Construction of Conjugated Organic Polymers for Efficient Photocatalytic Hydrogen Peroxide Generation with Adequate Utilization of Water Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of TPE-A-P
2.2. Synthesis of TPE-P
2.3. Photocatalytic Experiments
3. Results and Discussion
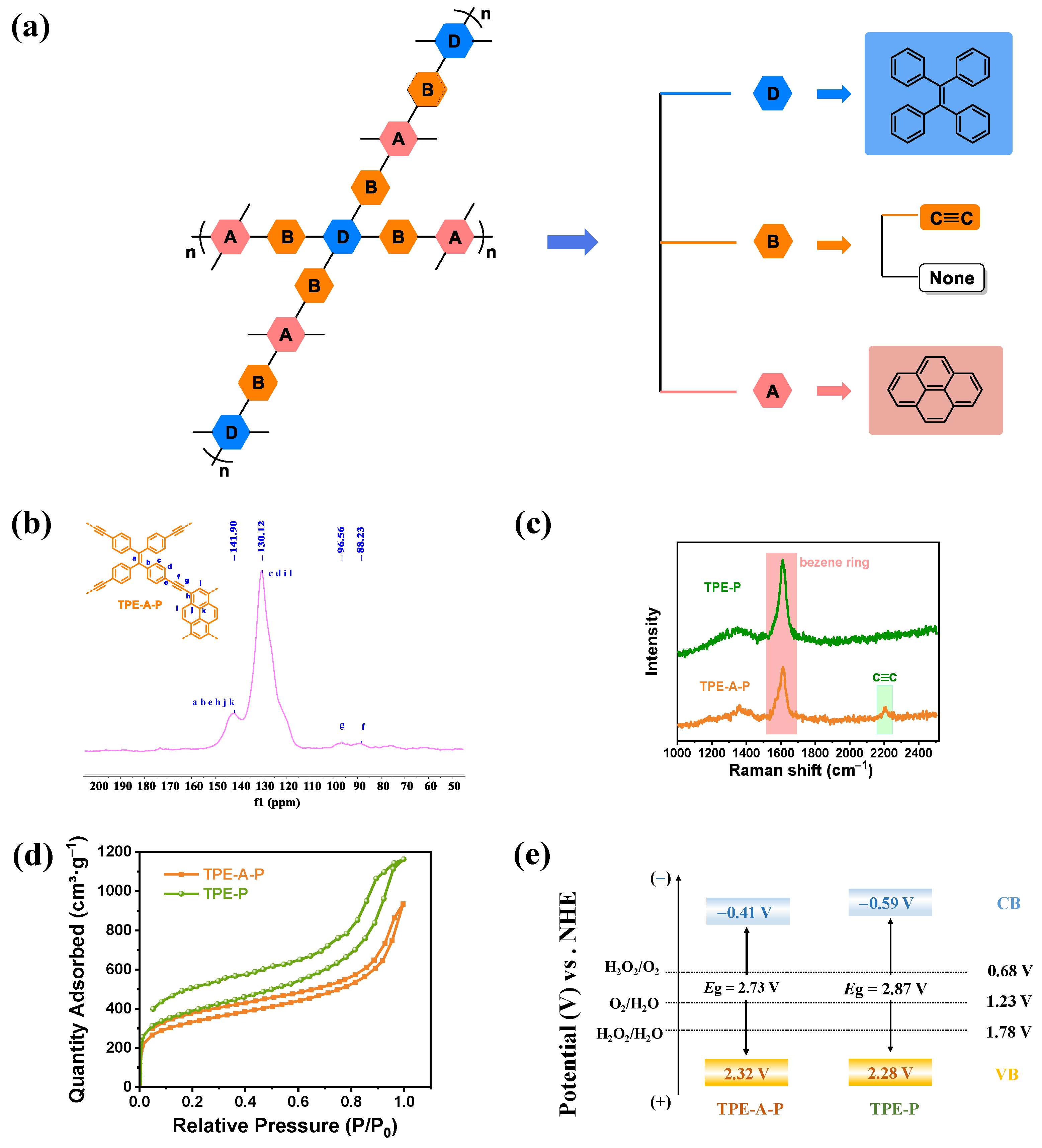
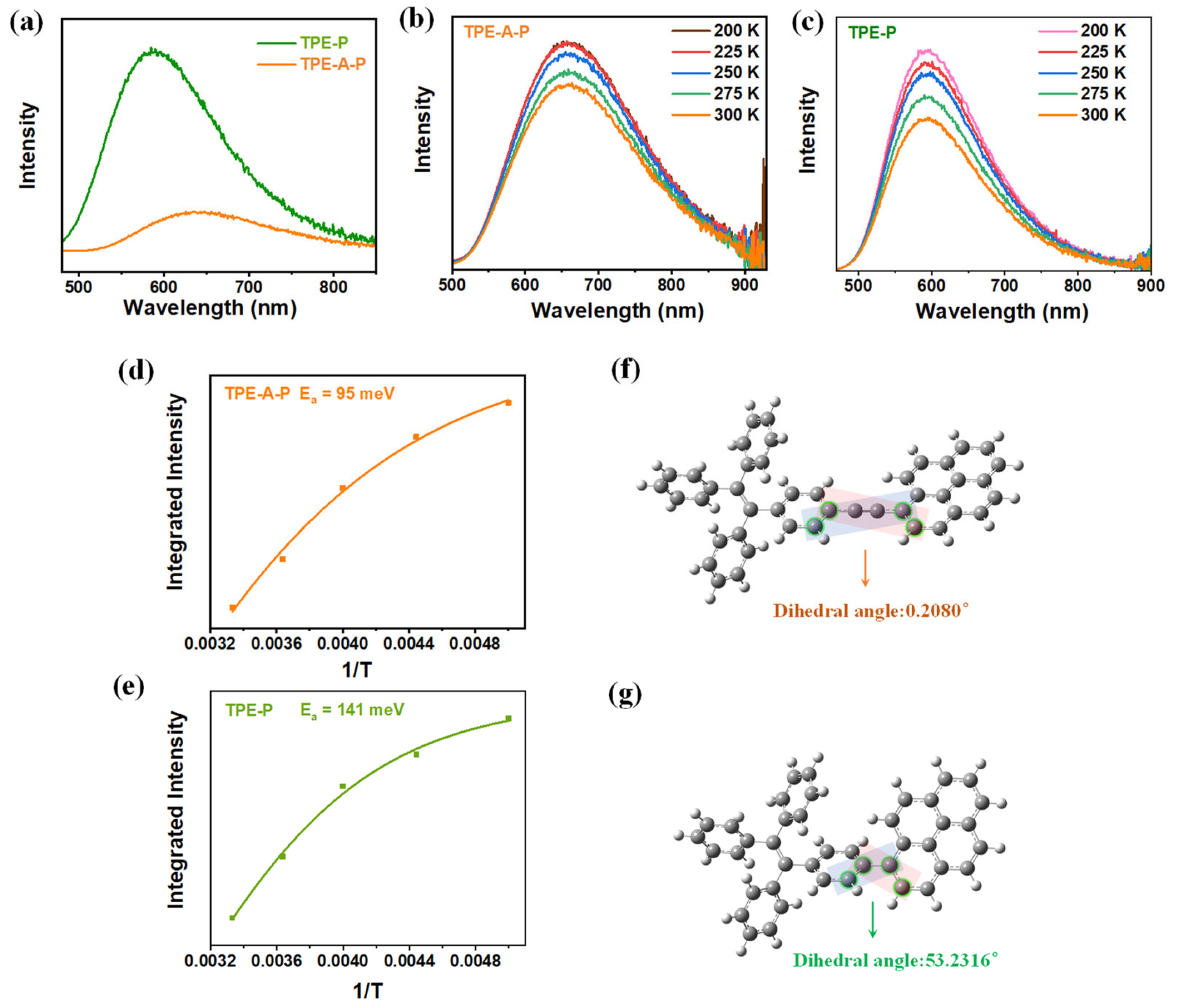
3.1. Chemical and Physical Characterization
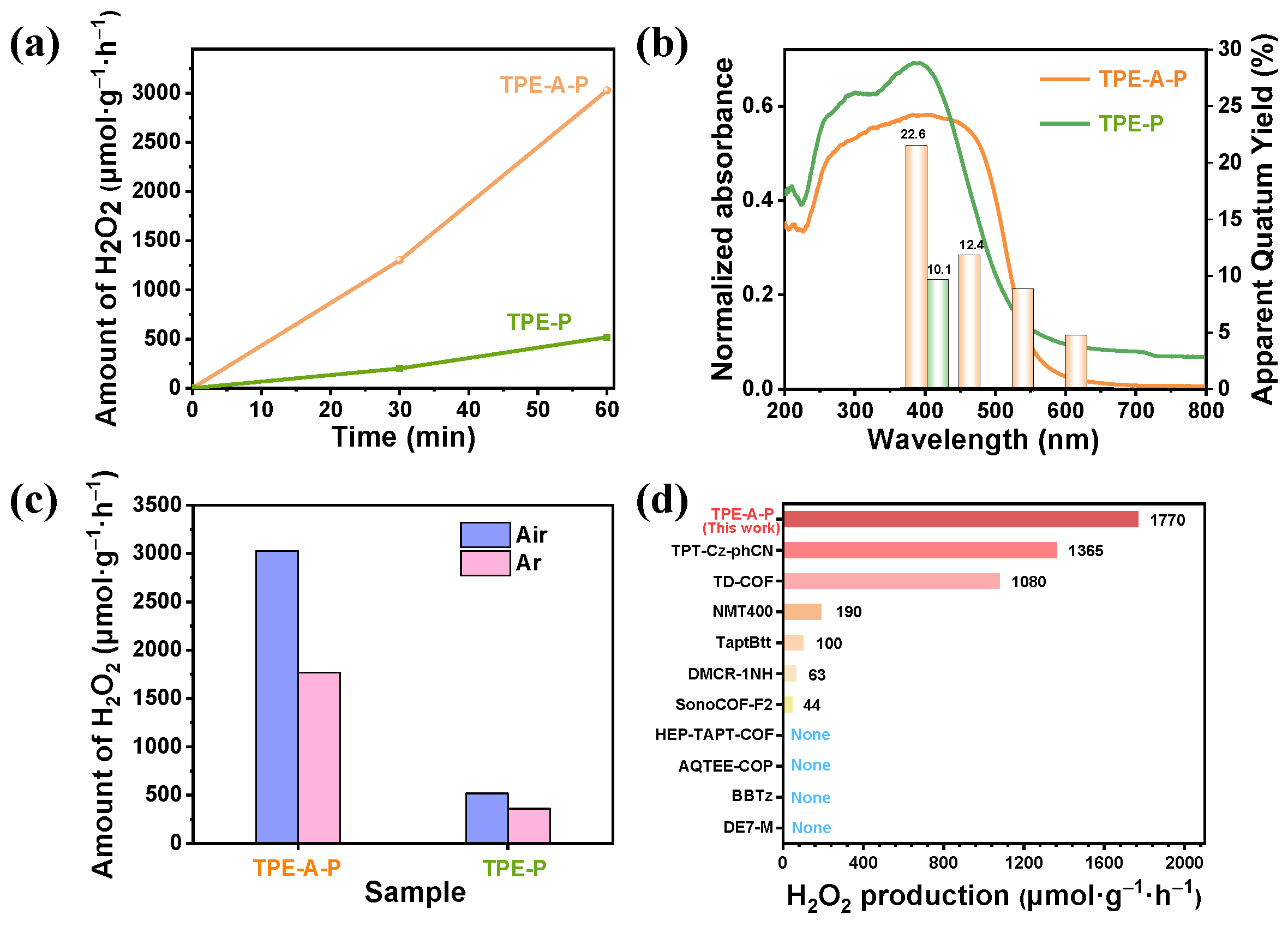
3.2. Photocatalytic Performance
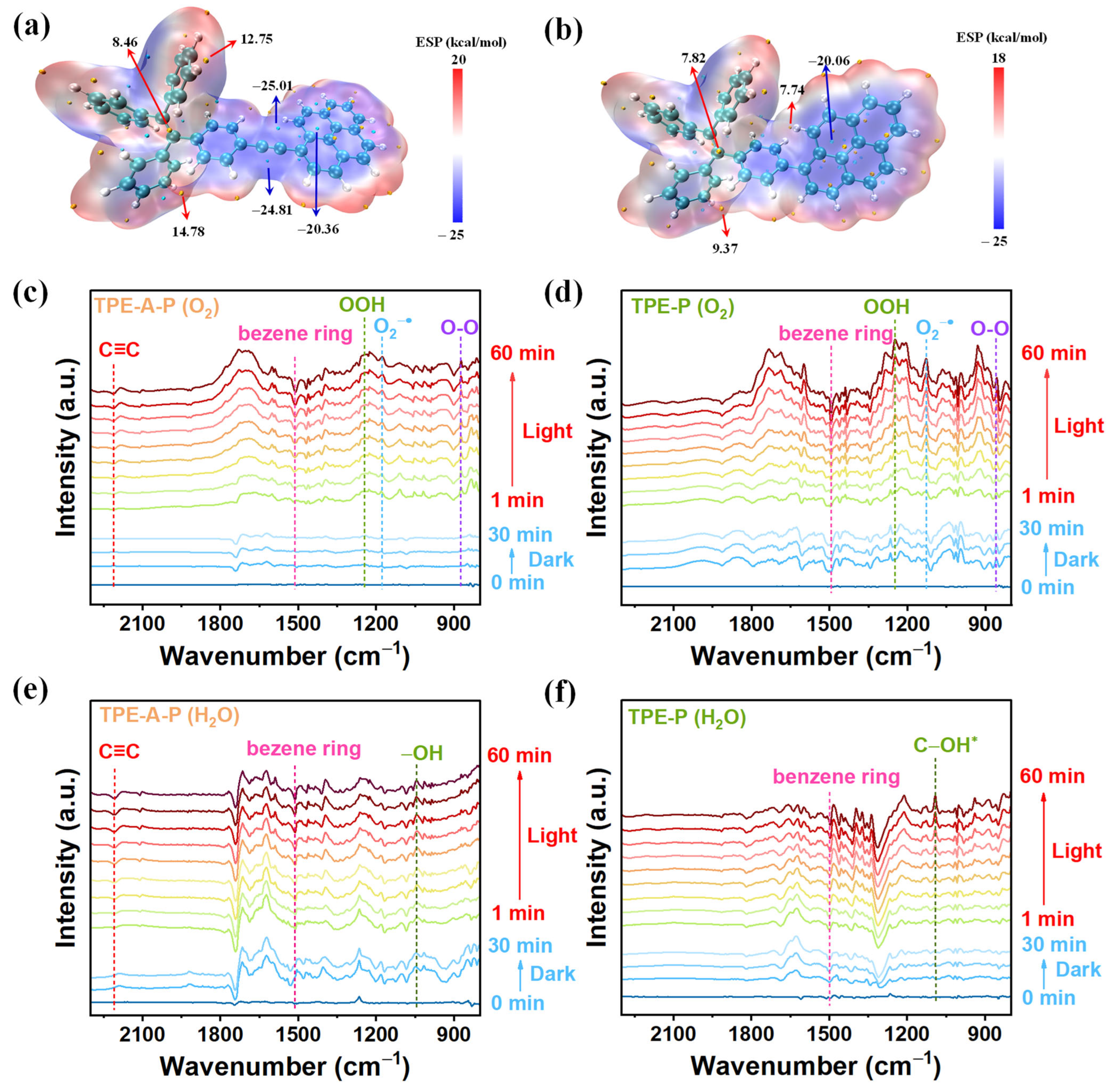
3.3. Pathways for Photosynthesis of H2O2
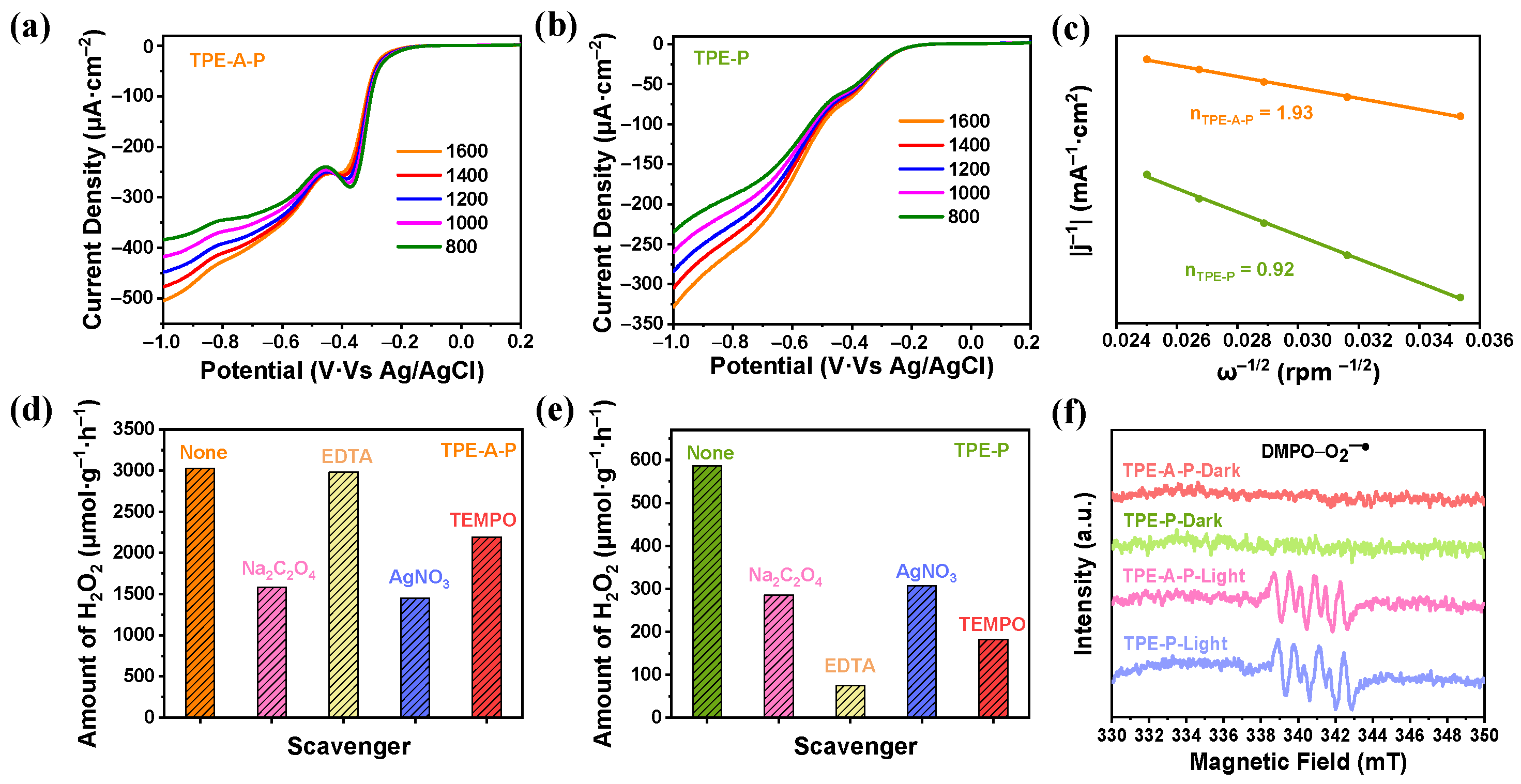
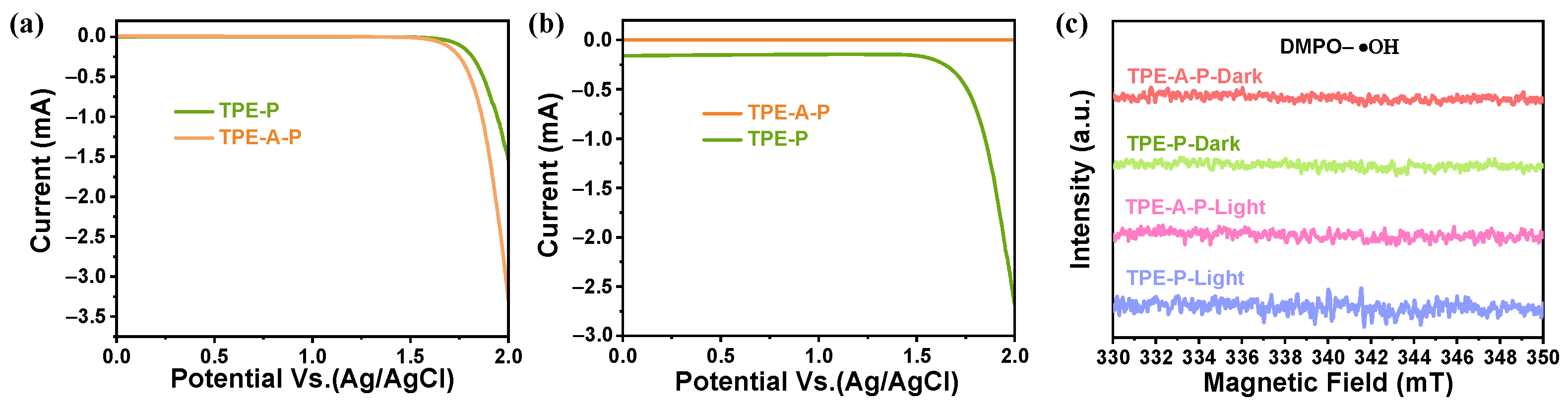
3.4. Active Sites for Both ORR and WOR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rani, E.; Talebi, P.; Cao, W.; Huttula, M.; Singh, H. Harnessing photo/electro-catalytic activity via nano-junctions in ternary nanocomposites for clean energy. Nanoscale 2020, 12, 23461–23479. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen peroxide: A key chemical for today’s sustainable development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Shi, Y.; Chen, Z.; Chen, R.; Chen, X.; Zheng, X.; Liu, Y.; Ding, R. Enhancement of solar water disinfection using H2O2 generated in situ by electrochemical reduction. Appl. Catal. B Environ. 2020, 267, 118730. [Google Scholar] [CrossRef]
- Mase, K.; Yoneda, M.; Yamada, Y.; Fukuzumi, S. Seawater usable for production and consumption of hydrogen peroxide as a solar fuel. Nat. Commun. 2016, 7, 11470. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Dai, C.; Lei, Z.; Chen, B.; Fang, X. Synthesis of hydrogen peroxide over Pd/SiO2/COR monolith catalysts by anthraquinone method. Catal. Today 2016, 276, 36–45. [Google Scholar] [CrossRef]
- Yue, J.; Song, L.; Fan, Y.F.; Pan, Z.X.; Yang, P.; Ma, Y.; Xu, Q.; Tang, B. Thiophene-containing covalent organic frameworks for overall photocatalytic H2O2 synthesis in water and seawater. Angew. Chem. Int. Ed. 2023, 62, e202309624. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.-H.; Kim, W.; Bokare, A.D.; Sung, N.-E.; Choi, W.Y. Solar production of H2O2 on reduced graphene oxide–TiO2 hybrid photocatalysts consisting of earth-abundant elements only. Energy Environ. Sci. 2014, 7, 4023–4028. [Google Scholar] [CrossRef]
- Chu, C.H.; Huang, D.H.; Zhu, Q.H.; Stavitski, E.; Spies, J.A.; Pan, Z.H.; Mao, J.; Xin, H.L.; Schmuttenmaer, C.A.; Hu, S.; et al. Electronic tuning of metal nanoparticles for highly efficient photocatalytic hydrogen peroxide production. ACS Catal. 2019, 9, 626–631. [Google Scholar] [CrossRef]
- Zeng, X.K.; Liu, Y.; Kang, Y.; Li, Q.Y.; Xia, Y.; Zhu, Y.L.; Hou, H.L.; Uddin, M.H.; Gengenbach, T.R.; Xia, D.H.; et al. Simultaneously tuning charge separation and oxygen reduction pathway on graphitic carbon nitride by polyethylenimine for boosted photocatalytic hydrogen peroxide production. ACS Catal. 2020, 10, 3697–3706. [Google Scholar] [CrossRef]
- Baek, J.H.; Gill, T.M.; Abroshan, H.; Park, S.; Shi, X.J.; Nørskov, J.; Jung, H.S.; Siahrostami, S.; Zheng, X.L. Selective and efficient Gd-doped BiVO4 photoanode for two-electron water oxidation to H2O2. ACS Energy Lett. 2019, 4, 720–728. [Google Scholar] [CrossRef]
- Xu, J.W.; Zheng, X.L.; Feng, Z.P.; Lu, Z.Y.; Zhang, Z.W.; Huang, W.; Li, Y.B.; Vuckovic, D.; Li, Y.Q.; Dai, S.; et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 2021, 4, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Wan, Y.Y.; Zhang, Y.; Qi, Z.M.; Wu, X.J.; Xu, H.X. Acetylene and diacetylene functionalized covalent triazine frameworks as metal-free photocatalysts for hydrogen peroxide production: A new two-electron water oxidation pathway. Adv. Mater. 2020, 32, 1904433. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.X.; Wen, C.; Pan, J.H.; Wang, J.W.; Tong, Y.J.; Wei, S.B.; Ke, Z.F.; Jiang, L.; Zhu, F.; Zhou, N.B.; et al. Visible-light driven efficient overall H2O2 production on modified graphitic carbon nitride under ambient conditions. Appl. Catal. B Environ. 2021, 285, 119726. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.P.; Liu, C.C.; Xia, D.H.; Ou, X.W.; Cai, Y.P.; Zhou, Y.; Jiang, J.; Han, B. Sulfone-modified covalent organic frameworks enabling efficient photocatalytic hydrogen peroxide generation via one-step two-electron O2 reduction. Angew. Chem. Int. Ed. 2023, 62, e202305355. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Zhang, F.L.; Wang, Y.X.; Huang, F.W.; Lang, X.J. Selective oxidation of sulfides with oxygen over a pyrene covalent organic framework photocatalyst with TEMPO. Appl. Catal. B Environ. 2024, 345, 123660. [Google Scholar] [CrossRef]
- Sun, J.; Sekhar Jena, H.; Krishnaraj, C.; Singh Rawat, K.; Abednatanzi, S.; Chakraborty, J.; Laemont, A.; Liu, W.; Chen, H.; Liu, Y.Y.; et al. Pyrene-based covalent organic frameworks for photocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. 2023, 62, e202216719. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.J.; Shen, M.H.; Shen, Y.; Wang, X.D.; Lin, W.; Pan, J.H.; He, J.; Ye, Y.X.; Yang, X.; Zhu, F.; et al. Spontaneous exciton dissociation in organic photocatalyst under ambient conditions for highly efficient synthesis of hydrogen peroxide. Proc. Natl. Acad. Sci. USA 2022, 119, e2202913119. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.J.; Deng, Y.C.; Shen, M.H.; Ye, Y.X.; Yang, X.; Zhu, F.; Ouyang, G.F. Regulation the reactive oxygen species on conjugated polymers for highly efficient photocatalysis. Appl. Catal. B Environ. 2022, 314, 121488. [Google Scholar] [CrossRef]
- Zhou, W.X.; Cheng, Y.Y.; Ye, Y.X.; Wei, X.Q.; Tong, Q.; Dong, L.; Ouyang, G.F. Metal-free photocatalytic CO2 reduction to CH4 and H2O2 under non-sacrificial ambient conditions. Angew. Chem. Int. Ed. 2023, 62, e202313392. [Google Scholar] [CrossRef]
- Ye, Y.X.; Pan, J.H.; Shen, Y.; Shen, M.H.; Yan, H.J.; He, J.; Yang, X.; Zhu, F.; Xu, J.Q.; He, J.G.; et al. A solar-to-chemical conversion efficiency up to 0.26% achieved in ambient conditions. Proc. Natl. Acad. Sci. USA 2021, 118, 2115666118. [Google Scholar] [CrossRef]
- Luo, J.; Liu, Y.N.; Fan, C.Z.; Tang, L.; Yang, S.J.; Liu, M.L.; Wang, M.; Feng, C.Y.; Ouyang, X.L.; Wang, L.L.; et al. Direct attack and indirect transfer mechanisms dominated by reactive oxygen species for photocatalytic H2O2 production on g-C3N4 possessing nitrogen vacancies. ACS Catal. 2021, 11, 11440–11450. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.Y.; Yin, Y.F.; Tian, W.M.; Zeng, G.; Li, H.T.; Ye, S.; Wu, L.M.; Liu, J. Molecular level modulation of anthraquinone-containing resorcinol-formaldehyde resin photocatalysts for H2O2 production with exceeding 1.2% efficiency. Angew. Chem. Int. Ed. 2023, 62, e202218318. [Google Scholar] [CrossRef]
- Haripriya, P.; Anjana, T.; Sreelakshmi, K.; Madhu, N.T.; Anjana, M.; Suneesh, P.V.; Ravi Kumar, D.V. Effect of alkali metal cation doping in graphitic carbon nitride towards photocatalytic generation of hydrogen peroxide under direct sunlight. Catal. Commun. 2024, 187, 106909. [Google Scholar] [CrossRef]
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Photocatalytic conversion of nitrogen to ammonia with water on surface oxygen vacancies of titanium dioxide. J. Am. Chem. Soc. 2017, 139, 10929–10936. [Google Scholar] [CrossRef]
- Xiao, C.L.; Hu, H.; Zhang, X.Y.; MacFarlane, D.R. Nanostructured gold/bismutite hybrid heterocatalysts for plasmon-enhanced photosynthesis of ammonia. ACS Sustain. Chem. Eng. 2017, 5, 10858–10863. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Shito, S.; Kofuji, Y.; Hashimoto, M.; Chishiro, K.; Hirakawa, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Nitrogen fixation with water on carbon-nitride-based metal-free photocatalysts with 0.1% solar-to-ammonia energy conversion efficiency. ACS Appl. Energy Mater. 2018, 1, 4169–4177. [Google Scholar] [CrossRef]
- Zhang, N.; Jalil, A.; Wu, D.; Chen, S.; Liu, Y.; Gao, C.; Ye, W.; Qi, Z.; Ju, H.; Wang, C.; et al. Refining defect states in W18O49 by Mo doping: A strategy for tuning N2 activation towards solar-driven nitrogen fixation. J. Am. Chem. Soc. 2018, 140, 9434–9443. [Google Scholar] [CrossRef]
- Li, X.H.; Chen, W.L.; Tan, H.Q.; Li, F.R.; Li, J.P.; Li, Y.G.; Wang, E.B. Reduced state of the graphene oxide@polyoxometalate nanocatalyst achieving high-efficiency nitrogen fixation under light driving conditions. ACS Appl. Mater. Interfaces 2019, 11, 37927–37938. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Zhang, T.; Jain, N.; Ching, H.V.; Jaworski, A.; Barcaro, G.; Monti, S.; Silvestre-Albero, J.; Celorrio, V.; Chouhan, L.; et al. An atomically dispersed Mn-photocatalyst for generating hydrogen peroxide from seawater via the water oxidation reaction (WOR). J. Am. Chem. Soc. 2023, 145, 16584–16596. [Google Scholar] [CrossRef]
- Yan, H.; Peng, Y.; Huang, Y.; Shen, M.; Wei, X.; Zou, W.; Tong, Q.; Zhou, N.; Xu, J.; Zhang, Y.; et al. Enhancing photosynthesis efficiency of hydrogen peroxide by modulating side chains to facilitate water oxidation at low-energy barrier sites. Adv. Mater. 2024, 36, 2311535. [Google Scholar] [CrossRef]
- Misra, P.; Sharma, T.K.; Kukreja, L.M. Temperature dependent photoluminescence processes in ZnO thin films grown on sapphire by pulsed laser deposition. Curr. Appl. Phys. 2009, 9, 179. [Google Scholar] [CrossRef]
- Xing, X.J.; Zhu, P.; Pang, E.; Zhao, S.J.; Tang, Y.; Hu, Z.Y.; Ouyang, Q.C.; Lan, M.H. D-A-D-structured boron-dipyrromethene with aggregation-induced enhanced phototherapeutic efficiency for near-infrared fluorescent and photoacoustic imaging-guided synergistic photodynamic and photothermal cancer therapy. Chin. Chem. Lett. 2023, 109452. [Google Scholar] [CrossRef]
- Kofuji, Y.; Isobe, Y.; Shiraishi, Y.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Carbon nitride–aromatic diimide–graphene nanohybrids: Metal-free photocatalysts for solar-to-hydrogen peroxide energy conversion with 0.2% efficiency. J. Am. Chem. Soc. 2016, 138, 10019–10025. [Google Scholar] [CrossRef]
- Kou, M.; Wang, Y.; Xu, Y.; Ye, L.; Huang, Y.; Jia, B.; Li, H.; Ren, J.; Deng, Y.; Chen, J.; et al. Molecularly engineered covalent organic frameworks for hydrogen peroxide photosynthesis. Angew. Chem. Int. Ed. 2022, 61, e202200413. [Google Scholar] [CrossRef]
- Sun, J.; Jeet, C.; Deng, M.; Laemont, A.; Feng, X.; Liu, Y.Y.; Van Der Voort, P. Metal–organic frameworks and covalent organic frameworks as photocatalysts for H2O2 production from oxygen and water. J. Mater. Chem. A 2023, 11, 21516–21540. [Google Scholar] [CrossRef]
- Shu, C.; Yang, X.J.; Liu, L.J.; Hu, X.L.; Sun, R.X.; Yang, X.; Cooper, A.I.; Tan, B.; Wang, X.Y. Mixed-linker strategy for the construction of sulfone-containing D–A–A covalent organic frameworks for efficient photocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. 2024, 136, e202403926. [Google Scholar] [CrossRef]
- Wu, C.B.; Teng, Z.Y.; Yang, C.; Chen, F.S.; Yang, H.B.; Wang, L.; Xu, H.X.; Liu, B.; Zheng, G.F.; Han, Q. Polarization engineering of covalent triazine frameworks for highly efficient photosynthesis of hydrogen peroxide from molecular oxygen and water. Adv. Mater. 2022, 34, 2110266. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.D.; Hou, H.S.; Liu, Z.Y.; Yang, C.K.; Long, J.L.; Huang, G.C.; Bi, J.H.; Yu, Y.; Li, L.Y. The hydration-initiated pathway of water oxidation over photoexcited covalent organic frameworks. ACS Catal. 2022, 12, 14911–14917. [Google Scholar] [CrossRef]
- Wang, H.Z.; Yang, C.; Chen, F.S.; Zheng, G.F.; Han, Q. A crystalline partially fluorinated triazine covalent organic framework for efficient photosynthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 2022, 61, e202202328. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Wu, W.D.; Wang, A.P.; Luo, J.; Liu, T.L. A highly stable, capacity dense carboxylate viologen anolyte towards long-duration energy storage. Angew. Chem. Int. Ed. 2023, 62, e202216662. [Google Scholar] [CrossRef]
- Yang, C.; Wan, S.J.; Zhu, B.C.; Yu, J.G.; Cao, S.W. Calcination-regulated microstructures of donor-acceptor polymers towards enhanced and stable photocatalytic H2O2 production in pure water. Angew. Chem. Int. Ed. 2022, 61, e202208438. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhan, Z.; Zhang, J.Q.; Hussain, I.; Tan, B. Immobilized covalent triazine frameworks films as effective photocatalysts for hydrogen evolution reaction. Nat. Commun. 2021, 12, 6596. [Google Scholar] [CrossRef]
- Lu, J.N.; Liu, J.J.; Dong, L.Z.; Lin, J.M.; Yu, F.; Liu, J.; Lan, Y.Q. Synergistic metal-nonmetal active sites in a metal-organic cage for efficient photocatalytic synthesis of hydrogen peroxide in pure water. Angew. Chem. Int. Ed. 2023, 62, e202308505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Pan, C.S.; Bian, G.M.; Xu, J.; Dong, Y.M.; Zhang, Y.; Lou, Y.; Liu, W.X.; Zhu, Y.F. H2O2 generation from O2 and H2O on a near-infrared absorbing porphyrin supramolecular photocatalyst. Nat. Energy 2023, 8, 361–371. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- GaussView, version 6.1.1; Shawnee: Mission, KS, USA, 2019.
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, X.; Truhlar, D.G. Minimally augmented Karlsruhe basis sets. Theor. Chem. Acc. 2011, 128, 295–305. [Google Scholar] [CrossRef]
- Papajak, E.; Zheng, J.; Xu, X.; Leverentz, H.R.; Truhlar, D.G. Perspectives on basis sets beautiful: Seasonal plantings of diffuse basis functions. J. Chem. Theory Comput. 2011, 7, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F.-W. Comparison of computational methods for atomic charges. Acta Phys. Chim. Sin. 2012, 28, 1–18. [Google Scholar]
- Zhu, L.; Zhang, J.; Guo, Y.; Yang, C.; Yi, Y.; Wei, Z. Small exciton binding energies enabling direct charge photogeneration towards low-driving-force organic solar cells. Angew. Chem. Int. Ed. 2021, 60, 15348–15353. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F.-W. Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Mol. Graph. 2012, 38, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, T.; Chen, Q. Intermolecular interaction characteristics of the all-carboatomic ring, cyclo[18]carbon: Focusing on molecular adsorption and stacking. Carbon 2021, 171, 514–523. [Google Scholar] [CrossRef]
- Liu, L.J.; Gao, M.-Y.; Yang, H.F.; Wang, X.Y.; Li, X.B.; Cooper, A.I. Linear conjugated polymers for solar-driven hydrogen peroxide production: The importance of catalyst stability. J. Am. Chem. Soc. 2021, 143, 19287–19293. [Google Scholar] [CrossRef]
- Cheng, J.Z.; Wan, S.J.; Cao, S.W. Promoting solar-driven hydrogen peroxide production over thiazole-based conjugated polymers via generating and converting singlet oxygen. Angew. Chem. Int. Ed. 2023, 62, e202310476. [Google Scholar] [CrossRef]
- Xu, X.H.; Sa, R.J.; Huang, W.; Sui, Y.; Chen, W.T.; Zhou, G.Y.; Li, X.D.; Li, Y.T.; Zhong, H. Conjugated organic polymers with anthraquinone redox centers for efficient photocatalytic hydrogen peroxide production from water and oxygen under visible light irradiation without any additives. ACS Catal. 2022, 12, 12954–12963. [Google Scholar] [CrossRef]
- Chen, D.; Chen, W.; Wu, Y.; Wang, L.; Wu, X.; Xu, H.; Chen, L. Covalent organic frameworks containing dual O2 reduction centers for overall photosynthetic hydrogen peroxide production. Angew. Chem. Int. Ed. 2023, 62, e202217479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yan, P.Y.; Li, B.Y.; Bahri, M.; Liu, L.J.; Zhou, X.; Clowes, R.; Browning, N.D.; Wu, Y.; Ward, J.W.; et al. Accelerated synthesis and discovery of covalent organic framework photocatalysts for hydrogen peroxide production. J. Am. Chem. Soc. 2022, 144, 9902–9909. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.C.; Wu, X.D.; Tang, L.; Chen, X.H.; Li, M.; Mou, Y.; Su, B.; Wang, S.B.; Feng, C.Y.; Liu, J.W.; et al. Dual donor-acceptor covalent organic frameworks for hydrogen peroxide photosynthesis. Nat. Commun. 2023, 14, 5238. [Google Scholar] [CrossRef]
- Das, P.; Chakraborty, G.; Roeser, J.; Vogl, S.; Rabeah, J.; Thomas, A. Integrating bifunctionality and chemical stability in covalent organic frameworks via one-pot multicomponent reactions for solar-driven H2O2 production. J. Am. Chem. Soc. 2023, 145, 2975–2984. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Huang, Y.; Ye, Y.-x. Construction of Conjugated Organic Polymers for Efficient Photocatalytic Hydrogen Peroxide Generation with Adequate Utilization of Water Oxidation. Materials 2024, 17, 2709. https://doi.org/10.3390/ma17112709
Liu Q, Huang Y, Ye Y-x. Construction of Conjugated Organic Polymers for Efficient Photocatalytic Hydrogen Peroxide Generation with Adequate Utilization of Water Oxidation. Materials. 2024; 17(11):2709. https://doi.org/10.3390/ma17112709
Chicago/Turabian StyleLiu, Qinzhe, Yuyan Huang, and Yu-xin Ye. 2024. "Construction of Conjugated Organic Polymers for Efficient Photocatalytic Hydrogen Peroxide Generation with Adequate Utilization of Water Oxidation" Materials 17, no. 11: 2709. https://doi.org/10.3390/ma17112709
APA StyleLiu, Q., Huang, Y., & Ye, Y.-x. (2024). Construction of Conjugated Organic Polymers for Efficient Photocatalytic Hydrogen Peroxide Generation with Adequate Utilization of Water Oxidation. Materials, 17(11), 2709. https://doi.org/10.3390/ma17112709



