Real-Time Spectroscopic Ellipsometry for Flux Calibrations in Multi-Source Co-Evaporation of Thin Films: Application to Rate Variations in CuInSe2 Deposition
Abstract
1. Introduction
2. Materials and Methods
3. Results
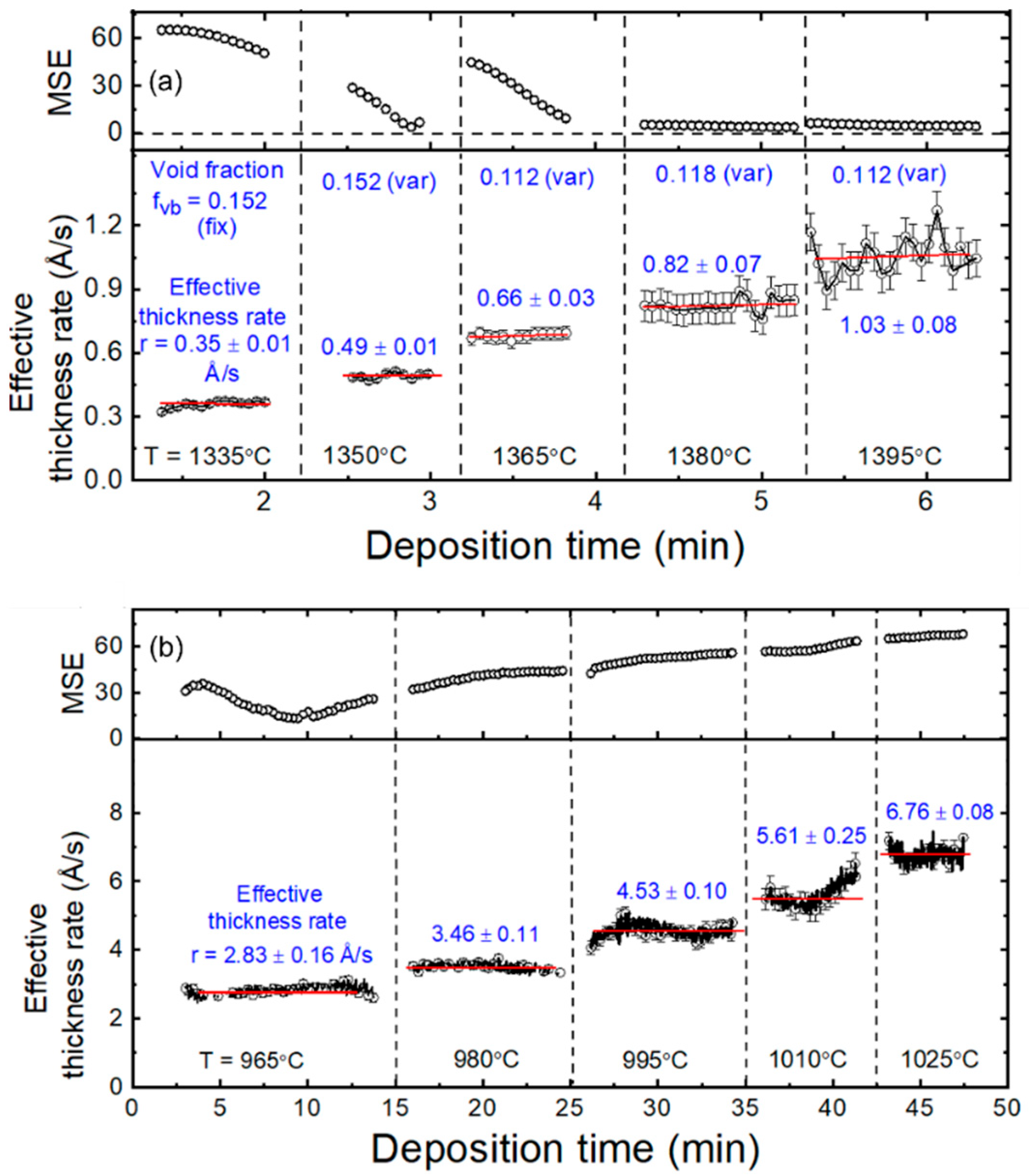
3.1. Real-Time Spectroscopic Ellipsometry: Cu and In2Se3 Calibration Depositions
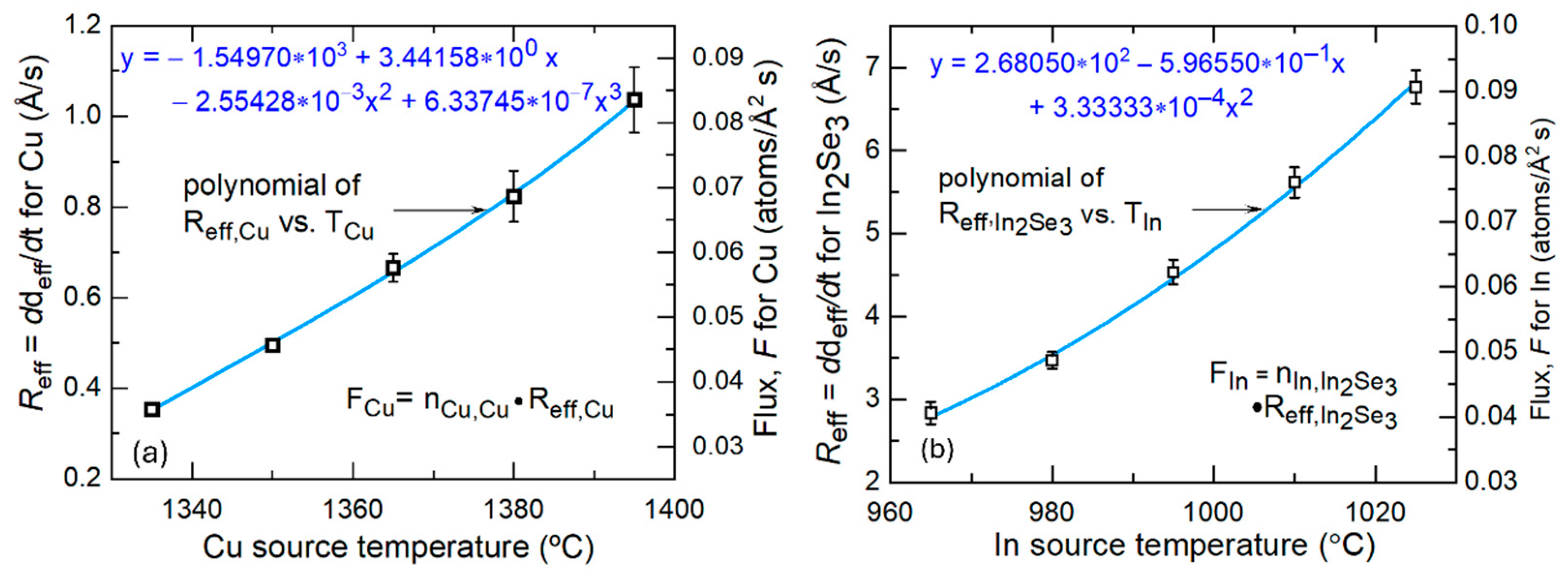
3.2. Cu and In Flux Analysis
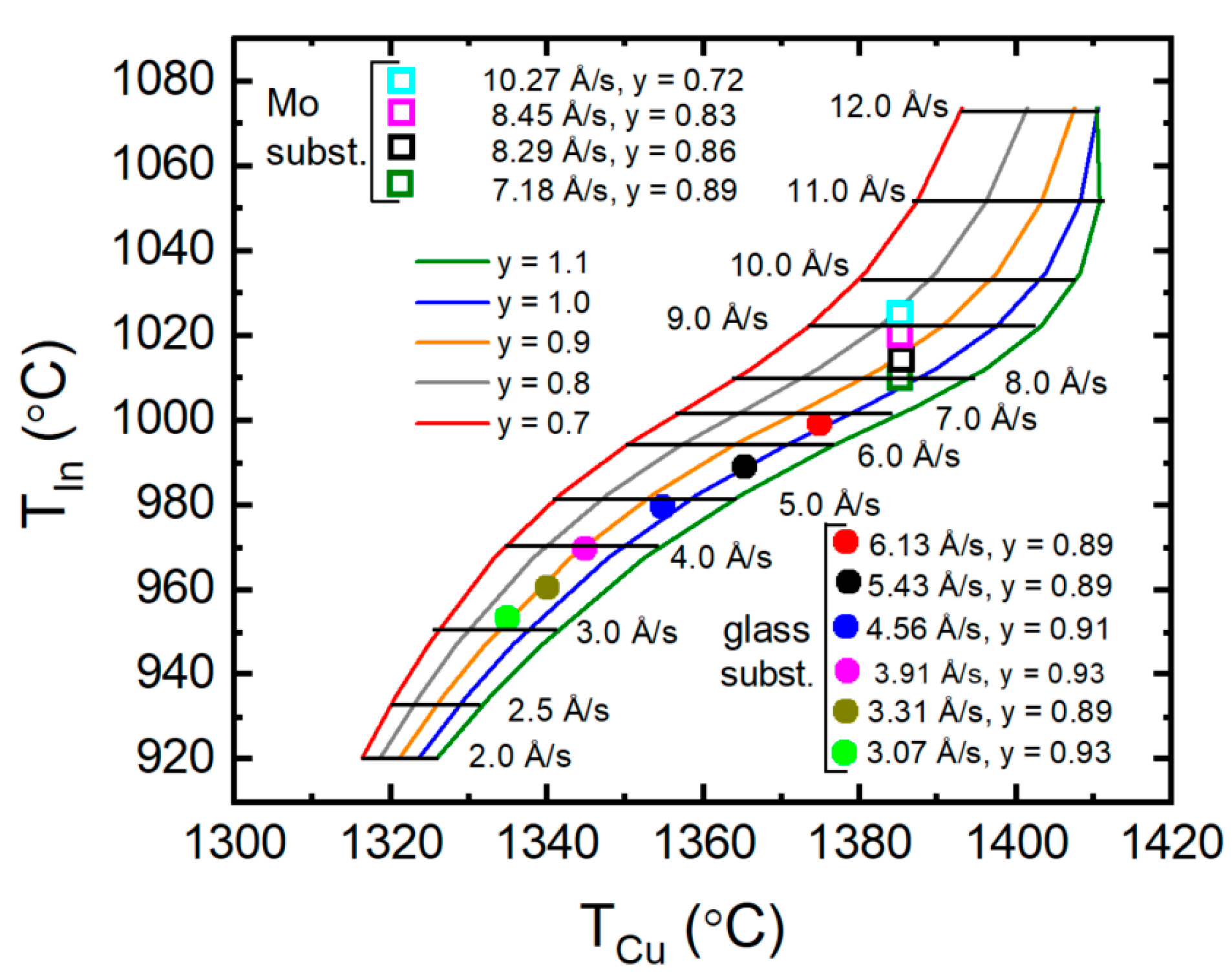
3.3. Development of Source Calibration for CIS Deposition
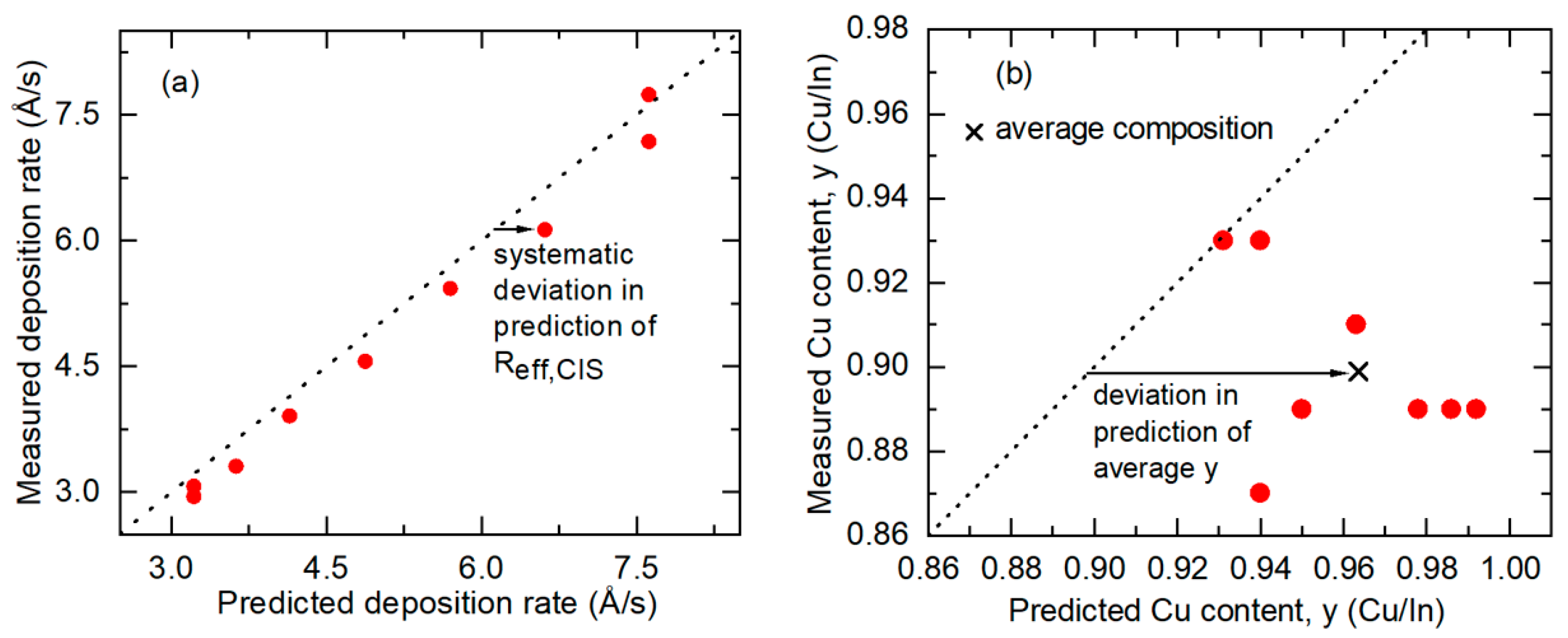
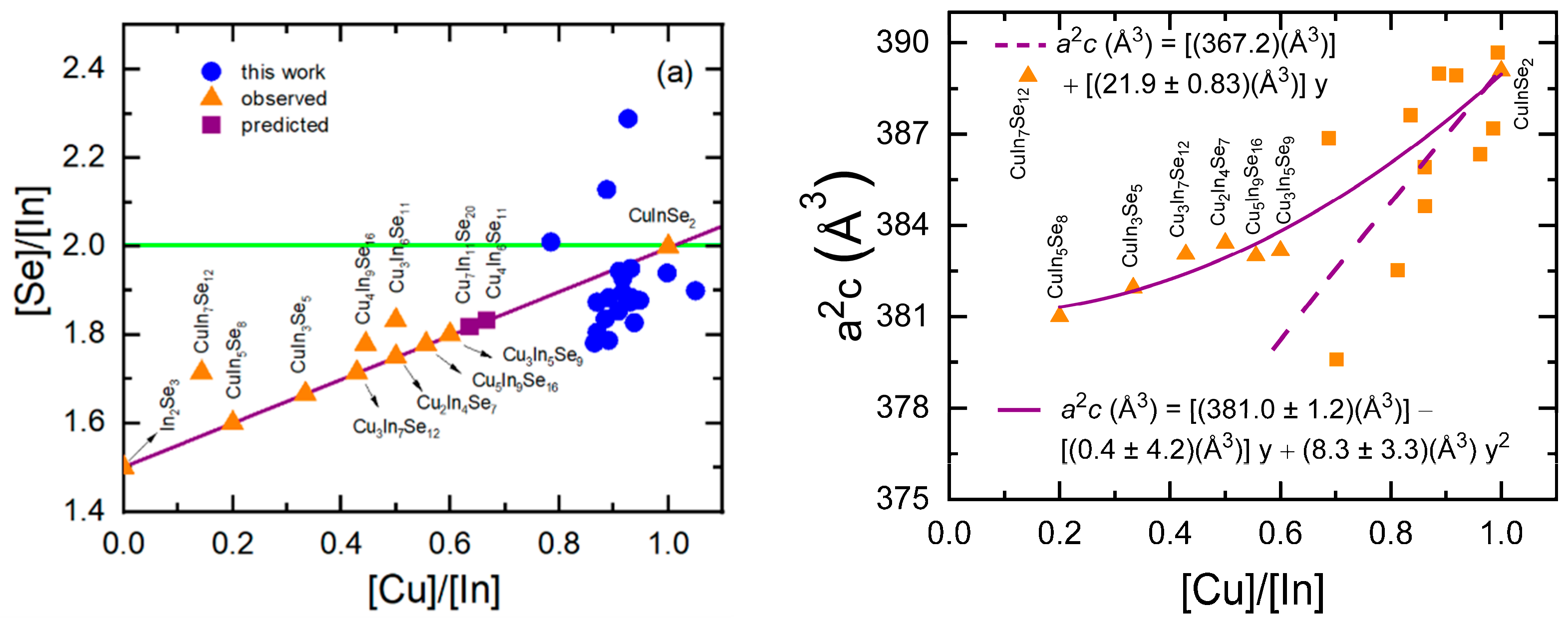
3.4. Atomic Concentration Corrections in Source Calibration for CIS Deposition
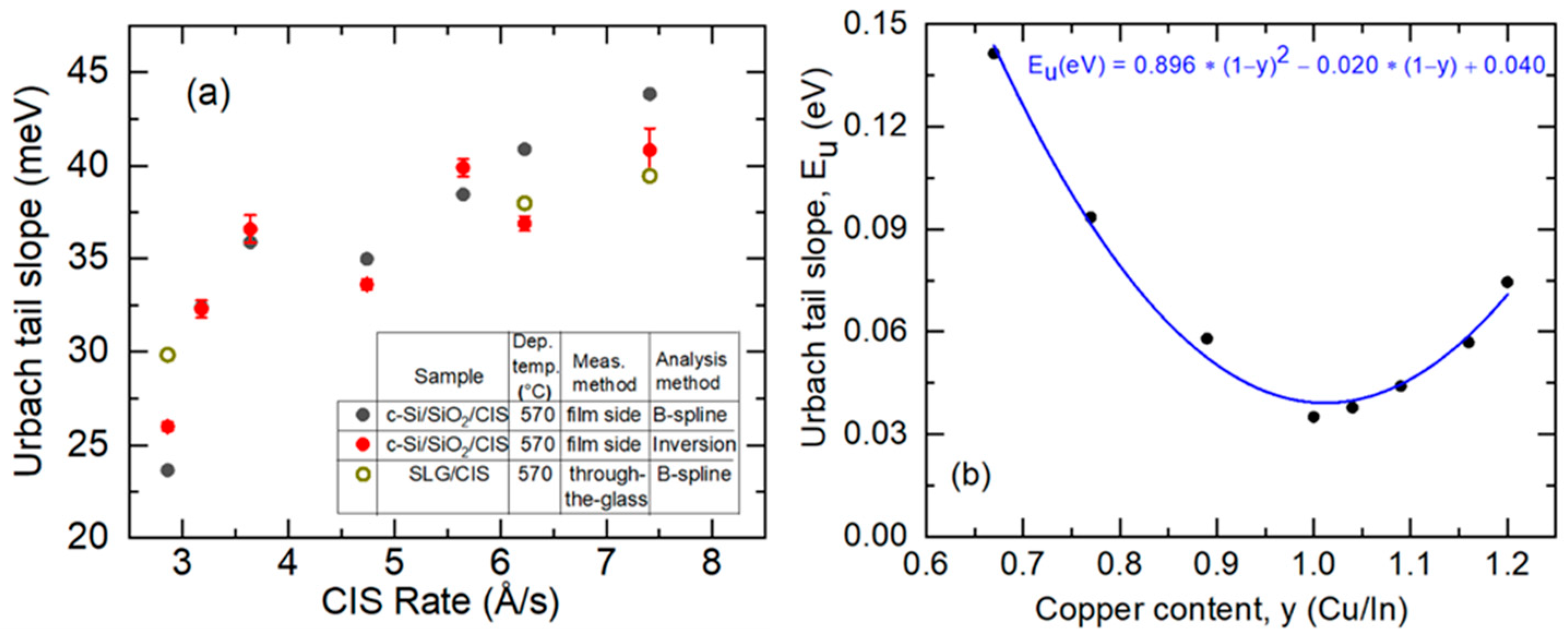
3.5. Structural and Photoelectronic Properties for CIS Films Deposited at Different Rates
4. Discussion
4.1. Calibration of CIS Deposition
4.2. Effect of Deposition Rate on the Properties of CIS
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vossen, J.L.; Kern, W. (Eds.) Thin Film Processes II; Academic: San Diego, CA, USA, 1991. [Google Scholar]
- Smith, D.L. Thin-Film Deposition: Principles and Practice; McGraw Hill: New York, NY, USA, 1995. [Google Scholar]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing; William Andrew: Norwich, NY, USA, 1998. [Google Scholar]
- Messier, R.; Troiler-McKinstry, S. Thin-film processes. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 9307–9313. [Google Scholar]
- Thornton, J.A. High rate thick film growth. Annu. Rev. Mater. Sci. 1977, 7, 239–260. [Google Scholar] [CrossRef]
- Messier, R.; Giri, A.P.; Roy, R.A. Revised structure zone model for thin film physical structure. J. Vac. Sci. Technol. A 1984, 2, 500–503. [Google Scholar] [CrossRef]
- Thompson, C.V. Structure evolution during processing of polycrystalline films. Annu. Rev. Mater. Sci. 2000, 30, 159–190. [Google Scholar] [CrossRef]
- Min, H.S. Characterization techniques for metal chalcogenide thin films: Review. Curr. Adv. Chem. Biochem. 2021, 1, 106–125. [Google Scholar]
- Miller, A.; MacKinnon, A.; Weaire, D. Beyond the binaries—The chalcopyrite and related semiconducting compounds. Solid State Phys. 1982, 36, 119–175. [Google Scholar]
- Shafarman, W.N.; Klenk, R.; McCandless, B.E. Device and material characterization of Cu(InGa)Se2 solar cells with increasing bandgap. J. Appl. Phys. 1996, 79, 7324–7328. [Google Scholar] [CrossRef]
- Marsillac, S.; Paulson, P.D.; Haimbodi, M.; Birkmire, R.; Shafarman, W. High-efficiency solar cells based on Cu(InAl)Se2 thin films. Appl. Phys. Lett. 2002, 81, 1350–1352. [Google Scholar] [CrossRef]
- Shafarman, W.N.; Siebentritt, S.; Stolt, L. Cu(InGa)Se2 solar cells. In Handbook of Photovoltaic Science and Engineering, 2nd ed.; Luque, A., Hegedus, S., Eds.; John Wiley & Sons: New York, NY, USA, 2011; pp. 546–599. [Google Scholar]
- Scheer, R.; Schock, H.-W. Chalcogenide Photovoltaics: Physics, Technologies, and Thin Film Devices; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Repins, I.; Vora, N.; Beall, C.; Wei, S.-H.; Yan, Y.; Romero, M.; Teeter, G.; Du, H.; To, B.; Young, M.; et al. Kesterites and chalcopyrites: A comparison of close cousins. In MRS Online Proceedings Library (OPL), Volume 1324: Symposium D–Compound Semiconductors for Energy Applications and Environmental Sustainability; Shahedipour-Sandvik, F., Bell, L.D., Jones, K., Simpkins, B., Schaadt, D., Contreras, M., Eds.; Materials Research Society: Warrenville, PA, USA, 2011; Paper mrss11-1324-d17-01. [Google Scholar]
- Repins, I.L.; Gomez, N.; Simpson, L.; Joshi, B. In situ sensors for CIGS deposition and manufacture. In MRS Online Proceedings Library (OPL), Volume 865: Symposium F–Thin-Film Compound Semiconductor Photovoltaics; Shafarman, W., Gessert, T., Niki, S., Siebentritt, S., Eds.; Materials Research Society: Warrenville, PA, USA, 2004; Article 153. [Google Scholar]
- Lu, C.; Blissett, C.D.; Diehl, G. An electron impact emission spectroscopy flux sensor for monitoring deposition rate at high background gas pressure with improved accuracy. J. Vac. Sci. Technol. A 2008, 26, 956–960. [Google Scholar] [CrossRef]
- Ibdah, A.-R.A.; Aryal, P.; Pradhan, P.; Marsillac, S.; Podraza, N.J.; Collins, R.W. Real time and in-situ spectroscopic ellipsometry of CuyIn1−xGaxSe2 for complex dielectric function determination and parameterization. In Spectroscopic Ellipsometry for Photovoltaics, Volume 1; Fujiwara, H., Collins, R., Eds.; Springer: Cham, Switzerland, 2018; Chapter 11; pp. 281–331. [Google Scholar]
- Pradhan, P.; Ibdah, A.-R.A.; Aryal, P.; Attygalle, D.; Podraza, N.J.; Marsillac, S.; Collins, R.W. Real time measurement, monitoring, and control of CuIn1−xGaxSe2 by spectroscopic ellipsometry. In Spectroscopic Ellipsometry for Photovoltaics, Volume 2; Fujiwara, H., Collins, R., Eds.; Springer: Cham, Switzerland, 2018; Chapter 6; pp. 209–253. [Google Scholar]
- Sapkota, D.R.; Pradhan, P.; Koirala, P.; Irving, R.; Phillips, A.B.; Ellingson, R.J.; Heben, M.J.; Marsillac, S.; Podraza, N.J.; Collins, R.W. Structural and optical properties of two-stage CuInSe2 thin films studied by real time spectroscopic ellipsometry. In Proceedings of the IEEE 46th Photovoltaic Specialists (PVSC), Chicago, IL, USA, 16–21 June 2019; IEEE: New York, NY, USA, 2019; pp. 943–948. [Google Scholar]
- Sapkota, D.R.; Pradhan, P.; Irving, R.; Grice, C.R.; Phillips, A.B.; Heben, M.J.; Marsillac, S.; Podraza, N.J.; Collins, R.W. Evaluation of CuInSe2 materials and solar cells co-evaporated at different rates based on real time spectroscopic ellipsometry calibrations. In Proceedings of the IEEE 48th Photovoltaic Specialists (PVSC), Fort Lauderdale, FL, USA, 20–25 June 2021; IEEE: New York, NY, USA, 2021; pp. 451–458. [Google Scholar]
- Fujiwara, H.; Koh, J.; Rovira, P.I.; Collins, R.W. Assessment of effective-medium theories in the analysis of nucleation and microscopic surface roughness evolution for semiconductor thin films. Phys. Rev. B 2000, 61, 10832–10844. [Google Scholar] [CrossRef]
- Kasap, S.O. Principles of Electronic Materials and Devices, 3rd ed.; McGraw-Hill: New York, NY, USA, 2006; pp. 303–305. [Google Scholar]
- Straumanis, M.E.; Yu, L.S. Lattice parameters, densities, expansion coefficients and perfection of structure of Cu and of Cu–In α phase. Acta Crystallogr. A 1969, 25, 676–682. [Google Scholar] [CrossRef]
- Pradhan, P.; Aryal, P.; Attygalle, D.; Ibdah, A.-R.; Koirala, P.; Li, J.; Bhandari, K.P.; Liyanage, G.K.; Ellingson, R.J.; Heben, M.J.; et al. Real time spectroscopic ellipsometry analysis of first stage CuIn1−xGaxSe2 growth: Indium-gallium selenide co-evaporation. Materials 2018, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Pfitzner, A.; Lutz, H.D. Redetermination of the crystal structure of γ-In2Se3 by twin crystal x-ray method. J. Solid State Chem. 1996, 124, 305–308. [Google Scholar] [CrossRef]
- Tonejc, A.; Popović, S.; Gržeta-Plenković, B. Phases, lattice parameters and thermal expansion of (GaxIn1−x)2Se3, 1 ≥ x ≥ 0, between room temperature and melting point. J. Appl. Cryst. 1980, 13, 24–30. [Google Scholar] [CrossRef]
- Knight, K.S. The crystal structures of CuInSe2 and CuInTe2. Mater. Res. Bull. 1992, 27, 161–167. [Google Scholar] [CrossRef]
- Paszkowicz, W.; Minikayev, R.; Piszora, P.; Trots, D.; Knapp, M.; Wojciechowski, T.; Bacewicz, R. Thermal expansion of CuInSe2 in the 11–1,073 K range: An X-ray diffraction study. Appl. Phys. A 2014, 116, 767–780. [Google Scholar] [CrossRef]
- Uprety, P.; Wang, C.; Koirala, P.; Sapkota, D.R.; Ghimire, K.; Junda, M.M.; Yan, Y.; Collins, R.W.; Podraza, N.J. Optical Hall effect of PV device materials. IEEE J. Photovolt. 2018, 8, 1793–1799. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.-H.; Zunger, A. Stabilization of ternary compounds via ordered arrays of defect pairs. Phys. Rev. Lett. 1997, 78, 4059–4062. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.-H.; Zunger, A.; Katayama-Yoshida, H. Defect physics of the CuInSe2 chalcopyrite semiconductor. Phys. Rev. B 1998, 57, 9642–9656. [Google Scholar] [CrossRef]
- Hahn, H.; Frank, G.; Klingler, W.; Meyer, A.-D.; Störger, G. Untersuchungen über ternäre Chalkogenide. V. Über einige ternäre Chalkogenide mit Chalkopyritstruktur. Z. Anorg. Allg. Chem. 1953, 271, 153–170. [Google Scholar] [CrossRef]
- Sapkota, D.R.; Koirala, P.; Pradhan, P.; Shrestha, N.; Junda, M.M.; Phillips, A.B.; Ellingson, R.J.; Heben, M.J.; Marsillac, S.; Podraza, N.J.; et al. Spectroscopic ellipsometry investigation of CuInSe2 as a narrow bandgap component of thin film tandem solar cells. In Proceedings of the 7th World Conference on Photovoltaic Energy Conversion (WCPEC; A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), Waikoloa, HI, USA, 10–15 June 2018; IEEE: New York, NY, USA, 2018; pp. 1943–1948. [Google Scholar]
- Sapkota, D.R. Characterization and Optimization of CuInSe2 Solar Cells Applicable for Tandem Devices. Doctoral Dissertation, University of Toledo, Toledo, OH, USA, 2022. [Google Scholar]
- Rincón, C.; Márquez, R. Defect physics of the CuInSe2 chalcopyrite semiconductor. J. Phys. Chem. Solids 1999, 60, 1865–1873. [Google Scholar] [CrossRef]
- Niki, S.; Suzuki, R.; Ishibashi, S.; Ohdaira, T.; Fons, P.J.; Yamada, A.; Oyanagi, H.; Wada, T.; Kimura, R.; Nakada, T. Anion vacancies in CuInSe2. Thin Solid Films 2001, 387, 129–134. [Google Scholar] [CrossRef]
- Lany, S.; Zunger, A. Light-and bias-induced metastabilities in Cu(In,Ga)Se2 based solar cells caused by the (VSe-VCu) vacancy complex. J. Appl. Phys. 2006, 100, 113725. [Google Scholar] [CrossRef]
- Oikkonen, L.E.; Ganchenkova, M.G.; Seitsonen, A.P.; Nieminen, R.M. Vacancies in CuInSe2: New insights from hybrid-functional calculations. J. Phys. Condens. Matter 2011, 23, 422202. [Google Scholar] [CrossRef] [PubMed]
- Rincón, C.; Wasim, S.M.; Marin, G.; Delgado, J.M.; Contreras, J. Effect of ordered arrays of native defects on the crystal structure of In- and Ga-rich Cu-ternaries. Appl. Phys. Lett. 2003, 83, 1328–1330. [Google Scholar] [CrossRef]
- Hamzah, Y.; Salomo, S. Structure and photoluminescence of the ordered vacancy compound Cu3In7Se12. Kontribusi Fis. Indones. 2002, 13, 211–213. [Google Scholar]
- Philip, R.R.; Pradeep, B. Structural analysis and optical and electrical characterization of the ordered defect compound CuIn5Se8. Semicond. Sci. Technol. 2003, 18, 768–773. [Google Scholar] [CrossRef]
- Xu, C.-M.; Xu, X.L.; Xu, J.; Yang, X.-J.; Zuo, J.; Kong, N.; Huang, W.-H.; Liu, H.-T. Composition dependence of the Raman A1 mode and additional mode in tetragonal Cu–In–Se thin films. Semicond. Sci. Technol. 2004, 19, 1201–1206. [Google Scholar] [CrossRef]
- Philip, R.R.; Pradeep, B.; Shripathi, T. Spin orbit and tetragonal crystalline field interaction in the valence band of CuInSe2-related ordered vacancy compound CuIn7Se12. Phys. Stat. Solidi 2005, 242, 1027–1035. [Google Scholar] [CrossRef]
- Fearheiley, M.L.; Bachmann, K.J.; Shing, Y.-H.; Vasquez, S.A.; Herrington, C.R. The lattice constants of CuInSe2. J. Electron. Mater. 1985, 14, 677–683. [Google Scholar] [CrossRef]
- Hanna, G.; Mattheis, J.; Laptev, V.; Yamamoto, Y.; Rau, U.; Schock, H.W. Influence of the selenium flux on the growth of Cu(In,Ga)Se2 thin films. Thin Solid Films 2003, 431–432, 31–36. [Google Scholar] [CrossRef]
- Aryal, K.; Rajan, G.; Ashrafee, T.; Ranjan, V.; Li, J.; Rockett, A.; Collins, R.W.; Marsillac, S. Effect of selenium evaporation rate on ultrathin Cu(In,Ga)Se2 films. In Proceedings of the IEEE 40th Photovoltaic Specialists (PVSC), Denver, CO, USA, 8–13 June 2014; IEEE: New York, NY, USA, 2014; pp. 314–317. [Google Scholar]
- Liu, H.; Wang, L.; Xiao, X.; Hemley, R.J. Anomalous high-pressure behavior of amorphous selenium from synchrotron x-ray diffraction and microtomography. Proc. Natl. Acad. Sci. USA 2008, 105, 13229–13234. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.L.; Tell, B.; Kasper, H.M.; Schiavone, L.M. Electronic structure of AgInSe2 and CuInSe2. Phys. Rev. B 1973, 7, 4485–4490. [Google Scholar] [CrossRef]
- Kazmerski, L.L.; Hallerdt, M.; Ireland, P.J.; Mickelsen, R.A.; Chen, W.S. Optical properties and grain boundary effects in CuInSe2. J. Vac. Sci. Technol. A 1983, 1, 395–398. [Google Scholar] [CrossRef]
- Neumann, H.; Tomlinson, R.D. Band-gap narrowing in n-type CuInSe2 single crystals. Solid State Commun. 1986, 57, 591–594. [Google Scholar] [CrossRef]
- Alonso, M.I.; Garriga, M.; Durante Rincón, C.A.; Hernández, E.; León, M. Optical functions of chalcopyrite CuGaxIn1−xSe2 alloys. Appl. Phys. A 2002, 74, 659–664. [Google Scholar] [CrossRef]
- Shirakata, S.; Chichibu, S.; Hideto, M.; Isomura, S.; Nakanishi, H.; Sugiyama, K. Electroreflectance of CuInSe2, CuIn3Se5 and Cu2In4Se7. In Ternary and Multinary Compounds: Proceedings of the 11th International Conference; Tomlinson, R.D., Hill, A.E., Pilkington, R.D., Eds.; CRC: Boca Raton, FL, USA, 1998; pp. 597–600. [Google Scholar]
- Chichibu, S.; Mizutani, T.; Murakami, K.; Shioda, T.; Kurafuji, T.; Nakanishi, H.; Niki, S.; Fons, P.J.; Yamada, A. Band gap energies of bulk, thin-film, and epitaxial layers of CuInSe2 and CuGaSe2. J. Appl. Phys. 1998, 83, 3678–3689. [Google Scholar] [CrossRef]
- Alonso, M.I.; Wakita, K.; Pascual, J.; Garriga, M.; Yamamoto, N. Optical functions and electronic structure of CuInSe2, CuGaSe2, CuInS2, and CuGaS2. Phys. Rev. B 2001, 63, 075203. [Google Scholar] [CrossRef]
- Shirakata, S.; Chichibu, S.; Isomura, S.; Nakanishi, H. Electroreflectance of CuInSe2 single crystals. Jpn. J. Appl. Phys. 1997, 36, L543–L546. [Google Scholar] [CrossRef]
- González, J.; Rincón, C. Optical absorption and phase transitions in CulnSe2 and CulnS2 single crystals at high pressure. J. Appl. Phys. 1989, 65, 2031–2034. [Google Scholar] [CrossRef]
- AbuShama, J.; Noufi, R.; Johnston, S.; Ward, S.; Wu, X. Improved performance in CuInSe2 and surface modified CuGaSe2 solar cells. In Proceedings of the IEEE 31st Photovoltaic Specialists Conference (PVSC), Lake Buena Vista, FL, USA, 3–7 January 2005; IEEE: New York, NY, USA, 2005; pp. 299–302. [Google Scholar]
- Gabor, A.M.; Tuttle, J.R.; Bode, M.H.; Franz, A.; Tennant, A.L.; Contreras, M.A.; Noufi, R.; Jensen, D.G.; Hermann, A.M. Band-gap engineering in Cu(In,Ga)Se2 thin films grown from (In,Ga)2Se3 precursors. Solar Energy Mater. Solar Cells 1996, 41–42, 247–260. [Google Scholar] [CrossRef]


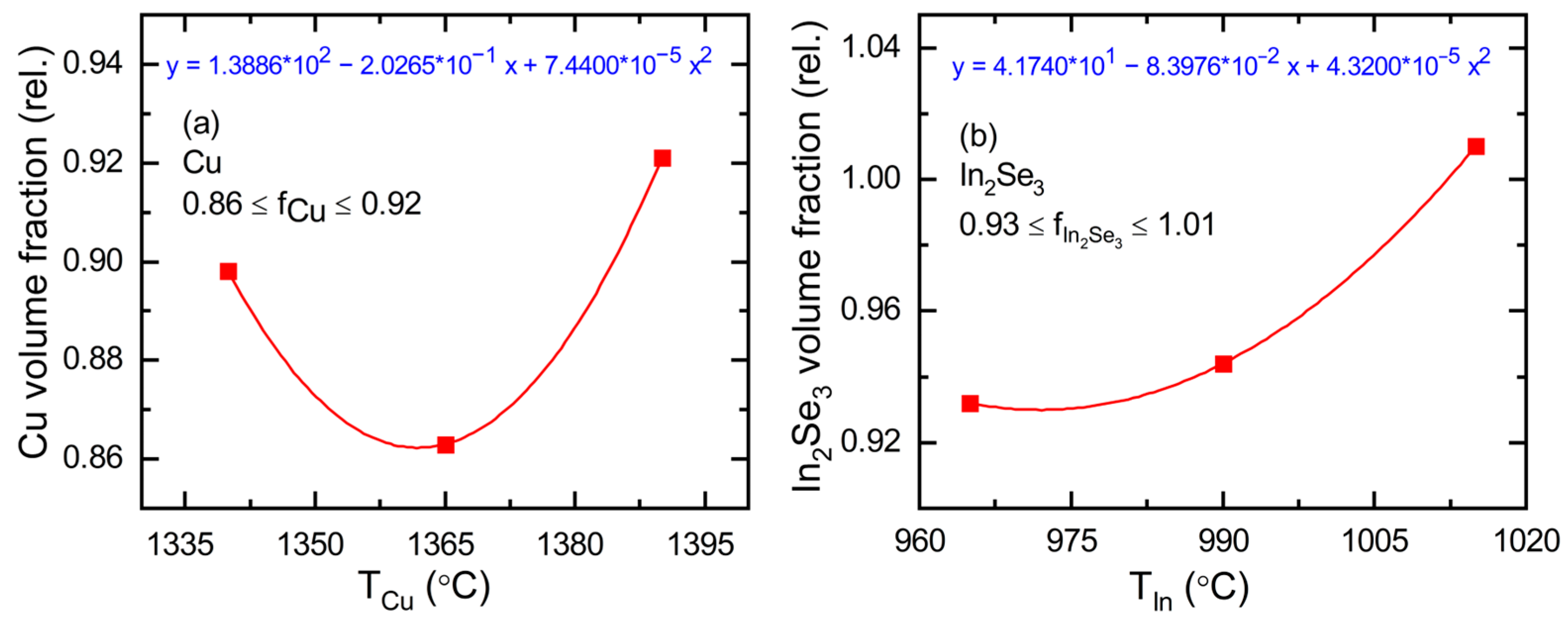
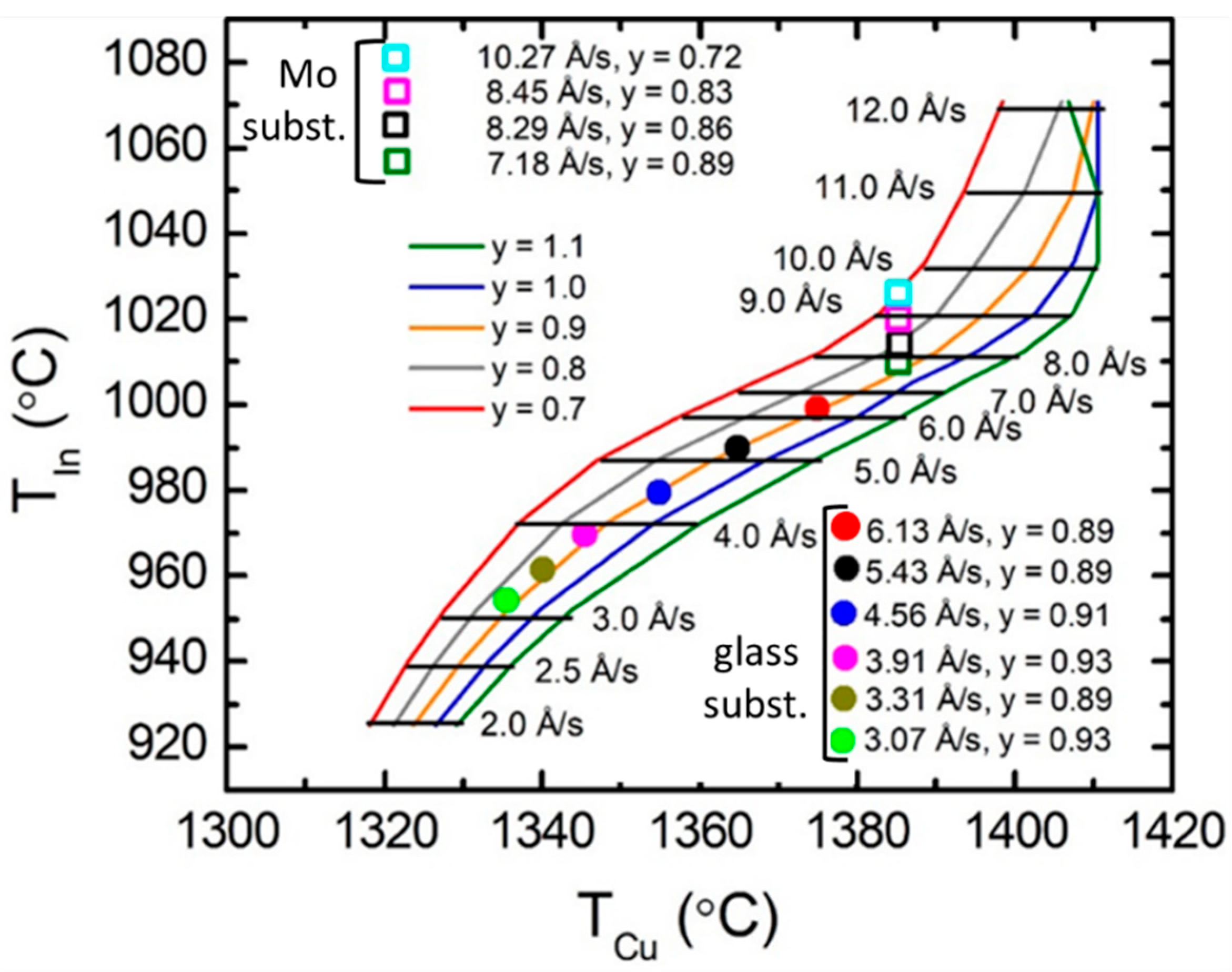

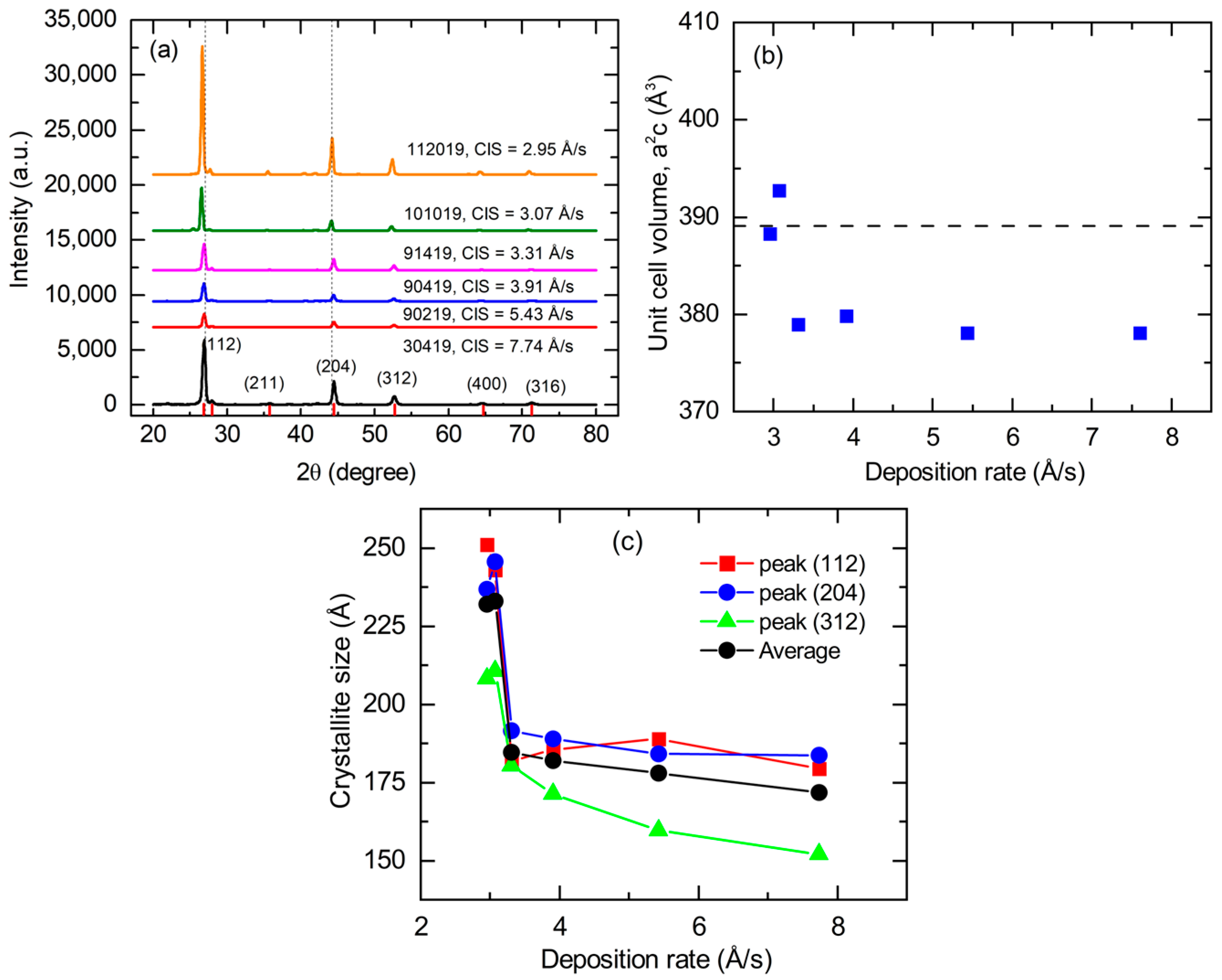

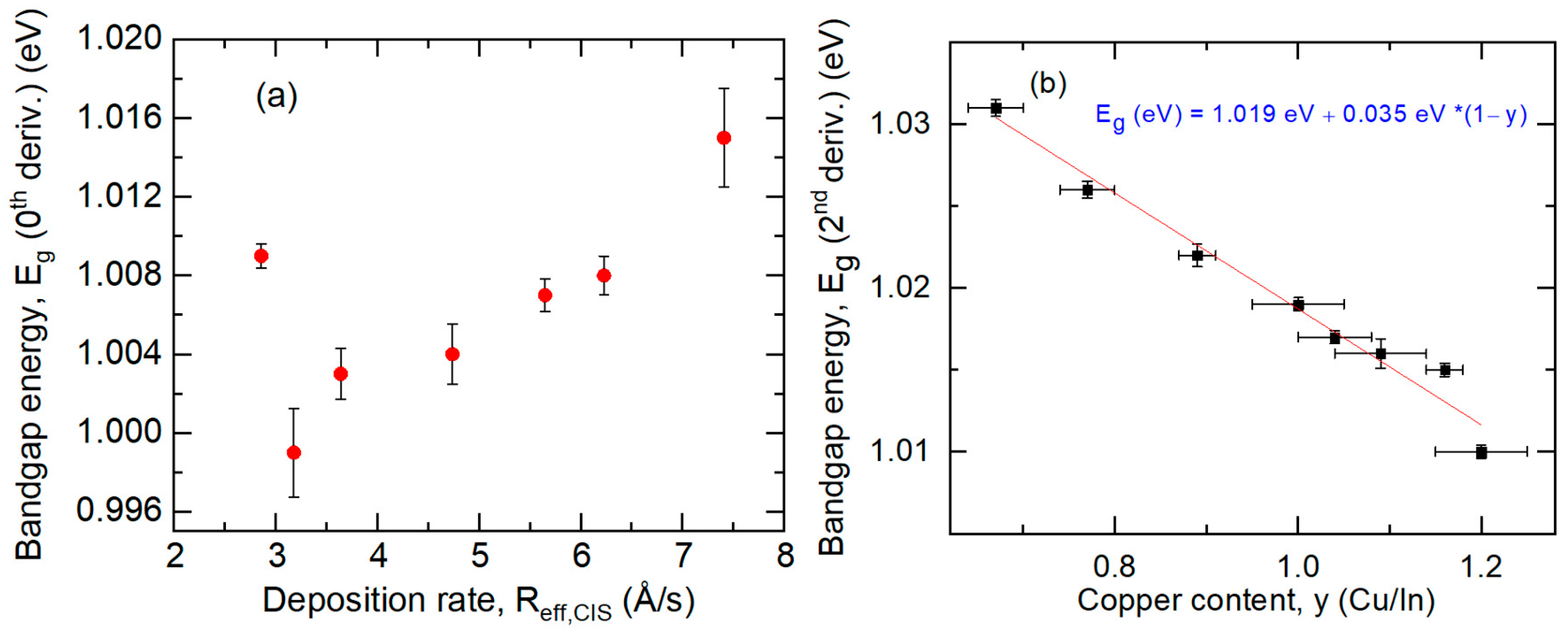

| Material and Its Thin Film Deposition Temperature T | Crystal Structure | Lattice Parameters at 20 °C [Ref.] (Å) | Number of Metal Atoms per Unit Cell | Concentration of Metal Atoms at RT (Å−3) | Thermal Expansion Coefficient α(T) = P1 (10−6 K−1) + P2 (K)/T2 [Ref.] | Concentration of Atoms at Temperature T (Å−3) |
|---|---|---|---|---|---|---|
| Cu T = 20 °C | face-centered cubic | a = 3.6147 [23] | 4 | 0.084692 | P1 = 14.87 [23] | 0.084692 |
| In2Se3 T = 570 °C | defect wurtzite | a = 7.1236 c = 19.381 [25] | 12 | 0.014069 | P1(a) = 5; P1(c) = 16 P2(a) ~ 0; P2(c) ~ 0 [26] | 0.013869 |
| CuInSe2 T = 570 °C | chalco- pyrite | a = 5.7810 c = 11.6422 [27] | Cu: 4y In: 4 (assumed) | Cu: 0.010281y In: 0.010281 | P1(a) = 9.15; P1(c) = 7.61 P2(a) ~ 0; P2(c) ~ 0 [28] | Cu: 0.010135y In: 0.010135 |
| Material, m, and Indept. Variable | cm,0 (°C) or dm,0 (°C) | cm,1 (°C s/Å) or dm,1 (°C Å2 s) | cm,2 (°C s2/Å2) or dm,2 (°C Å4 s2) | cm,3 (°C s3/Å3) or dm,3 (°C Å6 s3) |
|---|---|---|---|---|
| Cu; Reff,Cu,Cu | 1.29980 × 103 | 9.59360 × 101 | 2.00584 × 101 | −2.30861 × 101 |
| Cu; FCu | 1.29980 × 103 | 1.13276 ×103 | 2.79648 × 103 | −3.80034 × 104 |
| In2Se3; Reff,In,In2Se3 | 8.40985 × 102 | 6.85232 × 101 | −1.05532 × 101 | 6.57389 × 10−1 |
| In; FIn | 8.40985 × 102 | 4.94075 × 103 | −5.48649 × 104 | 2.46426 × 105 |
| Material, m, and property | am,0 (Å s−1) or bm,0 (Å−2 s−1) | am,1 (Å s−1 °C−1) or bm,1 (Å−2 s−1 °C−1) | am,2 (Å s−1 °C−2) or bm,2 (Å−2 s−1 °C−2) | am,3 (Å s−1 °C−3) or bm,3 (Å−2 s−1 °C−3) |
|---|---|---|---|---|
| Cu; Reff,Cu,Cu | −1.54970 × 103 | 3.44158 × 100 | −2.55428 × 10−3 | 6.33745 × 10−7 |
| Cu; FCu | −1.31247 × 102 | 2.91475 ×10−1 | −2.16327 × 10−4 | 5.36731 × 10−8 |
| In2Se3; Reff,In,In2Se3 | 2.68050 × 102 | −5.96550 × 10−1 | 3.33333 × 10−4 | -- |
| In; FIn | 3.71758 × 100 | −8.27355 × 10−3 | 4.62300 × 10−6 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapkota, D.R.; Ramanujam, B.; Pradhan, P.; Alaani, M.A.R.; Shan, A.; Heben, M.J.; Marsillac, S.; Podraza, N.J.; Collins, R.W. Real-Time Spectroscopic Ellipsometry for Flux Calibrations in Multi-Source Co-Evaporation of Thin Films: Application to Rate Variations in CuInSe2 Deposition. Materials 2024, 17, 4048. https://doi.org/10.3390/ma17164048
Sapkota DR, Ramanujam B, Pradhan P, Alaani MAR, Shan A, Heben MJ, Marsillac S, Podraza NJ, Collins RW. Real-Time Spectroscopic Ellipsometry for Flux Calibrations in Multi-Source Co-Evaporation of Thin Films: Application to Rate Variations in CuInSe2 Deposition. Materials. 2024; 17(16):4048. https://doi.org/10.3390/ma17164048
Chicago/Turabian StyleSapkota, Dhurba R., Balaji Ramanujam, Puja Pradhan, Mohammed A. Razooqi Alaani, Ambalanath Shan, Michael J. Heben, Sylvain Marsillac, Nikolas J. Podraza, and Robert W. Collins. 2024. "Real-Time Spectroscopic Ellipsometry for Flux Calibrations in Multi-Source Co-Evaporation of Thin Films: Application to Rate Variations in CuInSe2 Deposition" Materials 17, no. 16: 4048. https://doi.org/10.3390/ma17164048
APA StyleSapkota, D. R., Ramanujam, B., Pradhan, P., Alaani, M. A. R., Shan, A., Heben, M. J., Marsillac, S., Podraza, N. J., & Collins, R. W. (2024). Real-Time Spectroscopic Ellipsometry for Flux Calibrations in Multi-Source Co-Evaporation of Thin Films: Application to Rate Variations in CuInSe2 Deposition. Materials, 17(16), 4048. https://doi.org/10.3390/ma17164048








