Influence of the Type of Soft Segment on the Selected Properties of Polyurethane Materials for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
2.3. Measurements Methods
3. Results and Discussion
3.1. Physicochemical Properties
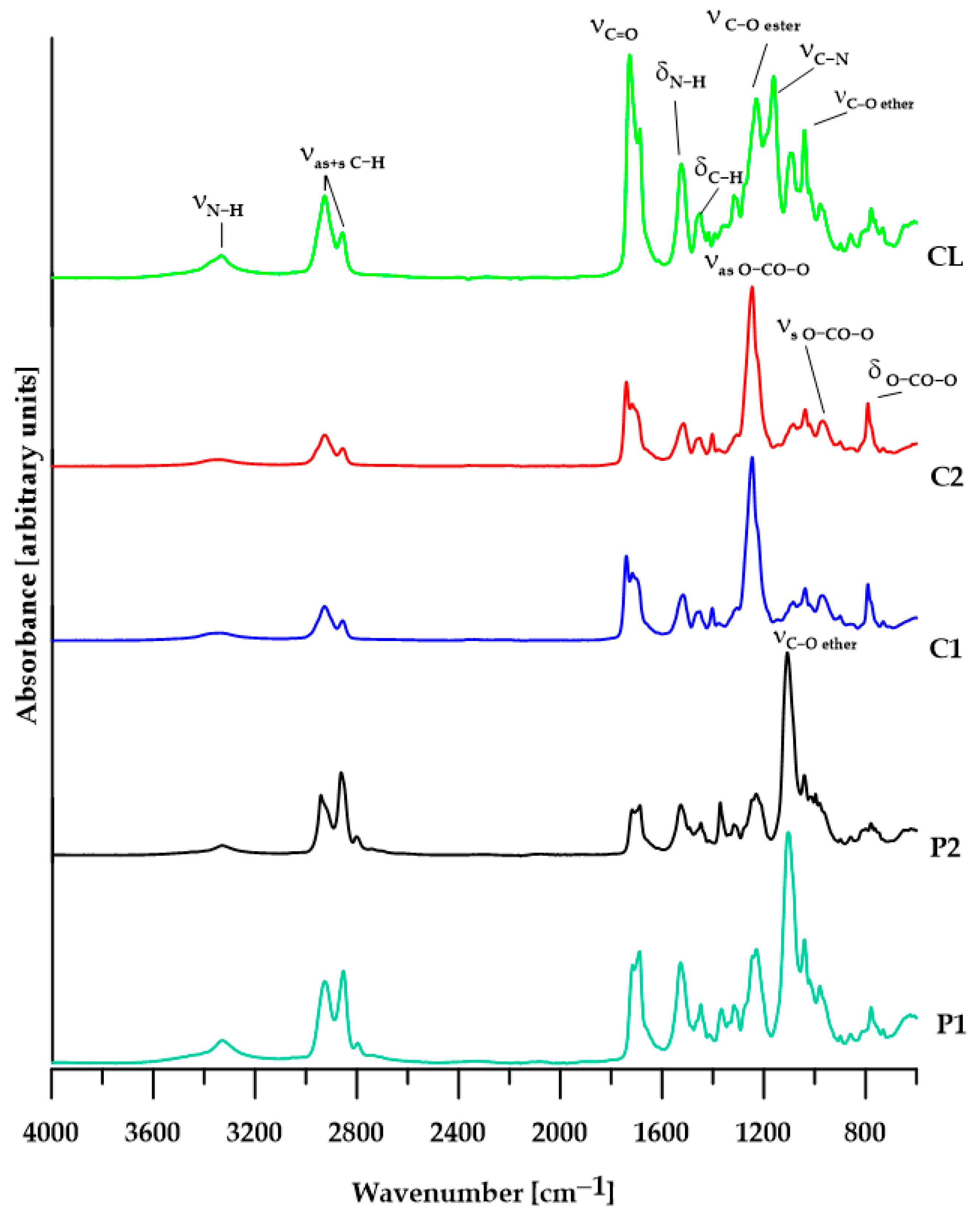
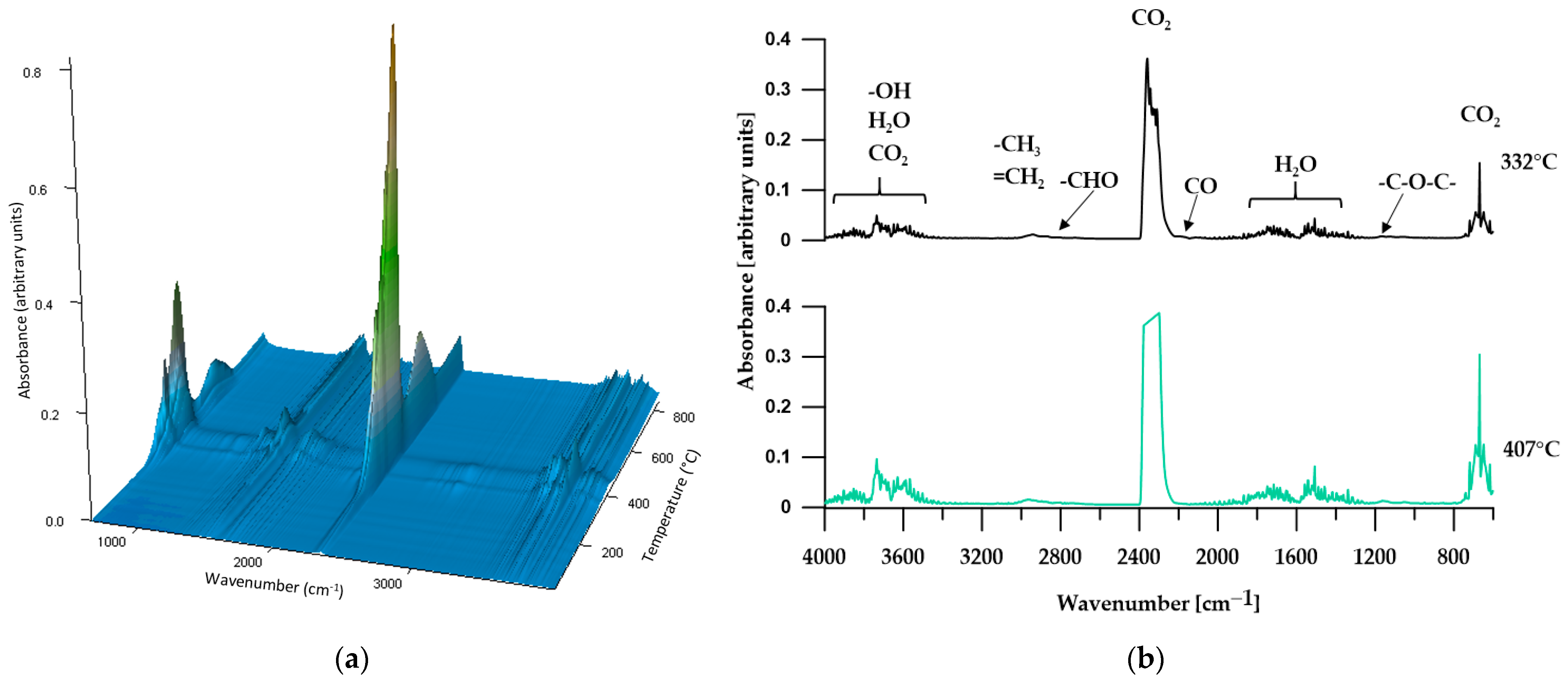
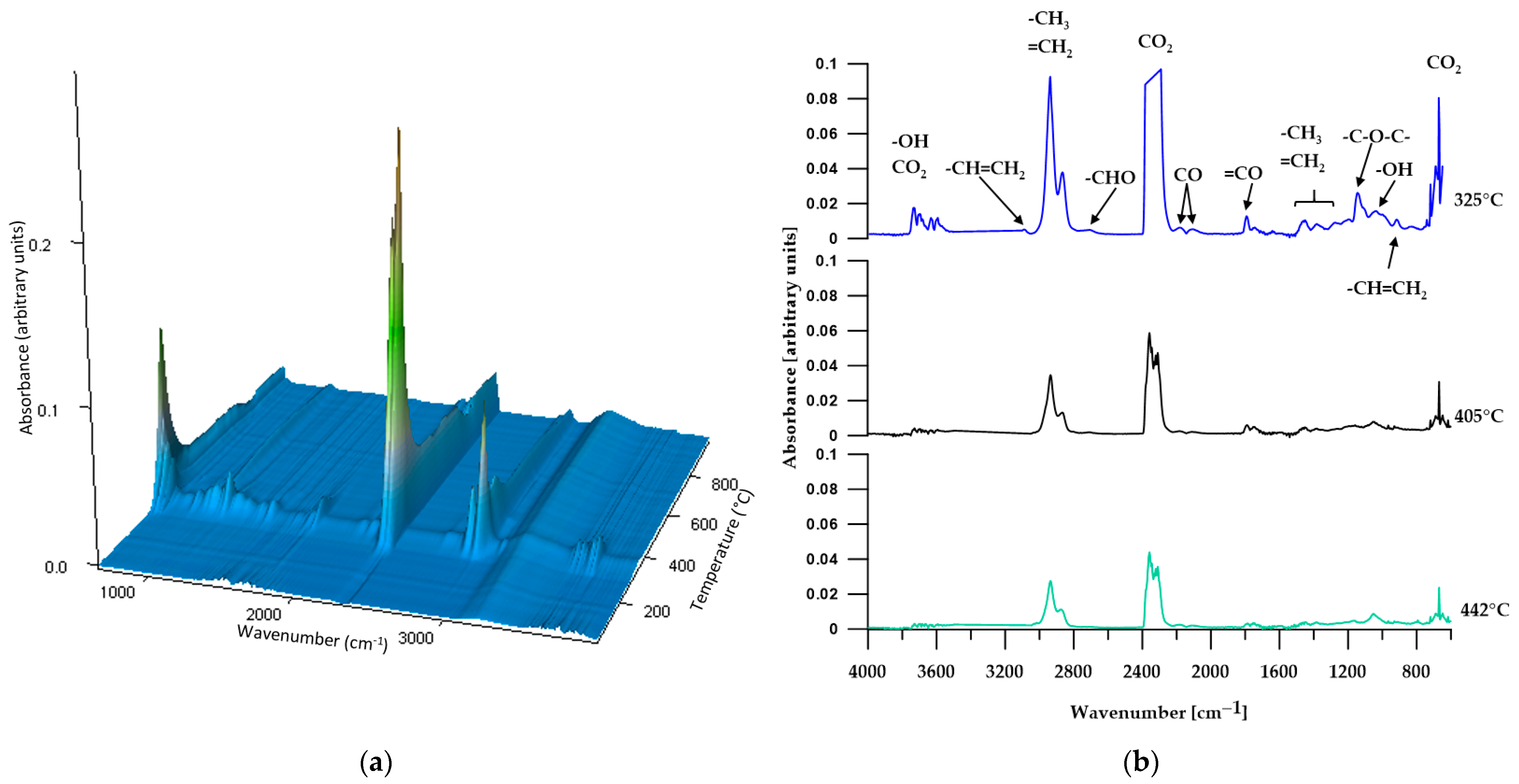
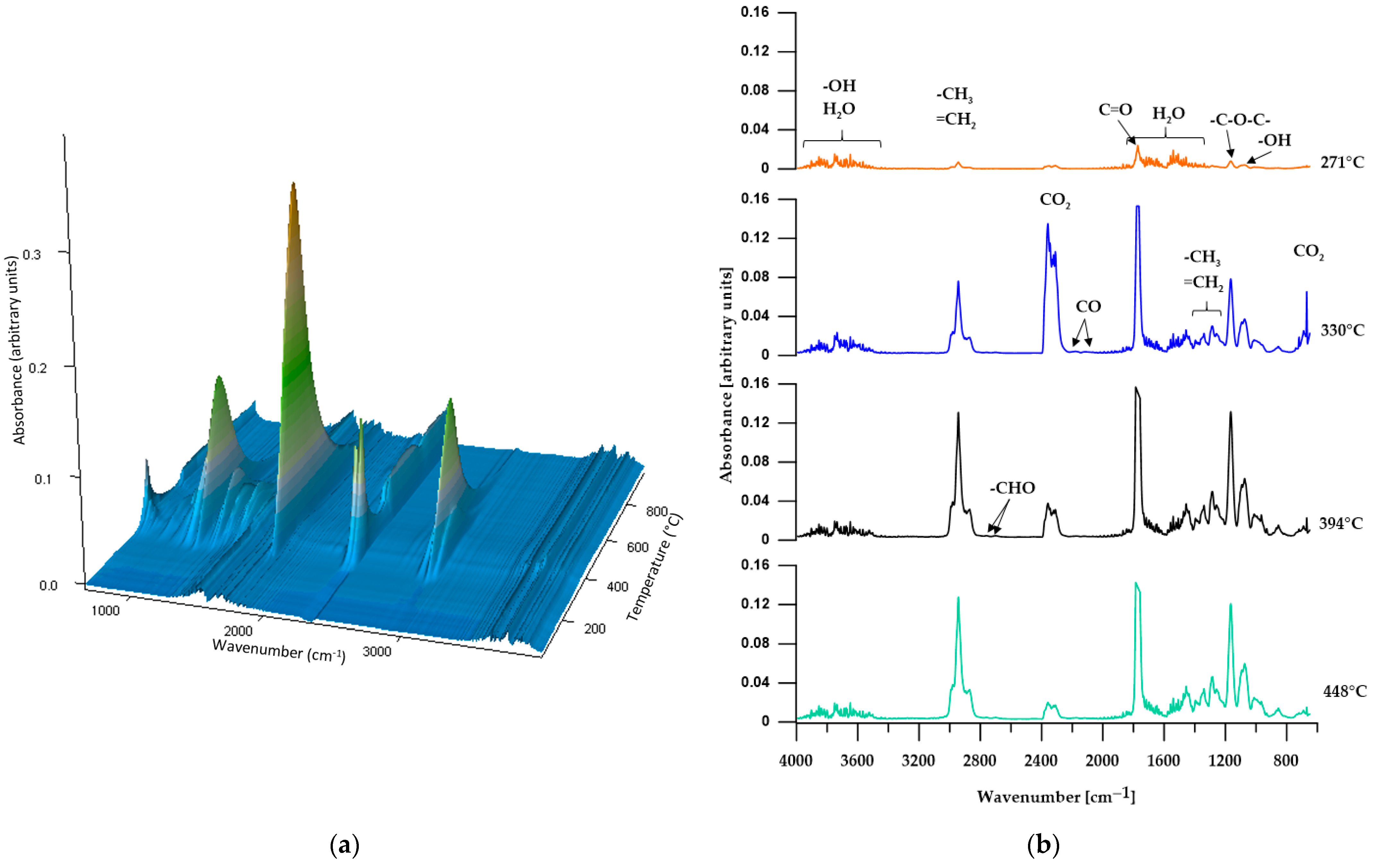
3.2. ATR/FTIR
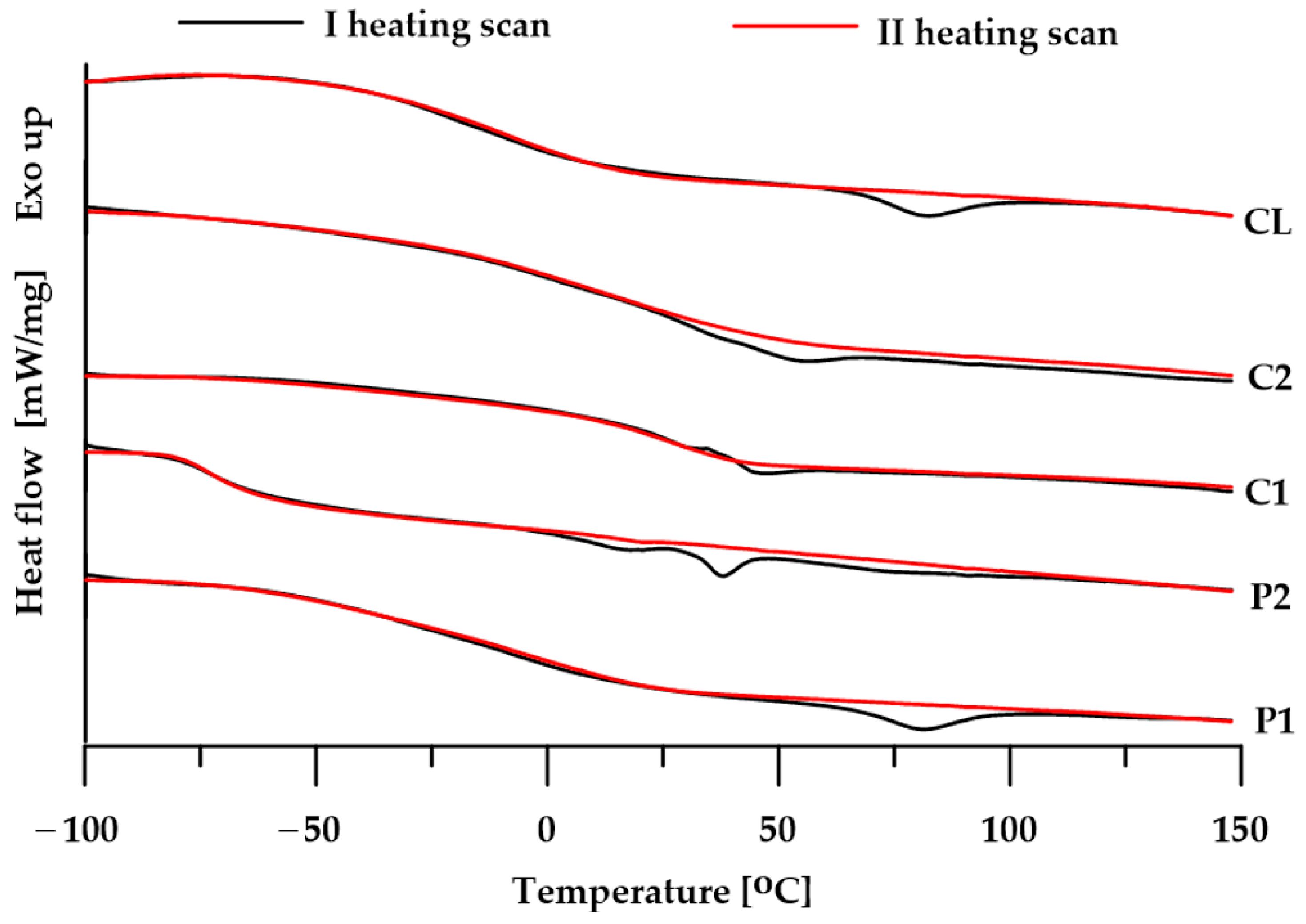
3.3. DSC
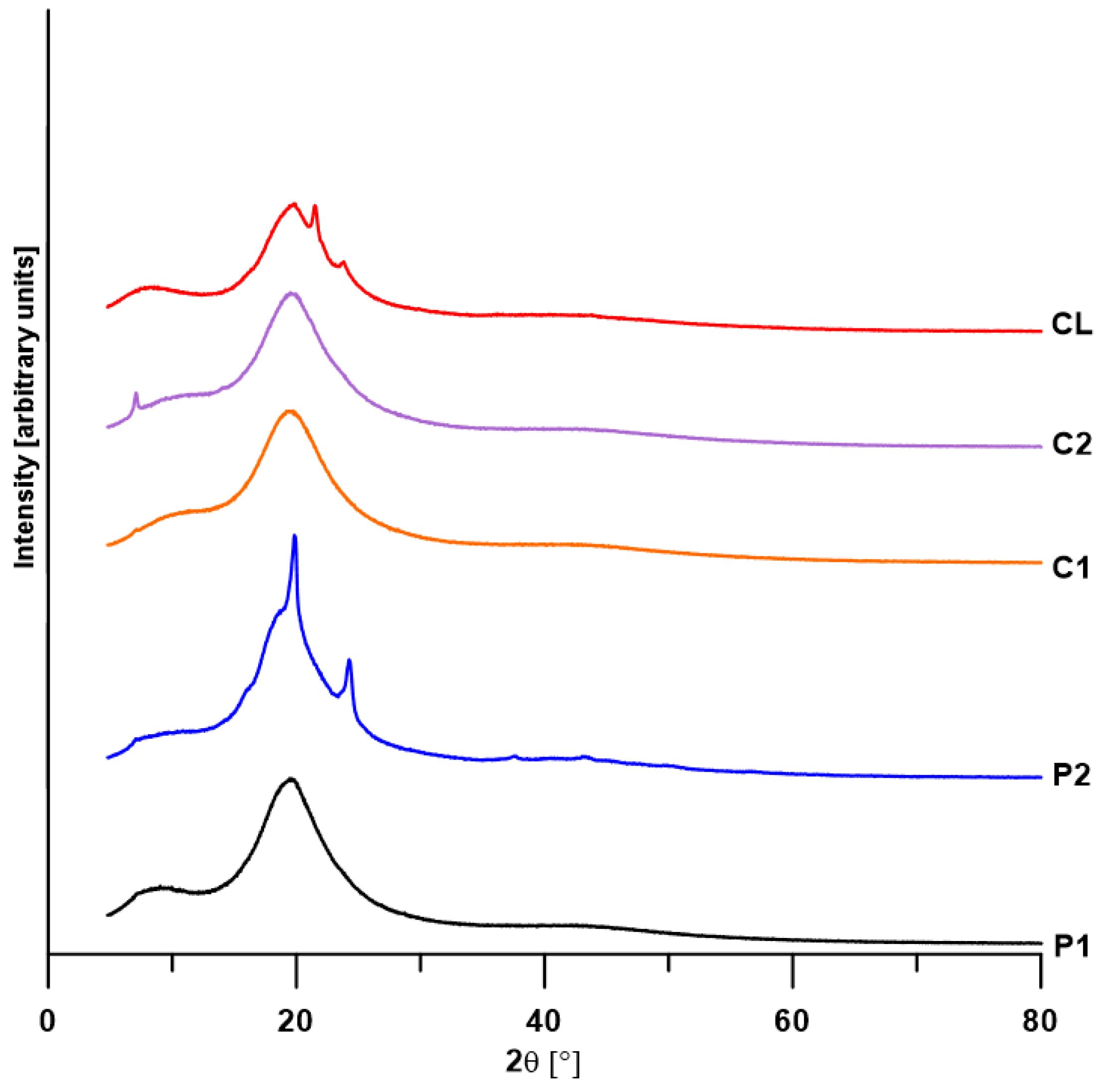
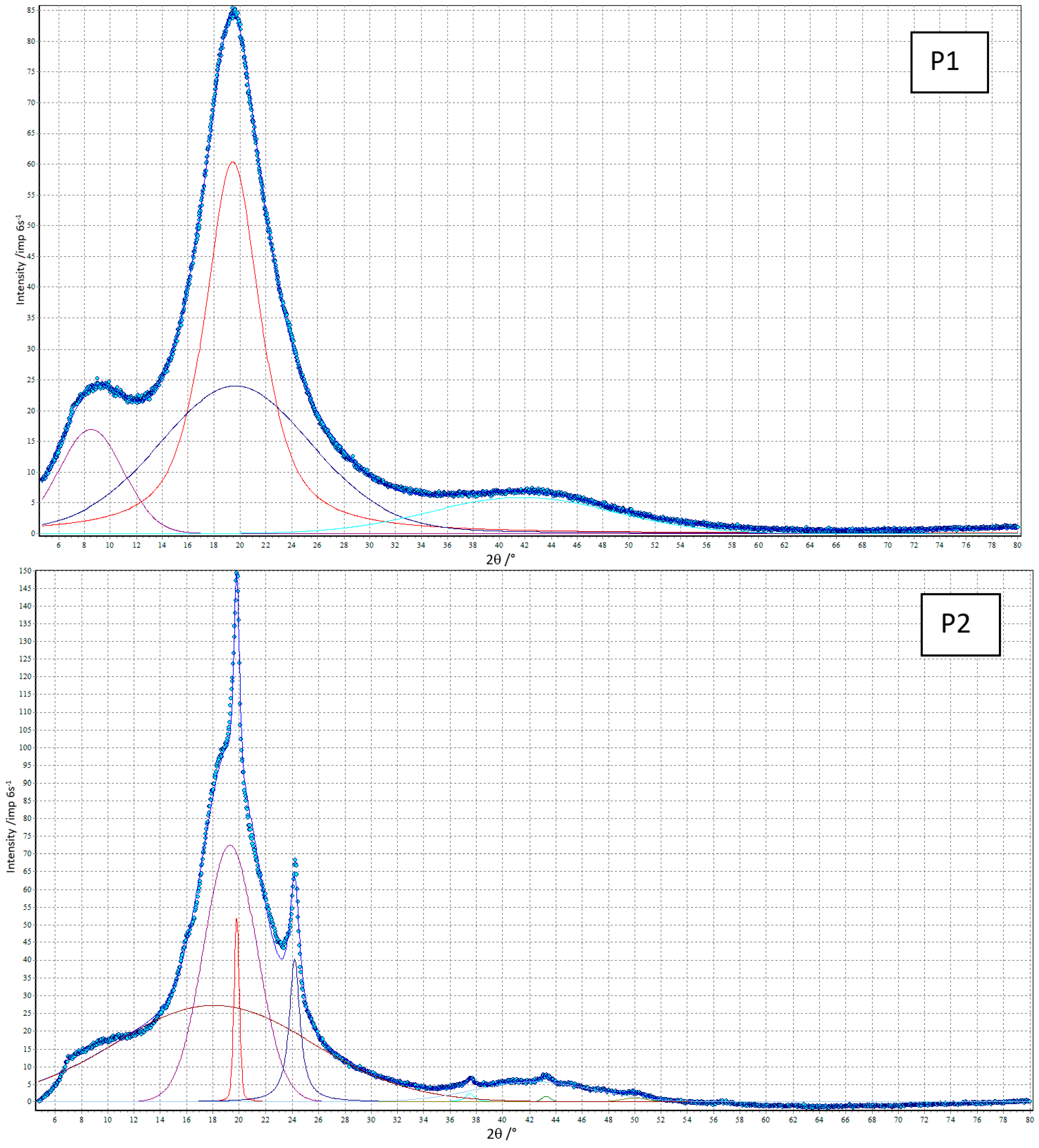
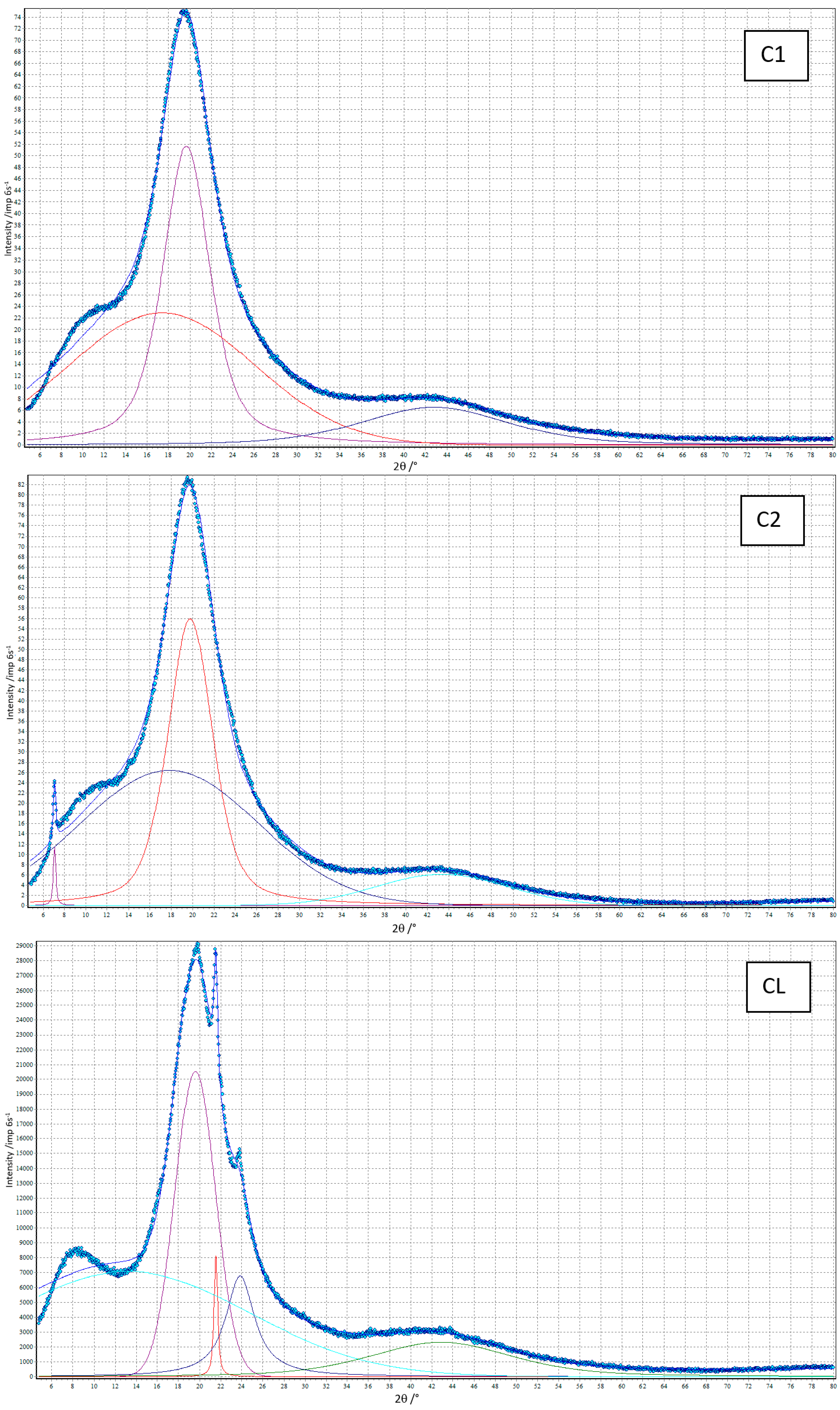
3.4. XRD
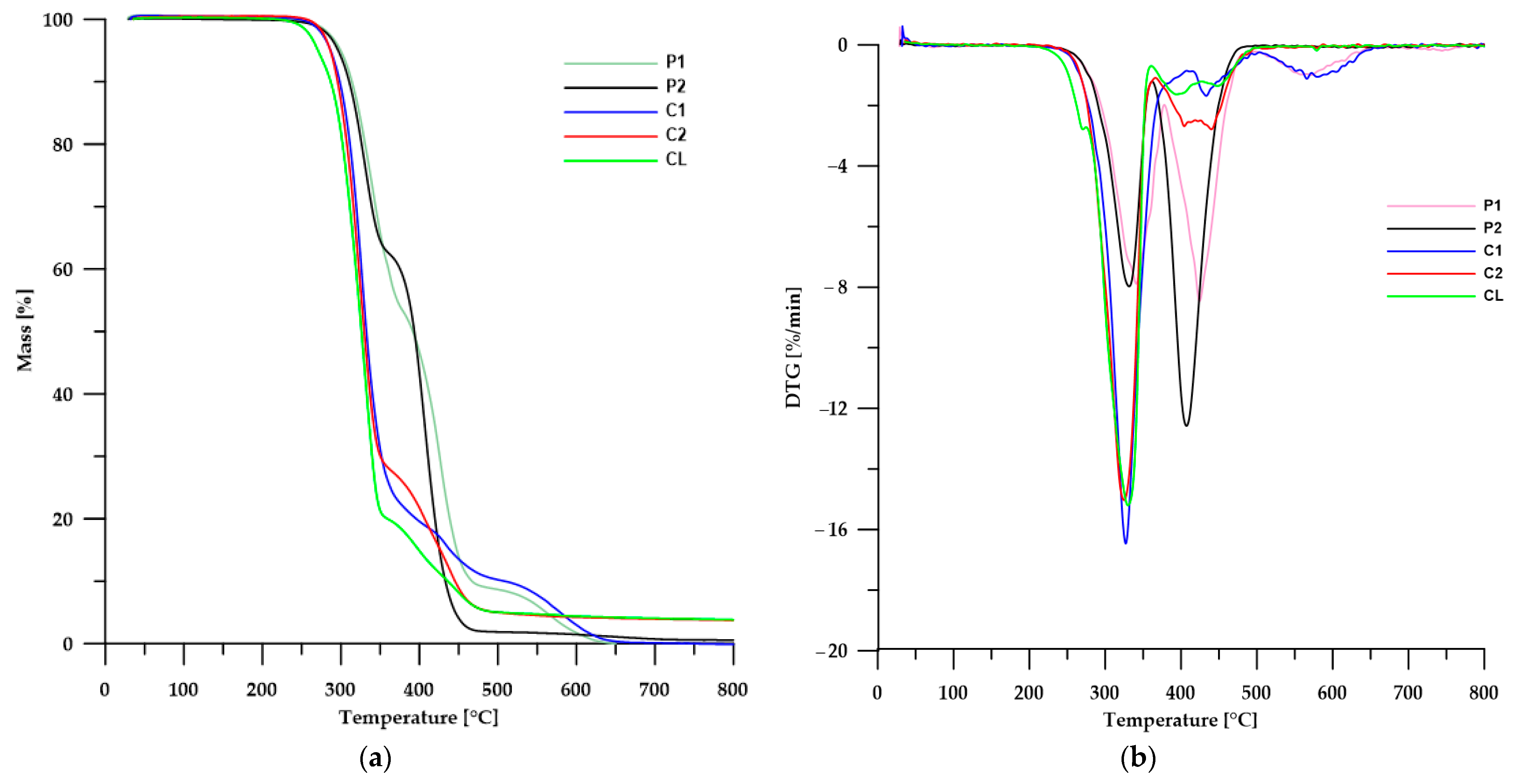
3.5. Thermal Stability
3.5.1. TGA
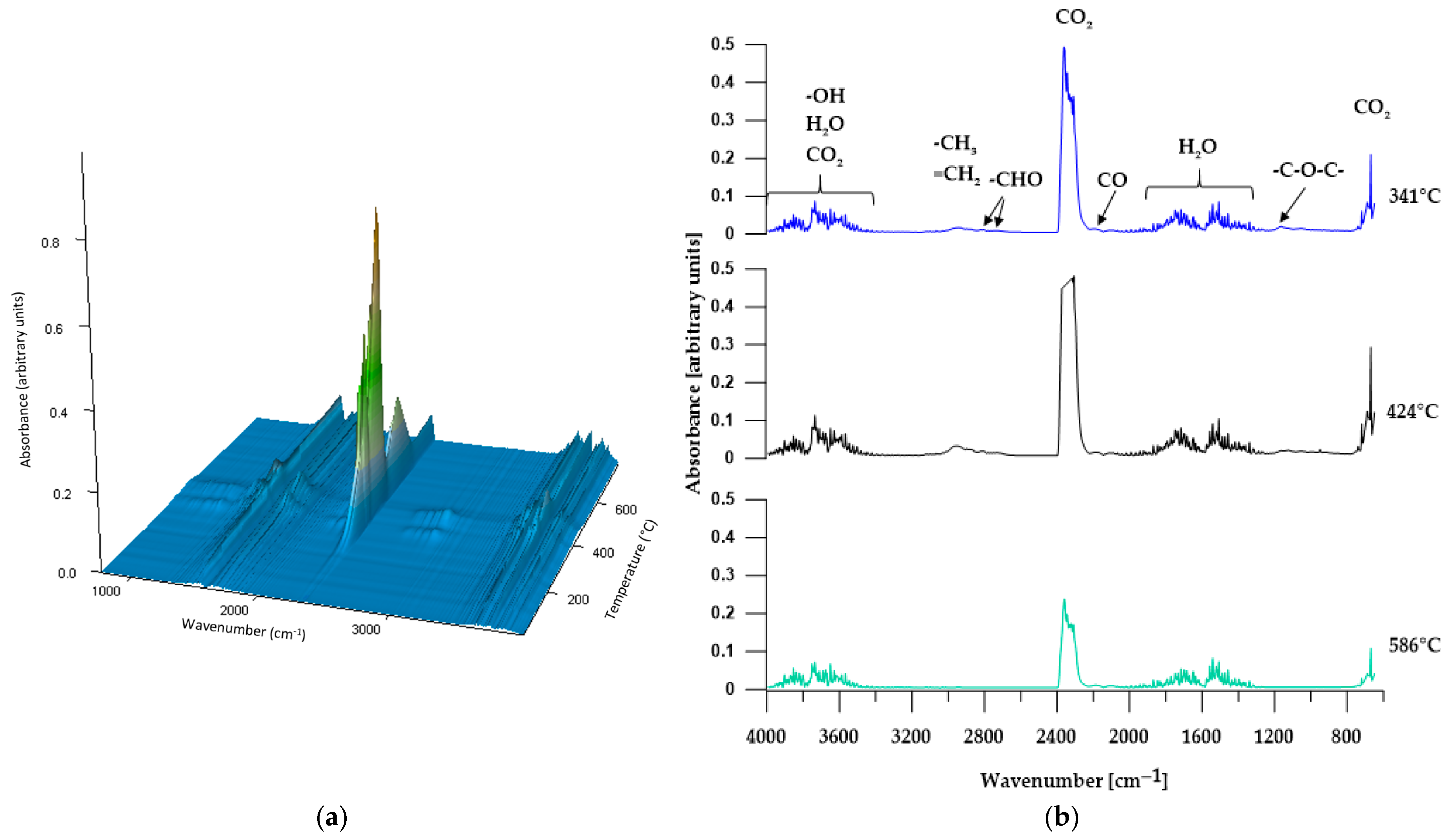
3.5.2. TGA-FTIR
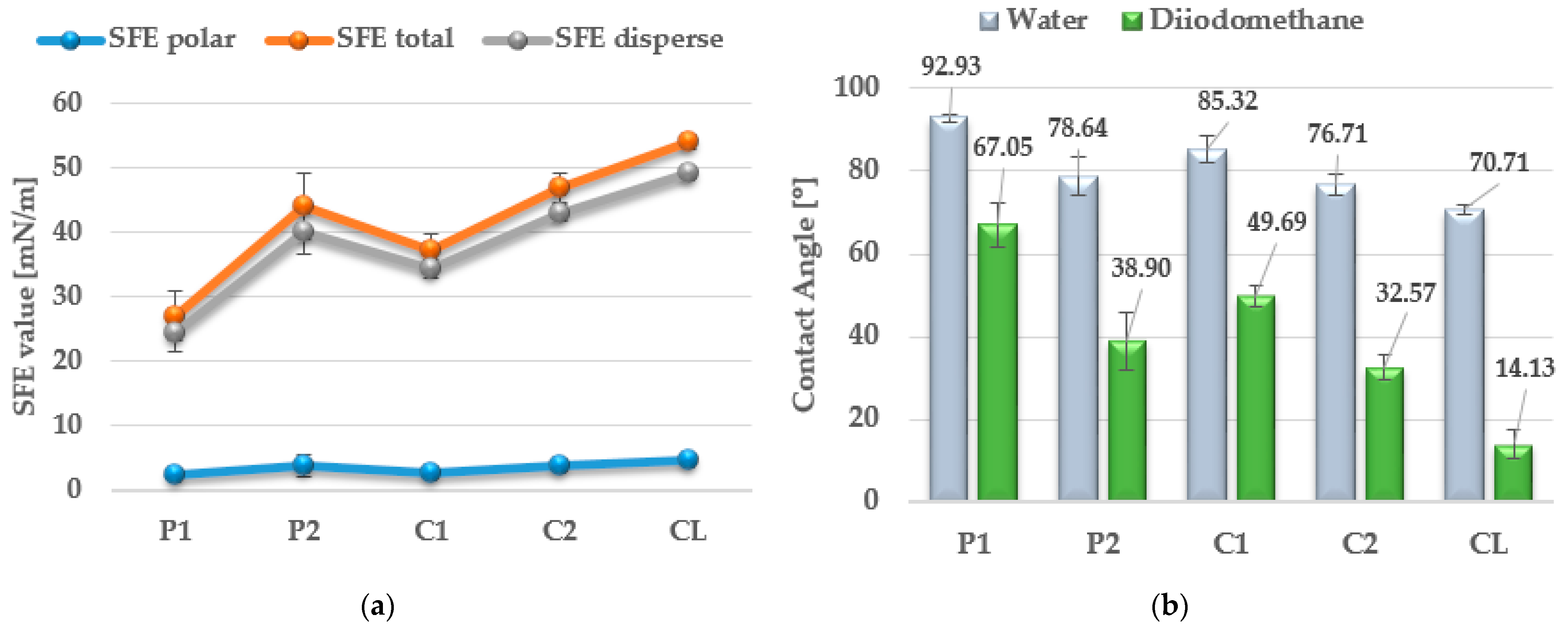
3.6. Contact Angles
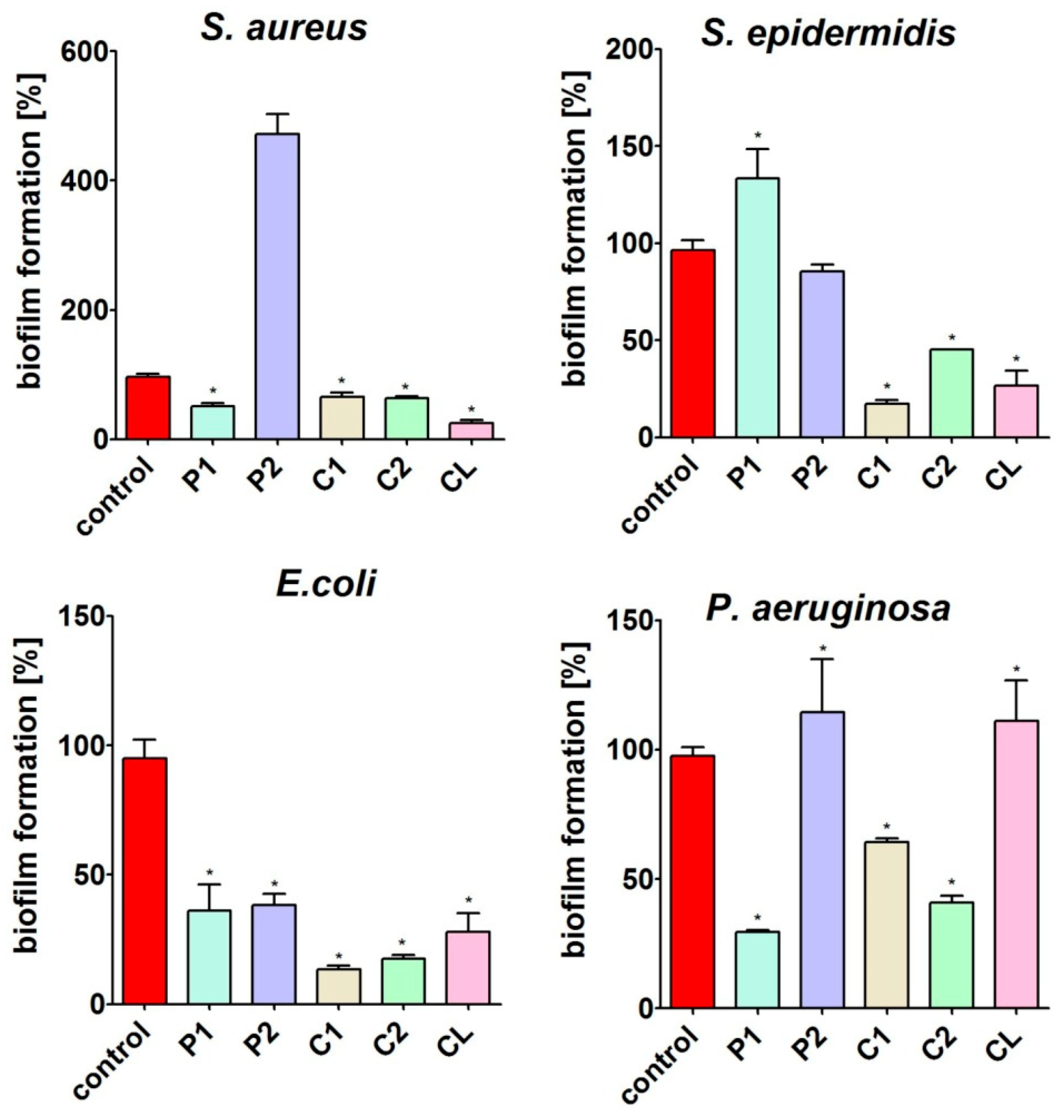
3.7. Antimicrobial Activity
3.7.1. Biofilm Formation
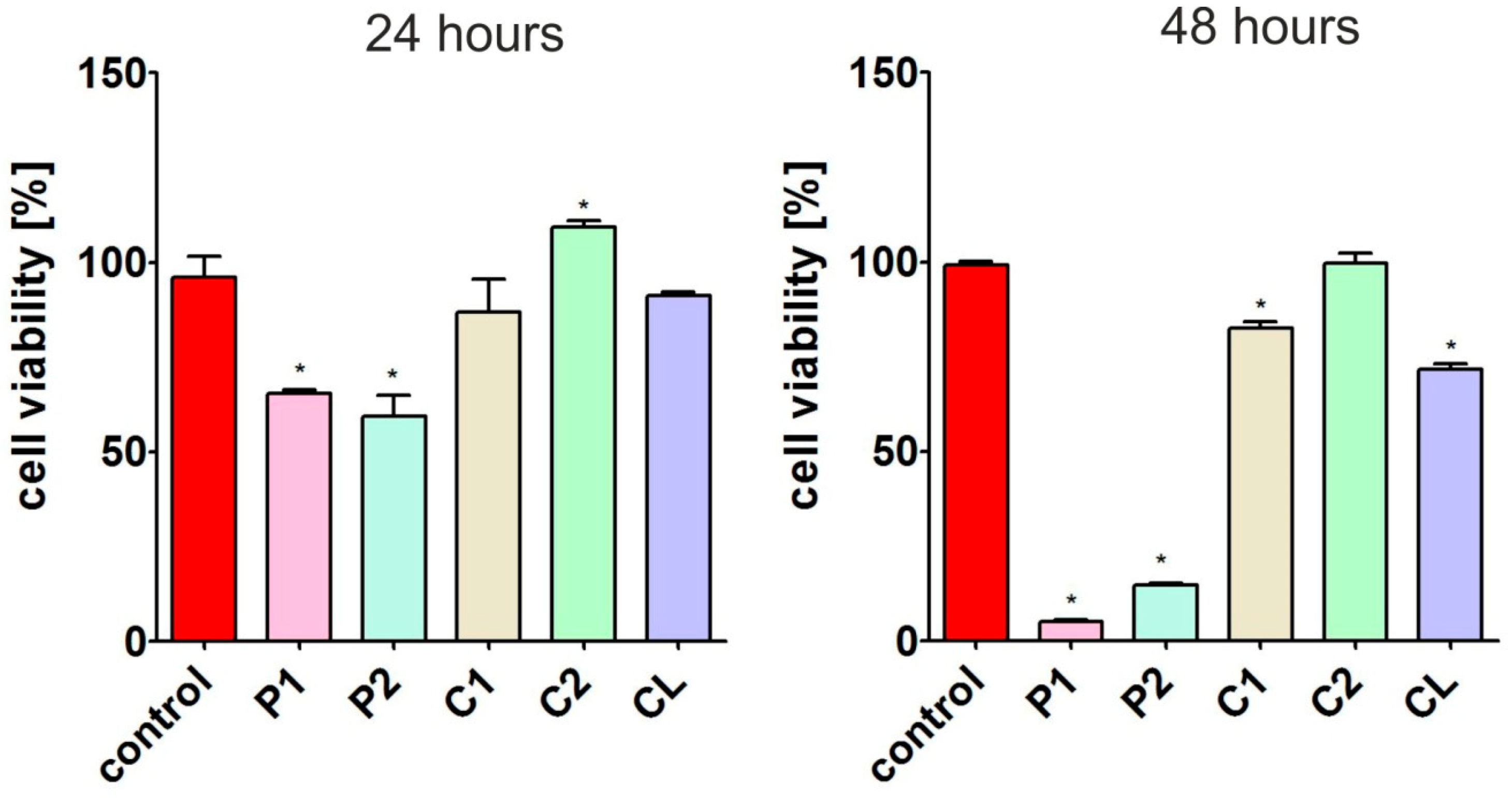
3.7.2. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wirpsza, Z. Polyurethanes—Chemistry, Technology, Application. Warszawa: WNT; Ellis Horwood Ltd.: Chichester, UK, 1991. [Google Scholar]
- Drobny, J.G. (Ed.) 9-Thermoplastic Polyurethane Elastomers. In Plastics Design Library, Handbook of Thermoplastic Elastomers, 2nd ed.; William Andrew Publishing: Norwich, NY, USA, 2014; pp. 233–253. [Google Scholar] [CrossRef]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Fink, J.K. (Ed.) Chapter 2—Poly(urethane)s. In Plastics Design Library, Reactive Polymers Fundamentals and Applications, 2nd ed.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 49–93. [Google Scholar] [CrossRef]
- Janik, H.; Sienkiewicz, M.; Kucinska-Lipka, J. 9-Polyurethanes. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2014; pp. 253–295. [Google Scholar] [CrossRef]
- Piegat, A.; El Fray, M. Thermoplastic Elastomers: Materials for Heart Assist Devices. Polim. Med. 2016, 46, 79–87. [Google Scholar] [CrossRef]
- Król, P.; Uram, Ł.; Król, B.; Pielichowska, K.; Walczak, M. Study of chemical, physico-mechanical and biological properties of 4,4′-methylenebis(cyclohexyl isocyanate)-based polyurethane films. Mater. Sci. Eng. C 2018, 93, 483–494. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Kultys, A.; Puszka, A. New thermoplastic polyurethane elastomers based on sulfur-containing chain extenders. Pol. J. Chem. Technol. 2013, 15, 65–70. [Google Scholar] [CrossRef]
- Mizera, K.; Sałasińska, K.; Ryszkowska, J.; Kurańska, M.; Kozera, R. Effect of the Addition of Biobased Polyols on the Thermal Stability and Flame Retardancy of Polyurethane and Poly(urea)urethane Elastomers. Materials 2021, 14, 1805. [Google Scholar] [CrossRef]
- Sobczak, M.; Dębek, C.; Olędzka, E.; Nałęcz-Jawecki, G.; Kołodziejski, W.L.; Rajkiewicz, M. Segmented polyurethane elastomers derived from aliphatic polycarbonate and poly(ester-carbonate) soft segments for biomedical applications. J. Polym. Sci. A Polym. Chem. 2012, 50, 3904–3913. [Google Scholar] [CrossRef]
- Hepburn, C. Polyurethane Elastomers; Elsevier Science Publishers Ltd.: London, UK, 1992. [Google Scholar]
- Karak, N. Vegetable Oil-Based Polymers. Properties, Processing and Applications; Woodhead Publishing Ltd.: Cambridge, UK, 2012. [Google Scholar]
- Oprea, S.; Joga, A.; Zorlescu, B.; Oprea, V. Effect of the hard segment structure on properties of resorcinol derivatives-based polyurethane elastomers. High Perform. Polym. 2014, 26, 859–866. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Li, J.; Cheng, J.; Zhang, J. Synthesis and properties of segmented polyurethanes with hydroquinone ether derivatives as chain extender. J. Polym. Res. 2015, 22, 149. [Google Scholar] [CrossRef]
- Padmavathy, T.; Srinivasan, K.S.V. Liquid crystalline polyurethanes—A review. J. Macromol. Sci. Polym. Rev. 2003, C43, 45–85. [Google Scholar] [CrossRef]
- Kultys, A.; Puszka, A. Transparent poly(thiourethane-urethane)s based on dithiol chain extender. J. Therm. Anal. Calorim. 2014, 117, 1427–1439. [Google Scholar] [CrossRef]
- Puszka, A.; Kultys, A. The influence of soft segments on some properties of new transparent segmented polyurethanes. Polym. Adv. Technol. 2017, 28, 1937–1944. [Google Scholar] [CrossRef]
- Liaw, D.J. The relative physical and thermal properties of polyurethane elastomers: Effect of chain extenders of bisphenols, diisocyanate, and polyol structures. J. Appl. Polym. Sci. 1997, 66, 1251–1265. [Google Scholar] [CrossRef]
- Tanzi, M.C.; Mantovani, D.; Petrini, P.; Guidoin, R.; Laroche, G. Chemical stability of polyether urethanes versus polycarbonate urethanes. J. Biomed. Mater. Res. 1997, 36, 550–559. [Google Scholar] [CrossRef]
- Khan, I.; Smith, N.; Jones, E.; Finch, D.S.; Cameron, R.E. Analysis and evaluation of a biomedical polycarbonate urethane tested in an in vitro study and an ovine arthroplasty model. Part I: Materials selection and evaluation. Biomaterials 2005, 26, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Hiltner, A.; Wiggins, M.J.; Schubert, M.A.; Collier, T.O.; Kao, W.J.; Mathur, A.B. Recent advances in biomedical polyurethane biostability and biodegradation. Polym. Int. 1998, 46, 163–171. [Google Scholar] [CrossRef]
- Pawłowski, P.; Rokicki, G. Synthesis of oligocarbonate diols from ethylene carbonate and aliphatic diols catalyzed by alkali metal salts. Polymer 2004, 45, 3125–3137. [Google Scholar] [CrossRef]
- Tang, Y.W.; Labow, R.S.; Santerre, J.P. Enzyme-induced biodegradation of polycarbonate-polyurethanes: Dependence on hard-segment chemistry. J. Biomed. Mater. Res. 2001, 57, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Guo, N.; You, Z.; Tan, Y. Design and Performance of Polyurethane Elastomers Composed with Different Soft Segments. Materials 2020, 13, 4991. [Google Scholar] [CrossRef] [PubMed]
- Romaskevic, T.; Sedlevicius, M.; Budriene, S.; Ramanavicius, A.; Ryskevic, N.; Miasojedovas, S.; Ramanaviciene, A. Assembly and Characterization of Polyurethane–Gold Nanoparticle Conjugates. Macromol. Chem. Phys. 2011, 212, 2291–2299. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Pikus, S.; Skrzypiec, K. The synthesis and characterization of new thermoplastic poly(carbonate-urethane) elastomers derived from HDI and aliphatic-aromatic chain extenders. Eur. Polym. J. 2009, 45, 2629–2643. [Google Scholar] [CrossRef]
- Christenson, E.M.; Anderson, J.M.; Hiltner, A. Antioxidant inhibition of poly(carbonate urethane) in vivo biodegradation. J. Biomed. Mater. Res. A 2006, 76, 480–490. [Google Scholar] [CrossRef]
- Špírková, M.; Hodan, J.; Kobera, L.; Kredatusová, J.; Kubies, D.; Machová, L.; Poręba, R.; Serkis, M.; Zhigunov, A.; Kotek, J. The influence of the length of the degradable segment on the functional properties and hydrolytic stability of multi-component polyurethane elastomeric films. Polym. Deg. Stab. 2017, 137, 216–228. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, H.; He, S.; Liu, X.; Liu, W.; Huang, M.; Zhu, C. Polyurethane as smart biocoatings: Effects of hard segments on phase structures and properties. Prog. Org. Coat. 2021, 150, 106000. [Google Scholar] [CrossRef]
- Hou, X.; Sun, L.; Wei, W.; Taylor, D.K.; Su, S.; Yu, H. Structure and performance control of high-damping bio-based thermoplastic polyurethane. J. Appl. Polym. Sci. 2022, 139, 52059. [Google Scholar] [CrossRef]
- Barrioni, B.R.; de Carvalho, S.M.; Oréfice, R.L.; de Oliveira, A.A.R.; Pereira, M.M. Synthesis and characterization of biodegradable polyurethane films based on HDI with hydrolyzable crosslinked bonds and a homogeneous structure for biomedical applications. Mater. Sci. Eng. C 2015, 52, 22–30. [Google Scholar] [CrossRef]
- Rueda, L.; Fernandez d’Arlas, B.; Corcuera, M.A.; Eceiza, A. Biostability of polyurethanes. Study from the viewpoint of microphase separated structure. Polym. Degrad. Stab. 2014, 108, 195–200. [Google Scholar] [CrossRef]
- Haryńska, A.; Kucinska-Lipka, J.; Sulowska, A.; Gubanska, I.; Kostrzewa, M.; Janik, H. Medical-Grade PCL Based Polyurethane System for FDM 3D Printing—Characterization and Fabrication. Materials 2019, 12, 887. [Google Scholar] [CrossRef] [PubMed]
- Wnuczek, K.; Puszka, A.; Podkościelna, B. Synthesis and Spectroscopic Analyses of New Polycarbonates Based on Bisphenol A-Free Components. Polymers 2021, 13, 4437. [Google Scholar] [CrossRef] [PubMed]
- ISO 1183-1A:2019; Plastics—Methods for Determining the Density of Noncellular Plastics—Part 1: Immersion Method, Liquid Pycnometer Method and Titration Method. ISO International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 1133-1:2011; Plastics—Determination of the Melt Mass-flow Rate (MFR) and Melt Volume-flow Rate (MVR) of Thermoplastics—Part 1: Standard Method; ISO International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 11357:2016; Plastics—Differential Scanning Calorimetry (DSC). International Organization of Standardization: Geneva, Switzerland, 2016.
- Rabiej, M.; Rabiej, S. Analysis of X-Ray Diffraction Pattern of Polymers by Means of WAXSFIT Computer Program; ATM: Bielsko-Biała, Poland, 2006. (In Polish) [Google Scholar]
- ISO 10993-5:2009; Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 10093-12:2012; Biological evaluation of medical devices—Part 12: Sample preparation and reference materials. International Organization for Standardization: Geneva, Switzerland, 2012.
- Klimek, K.; Strubińska, J.; Czernel, G.; Ginalska, G.; Gagoś, M. In vitro evaluation of antifungal and cytotoxic activities as also the therapeutic safety of the oxidized form of amphotericin B. Chem.-Biol. Interact. 2016, 256, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Puszka, A.; Sikora, J.W. New Segmented Poly(Thiourethane-Urethane)s Based on Poly(ε-Caprolactone)Diol Soft Segment: Synthesis and Characterization. Materials 2022, 15, 4940. [Google Scholar] [CrossRef] [PubMed]
- Puszka, A.; Sikora, J.W. Synthesis and Characterization of New Polycarbonate-Based Poly(thiourethane-urethane)s. Polymers 2022, 14, 2933. [Google Scholar] [CrossRef] [PubMed]


| TPU | Soft Segment | Amount of HMDI [mol] | Amount of DMD [mol] | Amount of Soft Segment [mol] |
|---|---|---|---|---|
| P1 | PTMO 1000 | 0.0105 | 0.00585 | 0.00415 |
| P2 | PTMO 2000 | 0.00770 | 0.00230 | |
| C1 | Desmphen C® 2100 | 0.00585 | 0.00415 | |
| C2 | Desmphen C® 2200 | 0.00770 | 0.00230 | |
| CL | PCL | 0.00770 | 0.00230 |
| TPU | ηred [dL/g] | Density [g/cm3] | MFR [g/10 min] | Transmittance [%] | Pressing Temperature [°C] | |
|---|---|---|---|---|---|---|
| 400 nm | 700 nm | |||||
| P1 | 2.17 | 1.10 | 13.93 ± 1.92 | 71.73 | 84.59 | 130 |
| P2 | 1.40 | 0.98 | 2.23 ± 0.31 | 18.18 | 70.51 | 135 |
| C1 | 5.47 | 1.14 | 13.57 ± 1.92 | 76.31 | 86.95 | 130 |
| C2 | 3.49 | 1.23 | 11.48 ± 1.30 | 67.76 | 82.73 | 130 |
| CL | 2.77 | 0.96 | 9.36 ± 1.21 | 55.77 | 69.87 | 145 |
| TPU | Tg [°C] | Tm [°C] | ΔH [J/g] | |
|---|---|---|---|---|
| Ist | IInd | |||
| P1 | −8 | −9 | 81 | 3.8 |
| P2 | −71 | −74 | 38 | 1.8 |
| C1 | 31 | 30 | 47 | 0.9 |
| C2 | 42 | 23 | 57 | 1.2 |
| CL | −17 | −11 | 83 | 3.3 |
| TPU | Degree of Crystallinity [%] | 2θ [°] | FWHM [°] | Area of Diffraction Peak [Arbitrary Units] |
|---|---|---|---|---|
| P1 | 0 | 8.52 | 5.88 | 25 |
| 19.45 | 5.12 | 100 | ||
| 19.65 | 13.93 | 87 | ||
| 41.74 | 16.76 | 24 | ||
| P2 | 16.1 | 18.06 | 17.77 | 100 |
| 19.31 | 4.72 | 71 | ||
| 19.81 a | 0.48 a | 6 a | ||
| 24.20 a | 0.97 a | 12 a | ||
| 37.49 a | 0.84 a | 1 a | ||
| 42.40 | 10.61 | 13 | ||
| 43.27 a | 0.80 a | 1 a | ||
| 49.96 | 2.29 | 1 | ||
| C1 | 0 | 17.39 | 20.34 | 100 |
| 19.66 | 5.21 | 72 | ||
| 42.79 | 16.26 | 26 | ||
| C2 | 0.5 | 7.08 a | 0.29 a | 1 a |
| 17.83 | 19.66 | 100 | ||
| 19.78 | 5.00 | 65 | ||
| 43.35 | 14.48 | 17 | ||
| CL | 1.5 | 13.19 | 27.83 | 100 |
| 19.59 | 4.51 | 55 | ||
| 21.54 a | 0.43 a | 3 a | ||
| 23.86 | 3.32 | 19 | ||
| 42.86 | 15.83 | 25 |
| TPU | T1 1 [°C] | T5 2 [°C] | T50 3 [°C] | Tmax 4 [°C] |
|---|---|---|---|---|
| P1 | 271 | 300 | 392 | 341; 424; 586 |
| P2 | 267 | 297 | 393 | 332; 407 |
| C1 | 266 | 287 | 332 | 327; 433; 596 |
| C2 | 270 | 287 | 330 | 325; 405; 442 |
| CL | 248 | 271 | 326 | 271; 330; 394; 448 |
| P1 | P2 | C1 | C2 | CL | |
|---|---|---|---|---|---|
| Water | 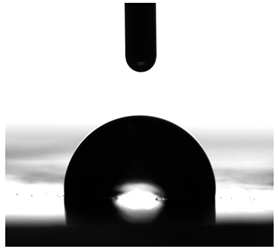 | 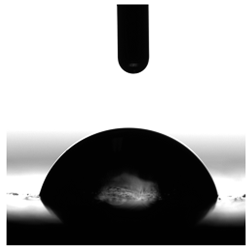 | 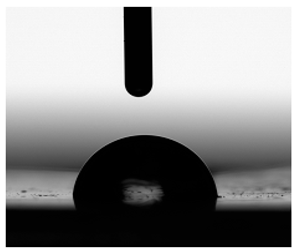 | 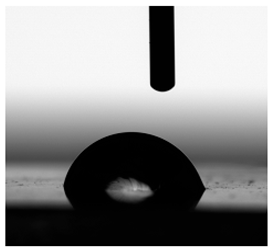 | 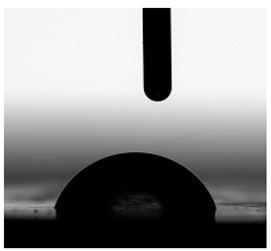 |
| Diiodomethane | 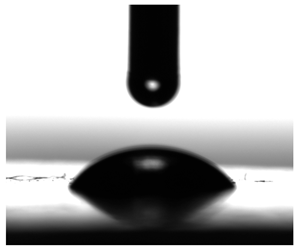 |  | 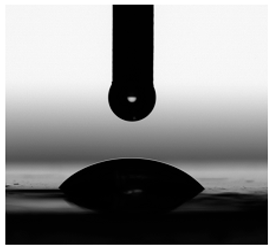 | 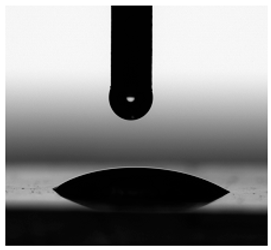 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puszka, A.; Sikora, J.W.; Nurzyńska, A. Influence of the Type of Soft Segment on the Selected Properties of Polyurethane Materials for Biomedical Applications. Materials 2024, 17, 840. https://doi.org/10.3390/ma17040840
Puszka A, Sikora JW, Nurzyńska A. Influence of the Type of Soft Segment on the Selected Properties of Polyurethane Materials for Biomedical Applications. Materials. 2024; 17(4):840. https://doi.org/10.3390/ma17040840
Chicago/Turabian StylePuszka, Andrzej, Janusz W. Sikora, and Aleksandra Nurzyńska. 2024. "Influence of the Type of Soft Segment on the Selected Properties of Polyurethane Materials for Biomedical Applications" Materials 17, no. 4: 840. https://doi.org/10.3390/ma17040840
APA StylePuszka, A., Sikora, J. W., & Nurzyńska, A. (2024). Influence of the Type of Soft Segment on the Selected Properties of Polyurethane Materials for Biomedical Applications. Materials, 17(4), 840. https://doi.org/10.3390/ma17040840








