Long-Term Corrosion of Eutectic Gallium, Indium, and Tin (EGaInSn) Interfacing with Diamond
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the Diamond Coatings
2.3. Aging/Corrosion Experiment of the Diamond/Liquid Metal Systems
2.4. Characterization
3. Results
3.1. Surface Morphology and Structure of Diamond Coatings after Contact with Liquid Metal
3.2. Corrosion Resistance and Penetration of the Diamond Coatings by the Liquid Metal
3.3. The Origin of the Solidification of the Liquid Metal?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, Y.; Xia, Y.; Ren, H.; Zhang, C.; Wang, M.; Tang, X.; Ding, S. Heat dissipation design and optimization of high-power LED lamps. Therm. Sci. Eng. Prog. 2023, 37, 101587. [Google Scholar] [CrossRef]
- Ryou, J.-H.; Choi, S. All-around diamond for cooling power devices. Nat. Electron. 2022, 5, 834–835. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Luo, J.; Zhuang, L.; Zou, D. Liquid metal (LM) and its composites in thermal management. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107216. [Google Scholar] [CrossRef]
- Sang, L. Diamond as the heat spreader for the thermal dissipation of GaN-based electronic devices. Funct. Diam. 2022, 1, 174–188. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Stadler, F.J.; Zhou, X. Critical Review on the Physical Properties of Gallium-Based Liquid Metals and Selected Pathways for Their Alteration. J. Phys. Chem. C 2021, 125, 20113–20142. [Google Scholar] [CrossRef]
- Ho, C.Y.; Powell, R.W.; Liley, P.E. Thermal Conductivity of the Elements. J. Phys. Chem. Ref. Data 1972, 1, 279–421. [Google Scholar] [CrossRef]
- Dong, H.; Wen, B.; Melnik, R. Relative importance of grain boundaries and size effects in thermal conductivity of nanocrystalline materials. Sci. Rep. 2014, 4, 7037. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.A. Nanocrystalline diamond. Diam. Relat. Mater. 2011, 20, 621–640. [Google Scholar] [CrossRef]
- Hicks, M.-L.; Pakpour-Tabrizi, A.C.; Jackman, R.B. Diamond Etching Beyond 10 μm with Near-Zero Micromasking. Sci. Rep. 2019, 9, 15619. [Google Scholar] [CrossRef]
- Einaga, Y. Boron-Doped Diamond Electrodes: Fundamentals for Electrochemical Applications. Acc. Chem. Res. 2022, 55, 3605–3615. [Google Scholar] [CrossRef]
- Kitanovski, A.; Egolf, P.W.; Poredos, A. Rotary magnetic chillers with permanent magnets. Int. J. Refrig. 2012, 35, 1055–1066. [Google Scholar] [CrossRef]
- Hidekazu, T.; Yutaka, T. Magnetic Air Conditioner. JP2015031472A, 16 February 2015. [Google Scholar]
- Yutaka, T. Magnetic Cooling and Heating Device. WO2015056322A1, 23 April 2015. [Google Scholar]
- Wang, W.; Wei, S.; Du, X.; Ding, Z.; Zhu, Q.; Qiao, Y.; Wang, X.; Guo, J. Fabrication of liquid metal/diamond hybrid thermal interface materials with high thermal conductivity and low flowability. J. Mater. Sci. Mater. Electron. 2023, 34, 1395. [Google Scholar] [CrossRef]
- Castner, H.Y. Process of and Apparatus for Electrolytic Decomposition of Alkaline Salts. US528322A, 30 October 1894. [Google Scholar]
- Zuraiqi, K.; Zavabeti, A.; Allioux, F.-M.; Tang, J.; Nguyen, C.K.; Tafazolymotie, P.; Mayyas, M.; Ramarao, A.V.; Spencer, M.; Shah, K.; et al. Liquid Metals in Catalysis for Energy Applications. Joule 2020, 4, 2290–2321. [Google Scholar] [CrossRef]
- Kaur, P.; Verma, G. A critical assessment of aluminum-water reaction for on-site hydrogen-powered applications. Mater. Today Energy 2024, 40, 101508. [Google Scholar] [CrossRef]
- du Preez, S.P.; Bessarabov, D.G. The effects of bismuth and tin on the mechanochemical processing of aluminum-based composites for hydrogen generation purposes. Int. J. Hydrogen Energy 2019, 44, 21896–21912. [Google Scholar] [CrossRef]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- Handschuh-Wang, S.; Gan, T.; Rauf, M.; Yang, W.; Stadler, F.J.; Zhou, X. The subtle difference between Galinstan (R) and eutectic GaInSn. Materialia 2022, 26, 101642. [Google Scholar] [CrossRef]
- Koh, A.; Hwang, W.Y.; Zavalij, P.; Chun, S.; Slipher, G.; Mrozek, R. Solidification and melting phase change behavior of eutectic gallium-indium-tin. Materialia 2019, 8, 100512. [Google Scholar] [CrossRef]
- Dobosz, A.; Plevachuk, Y.; Sklyarchuk, V.; Sokoliuk, B.; Gancarz, T. Thermophysical properties of the liquid Ga–Sn–Zn eutectic alloy. Fluid Phase Equilibria 2018, 465, 1–9. [Google Scholar] [CrossRef]
- Guminski, C. The Hg-Ta system (mercury-tantalum). J. Phase Equilibria 2001, 22, 684–685. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Murakami, Y. Chemical Reaction between Tantalum and Gallium under High Pressure. Mater. Trans. 2003, 44, 377–380. [Google Scholar] [CrossRef]
- Mingear, J.; Hartl, D. Liquid metal-induced corrosion of nickel-titanium alloys by gallium alloys for liquid metal-enabled shape memory applications. Corros. Sci. 2020, 167, 108524. [Google Scholar] [CrossRef]
- Dobosz, A.; Berent, K.; Bigos, A.; Gancarz, T. Interfacial phenomena between liquid alloy and Ni substrate covered by Ni–W layer. Mater. Lett. 2020, 277, 128299. [Google Scholar] [CrossRef]
- Naidich, J.V. The Wettability of Solids by Liquid Metals. In Progress in Surface and Membrane Science; Cadenhead, D.A., Danielli, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; Volume 14, pp. 353–484. [Google Scholar]
- Handschuh-Wang, S.; Wang, T.; Zhu, L.; Xu, Y.; Huang, L.; Gan, T.; Tang, Y.; Zhou, X. Corrosion-Resistant Functional Diamond Coatings for Reliable Interfacing of Liquid Metals with Solid Metals. ACS Appl. Mater. Interfaces 2020, 12, 40891–40900. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Gan, T.; Wang, T.; He, B.; Han, P.; Stadler, F.J.; Zhou, X. Vibration-Enabled Mobility of Liquid Metal. Adv. Funct. Mater. 2024, 34, 2313474. [Google Scholar] [CrossRef]
- Oh, E.; Kim, T.; Yoon, J.; Lee, S.; Kim, D.; Lee, B.; Byun, J.; Cho, H.; Ha, J.; Hong, Y. Highly Reliable Liquid Metal–Solid Metal Contacts with a Corrugated Single-Walled Carbon Nanotube Diffusion Barrier for Stretchable Electronics. Adv. Funct. Mater. 2018, 28, 1806014. [Google Scholar] [CrossRef]
- Andrews, J.B.; Mondal, K.; Neumann, T.V.; Cardenas, J.A.; Wang, J.; Parekh, D.P.; Lin, Y.; Ballentine, P.; Dickey, M.D.; Franklin, A.D. Patterned Liquid Metal Contacts for Printed Carbon Nanotube Transistors. ACS Nano 2018, 12, 5482–5488. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Zhu, L.; Gan, T.; Wang, T.; Wang, B.; Zhou, X. Interfacing of surfaces with gallium-based liquid metals—Approaches for mitigation and augmentation of liquid metal adhesion on surfaces. Appl. Mater. Today 2020, 21, 100868. [Google Scholar] [CrossRef]
- Wang, T.; Huang, L.; Liu, Y.; Li, X.; Liu, C.; Handschuh-Wang, S.; Xu, Y.; Zhao, Y.; Tang, Y. Robust Biomimetic Hierarchical Diamond Architecture with a Self-Cleaning, Antibacterial, and Antibiofouling Surface. ACS Appl. Mater. Interfaces 2020, 12, 24432–24441. [Google Scholar] [CrossRef]
- Huang, L.; Wang, T.; Li, X.; Wang, X.; Zhang, W.; Yang, Y.; Tang, Y. UV-to-IR highly transparent ultrathin diamond nanofilms with intriguing performances: Anti-fogging, self-cleaning and self-lubricating. Appl. Surf. Sci. 2020, 527, 146733. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Zhu, L.; Wang, T. Is There a Relationship between Surface Wettability of Structured Surfaces and Lyophobicity toward Liquid Metals? Materials 2020, 13, 2283. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Gorelik, V.S.; Pyatyshev, A.Y. Raman scattering in diamond nano- and microcrystals, synthesized at high temperatures and high pressures. Diam. Relat. Mater. 2020, 110, 108104. [Google Scholar] [CrossRef]
- McNamara, D.; Alveen, P.; Damm, S.; Carolan, D.; Rice, J.H.; Murphy, N.; Ivanković, A. A Raman spectroscopy investigation into the influence of thermal treatments on the residual stress of polycrystalline diamond. Int. J. Refract. Met. Hard Mater. 2015, 52, 114–122. [Google Scholar] [CrossRef]
- Erasmus, R.M.; Comins, J.D.; Mofokeng, V.; Martin, Z. Application of Raman spectroscopy to determine stress in polycrystalline diamond tools as a function of tool geometry and temperature. Diam. Relat. Mater. 2011, 20, 907–911. [Google Scholar] [CrossRef]
- Schügerl, F.B.; Kuzmany, H. Optical modes of trans-polyacetylene. J. Chem. Phys. 1981, 74, 953–958. [Google Scholar] [CrossRef]
- Kuzmany, H.; Pfeiffer, R.; Salk, N.; Günther, B. The mystery of the 1140 cm−1 Raman line in nanocrystalline diamond films. Carbon 2004, 42, 911–917. [Google Scholar] [CrossRef]
- Bernard, M.; Deneuville, A.; Muret, P. Non-destructive determination of the boron concentration of heavily doped metallic diamond thin films from Raman spectroscopy. Diam. Relat. Mater. 2004, 13, 282–286. [Google Scholar] [CrossRef]
- Schwarzová-Pecková, K.; Vosáhlová, J.; Barek, J.; Šloufová, I.; Pavlova, E.; Petrák, V.; Zavázalová, J. Influence of boron content on the morphological, spectral, and electroanalytical characteristics of anodically oxidized boron-doped diamond electrodes. Electrochim. Acta 2017, 243, 170–182. [Google Scholar] [CrossRef]
- Bernard, M.; Baron, C.; Deneuville, A. About the origin of the low wave number structures of the Raman spectra of heavily boron doped diamond films. Diam. Relat. Mater. 2004, 13, 896–899. [Google Scholar] [CrossRef]
- Cao, L.; Yu, D.; Xia, Z.; Wan, H.; Liu, C.; Yin, T.; He, Z. Ferromagnetic Liquid Metal Putty-Like Material with Transformed Shape and Reconfigurable Polarity. Adv. Mater. 2020, 32, 2000827. [Google Scholar] [CrossRef]
- Jang, S.; Jeghan, S.M.N.; Seon, E.; Tak, Y.; Kim, M.; Lee, G. Unraveling the corrosion kinetics of gallium-aluminum for efficient hydrogen production from water at zero CO2 emission. Int. J. Hydrogen Energy 2023, 48, 13390–13403. [Google Scholar] [CrossRef]
- Deng, Y.-G.; Liu, J. Corrosion development between liquid gallium and four typical metal substrates used in chip cooling device. Appl. Phys. A 2009, 95, 907–915. [Google Scholar] [CrossRef]
- Bagchi, D.; Raj, J.; Singh, A.K.; Cherevotan, A.; Roy, S.; Manoj, K.S.; Vinod, C.P.; Peter, S.C. Structure-Tailored Surface Oxide on Cu–Ga Intermetallics Enhances CO2 Reduction Selectivity to Methanol at Ultralow Potential. Adv. Mater. 2022, 34, 2109426. [Google Scholar] [CrossRef]
- Liu, S.; Qu, D.; McDonald, S.; Gu, Q.; Matsumura, S.; Nogita, K. Intermetallic formation mechanisms and properties in room-temperature Ga soldering. J. Alloys Compd. 2020, 826, 154221. [Google Scholar] [CrossRef]
- Naidich, Y.V.; Kolesnichenko, G.A. Investigation of the wetting of diamond and graphite by molten metals and alloys. Sov. Powder Metall. Met. Ceram. 1965, 3, 191–195. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Genzer, J.; Dickey, M.D. Shape-transformable liquid metal nanoparticles in aqueous solution. Chem. Sci. 2017, 8, 3832–3837. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Handschuh-Wang, S.; Shang, W.; Zhou, X. GaOOH Crystallite Growth on Liquid Metal Microdroplets in Water: Influence of the Local Environment. Langmuir 2022, 38, 14475–14484. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Hill, V.G.; Osborn, E.F. Polymorphism of Ga2O3 and the System Ga2O3—H2O. J. Am. Chem. Soc. 1952, 74, 719–722. [Google Scholar] [CrossRef]
- Rodríguez, C.I.M.; Álvarez, M.Á.L.; Rivera, J.d.J.F.; Arízaga, G.G.C.; Michel, C.R. α-Ga2O3 as a Photocatalyst in the Degradation of Malachite Green. ECS J. Solid State Sci. Technol. 2019, 8, Q3180. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Tabor, C.; Kalantar-Zadeh, K.; Dickey, M.D. Gallium Liquid Metal: The Devil's Elixir. Annu. Rev. Mater. Res. 2021, 51, 381–408. [Google Scholar] [CrossRef]
- Wolf, M.; de Oliveira, A.L.; Taccardi, N.; Maisel, S.; Heller, M.; Khan Antara, S.; Søgaard, A.; Felfer, P.; Görling, A.; Haumann, M.; et al. Dry reforming of methane over gallium-based supported catalytically active liquid metal solutions. Commun. Chem. 2023, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Ichirou. Liquid Metal Drying up? 2023. Available online: https://www.overclock.net/threads/liquid-metal-drying-up.1803176/ (accessed on 10 April 2024).
- Alsharif, M.H.; Hossain, M.S.; Jahid, A.; Khan, M.A.; Choi, B.J.; Mostafa, S.M. Milestones of Wireless Communication Networks and Technology Prospect of Next Generation (6G). Comput. Mater. Contin. 2022, 71, 4803–4818. [Google Scholar] [CrossRef]
- Xing, W.; Xu, Y.; Song, C.; Deng, T. Recent Advances in Thermal Interface Materials for Thermal Management of High-Power Electronics. Nanomaterials 2022, 12, 3365. [Google Scholar] [CrossRef] [PubMed]

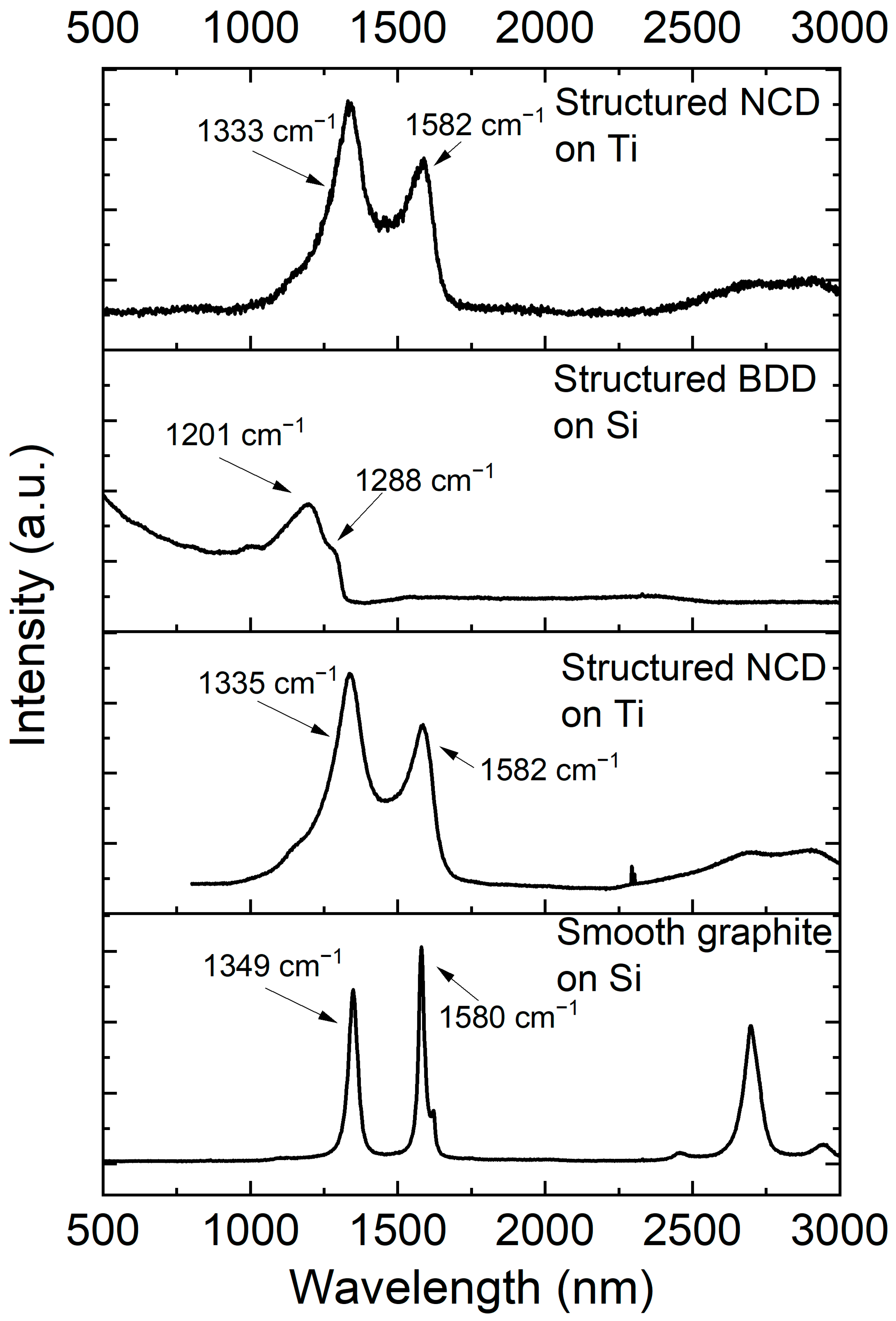
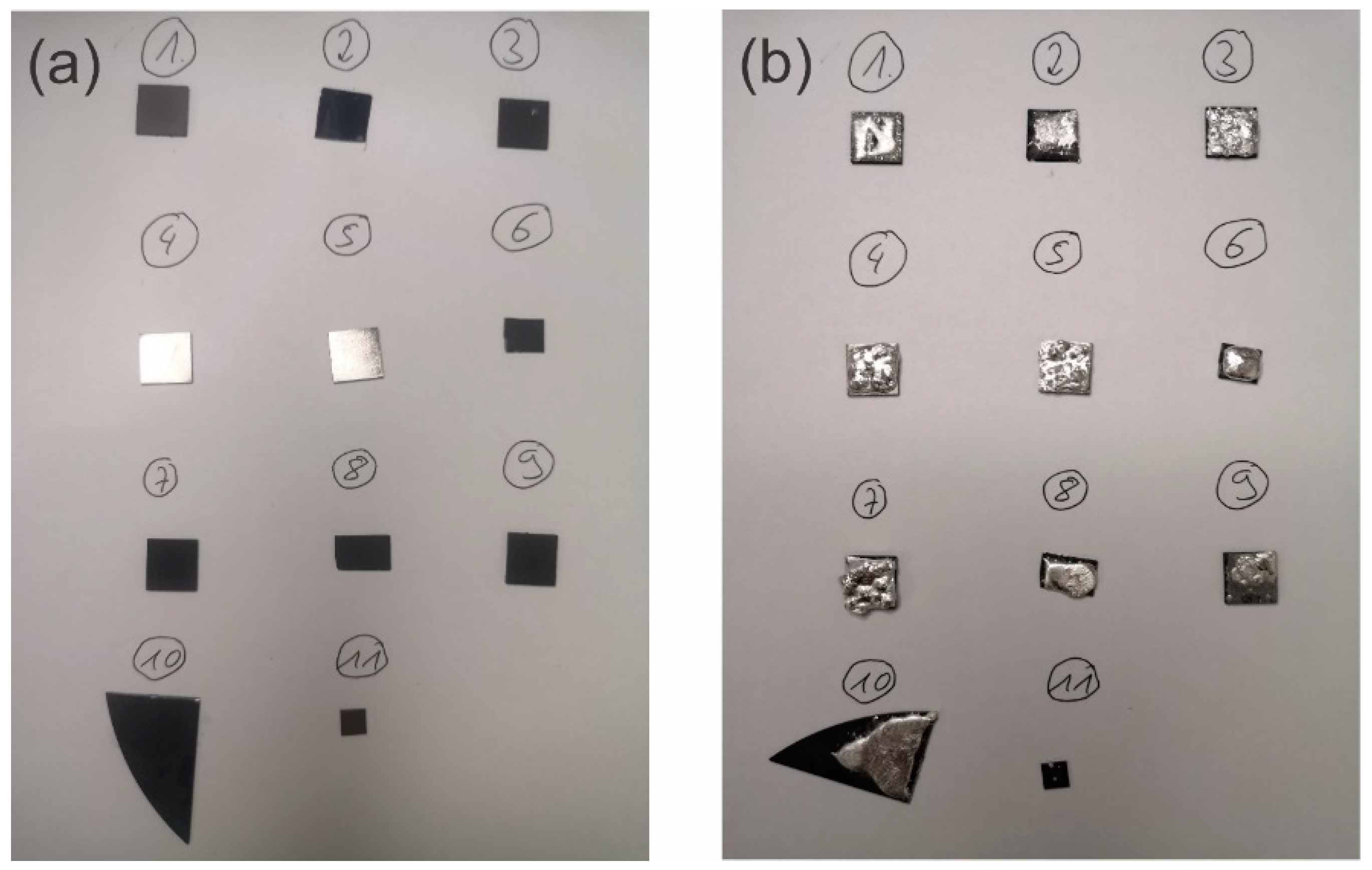



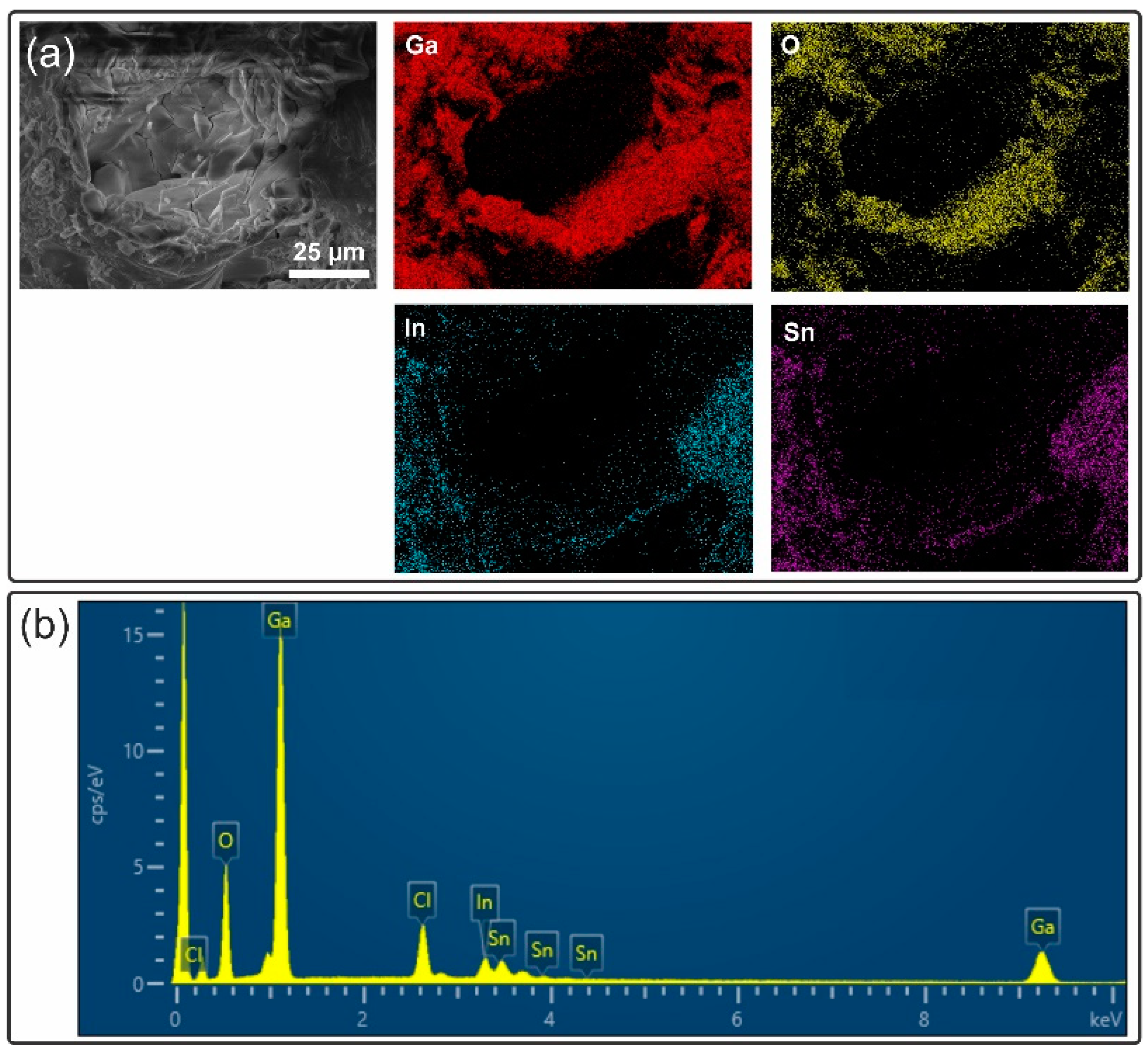
| Sample ID | Substrate | Diamond Coating |
|---|---|---|
| #1 | Titanium | Smooth nanocrystalline diamond |
| #2 | Si | Smooth graphite |
| #3 | Titanium | Structured nanocrystalline diamond |
| #4 | Titanium | Uncoated |
| #5 | Titanium | Uncoated |
| #6 | Si | Smooth BDD |
| #7 | Titanium | Structured nanocrystalline diamond |
| #8 | Si | Structured sub-microcrystalline BDD |
| #9 | Titanium | Structured nanocrystalline diamond |
| #10 | Si | BDD |
| #11 | Titanium | BDD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handschuh-Wang, S.; Wang, T.; Zhang, Z.; Liu, F.; Han, P.; Liu, X. Long-Term Corrosion of Eutectic Gallium, Indium, and Tin (EGaInSn) Interfacing with Diamond. Materials 2024, 17, 2683. https://doi.org/10.3390/ma17112683
Handschuh-Wang S, Wang T, Zhang Z, Liu F, Han P, Liu X. Long-Term Corrosion of Eutectic Gallium, Indium, and Tin (EGaInSn) Interfacing with Diamond. Materials. 2024; 17(11):2683. https://doi.org/10.3390/ma17112683
Chicago/Turabian StyleHandschuh-Wang, Stephan, Tao Wang, Zongyan Zhang, Fucheng Liu, Peigang Han, and Xiaorui Liu. 2024. "Long-Term Corrosion of Eutectic Gallium, Indium, and Tin (EGaInSn) Interfacing with Diamond" Materials 17, no. 11: 2683. https://doi.org/10.3390/ma17112683
APA StyleHandschuh-Wang, S., Wang, T., Zhang, Z., Liu, F., Han, P., & Liu, X. (2024). Long-Term Corrosion of Eutectic Gallium, Indium, and Tin (EGaInSn) Interfacing with Diamond. Materials, 17(11), 2683. https://doi.org/10.3390/ma17112683







