACS Sustain. Chem. Eng. 2021, 9, 980–986. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, H.; Xi, S.; Lee, W.S.V.; Xue, J. Understanding of Oxygen Redox in the Oxygen Evolution Reaction. Adv. Mater. 2022, 34, 2107956. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, D.; Srinivas, K.; Yu, H.; Ma, F.; Zhang, Z.; Wu, Y.; Li, X.; Wang, Y.; Chen, Y. Ni3S4/FeNi2S4 Heterostructure-Embedded Metal-Organic Framework-Derived Nanosheets Interconnected by Carbon Nanotubes for Boosting Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2024, 51, 820–827. [Google Scholar] [CrossRef]
- Xue, Q.; Wu, Y.; Hao, J.; Ma, L.; Dang, Y.; Zhu, J.-J.; Zhou, Y. NiO–NiMoO4 Nanocomposites on Multi-Walled Carbon Nanotubes as Efficient Bifunctional Electrocatalysts for Total Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, 31470–31477. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Dong, Q.; Ren, J.; Wang, H.; Wang, X.; Wang, R. In-Situ Self-Catalytically Grown CoNi-Doped C-N/CNT as a Binder-Free Electrocatalyst for High-Performance Oxygen Evolution Reaction. J. Alloys Compd. 2023, 969, 172289. [Google Scholar] [CrossRef]
- Deng, X.; Mi, Y.; Liu, Y.; Sun, Y.; Cheng, Y.; Wang, W. Co, N Co-Doped Carbon Nanosheets Coupled with NiCo2O4 as an Efficient Bifunctional Oxygen Catalyst for Zn-Air Batteries. Int. J. Hydrogen Energy 2023, 48, 13452–13459. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 Nanocrystals on Graphene as a Synergistic Catalyst for Oxygen Reduction Reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.H.; Thabet, H.K.; Aman, S.; Ahmad, N.; Tahir Farid, H.M.; El-Bahy, Z.M. One-Pot Formation of an RGO-Based ZnAl2O4 Nanocomposite for Electrochemical Studies toward Oxygen Evolution Reactions. Energy Fuels 2024, 38, 2273–2283. [Google Scholar] [CrossRef]
- Mondal, S.; Mohanty, B.; Nurhuda, M.; Dalapati, S.; Jana, R.; Addicoat, M.; Datta, A.; Jena, B.K.; Bhaumik, A. A Thiadiazole-Based Covalent Organic Framework: A Metal-Free Electrocatalyst toward Oxygen Evolution Reaction. ACS Catal. 2020, 10, 5623–5630. [Google Scholar] [CrossRef]
- Ghosh, A.; Mondal, M.; Nath Manna, R.; Bhaumik, A. Targeted Synthesis of a Metal-Free Thiadiazolate Based Nitrogen and Sulfur Rich Porous Organic Polymer for an Unprecedented Hydrogen Evolution in the Electrochemical Water Splitting. J. Colloid. Interface Sci. 2024, 658, 415–424. [Google Scholar] [CrossRef]
- Huang, M.; Sun, C.; Zhang, X.; Wang, P.; Xu, S.; Shi, X. The Surface Structure, Stability, and Catalytic Performances toward O2 Reduction of CoP and FeCoP2. Dalton Trans. 2022, 51, 10420–10431. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.-H.; Zheng, H.; Li, R.; Zhou, K. Oxygen-Rich Cobalt–Nitrogen–Carbon Porous Nanosheets for Bifunctional Oxygen Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2200763. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, J.; Huang, W.; Dong, X. Three Dimensional Carbon Substrate Materials for Electrolysis of Water. Sci. China Mater. 2018, 61, 1143–1153. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, W.; Chen, Y.; Xiao, W.; Liu, T.; Zhong, Z.; Luo, Z.; Ding, Z.; Zhang, Z. Transition Metal and N Doping on AlP Monolayers for Bifunctional Oxygen Electrocatalysts: Density Functional Theory Study Assisted by Machine Learning Description. ACS Appl. Mater. Interfaces 2022, 14, 1249–1259. [Google Scholar] [CrossRef]
- Moon, J.; Beker, W.; Siek, M.; Kim, J.; Lee, H.S.; Hyeon, T.; Grzybowski, B.A. Active Learning Guides Discovery of a Champion Four-Metal Perovskite Oxide for Oxygen Evolution Electrocatalysis. Nat. Mater. 2024, 23, 108–115. [Google Scholar] [CrossRef]
- Sun, C.; Duan, Z.; Wang, P.; Zhang, X.; Huang, M.; Cao, F.; Lin, W.; Wang, H.; Chen, Y.; Shi, X.-R. Modulation of Graphene and Graphdiyne by Metaln (n = 1–5) Adsorption and Nucleation and the Effect on Hydrogen Evolution Reaction. Appl. Surf. Sci. 2022, 580, 152197. [Google Scholar] [CrossRef]
- Ma, C.; Feng, J.; Xia, C.; Du, C.; Chen, X.; Pang, B.; Dong, H.; Yu, L.; Dong, L. Theoretical Insights into Multi-Metal Atoms Embedded Nitrogen-Doped Graphene as Efficient Bifunctional Catalysts for Oxygen Reduction and Evolution Reactions. Appl. Surf. Sci. 2022, 605, 154714. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Liu, J.; Zhao, J. Transition Metal Atoms Anchored on Square Graphyne as Multifunctional Electrocatalysts: A Computational Investigation. Mol. Catal. 2022, 531, 112706. [Google Scholar] [CrossRef]
- Wu, L.; Guo, T.; Li, T. Data-Driven High-Throughput Rational Design of Double-Atom Catalysts for Oxygen Evolution and Reduction. Adv. Funct. Mater. 2022, 32, 2203439. [Google Scholar] [CrossRef]
- Fang, Z.; Li, S.; Zhang, Y.; Wang, Y.; Meng, K.; Huang, C.; Sun, S. The DFT and Machine Learning Method Accelerated the Discovery of DMSCs with High ORR and OER Catalytic Activities. J. Phys. Chem. Lett. 2024, 15, 281–289. [Google Scholar] [CrossRef]
- Yao, W.; Qu, Y.; Zhou, M.; Wang, W.; Zhang, A.; Feng, Z.; Yan, H. Increasing the Bifunctional OER/ORR Activity of 3d Transition Metals Doped g-C3N3 by Controlling the Charge States. Mol. Catal. 2024, 554, 113807. [Google Scholar] [CrossRef]
- Ye, Q.; Yi, X.; Wang, C.-Z.; Zhang, T.; Liu, Y.; Lin, S.; Fan, H.J. Data-Driven Screening of Pivotal Subunits in Edge-Anchored Single Atom Catalysts for Oxygen Reactions. Adv. Funct. Mater. 2024, 2400107. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Xu, S.; Huang, M.; Wen, Y.; Shi, X.-R. DFT-Assisted Rational Design of CoMxP/CC (M = Fe, Mn, and Ni) as Efficient Electrocatalyst for Wide PH Range Hydrogen Evolution and Oxygen Evolution. Nano Res. 2022, 15, 8897–8907. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect Graphene as a Trifunctional Catalyst for Electrochemical Reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhu, J.; Gao, Z.; Li, S.; Mu, S.; Huang, Y. Ultranarrow Graphene Nanoribbons toward Oxygen Reduction and Evolution Reactions. Adv. Sci. 2018, 5, 1801375. [Google Scholar] [CrossRef]
- Li, D.; Zhang, A.; Feng, Z.; Wang, W. Theoretical Insights on the Charge State and Bifunctional OER/ORR Electrocatalyst Activity in 4d-Transition-Metal-Doped g-C3N4 Monolayers. ACS Appl. Mater. Interfaces 2024, 16, 5779–5791. [Google Scholar] [CrossRef]
- Wang, P.; Shi, X.-R.; Zhang, Y.; Wei, M. The Influence of Co Nucleation in CoNi Single Atom Alloy on Low-Temperature Methane Dry Reforming with DFT Simulations and Microkinetic Modeling. Mol. Catal. 2023, 549, 113515. [Google Scholar] [CrossRef]
- Cao, F.; Shi, X.R.; Wang, P.; Zhao, W.; Huang, M.; Hu, J.; Xu, S.; Zhao, G. Multistage Interface Engineered Cobalt Polysulfides Core-Shell Nanostructures for Dual Energy Storage Devices and Hydrogen Evolution. Vacuum 2023, 216, 112461. [Google Scholar] [CrossRef]
- Li, H.; Jin, Z.; Lu, N.; Pan, J.; Xu, J.; Yin, X.-B.; Zhang, M. Fe3O4 Nanoparticles Entrapped in the Inner Surfaces of N-Doped Carbon Microtubes with Enhanced Biomimetic Activity. Dalton Trans. 2024, 53, 6974–6982. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Li, K.; Wang, S.; De Bonis, E.; Cao, H.; Mertens, S.F.L.; Teng, C. Shape Control of Bimetallic MOF/Graphene Composites for Efficient Oxygen Evolution Reaction. J. Electroanal. Chem. 2023, 930, 117144. [Google Scholar] [CrossRef]
- Fan, F.; Huang, Q.; Rani, K.K.; Peng, X.; Liu, X.; Wang, L.; Yang, Z.; Huang, D.; Devasenathipathy, R.; Chen, D.-H.; et al. Interface and Doping Engineering of Co-Based Electrocatalysts for Enhanced Oxygen Reduction and Evolution Reactions. Chem. Eng. J. 2023, 470, 144380. [Google Scholar] [CrossRef]
- Xiao, Y.; Gong, W.; Zhao, M.; Zhang, M.; Lu, N. Surface-Engineered Prussian Blue Nanozymes as Artificial Receptors for Universal Pattern Recognition of Metal Ions and Proteins. Sens. Actuators B Chem. 2023, 390, 134006. [Google Scholar] [CrossRef]
- Jin, Z.; Li, H.; Zhang, L.; Pan, J.; Xu, J.; Yin, X.-B.; Zhang, M. Interfacing Ag2S Nanoparticles and MoS2 Nanosheets on Polypyrrole Nanotubes with Enhanced Catalytic Performance. Inorg. Chem. 2024, 63, 4260–4268. [Google Scholar] [CrossRef]
- Tu, Y.; Ren, P.; Deng, D.; Bao, X. Structural and Electronic Optimization of Graphene Encapsulating Binary Metal for Highly Efficient Water Oxidation. Nano Energy 2018, 52, 494–500. [Google Scholar] [CrossRef]
- Jing, S.; Wang, D.; Yin, S.; Lu, J.; Shen, P.K.; Tsiakaras, P. P-Doped CNTs Encapsulated Nickel Hybrids with Flower-like Structure as Efficient Catalysts for Hydrogen Evolution Reaction. Electrochim. Acta 2019, 298, 142–149. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.; Wu, A.; Cai, Z.; Wang, L.; Tian, C.; Zhang, X.; Fu, H. Anion-Modulated HER and OER Activities of 3D Ni–V-Based Interstitial Compound Heterojunctions for High-Efficiency and Stable Overall Water Splitting. Adv. Mater. 2019, 31, 1901174. [Google Scholar] [CrossRef]
- Deng, J.; Ren, P.; Deng, D.; Bao, X. Enhanced Electron Penetration through an Ultrathin Graphene Layer for Highly Efficient Catalysis of the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2100–2104. [Google Scholar] [CrossRef]
- Lu, J.; Yin, S.; Shen, P.K. Carbon-Encapsulated Electrocatalysts for the Hydrogen Evolution Reaction. Electrochem. Energy Rev. 2019, 2, 105–127. [Google Scholar] [CrossRef]
- Wang, S.; Huo, W.; Feng, H.; Xie, Z.; Shang, J.K.; Formo, E.V.; Camargo, P.H.C.; Fang, F.; Jiang, J. Enhancing Oxygen Evolution Reaction Performance in Prussian Blue Analogues: Triple-Play of Metal Exsolution, Hollow Interiors, and Anionic Regulation. Adv. Mater. 2023, 35, 2304494. [Google Scholar] [CrossRef]
- Jiang, M.; Fan, X.; Cao, S.; Wang, Z.; Yang, Z.; Zhang, W. Thermally Activated Carbon–Nitrogen Vacancies in Double-Shelled NiFe Prussian Blue Analogue Nanocages for Enhanced Electrocatalytic Oxygen Evolution. J. Mater. Chem. A Mater. 2021, 9, 12734–12745. [Google Scholar] [CrossRef]
- Guo, X.; Yue, K.; Zheng, J.; Yu, Z.; Wang, Y.; Liu, Y.; Liu, T.; Luo, J.; Tao, X.; Nai, J. Formation of Prussian Blue Analog Coronal Nanomaterials and Their Conversion into Mn–Co-Mixed Selenide for Enhanced Electrocatalytic Oxygen Evolution. Mater. Chem. Front. 2023, 7, 3728–3737. [Google Scholar] [CrossRef]
- Zhao, P.; Fu, S.; Cheng, L.; Jiao, Z.; Wu, M. Modulating Zeolitic Imidazolate Framework-67 and Its Derivatives as Advanced Oxygen Evolution Reaction Electrocatalysts. Coord. Chem. Rev. 2024, 498, 215452. [Google Scholar] [CrossRef]
- Chen, D.; Ji, X.; Zhou, X.; Sun, Q.; Xu, S.; Mao, L.; Guo, Z.; Guan, J.; Li, T.-T.; Qian, J. MOF-on-MOF-Derived Hollow FeNi3/N-Doped Carbon Nanorods for Efficient Oxygen Evolution. Chem. Eng. J. 2023, 470, 144418. [Google Scholar] [CrossRef]
- Lima, M.A.; Raimundo, R.A.; Araújo, A.J.; João, F.D.A.; Loureiro, F.J.A.; Macedo, D.A.; Morales, M.A. Nickel-Copper-Carbon Based Electrocatalysts for Oxygen Evolution Reaction: Sol-Gel Synthesis Using Chitosan. Int. J. Hydrogen Energy 2024, 51, 663–675. [Google Scholar] [CrossRef]
- Nasri, A.; Jaleh, B.; Khazalpour, S.; Nasrollahzadeh, M.; Shokouhimehr, M. Facile Synthesis of Graphitic Carbon Nitride/Chitosan/Au Nanocomposite: A Catalyst for Electrochemical Hydrogen Evolution. Int. J. Biol. Macromol. 2020, 164, 3012–3024. [Google Scholar] [CrossRef]
- Kayan, D.B.; İlhan, M.; Koçak, D. Chitosan-Based Hybrid Nanocomposite on Aluminium for Hydrogen Production from Water. Ionics 2018, 24, 563–569. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, L.; Huang, Q.; Guo, Y.; Miao, T.; Hu, Y.; Qian, J.; Huang, S. Chitosan Hydrogel Derived Carbon Foam with Typical Transition-Metal Catalysts for Efficient Water Splitting. Carbon 2021, 177, 160–170. [Google Scholar] [CrossRef]
- Lei, Y.; Jia, M.; Guo, P.; Liu, J.; Zhai, J. MoP Nanoparticles Encapsulated in P-Doped Carbon as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Catal. Commun. 2020, 140, 106000. [Google Scholar] [CrossRef]
- Pu, Z.; Zhao, J.; Amiinu, I.S.; Li, W.; Wang, M.; He, D.; Mu, S. A Universal Synthesis Strategy for P-Rich Noble Metal Diphosphide-Based Electrocatalysts for the Hydrogen Evolution Reaction. Energy Environ. Sci. 2019, 12, 952–957. [Google Scholar] [CrossRef]
- Ai, L.; Wang, Y.; Luo, Y.; Tian, Y.; Yang, S.; Chen, M.; Jiang, J. Robust Interfacial Ru-RuO2 Heterostructures for Highly Efficient and Ultrastable Oxygen Evolution Reaction and Overall Water Splitting in Acidic Media. J. Alloys Compd. 2022, 902, 163787. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, X.F.; Wu, Z.; Pei, Z.; Luan, D.; Lou, X.W. (David) Supporting Trimetallic Metal-Organic Frameworks on S/N-Doped Carbon Macroporous Fibers for Highly Efficient Electrocatalytic Oxygen Evolution. Adv. Mater. 2023, 35, 2207888. [Google Scholar] [CrossRef]
- Yan, H.; Jiang, Z.; Deng, B.; Wang, Y.; Jiang, Z. Ultrathin Carbon Coating and Defect Engineering Promote RuO2 as an Efficient Catalyst for Acidic Oxygen Evolution Reaction with Super-High Durability. Adv. Energy Mater. 2023, 13, 2300152. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Karmakar, A.; Koner, K.; Karak, S.; Sharma, R.K.; Roy, A.; Sen, P.; Dey, K.K.; Mahalingam, V.; Pathak, B.; et al. Functionality Modulation Toward Thianthrene-Based Metal-Free Electrocatalysts for Water Splitting. Adv. Mater. 2024, 36, 2310938. [Google Scholar] [CrossRef]
- Karak, S.; Koner, K.; Karmakar, A.; Mohata, S.; Nishiyama, Y.; Duong, N.T.; Thomas, N.; Ajithkumar, T.G.; Hossain, M.S.; Bandyopadhyay, S.; et al. Morphology Tuning via Linker Modulation: Metal-Free Covalent Organic Nanostructures with Exceptional Chemical Stability for Electrocatalytic Water Splitting. Adv. Mater. 2024, 36, 2209919. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Baig, N.; Bake, A.; Haroon, M.; Ashraf, M.; Al-Saadi, A.; Tahir, M.N.; Wooh, S. Robust Electrocatalysts Decorated Three-Dimensional Laser-Induced Graphene for Selective Alkaline OER and HER. Carbon 2023, 213, 118292. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Lee, J.; Park, J.-S. Biomass-Derived Bifunctional Electrocatalysts for Oxygen Reduction and Evolution Reaction: A Review. J. Energy Chem. 2022, 65, 149–172. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-Doped Carbon Nanomaterials as Non-Metal Electrocatalysts for Water Oxidation. Nat. Commun. 2013, 4, 2390. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.; Thangarasu, S.; Seo, S.B.; Mariappan, A.; Choi, Y.R.; Oh, T.H.; Kim, Y.A. N-Doped Defect-Rich Porous Carbon Nanosheets Framework from Renewable Biomass as Efficient Metal-Free Bifunctional Electrocatalysts for HER and OER Application. Renew. Energy 2024, 222, 119801. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Q.; Zhao, B.; Li, S.; Xiong, Y.; Xu, W. Few-Layer N-Doped Porous Carbon Nanosheets Derived from Corn Stalks as a Bifunctional Electrocatalyst for Overall Water Splitting. Fuel 2020, 280, 118567. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Wang, F.; Xuan, J. The Influence of the Type of N Dopping on the Performance of Bifunctional N-Doped Ordered Mesoporous Carbon Electrocatalysts in Oxygen Reduction and Evolution Reaction. J. Energy Chem. 2017, 26, 422–427. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Cao, D.; Cheng, D. Facile Preparation of Biomass-Derived Bifunctional Electrocatalysts for Oxygen Reduction and Evolution Reactions. Int. J. Hydrogen Energy 2018, 43, 8611–8622. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L.; Chen, Z.; Zhong, L.; Zhao, D.; Chi, X.; Zhao, X.; Li, L.; Lu, X.; Leng, K.; et al. Hierarchically Porous Carbon Plates Derived from Wood as Bifunctional ORR/OER Electrodes. Adv. Mater. 2019, 31, 1900341. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Jeong, H.K. Coffee Waste-Derived Porous Carbon for Hydrogen and Oxygen Evolution Reaction. Chem. Phys. Impact 2023, 6, 100175. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Díez, A.M.; Silva, C.G.; Faria, J.L.; Sanromán, M.Á.; Silva, A.M.T.; Pazos, M. Tuning Graphitic Carbon Nitride (g-C3N4) Electrocatalysts for Efficient Oxygen Evolution Reaction (OER). Fuel 2024, 360, 130575. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, T.; Zhou, H.; Wang, Y.; Yang, X.; Liang, W.; Wu, D.; Yuan, P.; Yu, T.; He, M.; et al. Activating Fe Activity and Improving Ni Activity via C3N4 Substrate in Alkaline Oxygen Evolution Catalyzed by Ni-Fe Phosphide. Appl. Catal. B 2024, 342, 123391. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Wang, R.; Zhang, B.; Song, B.; Guan, Y.; Li, S.; Xu, P. Surface Reconstruction of Se-Doped NiS2 Enables High-Efficiency Oxygen Evolution Reaction. J. Energy Chem. 2023, 84, 173–180. [Google Scholar] [CrossRef]
- Kang, T.; Kim, K.; Kim, M.; Kim, J. Electronic Structure Modulation of Nickel Hydroxide and Tungsten Nanoparticles for Fast Structure Transformation and Enhanced Oxygen Evolution Reaction Activity. Chem. Eng. J. 2021, 418, 129403. [Google Scholar] [CrossRef]
- Chen, T.; Li, B.; Song, K.; Wang, C.; Ding, J.; Liu, E.; Chen, B.; He, F. Defect-Activated Surface Reconstruction: Mechanism for Triggering the Oxygen Evolution Reaction Activity of NiFe Phosphide. J. Mater. Chem. A Mater. 2022, 10, 22750–22759. [Google Scholar] [CrossRef]
- Islam, M.; Tran, D.T.; Nguyen, T.H.; Dinh, V.A.; Kim, N.H.; Lee, J.H. Efficient Synergism of NiO-NiSe2 Nanosheet-Based Heterostructures Shelled Titanium Nitride Array for Robust Overall Water Splitting. J. Colloid. Interface Sci. 2022, 612, 121–131. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Q.; Yuan, A.; Shi, Q.; Jiang, L.; Yang, W.; Yang, T.; Hou, X. A Facile and Green Strategy for Mass Production of Dispersive FeCo-Rich Phosphides@N,P-Doped Carbon Electrocatalysts toward Efficient and Stable Rechargeable Zn-Air Battery and Water Splitting. J. Mater. Sci. Technol. 2024, 182, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Yang, Z.-J.; Yang, D.-H.; Zhao, L.; Shi, X.-R.; Yang, G.; Han, B.-H. FeCoP2 Nanoparticles Embedded in N and P Co-Doped Hierarchically Porous Carbon for Efficient Electrocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 8832–8843. [Google Scholar] [CrossRef]
- Qin, Y.; Chai, R.; Tan, Z.; Hou, X.; Li, J.; Wu, F. A Ni-Fe-V Trimetallic Phosphorus-Selenium Composite Supported on Carbon Cloth as Freestanding Electrocatalyst for Oxygen Evolution Reaction. Fuel 2024, 357, 129857. [Google Scholar] [CrossRef]
- He, X.; Qiao, T.; Zhang, Z.; Liu, H.; Wang, S.; Wang, X. Carbon Cloth Supporting Spinel CuMn0.5Co2O4 Nanoneedles with the Regulated Electronic Structure by Multiple Metal Elements as Catalysts for Efficient Oxygen Evolution Reaction. J. Colloid. Interface Sci. 2023, 649, 635–645. [Google Scholar] [CrossRef]
- Pan, F.; Huang, K.; Liu, P.; Li, R.; Lian, C.; Liu, H.; Hu, J. In Situ Electroactivated Fe-NiOOH Nanoclusters on Carbon Quantum Dots for Efficient Large-Scale Oxygen Production. Small Struct. 2022, 3, 2200094. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmad, T. Unveiling the Bifunctional Photo/Electrocatalytic Activity of In Situ Grown CdSe QDs on g-C3N4 Nanosheet Z-Scheme Heterostructures for Efficient Hydrogen Generation. ACS Catal. 2024, 14, 691–702. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Wang, B.; Liu, J.; Yu, M. From Biomass Chitin to Mesoporous Nanosheets Assembled Loofa Sponge-like N-Doped Carbon/g-C3N4 3D Network Architectures as Ultralow-Cost Bifunctional Oxygen Catalysts. Microporous Mesoporous Mater. 2017, 240, 216–226. [Google Scholar] [CrossRef]
- Mohana, P.; Swathi, S.; Yuvakkumar, R.; Ravi, G.; Thambidurai, M.; Nguyen, H.D. Co3O4/g-C3N4 Nanocomposite for Enriched Electrochemical Water Splitting. Int. J. Hydrogen Energy 2024, 49, 376–389. [Google Scholar] [CrossRef]
- Vignesh, S.; Nam, S.; Kim, H. Interfacial Engineering of α-Fe2O3 Coupled Co3O4 Heterostructures Anchored on g-C3N4 Structure for Enhanced Electrocatalytic Performance in Alkaline Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2024, 53, 1445–1456. [Google Scholar] [CrossRef]
- Ricciardi, B.; da Silva Freitas, W.; Mecheri, B.; Nisa, K.U.; Montero, J.; Ficca, V.C.A.; Placidi, E.; Alegre, C.; D’Epifanio, A. Hierarchical Porous Fe/Ni-Based Bifunctional Oxygen Electrocatalysts for Rechargeable Zinc-Air Batteries. Carbon 2024, 219, 118781. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, S.; Wang, P.; Shao, X.; Sun, X.; Hu, J.; Shi, X.-R. Bimetallic FeCo Phosphide Nanoparticles Anchored on N-Doped Carbon Foam for Wide PH Hydrogen Evolution Reaction. J. Alloys Compd. 2023, 931, 167570. [Google Scholar] [CrossRef]
- Liu, R.; Shi, X.-R.; Wen, Y.; Shao, X.; Su, C.; Hu, J.; Xu, S. Trimetallic Synergistic Optimization of 0D NiCoFe-P QDs Anchoring on 2D Porous Carbon for Efficient Electrocatalysis and High-Energy Supercapacitor. J. Energy Chem. 2022, 74, 149–158. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, X.-R.; Wang, P.; Bao, Z.; Huang, M.; Xu, Y.; Xu, S. Bio-Inspired Design of NiFeP Nanoparticles Embedded in (N,P) Co-Doped Carbon for Boosting Overall Water Splitting. Dalton Trans. 2023, 52, 6860–6869. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, B.; Guo, P.-F.; Wang, W.-J.; Wang, W.-T.; Wang, K.; He, Z.-H.; Liu, Z.-T. Core-Shell Trimetallic NiFeV Disulfides and Amorphous High-Valance NiFe Hydroxide Nanosheets Enhancing Oxygen Evolution Reaction. Chem. Eng. J. 2022, 430, 133047. [Google Scholar] [CrossRef]
- Chen, F.; Shen, C.; Zhu, Y.; Liu, Y.; Zhou, D.; Huang, L.; Shi, L.; Zhang, H.; Cao, S.; Jia, R. Magnetic Field Enhanced Surface Reconstruction of Fe2P and the Promotional Effect in Electrochemical Oxygen Evolution. Appl. Surf. Sci. 2024, 653, 159357. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S.; Jeong, H.; Im, Y.; Park, N.; Kang, M. Improved OH Adsorption and Effective Oxygen Evolution Reaction on Carbon-Capsulated Co0.1Ni0.9O@C/CP Electrode. Appl. Surf. Sci. 2024, 655, 159549. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Xing, P.; Dai, L.; Wang, Y. Iron-Doped Nickel Sulfide Nanoparticles Grown on N-Doped Reduced Graphene Oxide as Efficient Electrocatalysts for Oxygen Evolution Reaction. J. Electroanal. Chem. 2023, 936, 117323. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Shi, X.; Zhou, F.; Wang, R.; Li, X. Facile Fabrication of Ultrafine Nickel-Iridium Alloy Nanoparticles/Graphene Hybrid with Enhanced Mass Activity and Stability for Overall Water Splitting. J. Energy Chem. 2020, 49, 166–173. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, X.; Li, F.; Dong, Q.; Wen, B.; Sun, D.; Liang, W.; Lyu, X. Spherical ZnFe2O4 Nanoparticles on Nitrogen-Doped Graphene: A Synergistic Effect on Efficient Electrocatalytic Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2023, 6, 9985–9993. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Aulia, S.; Yeh, M.-H.; Hsiao, L.-Y.; Tarigan, A.M.; Ho, K.-C. Graphene Quantum Dots Induced Defect-Rich NiFe Prussian Blue Analogue as an Efficient Electrocatalyst for Oxygen Evolution Reaction. J. Colloid. Interface Sci. 2023, 648, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, X.; Zhang, Y.; Ye, J.; Mei, B.; Lin, S. Flower-like Iron Sulfide/Cobaltous Sulfide Heterostructure as Advanced Electrocatalyst for Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2023, 51, 550–557. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Huang, Z.; Li, H.; Taylor Isimjan, T.; Yang, X. Revealing Dynamic Structural Evolution of V and P Co-Doping-Induced Co Defects as Large-Current Water Oxidation Catalyst. Chem. Eng. J. 2023, 472, 144924. [Google Scholar] [CrossRef]
- Gao, D.; Zhu, W.; Chen, J.; Qin, K.; Ma, M.; Shi, J.; Wang, Q.; Fan, Z.; Shao, Q.; Liao, F.; et al. High-Entropy Effect Promoting Self-Healing Behavior of Two-Dimensional Metal Oxide Electrocatalysts for Oxygen Evolution Reaction. ACS Catal. 2024, 14, 3700–3711. [Google Scholar] [CrossRef]
- Shao, X.; Xu, S.; Wang, P.; Wen, Y.; Sun, X.; Hong, M.; Wu, K.; Shi, X.-R. Carbon-Incorporated Bimetallic Phosphide Nanospheres Derived from MOFs as Superior Electrocatalysts for Hydrogen Evolution. Dalt Trans. 2022, 51, 14517–14525. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhang, T.; Liu, F.; Zheng, M.; Shi, K.; Liu, J.; Zhang, Y.; Wang, H. Synthesis of Novel Hollow Carbon Nanotubes @ Co-Fe Alloy/Iron Phthalocyanine Electrocatalyst by Self-Assembly Method for OER and ORR Study. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133093. [Google Scholar] [CrossRef]
- An, Y.; Ren, Z.; Kong, Y.; Tian, Y.; Jiang, B.; Shaik, F. Fluorine-Based Multi-Halogen Atom Doped Vinasse Carbon Quantum Dots on Vertical Graphene: A Bifunctional Catalytic Electrode for Water Splitting. Int. J. Hydrogen Energy 2024, 58, 633–645. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, N.; Zhang, J.; Yan, R.; Li, J.; Wang, L.; Wang, N.; Lv, M.; Zhang, M. Ultrasensitive Aptamer-Based Protein Assays Based on One-Dimensional Core-Shell Nanozymes. Biosens. Bioelectron. 2020, 150, 111881. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Lu, N.; Wen, Y.; Liu, G.; Zhang, R.; Zhang, J.; Wang, F.; Liu, X.; Li, Q.; Tang, Z.; et al. Engineering Nanozymes Using DNA for Catalytic Regulation. ACS Appl. Mater. Interfaces 2019, 11, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, H.; Chen, Z.; Cao, G.; Xu, N.; Wu, H.; Wang, P. A Core-Shell Structured CoMoO4·nH2O@Co1−xFexOOH Nanocatalyst for Electrochemical Evolution of Oxygen. Electrochim. Acta 2020, 345, 136125. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tao, L.; Xiao, X.; Lv, X.; Jiang, X.; Wang, M.; Peng, Z.; Shen, Y. Core-Shell Structured NiCo2O4@FeOOH Nanowire Arrays as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. ChemCatChem 2018, 10, 4119–4125. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Guan, T.; Zhang, G.; Zhang, J.; Han, J.; Guan, S.; Wang, N.; Wang, J.; Li, K. Optimizing Band Structure of CoP Nanoparticles via Rich-defect Carbon Shell toward Bifunctional Electrocatalysts for Overall Water Splitting. Carbon. Energy 2023, 5, e268. [Google Scholar] [CrossRef]
- Nan, Y.; Liu, T.; Liu, W.; Cao, D.; Cheng, D. Constructing Chainmail-Structured CoP/C Nanospheres as Highly Active Anodic Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2024, 16, 16309–16316. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Xu, Q.; Chu, N.; Wang, H.; Lim, Y.V.; Cheng, J.; Huang, S.; Xu, T.; Li, X.; Wang, Y.; et al. Rational Construction of 3D Self-Supported MOF-Derived Cobalt Phosphide-Based Hollow Nanowall Arrays for Efficient Overall Water Splitting at Large Current Density. Small 2024, 2310012. [Google Scholar] [CrossRef]
- Lv, X.; Xu, W.; Tian, W.; Wang, H.; Yuan, Z. Activity Promotion of Core and Shell in Multifunctional Core–Shell Co2P@NC Electrocatalyst by Secondary Metal Doping for Water Electrolysis and Zn-Air Batteries. Small 2021, 17, 2101856. [Google Scholar] [CrossRef]
- Abbas, S.A.; Ma, A.; Seo, D.; Jung, H.; Lim, Y.J.; Mehmood, A.; Nam, K.M. Synthesis of Fe3C@C Core-Shell Catalysts with Controlled Shell Composition for Robust Oxygen Evolution Reaction. Appl. Surf. Sci. 2021, 551, 149445. [Google Scholar] [CrossRef]
- Wang, P.; Bai, P.; Mu, J.; Jing, J.; Wang, L.; Su, Y. N, S Codoped Carbon Matrix-Encapsulated CoFe/Co0.2Fe0.8S Heterostructure as a Highly Efficient and Durable Bifunctional Oxygen Electrocatalyst for Rechargeable Zinc-Air Batteries. J. Colloid. Interface Sci. 2023, 642, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alhakemy, A.Z.; Elseman, A.M.; Fayed, M.G.; Ahmed Amine Nassr, A.B.; El-Hady Kashyout, A.; Wen, Z. Hybrid Electrocatalyst of CoFe2O4 Decorating Carbon Spheres for Alkaline Oxygen Evolution Reaction. Ceram. Int. 2022, 48, 5442–5449. [Google Scholar] [CrossRef]
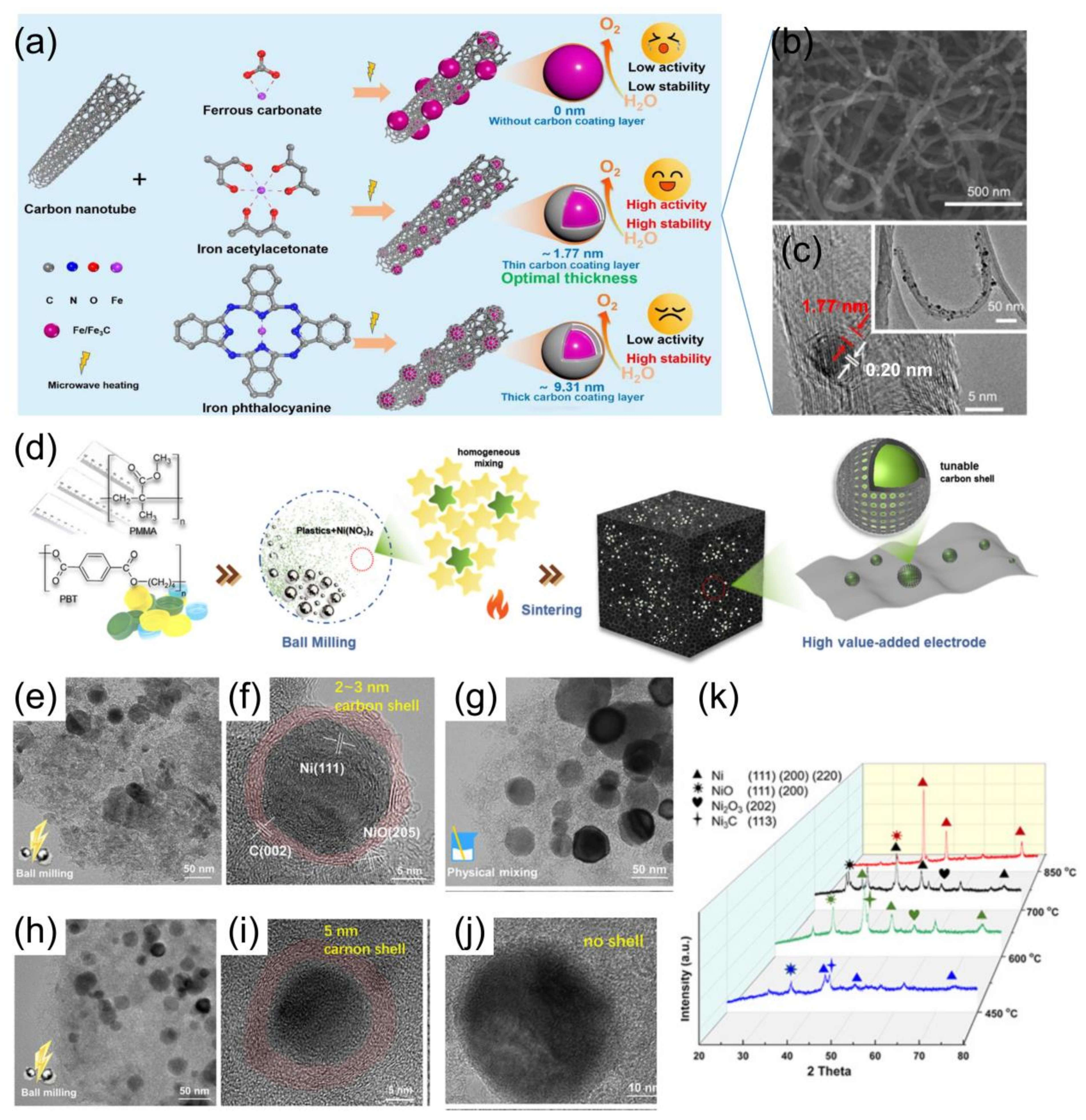
- Zhao, J.; Niu, Q.; Zhang, J.; Zhang, P. Core–Shell Construction of Metal@carbon by Mechanochemically Recycling Plastic Wastes: Towards an Efficient Oxygen Evolution Reaction. Green. Chem. 2023, 25, 8047–8056. [Google Scholar] [CrossRef]
- Gao, T.; Yu, S.; Chen, Y.; Li, X.; Tang, X.; Wu, S.; He, B.; Lan, H.; Li, S.; Yue, Q.; et al. Regulating the Thickness of the Carbon Coating Layer in Iron/Carbon Heterostructures to Enhance the Catalytic Performance for Oxygen Evolution Reaction. J. Colloid. Interface Sci. 2023, 642, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huo, W.; Fang, F.; Xie, Z.; Shang, J.K.; Jiang, J. High Entropy Alloy/C Nanoparticles Derived from Polymetallic MOF as Promising Electrocatalysts for Alkaline Oxygen Evolution Reaction. Chem. Eng. J. 2022, 429, 132410. [Google Scholar] [CrossRef]
- Ma, L.; Wei, Z.; Zhao, C.; Meng, X.; Zhang, H.; Song, M.; Wang, Y.; Li, B.; Huang, X.; Xu, C.; et al. Hierarchical Superhydrophilic/Superaerophobic 3D Porous Trimetallic (Fe, Co, Ni) Spinel/Carbon/Nickel Foam for Boosting Oxygen Evolution Reaction. Appl. Catal. B 2023, 332, 122717. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y. Degradation of Carbon Materials in Electrocatalysis. Curr. Opin. Electrochem. 2022, 36, 101159. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, P.; Xia, M.; Liu, W.; Gao, J.; Sun, L.; Hou, J. Identification of the Origin for Reconstructed Active Sites on Oxyhydroxide for Oxygen Evolution Reaction. Adv. Mater. 2023, 35, 2209307. [Google Scholar] [CrossRef]
- Righi, G.; Plescher, J.; Schmidt, F.-P.; Campen, R.K.; Fabris, S.; Knop-Gericke, A.; Schlögl, R.; Jones, T.E.; Teschner, D.; Piccinin, S. On the Origin of Multihole Oxygen Evolution in Haematite Photoanodes. Nat. Catal. 2022, 5, 888–899. [Google Scholar] [CrossRef]
- Haase, F.T.; Bergmann, A.; Jones, T.E.; Timoshenko, J.; Herzog, A.; Jeon, H.S.; Rettenmaier, C.; Cuenya, B.R. Size Effects and Active State Formation of Cobalt Oxide Nanoparticles during the Oxygen Evolution Reaction. Nat. Energy 2022, 7, 765–773. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Yao, H.; Wang, P.; Zhu, M.; Shi, X.-R.; Xu, S. Carbon-Based Composites for Oxygen Evolution Reaction Electrocatalysts: Design, Fabrication, and Application. Materials 2024, 17, 2265. https://doi.org/10.3390/ma17102265
Gao C, Yao H, Wang P, Zhu M, Shi X-R, Xu S. Carbon-Based Composites for Oxygen Evolution Reaction Electrocatalysts: Design, Fabrication, and Application. Materials. 2024; 17(10):2265. https://doi.org/10.3390/ma17102265
Chicago/Turabian StyleGao, Chang, Haiyu Yao, Peijie Wang, Min Zhu, Xue-Rong Shi, and Shusheng Xu. 2024. "Carbon-Based Composites for Oxygen Evolution Reaction Electrocatalysts: Design, Fabrication, and Application" Materials 17, no. 10: 2265. https://doi.org/10.3390/ma17102265






