Abstract
We report a one-pot synthesis of well-defined A5B and A8B miktoarm star-shaped polymers where N,N-dimethylaminoethyl methacrylate (DMAEMA) and various cyclic esters such as ε-caprolactone (ε-CL), lactide (LA) and glycolide (GA) were used for the synthesis. Miktopolymers were obtained by simultaneously carrying out atom transfer radical polymerization (ATRP) of DMAEMA, ring-opening polymerization (ROP) of cyclic esters, and click reaction between the azide group in gluconamide-based (GLBr5-Az) or lactonamide-based (GLBr8-Az) ATRP initiators and 4-pentyn-1-ol. The relatively low dispersity indices of the obtained miktoarm stars (Đ = 1.2–1.6) indicate that control over the polymerization processes was sustained despite almost complete monomers conversions (83–99%). The presence of salts from phosphate-buffered saline (PBS) in polymer solutions affects the phase transition, increasing cloud point temperatures (TCP) values. The critical aggregation concentration (CAC) values increased with a decreasing number of average molecular weights of the hydrophobic fraction. Hydrolytic degradation studies revealed that the highest reduction of molecular weight was observed for polymers with PCL and PLGCL arm. The influence of the composition on the miktopolymers hydrophilicity was investigated via water contact angle (WCA) measurement. Thermogravimetric analysis (TGA) disclosed that the number of arms and their composition in the miktopolymer affects its weight loss under the influence of temperature.
1. Introduction
The main goal of one-pot synthesis is to improve the efficiency of the chemical reaction by avoiding a long process of separation and purification of intermediate compounds while increasing the yield. More than a hundred years have passed since Sir Robert Robinson performed the first one-pot reaction, namely the synthesis of tropinone, and since then, a significant number of one-pot strategies have been developed [1,2,3,4,5]. In organic synthesis, one example of a one-pot reaction is a multicomponent reaction (MCR). In MCR, at least three starting materials react sequentially with each other in a single reaction vessel, where the order of reaction depends on the chemical affinity of the reagents and formed intermediates [6]. Thanks to this method, Heck and Dömling obtained a 2,4-disubstituted thiazoles complex using oxo components, primary amines, thiocarboxylic acids, and a special isocyanide [7]. Another example is domino reactions (cascade reactions, tandem reactions), which allow the efficient synthesis of complex molecules from relatively simple substrates in a single reaction vessel without the need to purify intermediates [8]. Konieczny et al. obtained 4,7-dihydroxythioaurone derivatives by a one-pot reaction of benzaldehydes with 4-acetyl-2-oxo-benz[1,3]oxathioles and piperidine acetate in DMSO [9]. In the case of tandem reactions, variable synthesis conditions can be used [10].
In polymer synthesis, a one-pot reaction simultaneously allows for the synthesis and functionalization of polymers and acquires a relatively complex structure in a one-step procedure [11,12,13]. The advantage of this method is the avoidance of a long process of separation and purification of intermediate compounds. Polymers synthesized by this method are usually obtained using various polymerization techniques, such as ring-opening polymerization (ROP) or/and various controlled/“pseudo-living” radical polymerization (CRP) techniques, including atom transfer radical polymerization (ATRP), nitroxide-mediated radical polymerization (NMP) and radical polymerization with reversible fragmentation combined with chain transfer (RAFT) [14,15,16]. The literature describes a few examples of the one-pot synthesis of well-defined polymers containing PDMAEMA and aliphatic polyester segments. For example, Mespouille et al. synthesized a well-defined amphiphilic block copolymer PCL-b-PDMAEMA with the one-pot method by combining ATRP and click reactions [17]. Huang et al. obtained star-shaped diblock and triblock copolymers with PCL, PDMAEMA, and/or POEGMA segments via one-pot ATRP using bromine end-capped six-arm PCL as a macroinitiator [18]. Zhang et al. obtained a miktoarm star-shaped polymer consisting of PDMAEMA, PCL, and polystyrene (PS) or polyethylene oxide (PEO) arms. They combined techniques such as ROP, ATRP, and click reactions [19].
Progress in the development of polymerization methods has led to the possibility of obtaining more complex polymer structures, such as block polymers [20], polymer brushes [21], and star polymers [22], which have completely different properties from their linear analogs. Structures with a star topology include, among others, miktopolymers, which contain three or more arms with different chemical compositions, connected to one central core [23]. Currently, they are obtained using the coupling-onto, in–out, arm-first, and core-first methods. Nowadays, designing macromolecules with predetermined properties, and the resulting applications, is desired; hence, the ability to control these properties by varying the polymer arms is essential. Due to their unique properties, which can be adjusted not only by the selection of polymer arms but also by the type of the core, miktoarm polymers are used, among others, in medicine, pharmacy, and biotechnology.
In our previous works concerning the degradation of PDMAEMA/polyester block copolymers, we have shown that enzymatic degradation using Novozyme 435 primarily involved the ester bonds in PDMAEMA side chains, and the rate of polyester degradation decreased with the increase in the chain length of PDMAEMA [24]. Whereas in the case of PDMAEMA/polyester miktoarm stars obtained via an in–out approach, enzymatic degradation performed using lipase from Pseudomonas cepacia showed an increased degradation rate for the polymer with PCL arms [25].
Our previous research was focused on the synthesis of miktoarm star-shaped polymers via a coupling-onto [26] or in–out method [25]. In this study, a series of well-defined miktoarm star-shaped copolymers based on DMAEMA and cyclic esters were obtained by facile one-pot synthesis using a combination of techniques such as ATRP, ROP, and click reaction. Miktoinitiators based on the amide derivative of D-gluconic acid containing one azide group and five bromoester groups (GLBr5-Az) [26,27], and the amide derivative of lactobionic acid containing one azide group and eight bromoester groups (GLBr8-Az) have been synthesized and characterized by our group previously [28].
Herein, we present the synthetic pathway to obtain amphiphilic pH- and thermoresponsive miktoarm stars, which additionally are semi-degradable due to the presence of the polyester arm. In this work, compositional properties of the obtained miktoarm stars were evaluated based on their behavior in water solution, WCA measurements on their surface, thermal stability, and hydrolytic degradation rate. It is worth noticing that the one-pot strategy enabled us to obtain polymers with complex structures with high reaction efficiency, and in a relatively short time.
2. Materials and Methods
2.1. Materials
N,N′-dimethylaminoethyl methacrylate (DMAEMA, Aldrich, St. Louis, MO, USA, 98%) was stored over molecular sieves in a freezer under nitrogen. ε-Caprolactone (CL, Alfa Aesar, 99%, Warsaw, Poland) and toluene were distilled before use and stored over molecular sieves. 4-Pentyn-1-ol (PentAl, Aldrich, 97%), lactide (LA, Aldrich, 99%), N,N,N′,N″,N″-Pentamethyldiethylenetriamine (PMDETA, Aldrich, 99%), glycolide (GA, Aldrich, 99%), and pyrene (Acros, extra dry, 99.5%, Geel, Belgium) were used directly without purification. Copper(I) chloride (CuCl, Fluka, 98%, Steinheim, Germany) was purified by stirring in glacial acetic acid followed by filtration and washing with ethanol and diethyl ether. Subsequently, the solid was dried under a vacuum. Tin(II) bis(2-ethylhexanoate) (Sn (Oct)2, Alfa Aesar, 96%) was distilled prior to use. An amount of 0.01 M Phosphate buffer saline (PBS, pH 7.4, Sigma-Aldrich) was prepared according to the following procedure: one tablet was dissolved in 200 mL of deionized water, yielding 0.01 M phosphate-buffered saline (0.0027 M potassium chloride and 0.137 M sodium chloride). Gluconamide-based (GLBr5-Az) and lactonamide-based (GLBr8-Az) initiators were synthesized earlier [27,28], and methylene chloride (CH2Cl2) and heptane were used as received.
2.2. One-Pot Synthesis of Amphiphilic Miktoarm Star-Shaped Polymers
Monomers (DMAEMA, CL, LA, GA), ligand (PMDETA), ATRP initiator (GLBr5-Az, LABr8-Az), ROP initiator (PentAl) and toluene were placed in a Schlenk flask (in the case of SMS1 and NMS1 the mixture of reagents in Schlenk flask was degassed by three freeze–pump–thaw cycles; in the case of SMS2-SMS4 and NMS2-NMS4, the reaction mixture was purged with inert gas). After that CuCl and Sn(Oct)2 were added, and the reaction flask was immersed in an oil bath at 130 °C. After a predetermined time, the reaction was stopped by exposing the reaction mixture to air. The reaction mixture was diluted with CH2Cl2, passed through a neutral alumina column to remove the copper catalyst, and concentrated in a rotary evaporator with a vacuum pressure gauge. The polymers were precipitated in n-heptane and dried under vacuum at room temperature to constant mass. Miktopolymers were obtained with yields above 50%. The compositions of the reaction mixtures for the syntheses are given in Tables S1 and S2.
2.3. Characterization of Miktoarm Star-Shaped Polymers
In the one-pot polymerization of DMAEMA and CL, the conversions were determined by gas chromatography (GC). The chromatograph (6850 Network GC System, Agilent Technologies, Santa Clara, CA, USA) was equipped with a DB-WAX column 30 m × 0.32 mm × 0.25 µm (Agilent Technologies, Santa Clara, CA, USA) and flame ionization detector. Injector and detector temperatures were kept constant at 250 °C (conditions: anisole as internal standard, column initial temperature 40 °C; column final temperature 200 °C). The conversions were calculated by detecting the decrease in the monomer peak area relative to the standard peak area. In the one-pot polymerization of DMAEMA and LA or GA, the conversions were calculated using 1H NMR spectra of the reaction mixtures in CDCl3. 1H NMR spectra of the polymers were collected on Varian Inova 600 MHz spectrometer (Palo Alto, Santa Clara, CA, USA) at 25 °C using CDCl3 as a solvent and TMS as an internal standard. The apparent number-average molecular weights (Mn, SEC) were determined by size-exclusion chromatograph (SEC, 1100 Agilent 1260 Infinity, Santa Clara, CA, USA,) equipped with an isocratic pump, autosampler, degasser, thermostatic box for columns and differential refractometer MDS RI Detector. Addon Rev. B.01.02 data analysis software (Agilent Technologies, Hongkong, China) was used for data collecting and processing. The SEC calculated molecular weight was based on calibration using linear polystyrene standards (Mp = 580–3,000,000 g/mol). Pre-column guard 5 μm 50 × 7.5 mm and double PLGel 5 μm MIXED-C and MIXED-D 300 × 7.5 mm column were used for separation. The measurements were carried out in THF (HPLC-grade) as the solvent at 40 °C with a flow rate of 0.8 mL/min. UV–vis spectroscopy (Evolution 300 Spectrophotometer, Thermo Scientific, Waltham, MA, USA) was used to determine the TCP. Optical absorbances of polymers in aqueous solutions (1 mg/mL) were measured at 500 nm at a temperature ranging from 40 to 100 °C with a heating rate of 2 °C/min. TCP was defined as the temperature at which an extremum of the first derivation is located by differentiating the transmittance vs. temperature curve. The critical aggregation concentration (CAC) values of miktoarm polymers water solutions were determined using pyrene as a fluorescence probe. First, the predetermined pyrene solution (5 µM) in ethanol was added to a series of vials, which were further stirred at room temperature in the dark for 24. Next, the miktoarm star-shaped polymers were dissolved in deionized water, and proper solutions within the range 1 × 10−3 to 1 mg/mL were added to the vials with pyrene. Final mixtures were stirred in the dark for 24 h before measurement The fluorescence emission spectra (λex = 337 nm) of polymer/pyrene solutions were measured on a fluoroSENS Pro-11 spectrofluorimeter (CAMLIN, Lisburn, Ireland). The CAC value was defined as the point of intersection of two lines in the plot of intensity ratio (I1/I3) from pyrene emission spectra vs. the logarithm of the polymer concentration (logC, where C is concentration in mg/mL). The water contact angle (WCA) was determined using the sessile drop method utilizing the OCA 15EC (Data Physics, Filderstadt, Germany) goniometer. Drops (4 µL) of deionized water were placed on the polymer surfaces using an end-flat micrometric syringe (B.Braun Inject-F, luer solo). Water droplets were dropped onto at least three different sites of each lozenge surface. Dynamic results were recorded, and the average value of the contact angle was obtained for each sample. The experimental error was ±2°. WCA was determined using the image analysis provided with the SCA20 software and calculated with the Owens, Wendt, Rabel, and Kaelble (OWRK) method. The polymeric samples were in the form of lozenges with a diameter equal to 12 mm and a height of 1 mm, which were obtained by pressing the polymer in a press with a pressure of 10 tons. Thermogravimetric analysis (TGA) was accomplished using a TGA 8000 thermogravimetric analyzer (PerkinElmer, Rodgau, Germany) at a heating rate of 10 °C/min over a temperature range from 30 to 600 °C under a nitrogen flow.
2.4. Hydrolytic Degradation
First, approximately 5 ± 0.25 mg of each polymeric sample was weighed in glass vials. Next, 5 mL of PBS solution at pH 7.4 or 5.0, with the addition of sodium azide (23 µmoles of NaN3 per 5 mL of PBS), was added. The vials and their contents were sealed and placed on Orbital Shaker-Incubator ES-80 set to 150 rpm at a temperature of 37 ± 2 °C. Samples were taken out of the incubator at predetermined time intervals, i.e., 1, 2, 4, 6, and 9 weeks, then frozen and lyophilized. Samples were analyzed in triplicate.
3. Results and Discussion
3.1. One-Pot Synthesis of Miktoarm Stars
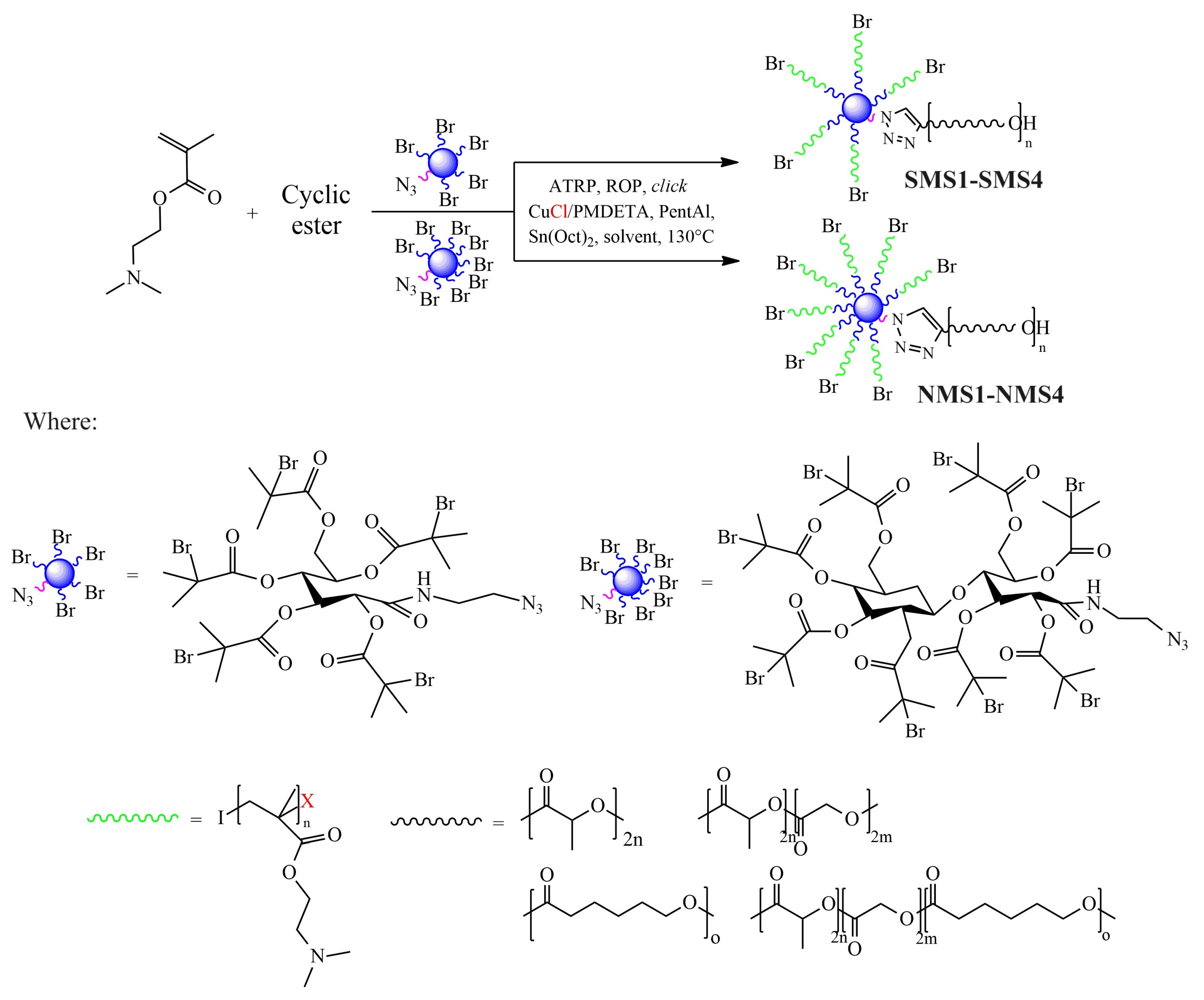
Well-defined A5B-type six-armed miktoarm star-shaped (SMS) polymers and A8B-type nine-armed miktoarm star-shaped (NMS) polymers were obtained through one-pot synthesis combining ATRP, ROP and click reaction (Scheme 1). Although there was a difference in the number of initiating bromoester groups (5 vs. 8), in all cases, after two hours, the conversions of monomers were in the range of 83–99%.
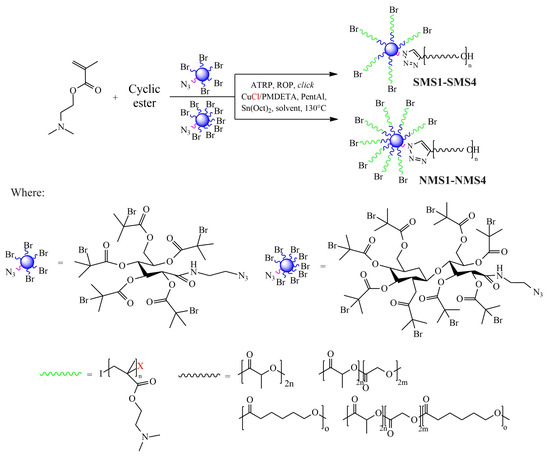
Scheme 1.
Schematic representation for the synthesis of amphiphilic star-shaped miktoarm polymers.
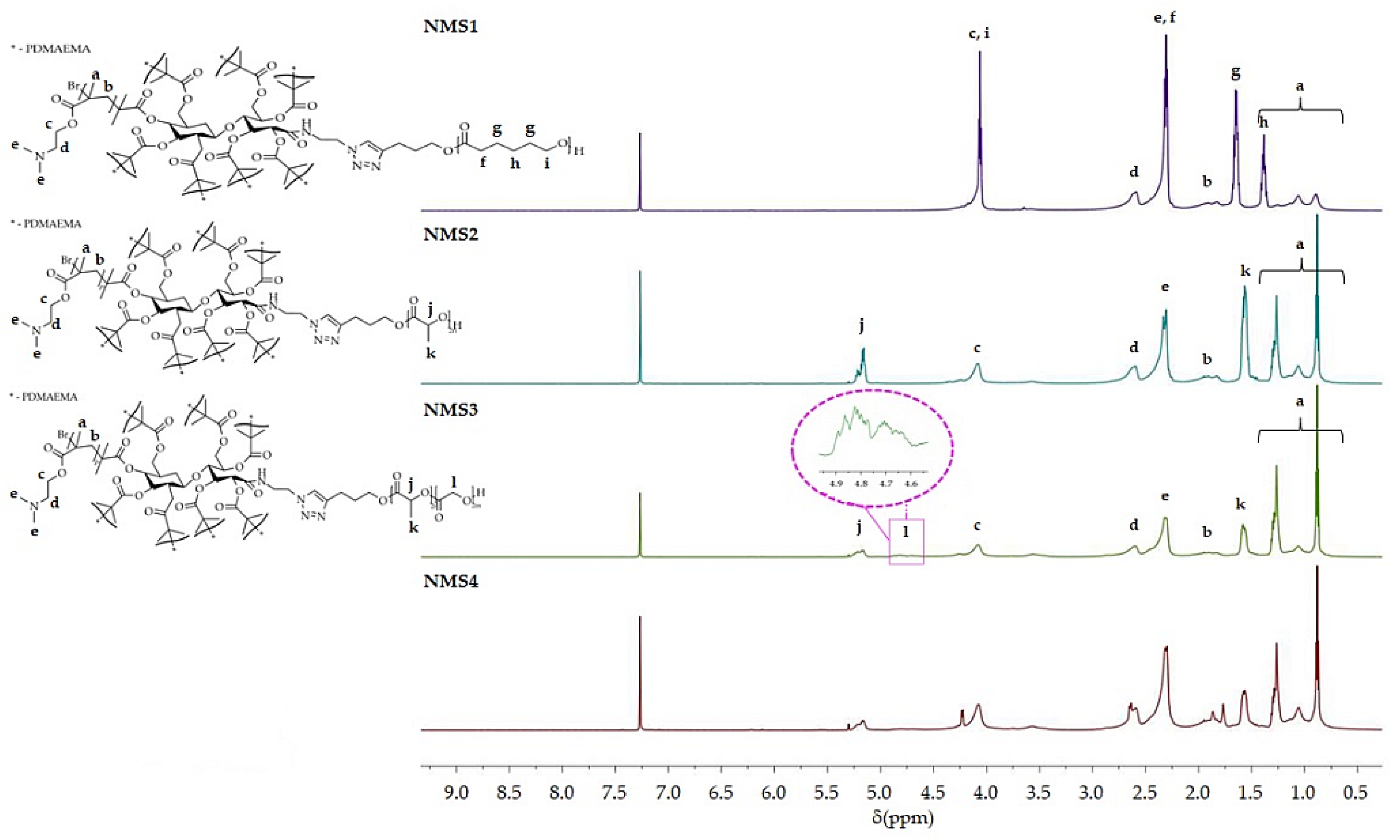
The characterization of the obtained six-arm star-shaped (SMS1-SMS4) and nine-arm star-shaped (NMS1-NMS4) copolymers are presented in Table 1. The structures of polymers were confirmed by 1H NMR analysis. 1H NMR spectra of the reaction mixtures were used to calculate the degree of polymerization (DP) of cyclic (di)esters in PLA, PLGA, and PCLGA, while GC analysis was used to calculate the DP of PCL. Figure 1 presents 1H NMR spectra of miktopolymers with characteristic peaks belonging to PDMAEMA and a proper polyester segment. Each spectrum contained signals from PDMAEMA, i.e., protons from the main chain (methyl groups at the range from 0.5 to 1.2 ppm, and methylene groups at the range from 1.8 to 2.0 ppm) and protons belonging to the pendant groups (two broad singlets from -N(CH3)2 at 2.2 ppm, and -CH2- near to carbonyl group at 2.5 ppm). In addition, at 3.65 ppm, there were protons from the methylene group close to the amine. In the case of copolymers with PLA, protons from methylidyne and methyl groups were observed at 5.2 ppm and 1.55 ppm, respectively. PCL gives four characteristic signals, i.e., a multiplet from methylene groups in the main chain at δ = 1.65 ppm and δ = 1.4 ppm, a triplet from the methylene group linked to the carbonyl group at δ = 2.31 ppm, and a triplet from the methylene group bonded to oxygen at δ = 4.06 ppm. However, a triplet from the methylene group bonded to the hydroxyl group at the chain-end at the shift δ = 3.66 ppm. The signal of the triazole protons overlaps with the signal from the deuterated solvent.

Table 1.
Characterization of miktoarm stars with six (SMS) or nine (NMS) arms obtained via one-pot synthesis.
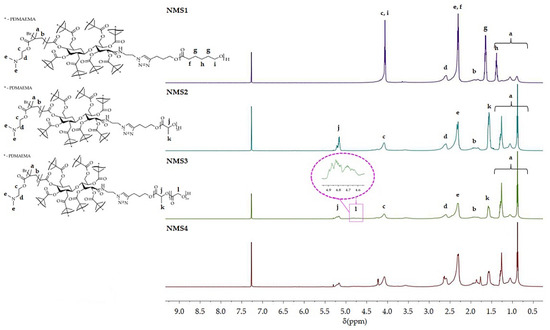
Figure 1.
1H NMR (600 MHz, CDCl3) spectra of nine-arm star-shaped miktopolymers.
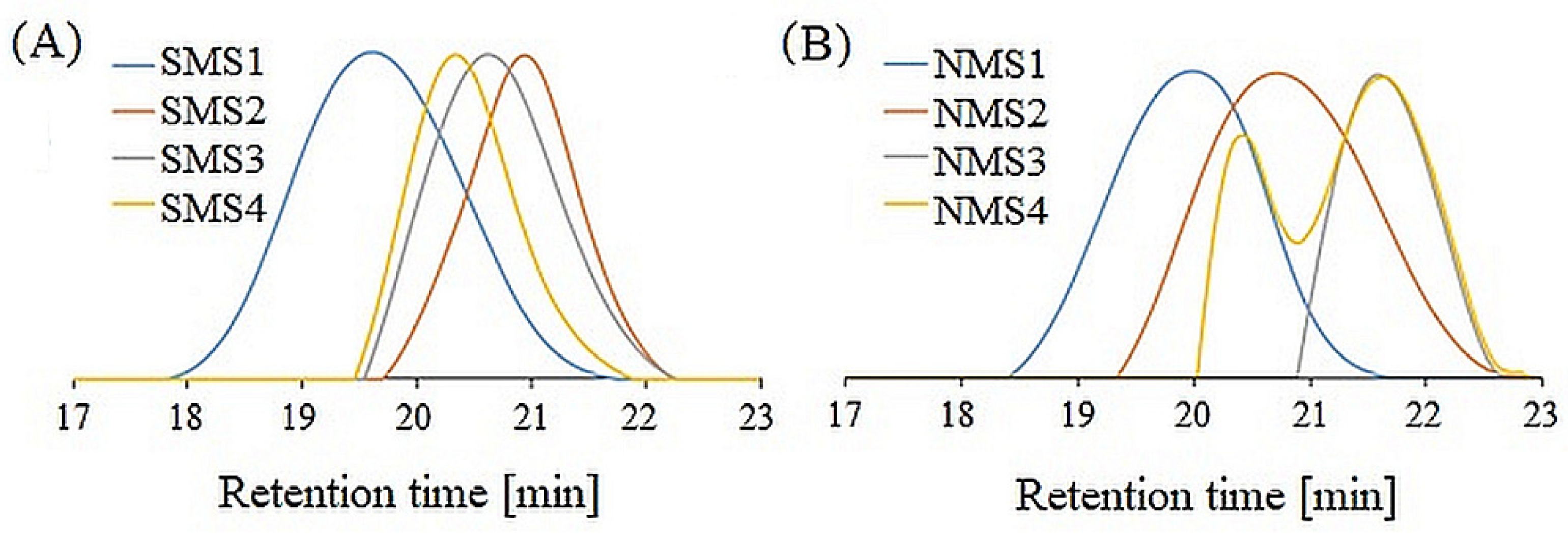
The SEC traces of miktoarm star-shaped copolymers are shown in Figure 2. Rather low dispersity values (Ð) (1.1–1.5) for the majority of samples indicate that the control over the polymerization processes was sustained. The exception is nine-arm NMS4 with terpolyester (PLGCL) arm, whose SEC eluogram shows a bimodal curve, which indicates star–star coupling as a result of the recombination of polymethacrylate arms. Presumably, the coupling reactions occurred due to high arm density and high monomer conversion [29].
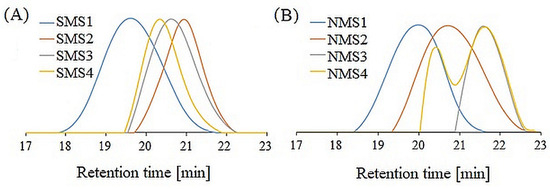
Figure 2.
SEC traces of six-arm (A) and nine-arm miktopolymers (B).
The number-average molecular weights determined using a refractive index detector (Mn; SEC) for six-arm miktopolymers ranged from 4800 to 14,200 g/mol and were lower than theoretical ones (Mn; the = 25,000–27,000 g/mol). A similar dependence was found for nine-arm miktopolymers, where Mn; SEC ranged from 3000 to 11,900 g/mol and corresponding Mn; theo = 24,000–28,000 g/mol. The differences in the hydrodynamic volumes of the tested samples and the PS standard result from both the chemical nature (PDMAEMA/polyester samples are amphiphilic and PS is hydrophobic) and topology (star-shaped vs. linear) were determined. Moreover, the molecular structures with five or eight short PDMAEMA arms and one long polyester arm probably influenced their radius of gyration [30], causing the shift toward higher retention time values, as was observed in our previous studies [26].
3.2. Miktoarm Stars Behavior in Water Solution
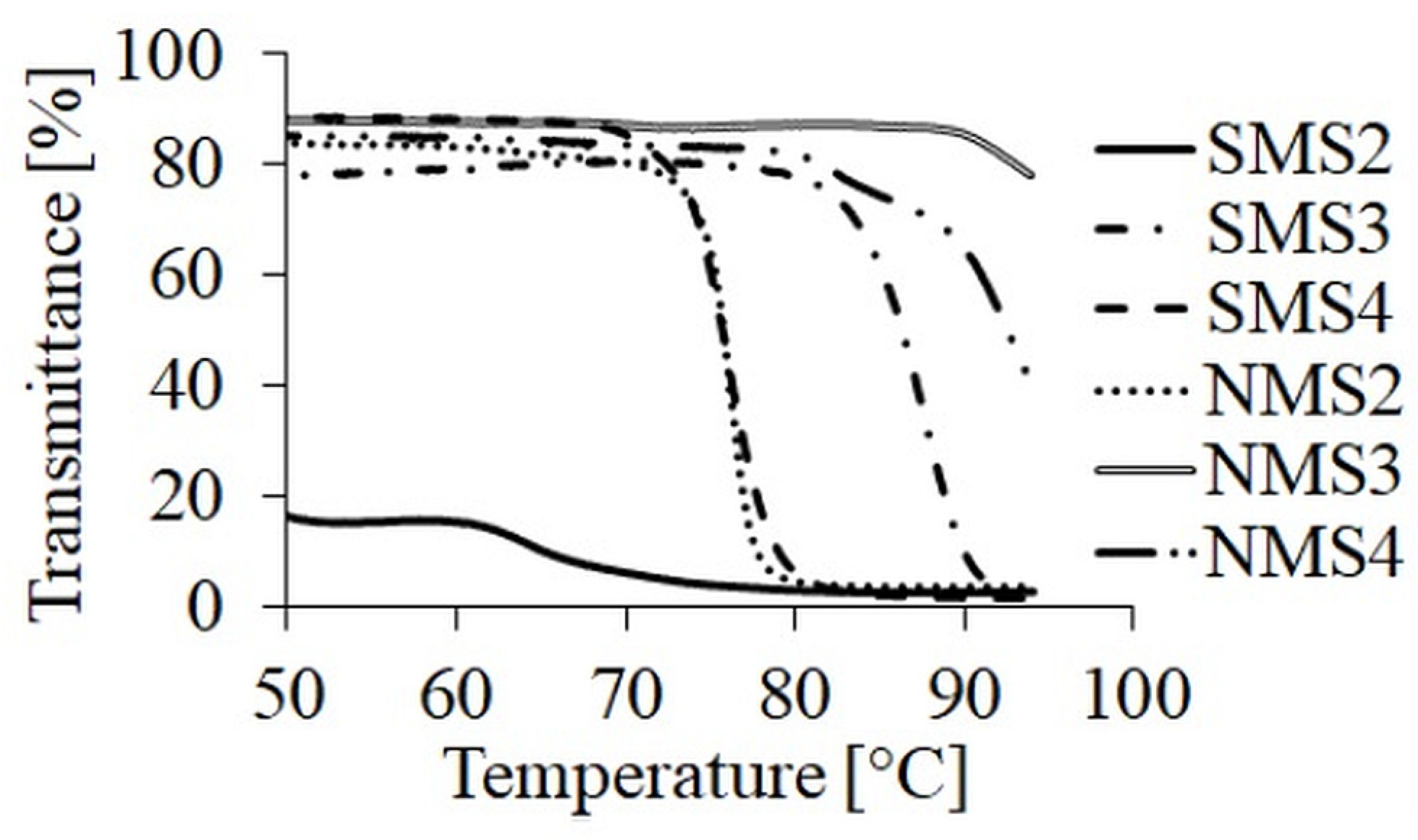
The behavior of obtained miktoarm star-shaped polymers was investigated in water and PBS (pH 7.4) solutions, depending on their composition and architecture, by determination of the cloud point temperature (TCP) of polymeric solutions by UV-vis (Table 1). The first derivative method was used to receive the TCP value.
Polymer samples dissolved in demineralized water did not show a visible and sharp phase transition point as opposed to the same samples dissolved in 0.01 M PBS. These results are in a good agreement with our previous studies [26]. Salts contained in PBS may cause an increase in ionic strength, leading to the replacement of water molecules with dissociated salt ions, which may lead to the appearance of the phase transition point of the tested polymer solutions [31]. The dependences of TCP on polymer composition and topology are shown in Figure 3. The presence in the structure of hydrophobic groups had a significant impact on whether the sample showed characteristic TCP. In the case of miktoarm polymers solutions in PBS, the introduction of the GA repeating units into the polyester arm increased the TCP value. The comparison of polymers with the same composition but a different number of arms let us to a conclusion that the additional three PDMAEAM chains increased the stability of a random coil structure in the solution by suppressing the occurrence of hydrophobic interaction between polymeric chains.
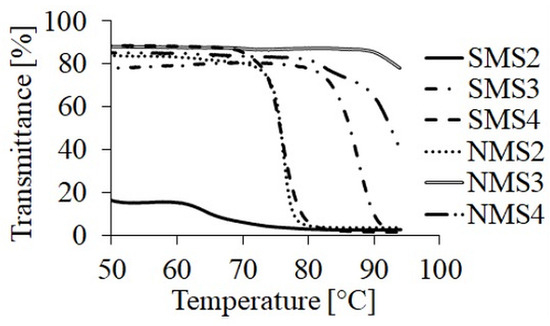
Figure 3.
TCP curves of miktopolymers in PBS at pH 7.4.
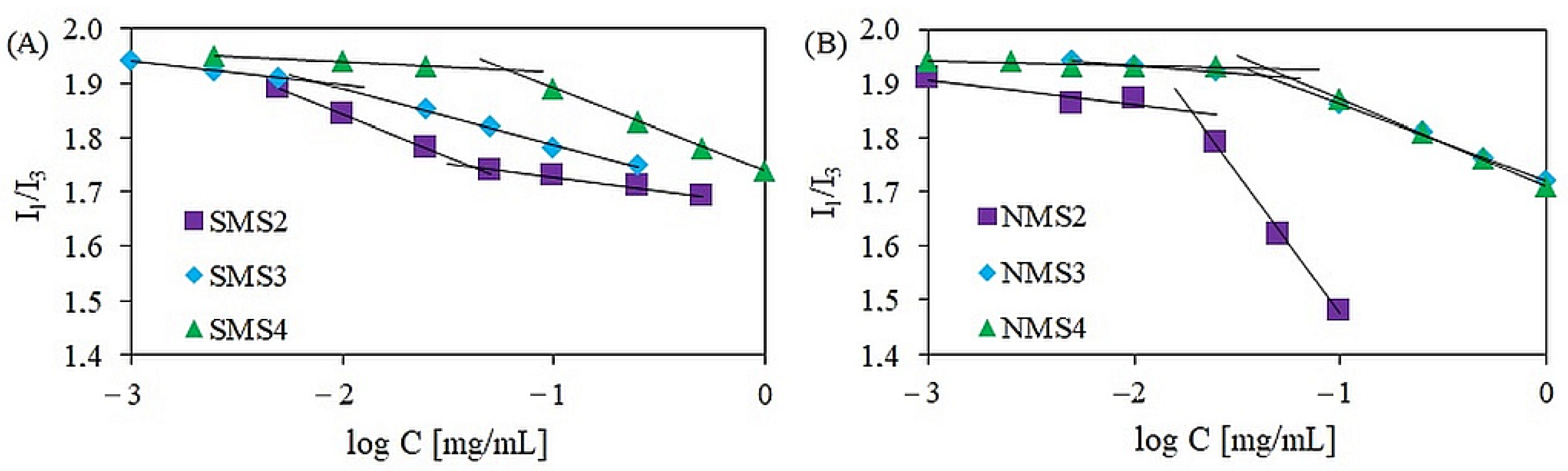
The critical aggregation concentration (CAC) values were monitored by fluorimetry in the presence of pyrene as a hydrophobic fluorescent probe. The CAC values of amphiphilic polymers were related to the ratio of hydrophilic blocks to hydrophobic ones. As shown in Figure 4, the ratio of I1 to I3 varied in response to different copolymer concentrations. The CAC values of amphiphilic six-arm and nine-arm star-shaped miktopolymers increased with increasing hydrophilic fraction content. The lowest CAC values were obtained for polymers in which the number-average molecular weight of the polyester arm was the highest. These results are consistent with those obtained earlier by our group for six-armed A5B type miktoarm polymers of the same composition but with different arm lengths, synthesized using the coupling-onto method [26].
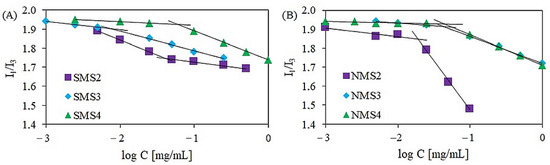
Figure 4.
The plot of intensity versus log C for six-arm (A) and nine-arm (B) miktopolymers.
3.3. Thermal Stability
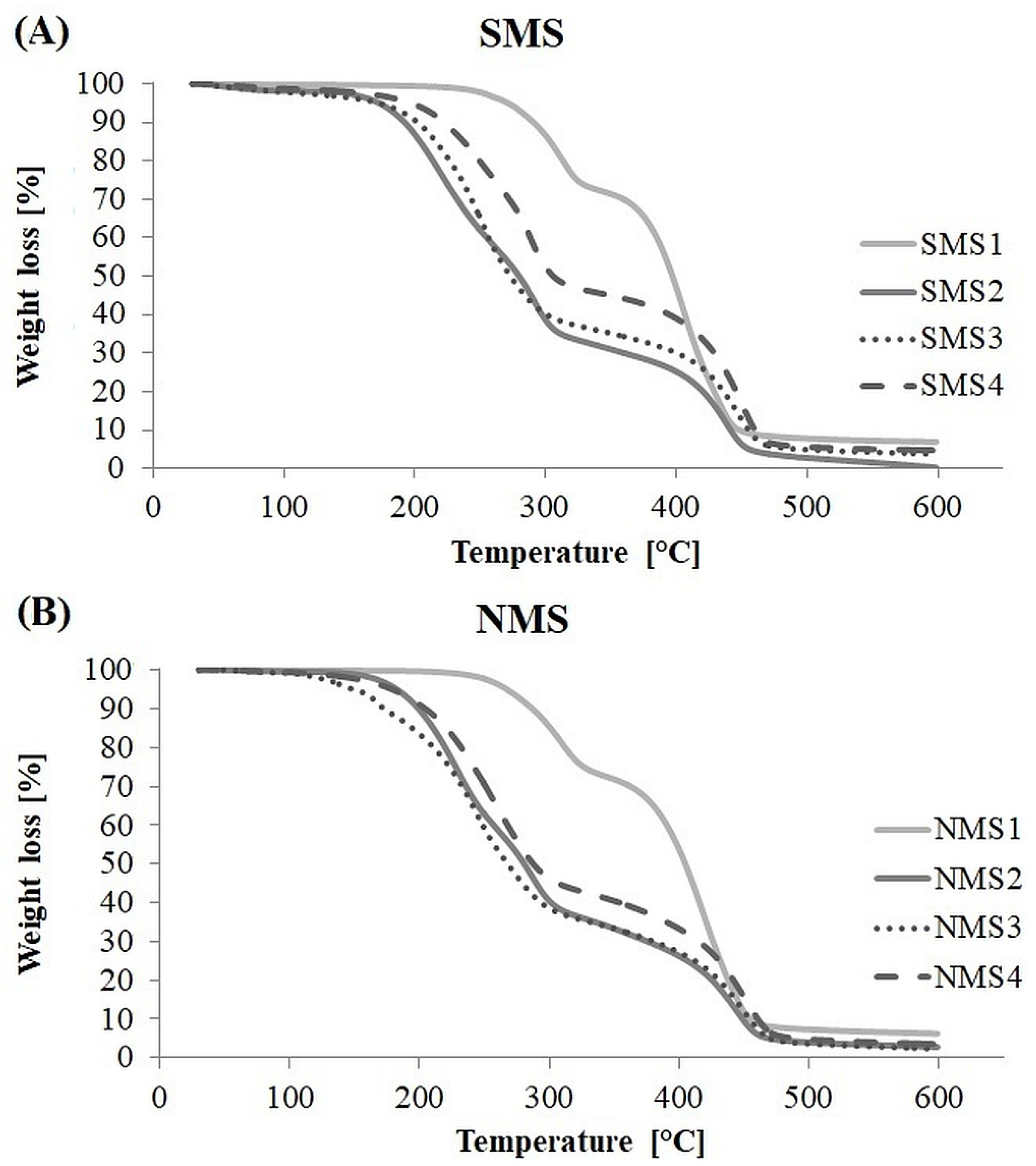
The data from the TGA analysis are shown in Figure 5. In most cases, the TGA curves showed three decomposition stages at temperature range 200–350 °C and 350–500 °C, attributed to the presence of PDMAEMA and polyester arm in the polymer structure (Figure S1). The first stage indicates thermal decomposition of PDMAEMA side groups, whereas in the second stage, destruction of the PDMAEMA main chain overlaps with the thermal decomposition of a polyester arm [32,33,34,35]. Miktoarm polymers with a PCL arm, that is, SMS1 and NMS1, showed considerable thermal resistance in comparison to all tested samples. SMS1 and NMS1 were stable to the same temperature equal 267 °C, and for both the first derivative peak temperature (Tp) for the first TGA slope was ca. 310 °C, and the second ca. 410–418 °C. Miktopolymers with a PLA arm, that is, SMS2 and NMS2, showed the most visible three decomposition stages with Tp1 equal to 223 °C (SMS2) or 232 °C (NMS2), Tp2 equal to 295 °C (SMS2) and 287 °C (NMS2), and Tp3 equal to 437 °C or 444 °C. In the case of miktopolymers with a PLGA and PLGCL arm in the structure, the decomposition of nine-arm miktopolymers samples started at temperature of ca. twenty degrees lower (NMS3: 149 °C and NMS4: 179 °C) than for six-arm analogs (SMS3:174 °C SMS4: 198 °C). Since NMS have shorter PDMAEMA arms than SMS, this indicates that the shorter the PDMAEMA arm, the faster weight loss begun. At the same time, the final sample weight loss occurred at slightly higher temperatures for nine-arm miktopolymers than for six-arm analogs with a PCL or PLA arm, indicating that miktopolymers with greater number of PDMAEMA arms are less prone to decomposition under temperature increase in an inert atmosphere. The resistance of the sample to the weight loss also depended on the composition of the polyester arm, and the decrease was as follows: PCL > PLGCL > PLGA > PLA.
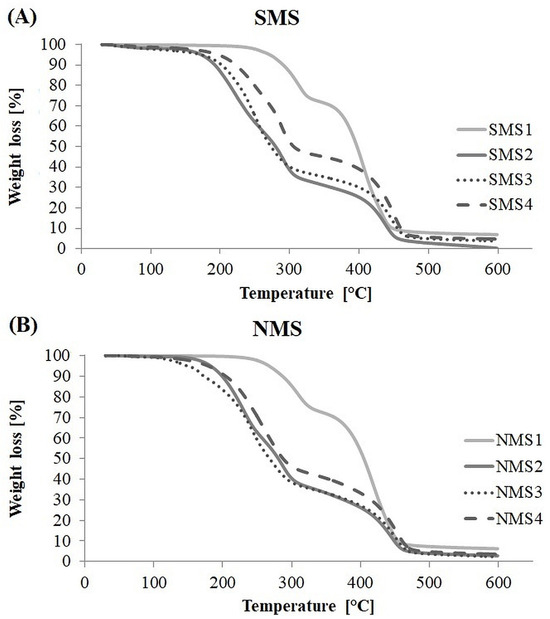
Figure 5.
TGA curves of six-arm (A) and nine-arm (B) miktopolymers.
3.4. Wettability and Hydrophilicity
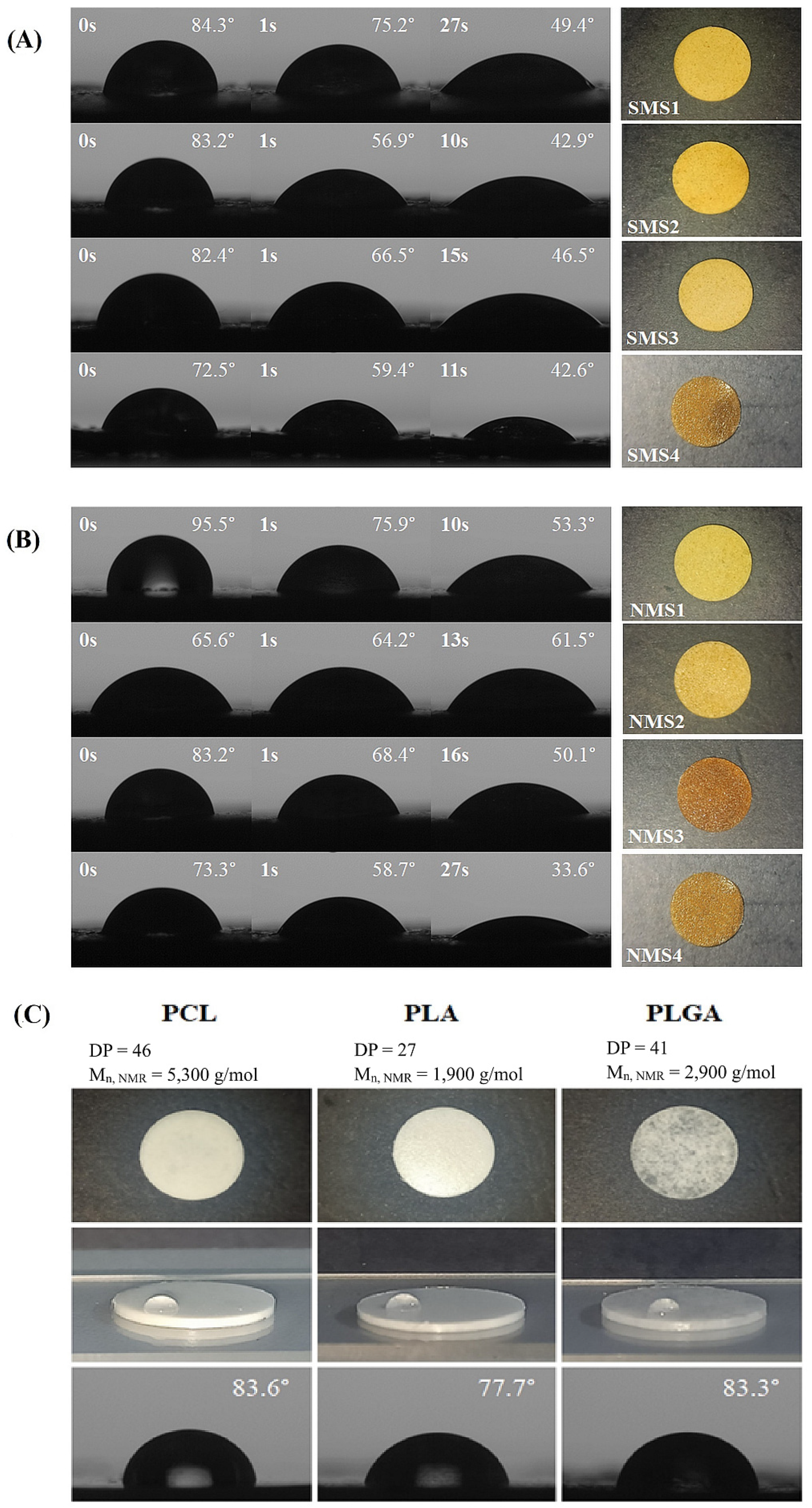
The difference in the hydrophilicity of miktopolymers, which impacts the hydrolytic degradation course, was evaluated by water contact angle (WCA) measurements. Additionally, WCA measurements on PCL, PLA and PLGA lozenges were performed, and the obtained contact angle values corresponded to literature data [36,37,38]. As can be seen in Figure 6, values of WCA obtained for miktoarm polymers decreased with the measurement time. This indicates that due to the increased affinity of PDMAEMA chains to the water, the dynamic reorganization of the macromolecules occurred at the interface between the sample surface and water. All tested samples eventually showed stable values of contact angle in the range 33.6° < WCA < 61.5°, indicating that despite the presence of a relatively long hydrophobic polyester arm, the sample in a solid state retained its hydrophilic character. The surface of the NMS2 lozenge appeared to be the most stable, with the angle decreasing by 4° compared to the samples of the other miktopolymers. Samples with PCL arms, despite having the highest molar fraction of DMAEMA, displayed the highest values of WCA. Although both miktopolymers had the same PCL chain length, the PDMAEMA arms of NMS1 were shorter than SMS1. In contrast, SMS4 and NMS4 with PLGCL arms with the lowest DPCL, DPLA and DPGA values, in comparison with polyester arms of other miktopolymers, showed the lowest WCA values. These results demonstrate that both the composition and the chain length can be used in order to tailor the surface hydrophylicity.
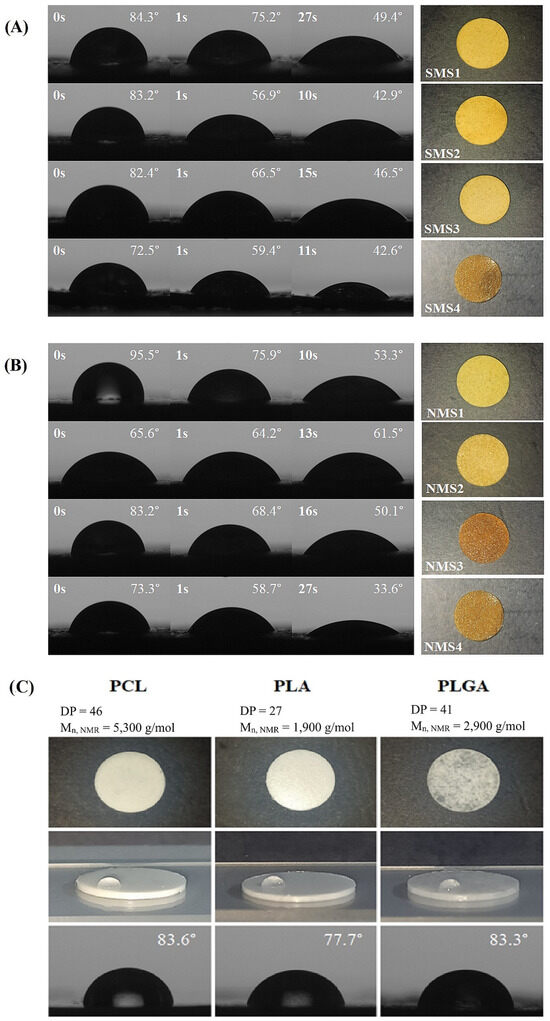
Figure 6.
WCA images and photos of six-arm (A) and nine-arm (B) miktopolymers as well as PCL, PLA, and PLGA (C) in the form of lozenges.
3.5. Hydrolytic Degradation
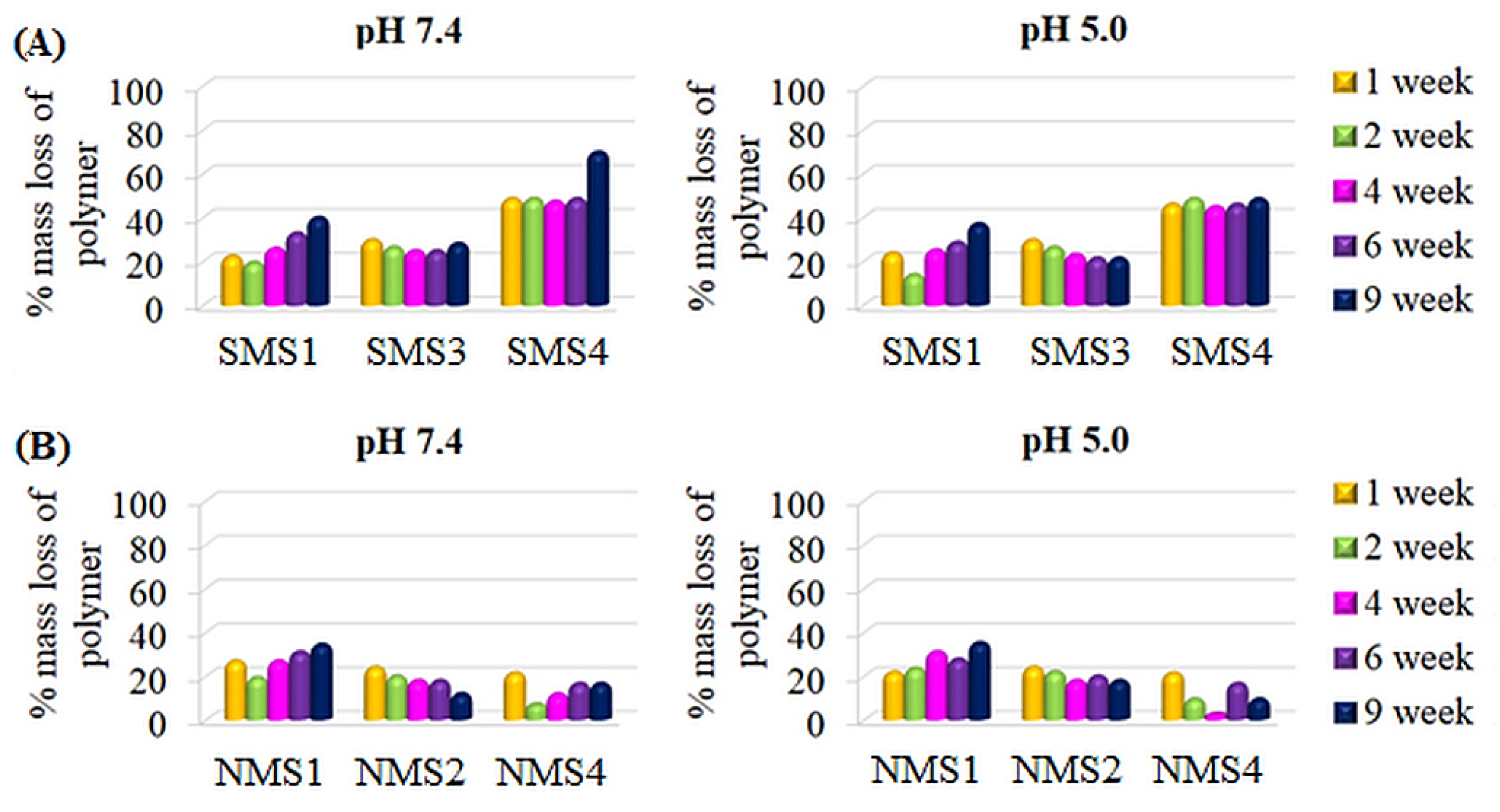
In the case of the A5B-type miktopolymers, the degradation rate decreased as follows: PDMAEMA5PLGCL (SMS4) > PDMAEMA5PCL (SMS1) > PDMAEMA5PLGA (SMS3) (Figure 7A). The comparison of SMS3 (FDMAEMA = 0.32; Mn, theo = 25,200 g/mol) with SMS4 (FDMAEMA = 0.41; Mn, theo = 24,000 g/mol) led us to a conclusion that the polymer with lower theoretical molecular weight and higher content of hydrophilic fraction degraded faster. Moreover, comparing SMS1 (FDMAEMA = 0.49; Mn, theo = 26,100 g/mol) with SMS3, miktopolymers with higher molecular weight and higher values of FDMAEMA degraded slower than SMS3 until 4 weeks. This indicates that at the beginning of the hydrolysis process, the water uptake and ester bond cleavage were faster in macromolecules with lower molecular weights. However, ultimately, macromolecules with the higher content of hydrophilic fraction reached higher values of percentage molar mass loss after 9 weeks.
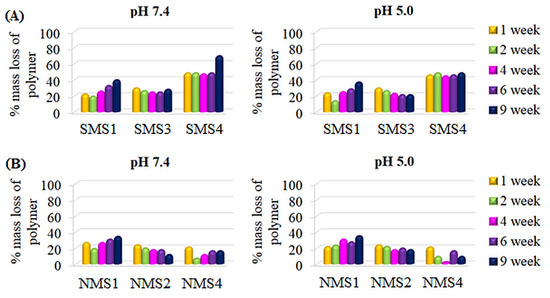
Figure 7.
Percentage Mn,SEC loss of A5B (A) and A8B (B) type miktopolymers.
Slightly different behavior was observed in the case of the nine-arm compounds. The degradation course of the NMS1 sample, containing a PCL arm with the highest hydrophilic content (FDMAEMA = 0.49, Mn, theo = 26,200 g/mol) but higher theoretical molecular weight than NMS4 (FDMAEMA = 0.41, Mn, theo = 24,200 g/mol), showed the fastest molecular weight reduction. Contrastingly, NMS4, with the PLGCL arm, showed the slowest molecular weight reduction (Figure 7B), which could be a result of the imperfect molecular structure of the macromolecule caused by star–star coupling during NMS4 synthesis manifested as a bimodal signal on the SEC chromatogram (Figure 2B). The formation of large macromolecules, which consist of several stars linked by the common PDMAEMA arms, could slow down the water diffusion inside the particle aggregates created by amphiphilic macromolecules in water and be followed by a longer time of polyester degradation entrapped inside the particle aggregate. Moreover, for SMS2 and NMS3, an initial increase in Mn, SEC value was observed, and then a slight decrease (Figure S2). However, the intensity of the RID signal corresponding to the polymeric sample gradually decreased with the degradation time. First, we concluded that this follows a gradual increase in PDMAEMA content in polymeric samples caused by polyester degradation and enhanced interactions of polymer with the column, or hydrolysis of (2-hydroxyethyl)dimethylamine groups leading to the formation of methacrylic acid repeating units. However, 1H NMR analysis excluded the hydrolysis of amine groups from polymethacrylate arms at pH 7.4, because the ratio of the intensity of protons within methyl groups bonded to amine, to the intensity of signals corresponding to the protons in methyl groups belonging to polymethacrylate backbone was not changed. It was, however, observed that signals of all protons shifted to higher values of δ, which can be seen in Figure 8. This could be caused by the small amount of salts that were still present in samples after degradation. In addition, it was observed that despite the increase in molecular weights determined by SEC analysis, the proton signals in the polyester chain in 1H NMR spectra were decreasing, indicating that the degradation of the polyester arm progressed over time.
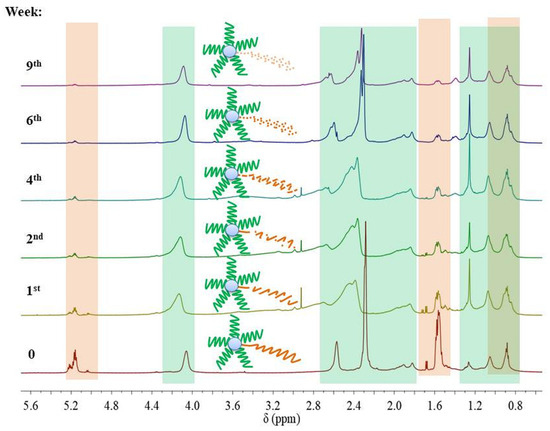
Figure 8.
1H NMR spectra (600 MHz, CDCl3) for SMS2 and its samples after degradation in PBS at pH 7.4, where the green area indicates signals from PDMAEMA arms and the orange area indicates signals from the polyester part. Chemical shifts and integration values of signals assigned to SMS2 are presented in Table S3.
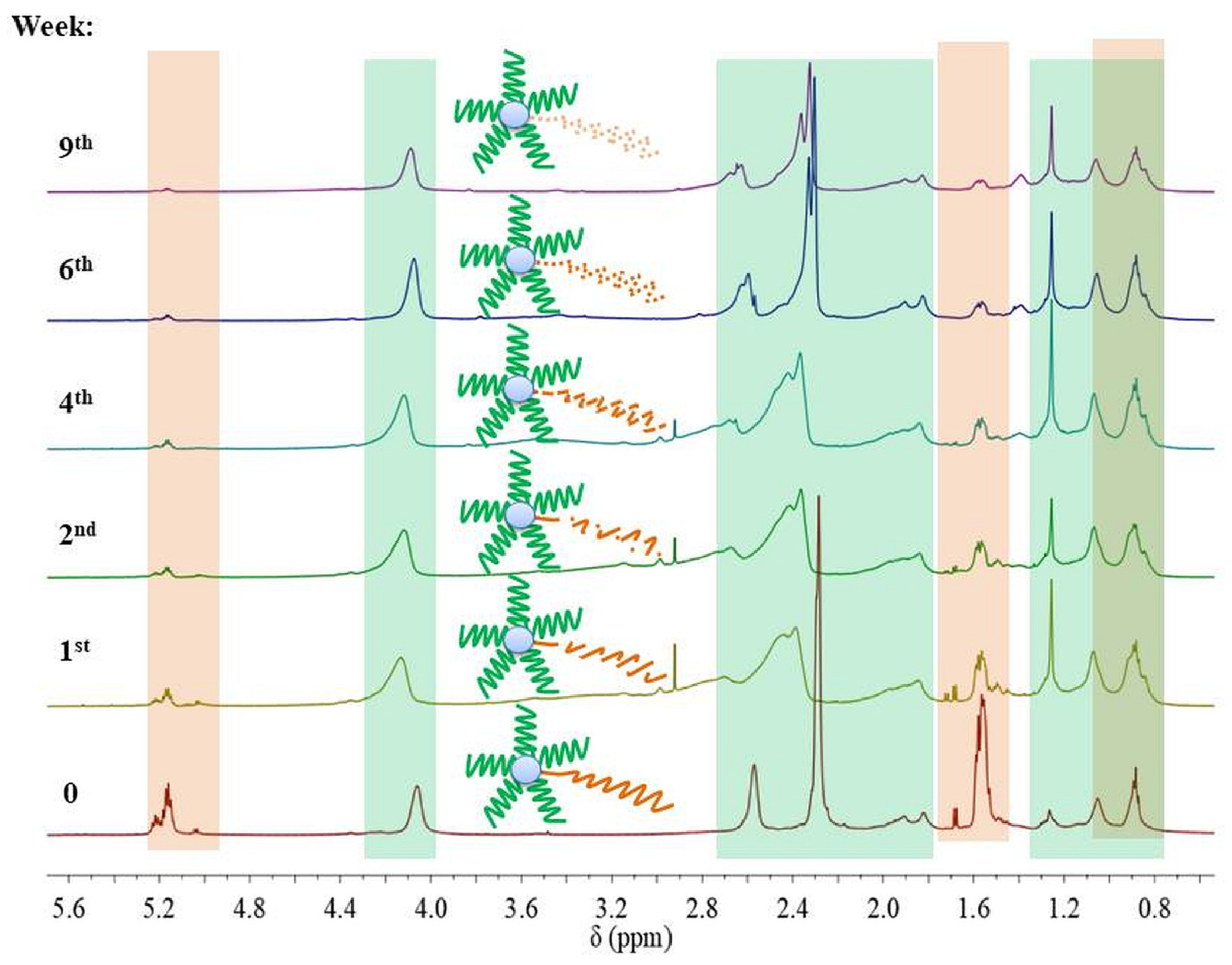
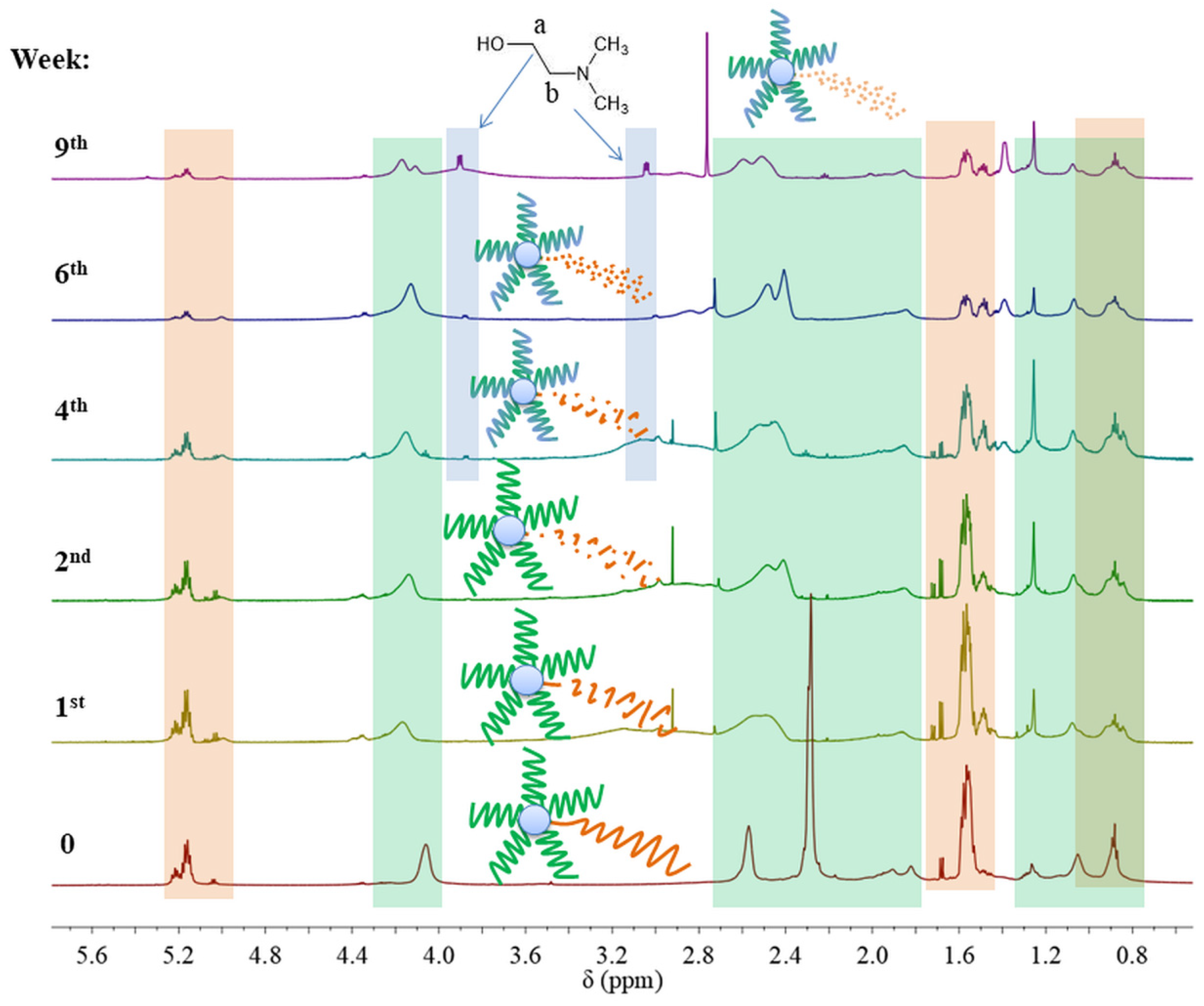
However, 1H NMR spectra of samples SMS2-SMS4 and NMS2-NMS4 that were subjected to a pH 5.0 revealed that the ratio of signal intensities of protons from methyl groups in the amine to intensities of protons from methyl groups in the main chain changed. This could indicate that the hydrolysis of DMAEMA occurred only in miktopolymers with LA repeating units in the polyester arm. In addition, the signals of protons from groups linked to the nitrogen atom began to separate. Moreover, at about the fourth week of degradation, two new triplets appeared at 3.08 and 3.90 ppm, indicating the presence of N, N-dimethylethanolamine (DMAE) formed during the hydrolysis of DMAEMA-pending groups (Figure 9). The proton signals from the PLA arm decreased gradually in SMS2 and NMS2 samples, whereas signals from PLGCL (SMS4 and NMS4) and from PLGA (SMS3 and NMS3) disappeared completely after one or two weeks, respectively (Figure S3–S9).
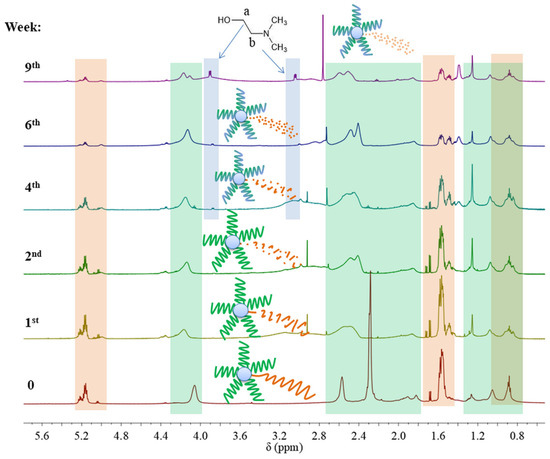
Figure 9.
1H NMR spectra (600 MHz, CDCl3) for SMS2 and its samples after degradation in PBS at pH 5.0 where the green area indicates signals from PDMAEMA arms and the orange area indicates signals from the polyester part. Chemical shifts and integration values of signals assigned to SMS2 are presented in Table S3.
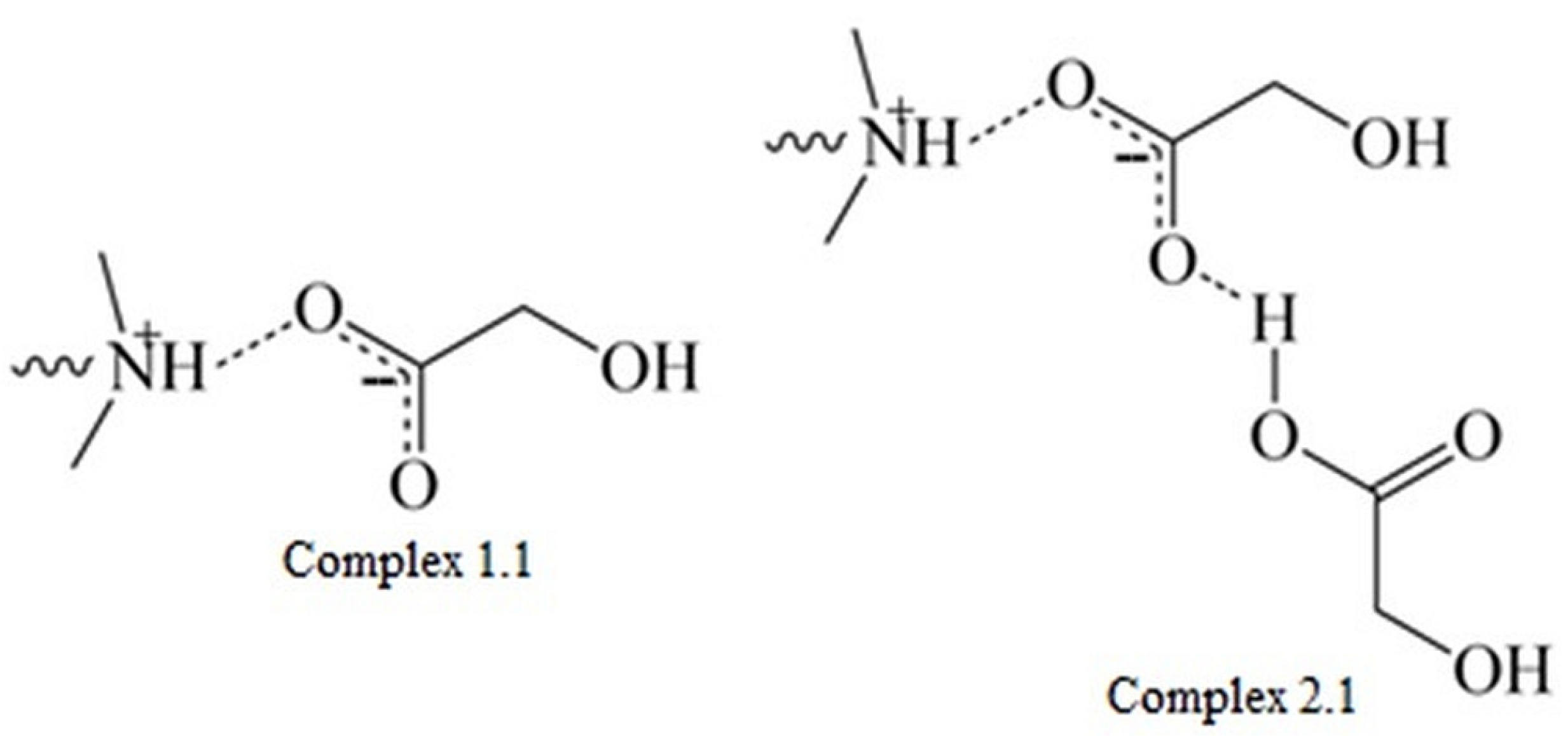
In the case of miktoarm polymers with a PLGA or PLGCL arm, a broad signal ranging from 4.0 to 3.5 ppm was observed and then shifted towards higher ppm values. We assume that the shift of this broad signal was the result of ester formation due to reaction between glycolic acid and DMAE. Due to the high susceptibility and speed of degradation of chain fragments composed of GA repeating units, a large fraction of glycolic acid was formed in the first two to four weeks, which then could react with DMAE formed at a later stage—the signal at 3.5 ppm disappeared and appeared at around 4.0 ppm. The signal in the range from 4.7 to 4.0 ppm belongs to PLA, PLGA, or PLGCL oligoesters. The signal shift toward the upfield can be attributed to the degradation of these oligomers and the formation of corresponding acids. Moreover, taking into account the values of the dissociation constants for hydroxyl acids formed during hydrolysis of esters, it can be concluded that glycolic acid (Ka = 1.48 × 10−4) and lactic acid (Ka = 1.41 × 10−4) are stronger acids than caproic acid (Ka = 1.4 × 10−5). This indicates the interaction of the acids formed during the degradation with the tertiary amine in the polymethacrylate side chain, forming complexes (1.1) and (2.1) (Figure 10).
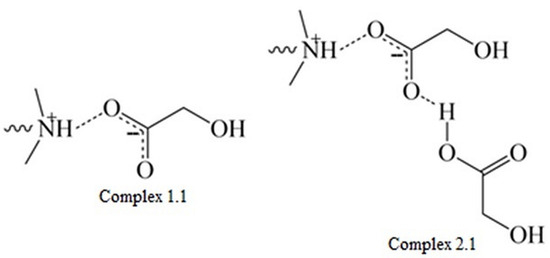
Figure 10.
The interaction of the acids formed during degradation with a tertiary amine in the polymethacrylate chain.
Complex (1.1) is formed by the interaction of one acid molecule with an amine, and complex (2.1) results from the interaction of another acid molecule with the already-formed complex (1.1). The formation of these complexes is controlled by various mechanisms. Complexation of (1.1) is controlled by the acid–base ion evaporation reaction and is highly dependent on the pKa. The complexation of (2.1), on the other hand, occurs through the formation of hydrogen bonds, which should not be directly dependent on pKa [39,40]. Thus, the observed increase in molecular weight values determined by SEC, despite the hydrolysis of the polyester chains, is most likely due to interactions between the tertiary amine groups present in the polymethacrylate arms and the hydroxy acids formed during hydrolysis.
4. Conclusions
The use of a one-pot synthesis approach allowed the preparation of a wide range of amphiphilic miktoarm polymers that respond to temperature stimuli in water and PBS within the temperature range that excludes these polymers from drug delivery system applications. The CAC values of miktoarm polymers depend on the content of hydrophobic segments and are in the range of 0.008–0.060 mg/mL. TGA analysis revealed that the number of arms and their composition affected the values of temperatures at which the weight loss occurred and the most stable miktoarm polymers are those with a PCL arm. Miktoarm polymers in a solid state demonstrated dynamic surface changes on exposure to water contact. However, the final stable values of WCA indicate the hydrophilic character of all samples and were the lowest for miktoarm polymers with PLGCL arms. The hydrolytic degradation results indicate that the presence of arms with amine functional groups in the structure of miktoarm polymers facilitates the process of polyester degradation and increases the spectrum of potential applications of the obtained polymers. The obtained amphiphilic miktopolymers, due to the presence of biodegradable polyester arm and thermo-/pH-sensitive polymethacrylate arms, can be used in medicine, agriculture, or cosmetology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma17112684/s1, Table S1. The compositions of the reaction mixtures for the performed syntheses of A5B type miktoarm star-shaped polymers. Table S2. The compositions of the reaction mixtures for the performed syntheses of A8B type miktoarm star-shaped polymers. Figure S1. The thermogravimetric analysis (TGA) and derivative thermogravimetric (DTG) curves of the miktoarm polymers in a nitrogen atmosphere. Figure S2. Plots of Mn,SEC versus the degradation time for SMS2 and NMS3. Figure S3. 1H NMR (600 MHz, CDCl3) spectra of SMS1 with assigned chemical shifts and integration values of signals at pH 7.4 (A), and pH 5.0 (B). Figure S4. 1H NMR (600 MHz, CDCl3) spectra of NMS1 with assigned chemical shifts and integration values of signals at pH 7.4 (A), and pH 5.0 (B). Figure S5. 1H NMR (600 MHz, CDCl3) spectra of NMS2 with assigned chemical shifts and integration values of signals at pH 7.4 (A), and pH 5.0 (B). Figure S6. 1H NMR (600 MHz, CDCl3) spectra of SMS3 with assigned chemical shifts and integration values of signals at pH 7.4 (A), and pH 5.0 (B). Figure S7. 1H NMR (600 MHz, CDCl3) spectra of NMS3 with assigned chemical shifts and integration values of signals at pH 7.4 (A), and pH 5.0 (B). Figure S8. 1H NMR (600 MHz, CDCl3) spectra of SMS4 with assigned chemical shifts and integration values of signals at pH 7.4 (A), and pH 5.0 (B). Figure S9. 1H NMR (600 MHz, CDCl3) spectra of NMS4 with assigned chemical shifts and integration values of signals at pH 7.4 (A), and pH 5.0 (B). Table S3. Chemical shifts and integration values of signals assigned to SMS2 at pH 7.4, and pH 5.0.
Author Contributions
A.M. Conceptualization; M.K., T.F., P.D. and P.L. Data curation; A.M. and M.K. Formal analysis; A.M. Funding acquisition; M.K., T.F., P.D., A.M. and P.L. Investigation; A.M. and M.K. Methodology; A.M. Project administration; A.M. Supervision; A.M., M.K., T.F. and P.D. Visualization; A.M, M.K., T.F. and P.D. Writing—original draft; A.M., M.K. and D.N. Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre (POLAND), grant number 2016/23/D/ST5/01312, and 1st degree pro-quality Silesian University of Technology Rector’s grant number 04/040/RGJ24/0274.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The author declares no conflict of interest.
References
- Robinson, R. LXIII.-A synthesis of tropinone. J. Chem. Soc. Trans. 1917, 111, 762–768. [Google Scholar] [CrossRef]
- Lundberg, P.; Hawker, C.J.; Hult, A.; Malkoch, M. Click Assisted One-Pot Multi-Step Reactions in Polymer Science: Accelerated Synthetic Protocols. Macromol. Rapid Commun. 2008, 29, 998–1015. [Google Scholar] [CrossRef]
- Medley, J.W.; Movassaghi, M. Robinson’s landmark synthesis of tropinone. Chem. Commun. 2013, 49, 10775–10777. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Navas, J.; Sabater, M.J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118, 1410–1459. [Google Scholar] [CrossRef]
- Ugi, I. Recent progress in the chemistry of multicomponent reactions. Pure Appl. Chem. 2001, 73, 187–191. [Google Scholar] [CrossRef]
- Heck, S.; Dömling, A. A versatile multi-component one-pot thiazole synthesis. Synlett 2000, 2000, 424–426. [Google Scholar] [CrossRef]
- Tietze, L.F.; Rackelmann, N. Domino reactions in the synthesis of heterocyclic natural products and analogs. Pure Appl. Chem. 2004, 76, 1967–1983. [Google Scholar] [CrossRef]
- Konieczny, M.T.; Konieczny, W.; Wolniewicz, S.; Wierzba, K.; Suda, Y. New domino reaction. One pot synthesis of 4, 7-dihydroxythioaurone derivatives from benzaldehydes and 4-acetyl-2-oxo-benz [1, 3] oxathiole. Tetrahedron 2005, 61, 8648–8655. [Google Scholar] [CrossRef]
- Evans, P.A.; Robinson, J.E. Regio- and Diastereoselective Tandem Rhodium-Catalyzed Allylic Alkylation/Pauson-Khand Annulation Reactions. J. Am. Chem. Soc. 2001, 123, 4609–4610. [Google Scholar] [CrossRef]
- Gao, C.; Zheng, X. Facile synthesis and self-assembly of multihetero-arm hyperbranched polymer brushes. Soft Matter 2009, 5, 4788–4796. [Google Scholar] [CrossRef]
- Shahrokhinia, A.; Biswas, P.; Reuther, J.F. Orthogonal synthesis and modification of polymer materials. J. Polym. Sci. 2021, 59, 1748–1786. [Google Scholar] [CrossRef]
- Xia, X.; Gao, T.; Li, F.; Suzuki, R.; Isono, T.; Satoh, T. Multidimensional Control of Repeating Unit/Sequence/Topology for One-Step Synthesis of Block Polymers from Monomer Mixtures. J. Am. Chem. Soc. 2022, 144, 17905–17915. [Google Scholar] [CrossRef] [PubMed]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E.; Copolymers, D.R. New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Rizzardo, E.; Chiefari, J.; Mayadunne, R.; Moad, G.; Thang, S. Tailored Polymer Architectures by Reversible Addition-Fragmentation Chain Transfer. Macromol. Symp. 2001, 174, 209–212. [Google Scholar] [CrossRef]
- Mespouille, L.; Vachaudez, M.; Suriano, F.; Gerbaux, P.; Flammang, R.; Dubois, P.; Coulembier, O.; Dege, P. One-Pot Synthesis of Well-Defined Amphiphilic and Adaptative Block Copolymers via Versatile Combination of “Click” Chemistry and ATRP. Macromol. Rapid Commun. 2007, 28, 2151–2158. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, Y.; Lang, M. Synthesis of star-shaped PCL-based copolymers via one-pot ATRP and their self-assembly behavior in aqueous solution. Macromol. Res. 2012, 20, 597–604. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Liu, S. One-Pot Synthesis of ABC Miktoarm Star Terpolymers by Coupling ATRP, ROP, and Click Chemistry Techniques. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3066–3077. [Google Scholar]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.G.; Mays, J.W. Block copolymers: Synthesis, self-assembly, and applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Davis, K.; Matyjaszewski, K. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany; Springer: New York, NY, USA, 2002; Volume 159. [Google Scholar]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Blankenship, J.R.; Levi, A.E.; Fu, Q.; Hudson, Z.M.; Bates, C.M. Miktoarm Star Polymers: Synthesis and Applications. Chem. Mater. 2022, 34, 6188–6209. [Google Scholar] [CrossRef]
- Kupczak, M.; Mielańczyk, A.; Neugebauer, D. The Influence of Polymer Composition on the Hydrolytic and Enzymatic Degradation of Polyesters and Their Block Copolymers with PDMAEMA. Materials 2021, 14, 3636. [Google Scholar] [CrossRef] [PubMed]
- Kupczak, M.; Mielańczyk, A.; Neugebauer, D. PDMAEMA/polyester miktopolymers: Synthesis via in-out approach, physicochemical characterization and enzymatic degradation. Materials 2021, 14, 1277. [Google Scholar] [CrossRef] [PubMed]
- Mielańczyk, A.; Kupczak, M.; Klymenko, O.; Mielańczyk, Ł.; Arabasz, S.; Madej, K.; Neugebauer, D. The structure-self-assembly relationship in PDMAEMA/polyester miktoarm stars. Polym. Chem. 2022, 13, 4763–4775. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Kupczak, M.; Burek, M.; Mielańczyk, Ł.; Klymenko, O.; Wandzik, I.; Neugebauer, D. Functional (mikto)stars and star-comb copolymers from D-gluconolactone derivative: An efficient route for tuning the architecture and responsiveness to stimuli. Polymer 2018, 146, 331–343. [Google Scholar] [CrossRef]
- Goc, K.; Mielańczyk, A.; Kupczak, M.; Milewska, M.; Babilas, D.; Milewski, A.; Neugebauer, D. Towards green synthesis of stimuli-responsive star-shaped copolymers with lactobionic or D-gluconic amide in the core. Manuscr. Prep. 2023. (under peer review). [Google Scholar]
- Van Ravensteijn, B.G.P.; Bou Zerdan, R.; Helgeson, M.E.; Hawker, C.J. Minimizing Star-Star Coupling in Cu(0)-Mediated Controlled Radical Polymerizations. Macromolecules 2019, 52, 601–609. [Google Scholar] [CrossRef]
- Netopilík, M.; Podzimek, S. Retention Mechanism of Branched Macromolecules in Size Exclusion Chromatography. ACS Omega 2020, 5, 14254–14260. [Google Scholar] [CrossRef]
- Kunz, W.; Henle, J.; Ninham, B.W. ‘Zur Lehre von der Wirkung der Salze’ (about the science of the effect of salts): Franz Hofmeister’ s historical papers. Curr. Opin. Colloid Interface Sci. 2004, 9, 19–37. [Google Scholar] [CrossRef]
- Stawski, D.; Nowak, A. Thermal properties of poly(N,N-dimethylaminoethyl methacrylate). PLoS ONE 2019, 14, e0217441. [Google Scholar] [CrossRef]
- Persenaire, O.; Alexandre, M.; Degée, P.; Dubois, P. Mechanisms and kinetics of thermal degradation of poly(ε-caprolactone). Biomacromolecules 2001, 2, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Feng, L.; Bian, X.; Li, G.; Chen, X. Evaluation of PLA content in PLA/PBAT blends using TGA. Polym. Test. 2020, 81, 106211. [Google Scholar] [CrossRef]
- Palacios, J.; Albano, C.; González, G.; Castillo, R.V.; Karam, A.; Covis, M. Characterization and thermal degradation of poly(d,l-lactide-co-glycolide) composites with nanofillers. Polym. Eng. Sci. 2013, 53, 1414–1429. [Google Scholar] [CrossRef]
- Ghanem, A.F.; Yassin, M.A.; Cosquer, R.; Gouanvé, F.; Espuche, E.; Abdel Rehim, M.H. Polycaprolactone composite films infused with hyperbranched polyester/reduced graphene oxide: Influence on biodegradability, gas/water transport and antimicrobial properties for sustainable packaging. RSC Adv. 2024, 14, 5740–5753. [Google Scholar] [CrossRef] [PubMed]
- Laput, O.; Vasenina, I.; Salvadori, M.C.; Savkin, K.; Zuza, D.; Kurzina, I. Low-temperature plasma treatment of polylactic acid and PLA/HA composite material. J. Mater. Sci. 2019, 54, 11726–11738. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Luo, R.; Chen, S.; Li, X.; Yuan, S.; Wang, J.; Huang, N. In vitro hemocompatibility and cytocompatibility of dexamethasone-eluting PLGA stent coatings. Appl. Surf. Sci. 2015, 328, 154–162. [Google Scholar] [CrossRef]
- Tamadat, J.A.; King, C.J. Extraction of carboxylic acids with amine extractants. 2. Chemical interactions and interpretation of data. Ind. Eng. Chem. Res 1990, 29, 1327–1333. [Google Scholar] [CrossRef]
- Procházka, J.; Heyberger, A.; Bízek, V.; Koušová, M.; Volaufová, E. Amine Extraction of Hydroxycarboxylic Acids. 2. Comparison of Equilibria for Lactic, Malic, and Citric Acids. Ind. Eng. Chem. Res. 1994, 33, 1565–1573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).