Oxidation Performance of Nano-Layered (AlTiZrHfTa)Nx/SiNx Coatings Deposited by Reactive Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
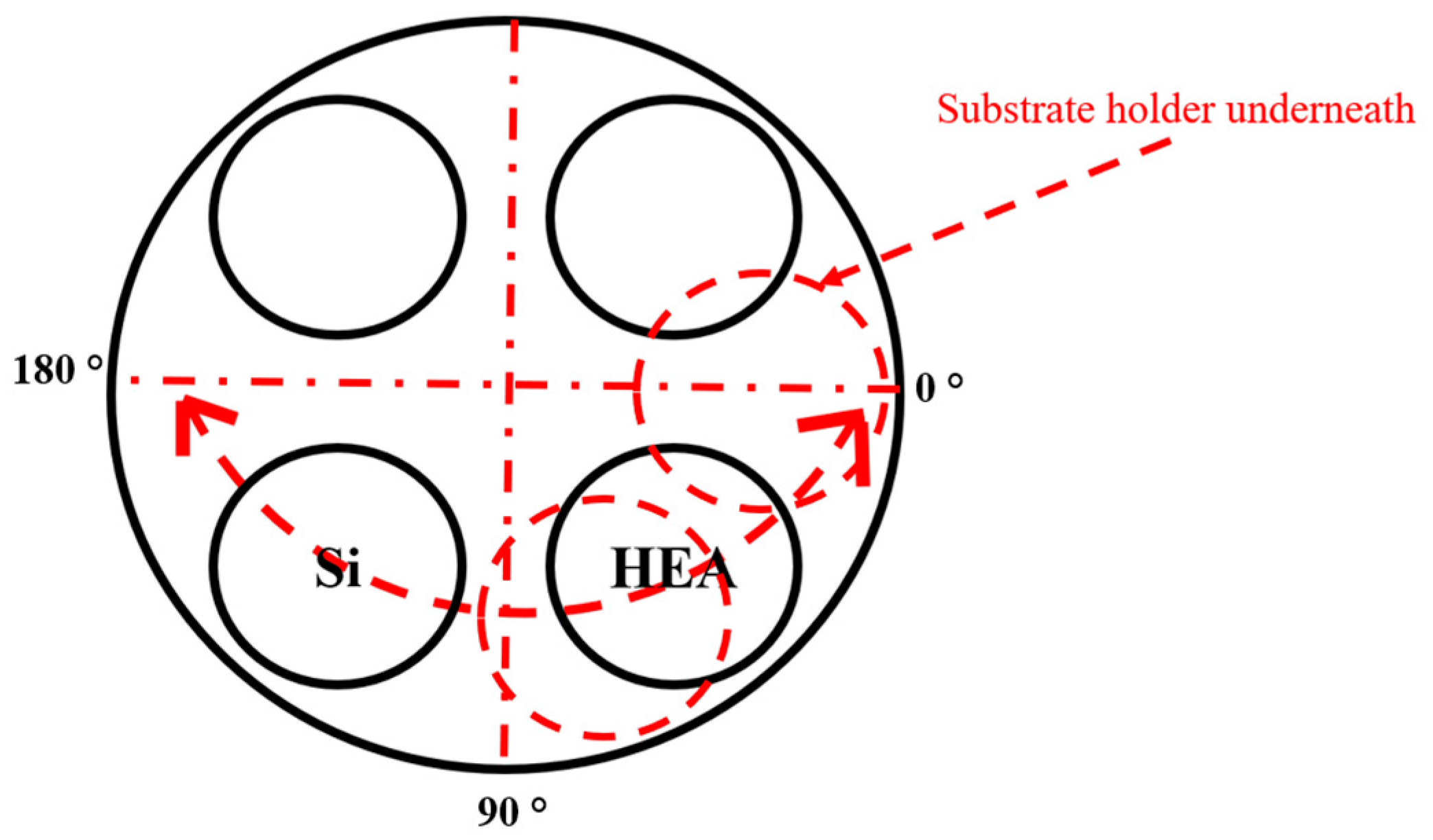
2.1. Deposition of the Coatings
2.2. Sample Analysis
2.2.1. Structure and Microstructure Characterization
2.2.2. Mechanical Properties
2.2.3. High-Temperature Oxidation Tests
3. Results and Discussion
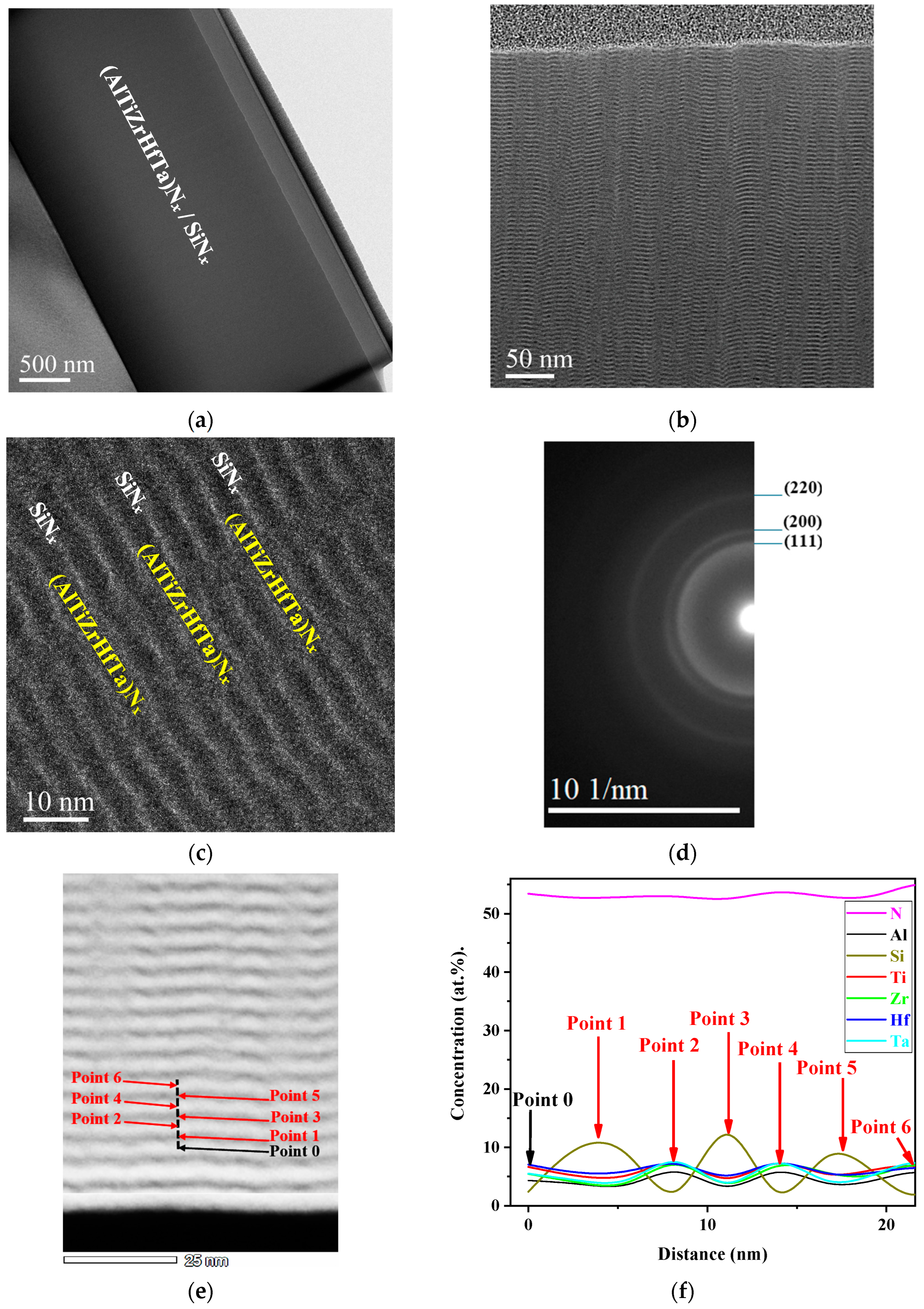
3.1. Microstructure of (AlTiZrHfTa)Nx/SiNx Thin Coatings
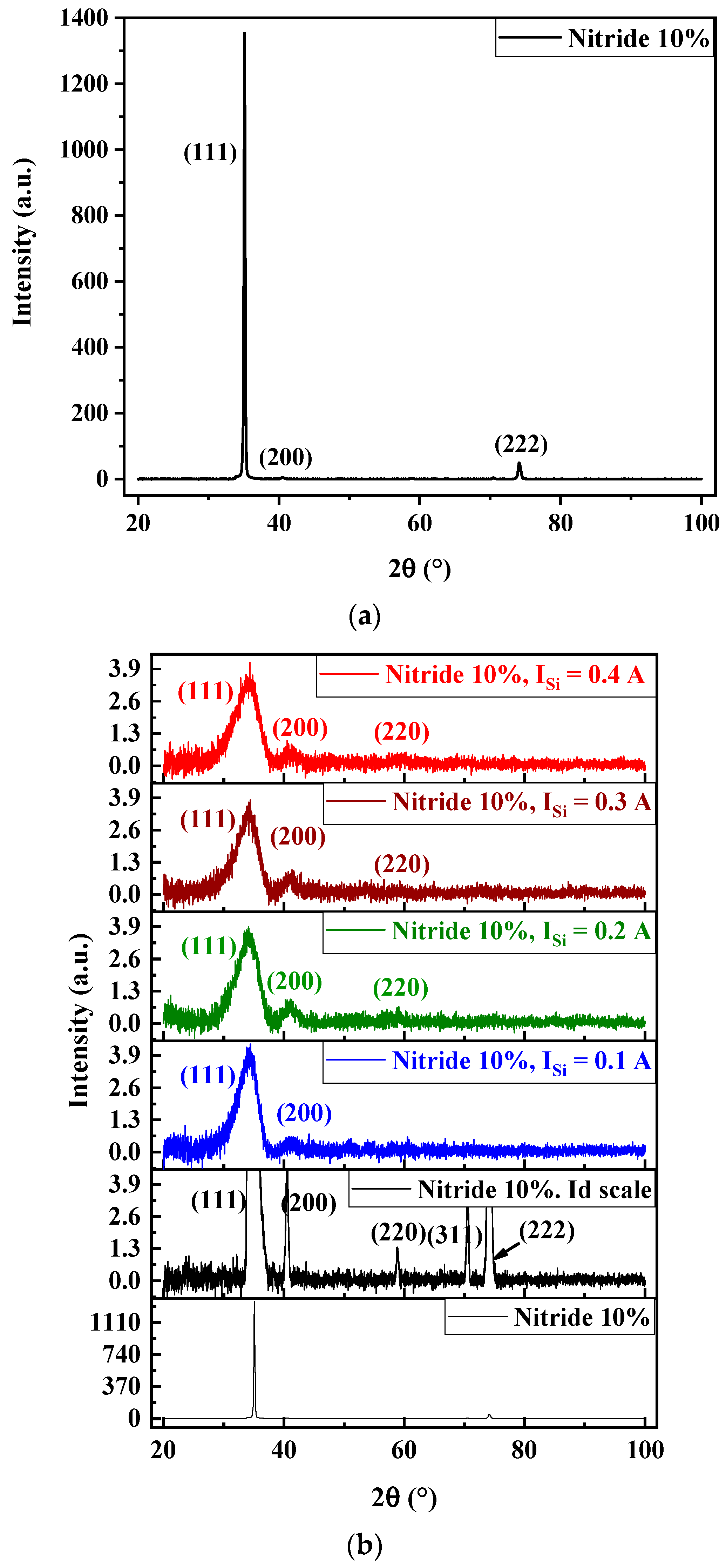
3.2. Structure and Microstructure
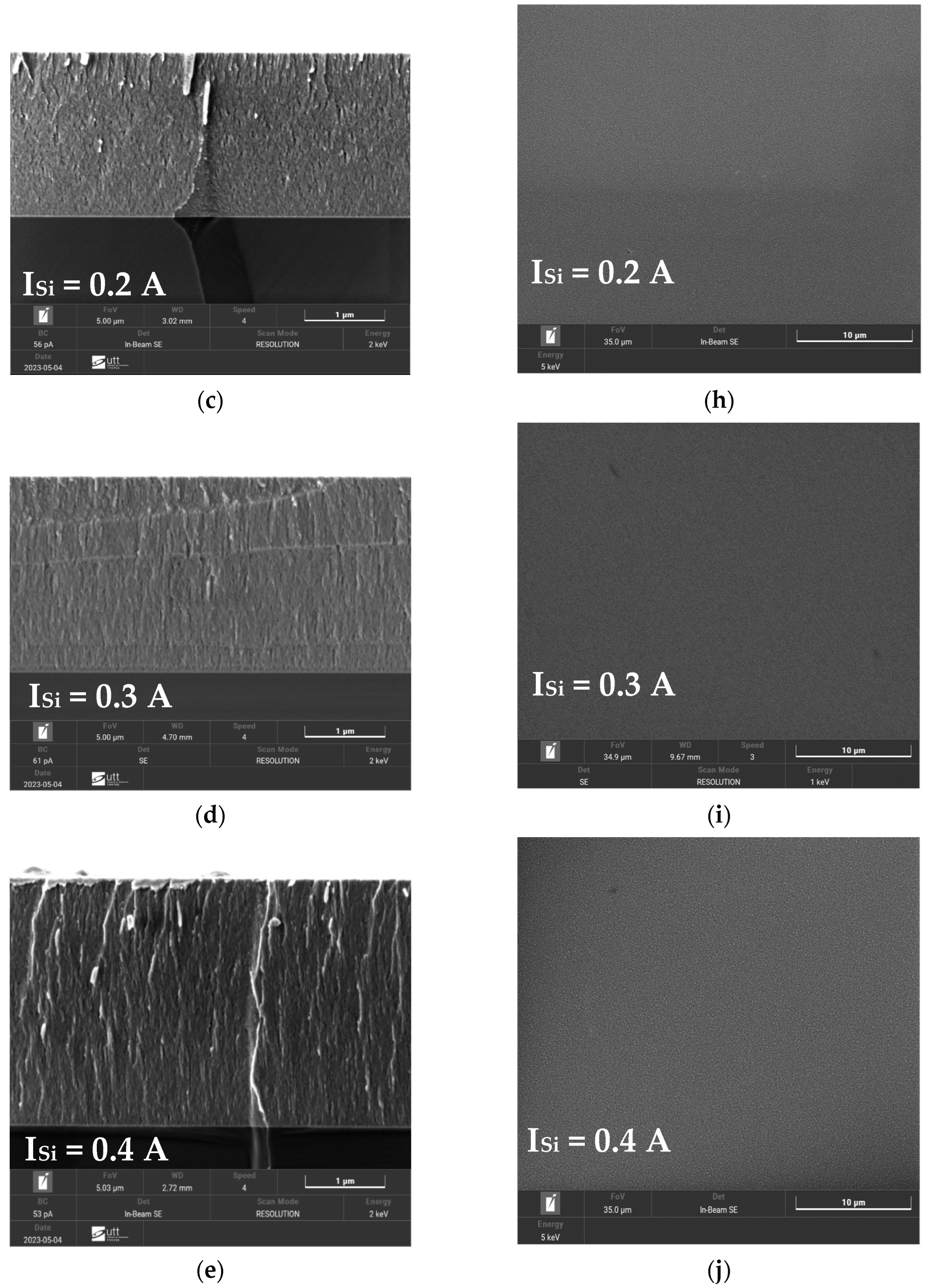
3.3. Morphology of FCC (AlTiZrHfTa)Nx/a-SiNx Thin Coatings
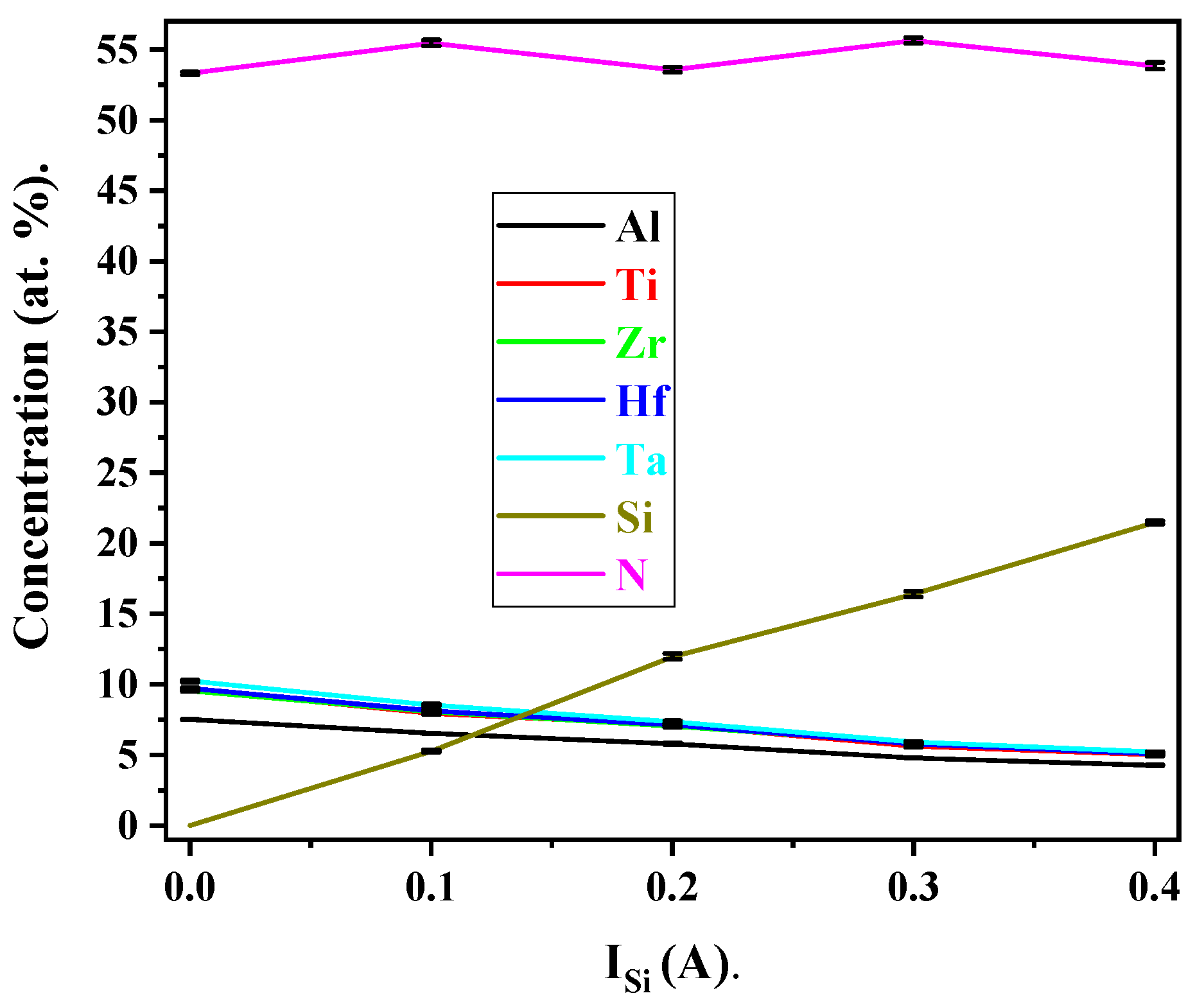
3.4. Chemical Composition
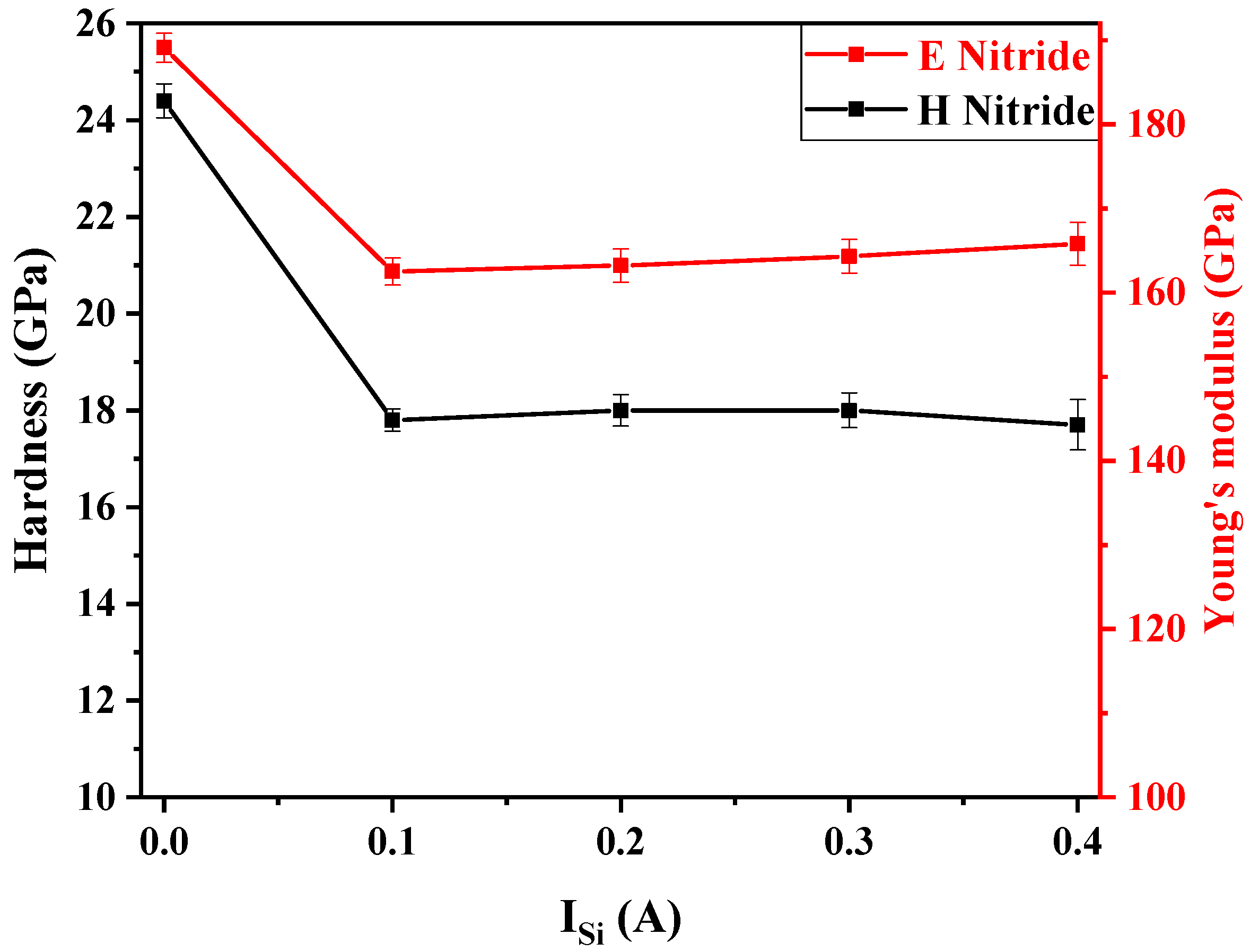
3.5. Mechanical Properties
Hardness and Young’s Modulus of FCC(AlTiZrHfTa)Nx/a-SiNx Thin Coatings
3.6. High-Temperature Oxidation Property
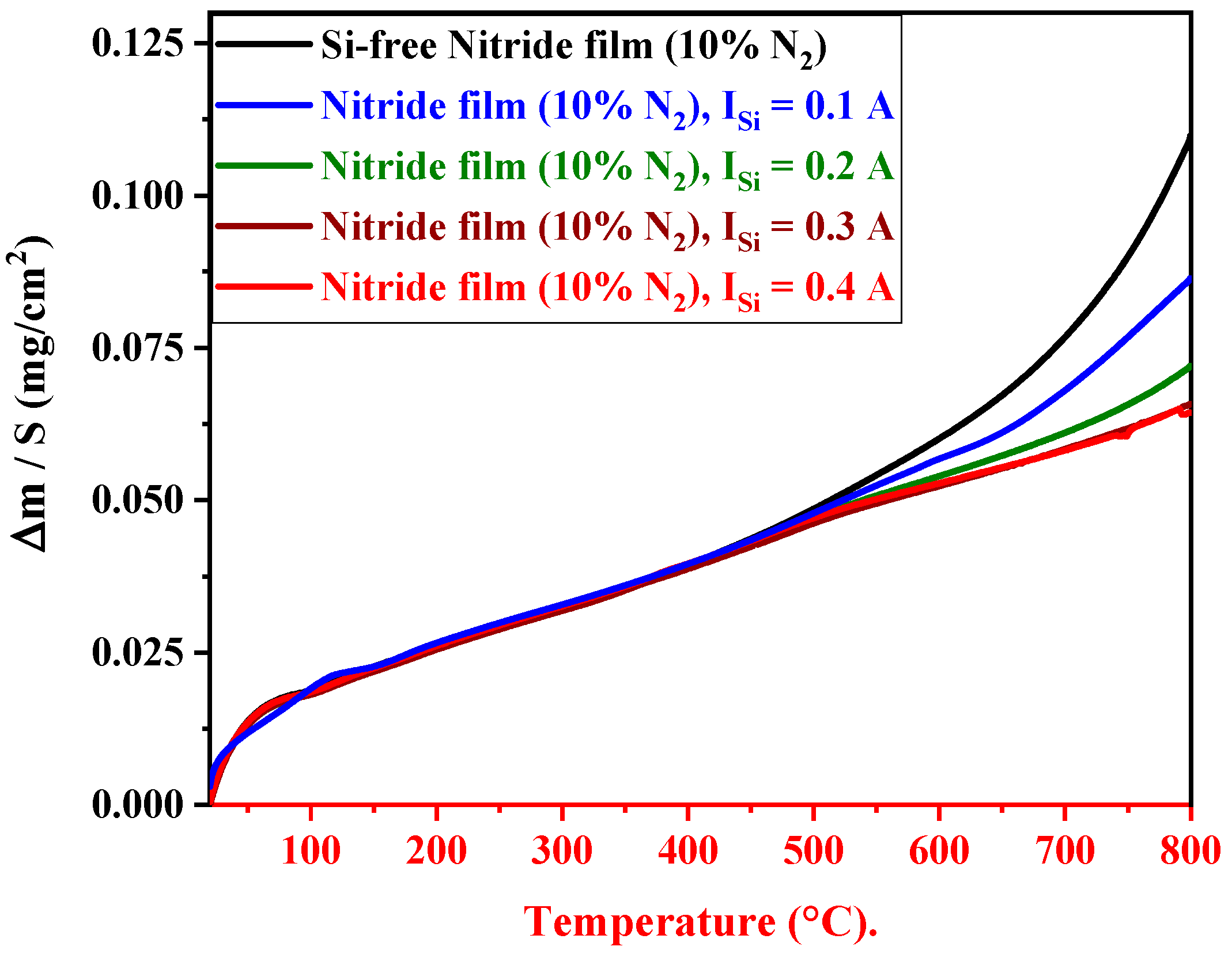
3.6.1. Oxidation Resistance of FCC(AlTiZrHfTa)Nx/a-SiNx Coatings
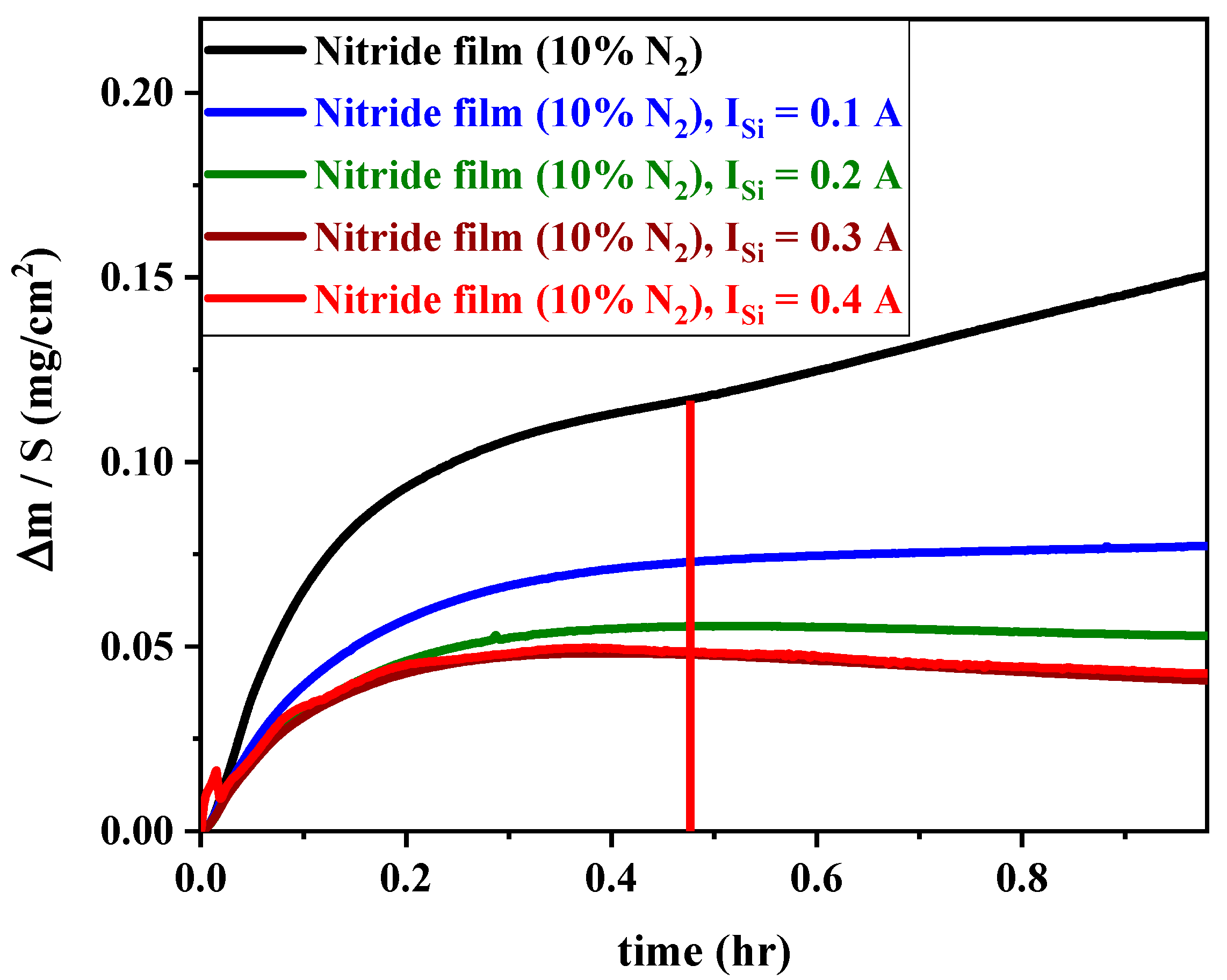
3.6.2. Oxidation Kinetics of FCC(AlTiZrHfTa)Nx/a-SiNx Coatings
Structure Analysis of Oxidized FCC(AlTiZrHfTa)Nx/a-SiNx Coatings
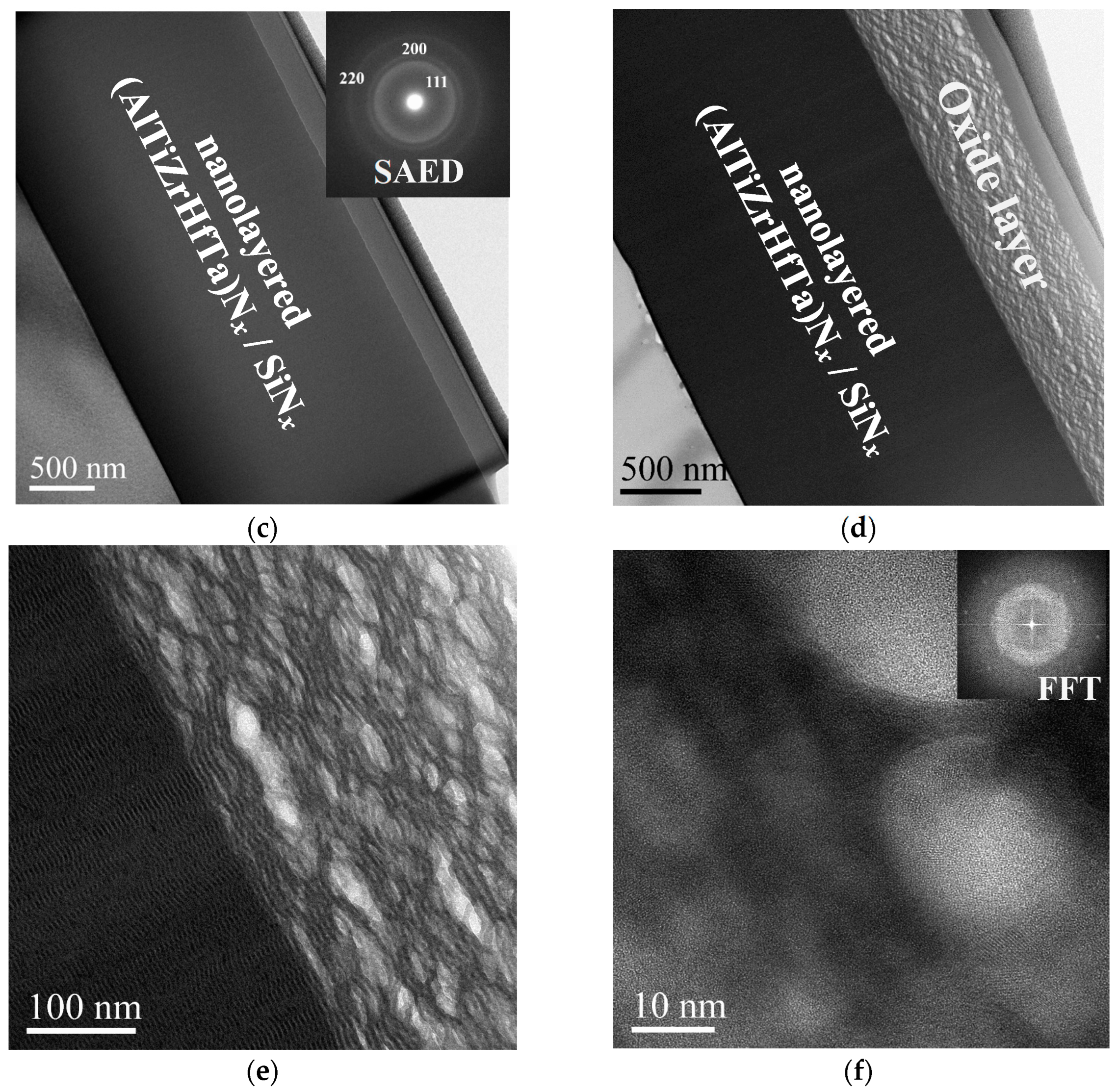
Morphology Analysis of Oxidized FCC(AlTiZrHfTa)Nx/a-SiNx Coatings
Microstructure Investigation of Oxidized FCC(AlTiZrHfTa)Nx/a-SiNx RHECs
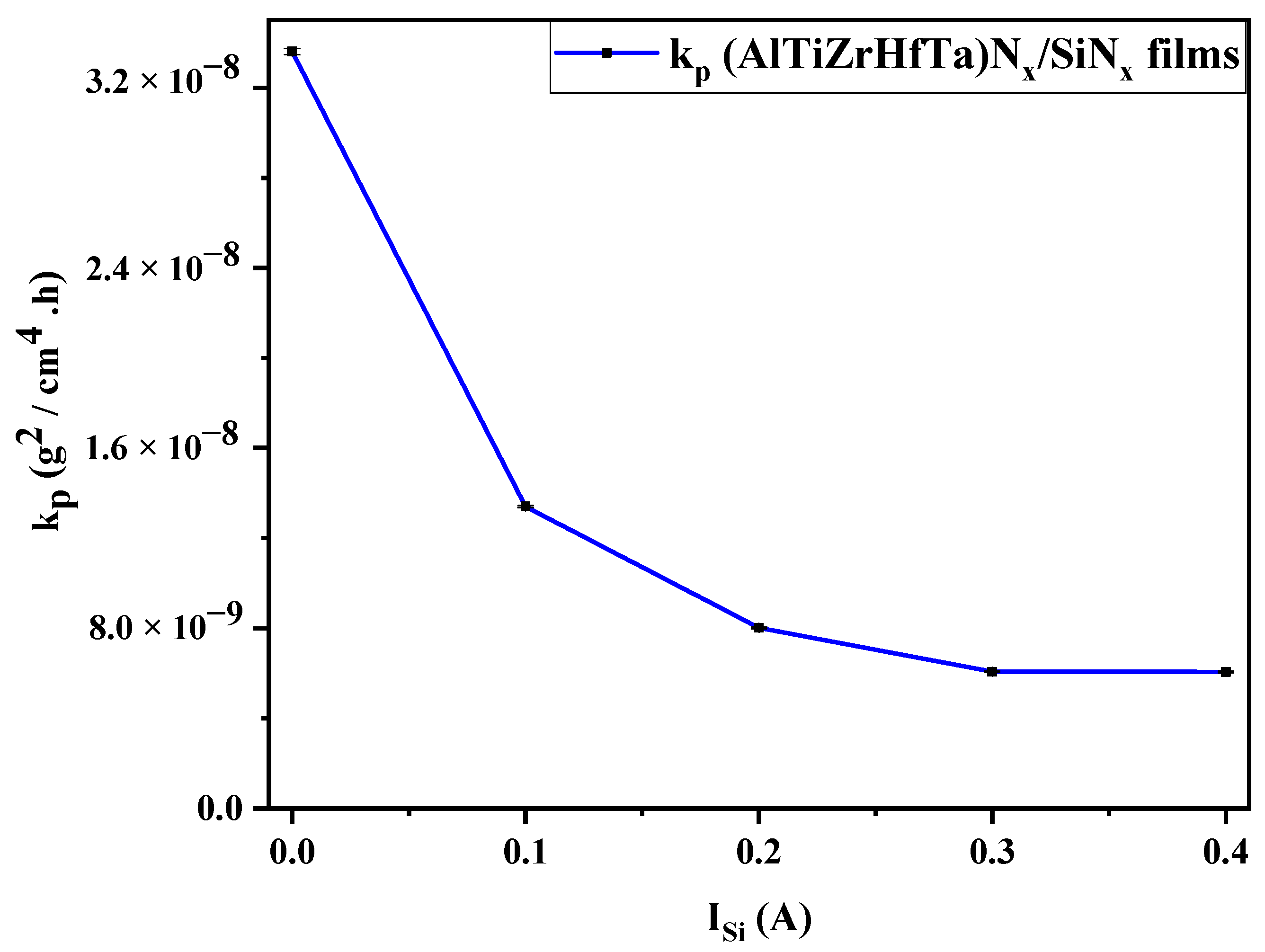
Oxidation Rate through kp Analysis
Activation Energy Ea
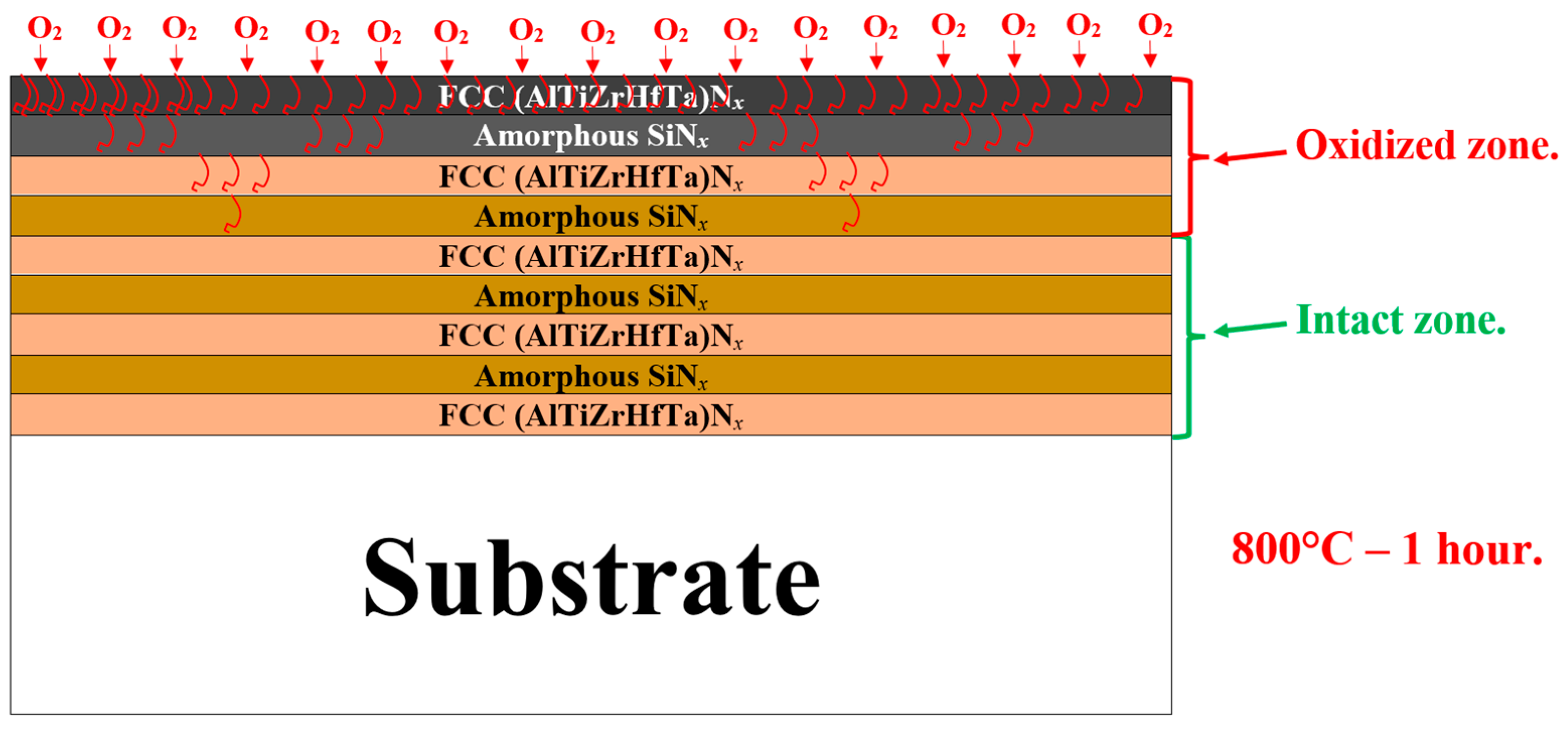
3.6.3. Discussion of the Oxidation Mechanisms
4. Conclusions
- The deposition of nano-layered FCC (AlTiZrHfTa)Nx/a-SiNx coatings results in a density increase in the nitride coatings.
- The deposition of the nano-layered FCC (AlTiZrHfTa)Nx/a-SiNx coatings leads to the decrease in hardness and Young’s modulus up to H = 17.7 ± 0.5 GPa and E = 162.5 ± 1.6 GPa. The softening of the coatings results from the formation of the amorphous SiNx nano-layers, hindering the growth of the FCC (AlTiZrHfTa)Nx nano-layers.
- The deposition of the nano-layered FCC (AlTiZrHfTa)Nx/a-SiNx coating improved the oxidation resistance at 800 °C. The increase in ISi significantly decreased the parabolic rate constant kp from 3.36 × 10−8 g2 cm−4 h−1 for FCC (AlTiZrHfTa)Nx coating to 6.06 × 10−9 g2 cm−4 h−1 for FCC (AlTiZrHfTa)Nx/a-SiNx coatings at 800 °C.
- The activation energy Ea has increased from 90.8 kJ·mol−1 for the FCC (AlTiZrHfTa)Nx coating to 126.52 kJ·mol−1 for the FCC (AlTiZrHfTa)Nx/a-SiNx coating obtained for ISi = 0.2 A. This trend reflects an oxidation resistance improvement due to the formation of the amorphous SiNx nano-layer in alternance with FCC (AlTiZrHfTa)Nx.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holleck, H. Material Selection for Hard Coatings. J. Vac. Sci. Technol. A 1986, 4, 2661–2669. [Google Scholar] [CrossRef]
- Sundgren, J.-E.; Hentzell, H.T.G. A Review of the Present State of Art in Hard Coatings Grown from the Vapor Phase. J. Vac. Sci. Technol. A 1986, 4, 2259–2279. [Google Scholar] [CrossRef]
- Panjan, P.; Navinšek, B.; Cvelbar, A.; Zalar, A.; Milošev, I. Oxidation of TiN, ZrN, TiZrN, CrN, TiCrN and TiN/CrN Multilayer Hard Coatings Reactively Sputtered at Low Temperature. Thin Solid Film. 1996, 281–282, 298–301. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Selvakumar, N.; Deepthi, B.; Rajam, K.S. A Comparative Study of Reactive Direct Current Magnetron Sputtered CrAlN and CrN Coatings. Surf. Coat. Technol. 2006, 201, 2193–2201. [Google Scholar] [CrossRef]
- Uchida, M.; Nihira, N.; Mitsuo, A.; Toyoda, K.; Kubota, K.; Aizawa, T. Friction and Wear Properties of CrAlN and CrVN Films Deposited by Cathodic Arc Ion Plating Method. Surf. Coat. Technol. 2004, 177–178, 627–630. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y.; Zou, Z.; Wu, D. Microstructures and Properties of Low-Chromium High Corrosion-Resistant TiC–VC Reinforced Fe-Based Laser Cladding Layer. J. Alloys Compd. 2015, 622, 62–68. [Google Scholar] [CrossRef]
- Choi, P.-P.; Povstugar, I.; Ahn, J.-P.; Kostka, A.; Raabe, D. Thermal Stability of TiAlN/CrN Multilayer Coatings Studied by Atom Probe Tomography. Ultramicroscopy 2011, 111, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Barshilia, H.C.; Deepthi, B.; Arun Prabhu, A.S.; Rajam, K.S. Superhard Nanocomposite Coatings of TiN/Si3N4 Prepared by Reactive Direct Current Unbalanced Magnetron Sputtering. Surf. Coat. Technol. 2006, 201, 329–337. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Shang, L.; Cao, X.; Zhang, G.; Yu, Y.; Li, W.; Zhang, S.; Hu, H. Mechanical and High-Temperature Tribological Properties of CrAlN/TiSiN Multilayer Coating Deposited by PVD. Ceram. Int. 2021, 47, 29285–29294. [Google Scholar] [CrossRef]
- Fu, T.; Zhang, Z.; Peng, X.; Weng, S.; Miao, Y.; Zhao, Y.; Fu, S.; Hu, N. Effects of Modulation Periods on Mechanical Properties of V/VN Nano-Multilayers. Ceram. Int. 2019, 45, 10295–10303. [Google Scholar] [CrossRef]
- Çomakli, O. Influence of CrN, TiAlN Monolayers and TiAlN/CrN Multilayer Ceramic Films on Structural, Mechanical and Tribological Behavior of β-Type Ti45Nb Alloys. Ceram. Int. 2020, 46, 8185–8191. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Yang, Y.-J.; Weng, S.-Y. Effect of Interlayer Design on the Mechanical Properties of AlTiCrN and Multilayered AlTiCrN/TiSiN Hard Coatings. Surf. Coat. Technol. 2020, 389, 125637. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Chen, H.-W.; Chao, P.-S.; Duh, J.-G.; Lee, J.-W. Microstructure Control in TiAlN/SiNx Multilayers with Appropriate Thickness Ratios for Improvement of Hardness and Anti-Corrosion Characteristics. Vacuum 2013, 87, 195–199. [Google Scholar] [CrossRef]
- Chen, S.N.; Zhao, Y.M.; Zhang, Y.F.; Chen, L.; Liao, B.; Zhang, X.; Ouyang, X.P. Influence of Carbon Content on the Structure and Tribocorrosion Properties of TiAlCN/TiAlN/TiAl Multilayer Composite Coatings. Surf. Coat. Technol. 2021, 411, 126886. [Google Scholar] [CrossRef]
- Yue, J.; Li, G. Microstructure and Mechanical Properties of TiAlN/Si3N4 Nano-Multilayers Synthesized by Reactive Magnetron Sputtering. J. Alloys Compd. 2009, 481, 710–713. [Google Scholar] [CrossRef]
- Vepřek, S.; Reiprich, S. A Concept for the Design of Novel Superhard Coatings. Thin Solid Film. 1995, 268, 64–71. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-Entropy Alloy: Challenges and Prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Guo, S. Phase Selection Rules for Cast High Entropy Alloys: An Overview. Mater. Sci. Technol. 2015, 31, 1223–1230. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase Stability in High Entropy Alloys: Formation of Solid-Solution Phase or Amorphous Phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J. Breakthrough Applications of High-Entropy Materials. J. Mater. Res. 2018, 33, 3129–3137. [Google Scholar] [CrossRef]
- Pickering, E.J.; Carruthers, A.W.; Barron, P.J.; Middleburgh, S.C.; Armstrong, D.E.J.; Gandy, A.S. High-Entropy Alloys for Advanced Nuclear Applications. Entropy 2021, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, W.; Liu, P.; Zhang, K.; Ma, F.; Chen, X.; Feng, R.; Liaw, P.K. Silicon-Content-Dependent Microstructures and Mechanical Behavior of (AlCrTiZrMo)-Six-N High-Entropy Alloy Nitride Films. Mater. Des. 2021, 203, 109553. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Li, C.-E.; Chiang, S.-C.; Huang, Y.-C. 4-Nm Thick Multilayer Structure of Multi-Component (AlCrRuTaTiZr)Nx as Robust Diffusion Barrier for Cu Interconnects. J. Alloys Compd. 2012, 515, 4–7. [Google Scholar] [CrossRef]
- Milošev, I.; Strehblow, H.-H.; Navinšek, B. Comparison of TiN, ZrN and CrN Hard Nitride Coatings: Electrochemical and Thermal Oxidation. Thin Solid Film. 1997, 303, 246–254. [Google Scholar] [CrossRef]
- Kehal, A.; Saoula, N.; Abaidia, S.-E.-H.; Nouveau, C. Effect of Ar/N2 Flow Ratio on the Microstructure and Mechanical Properties of Ti-Cr-N Coatings Deposited by DC Magnetron Sputtering on AISI D2 Tool Steels. Surf. Coat. Technol. 2021, 421, 127444. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and Exploration of Refractory High Entropy Alloys—A Review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Sheikh, S.; Gan, L.; Tsao, T.-K.; Murakami, H.; Shafeie, S.; Guo, S. Aluminizing for Enhanced Oxidation Resistance of Ductile Refractory High-Entropy Alloys. Intermetallics 2018, 103, 40–51. [Google Scholar] [CrossRef]
- El Garah, M.; Soubane, D.; Sanchette, F. Review on Mechanical and Functional Properties of Refractory High-Entropy Alloy Films by Magnetron Sputtering. Emergent Mater. 2023, 7, 77–101. [Google Scholar] [CrossRef]
- DiStefano, J.R.; Hendricks, J.W. Oxidation Rates of Niobium and Tantalum Alloys at Low Pressures. Oxid. Met. 1994, 41, 365–376. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Effect of Al, Cr and Ta Additions on the Oxidation Behaviour of Nb–Ti–Si in Situ Composites at 800 °C. Mater. Sci. Eng. A 2006, 416, 269–280. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, G.M.; Kim, C.J. Oxidation Behavior of TiAl-X (X = Cr, V, Si, Mo or Nb) Intermetallics at Elevated Temperature. Scr. Metall. Mater. 1995, 33, 1117–1125. [Google Scholar] [CrossRef]
- Moricca, M.D.P.; Varma, S.K. High Temperature Oxidation Characteristics of Nb–10W–XCr Alloys. J. Alloys Compd. 2010, 489, 195–201. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.-J.; Müller, J.; Butz, B.; Chen, H.; Kauffmann, A.; Heilmaier, M. On the Oxidation Mechanism of Refractory High Entropy Alloys. Corros. Sci. 2019, 159, 108161. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Deng, M.-J.; Chang, Z.-C.; Kuo, B.-H.; Chen, E.-C.; Chang, S.-Y.; Shieu, F.-S. Oxidation Resistance and Characterization of (AlCrMoTaTi)-Six-N Coating Deposited via Magnetron Sputtering. J. Alloys Compd. 2015, 647, 179–188. [Google Scholar] [CrossRef]
- Bagdasaryan, A.A.; Pshyk, A.V.; Coy, L.E.; Konarski, P.; Misnik, M.; Ivashchenko, V.I.; Kempiński, M.; Mediukh, N.R.; Pogrebnjak, A.D.; Beresnev, V.M.; et al. A New Type of (TiZrNbTaHf)N/MoN Nanocomposite Coating: Microstructure and Properties Depending on Energy of Incident Ions. Compos. Part B Eng. 2018, 146, 132–144. [Google Scholar] [CrossRef]
- Bagdasaryan, A.A.; Pshyk, A.V.; Coy, L.E.; Kempiński, M.; Pogrebnjak, A.D.; Beresnev, V.M.; Jurga, S. Structural and Mechanical Characterization of (TiZrNbHfTa)N/WN Multilayered Nitride Coatings. Mater. Lett. 2018, 229, 364–367. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Preparation, Structure, and Properties of High-Entropy Alloy Multilayer Coatings for Nuclear Fuel Cladding: A Case Study of AlCrMoNbZr/(AlCrMoNbZr)N. J. Nucl. Mater. 2018, 512, 15–24. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Wang, L.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Interface Stability, Mechanical and Corrosion Properties of AlCrMoNbZr/(AlCrMoNbZr)N High-Entropy Alloy Multilayer Coatings under Helium Ion Irradiation. Appl. Surf. Sci. 2019, 485, 108–118. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, R.; Zhang, G.; Liu, Z.; Cai, B.; Jiang, A. Microstructure and Properties of the AlCrMoZrTi/(AlCrMoZrTi)N Multilayer High-Entropy Nitride Ceramics Films Deposited by Reactive RF Sputtering. Ceram. Int. 2022, 48, 16901–16911. [Google Scholar] [CrossRef]
- Touaibia, D.E.; Achache, S.; Bouissil, A.; Ghanbaja, J.; Migot, S.; Yazdi, M.A.P.; Schuster, F.; Panicaud, B.; Sanchette, F.; El Garah, M. Oxidation Resistance and Mechanical Properties of AlTiZrHfTa(-N) High Entropy Films Deposited by Reactive Magnetron Sputtering. J. Alloys Compd. 2023, 969, 172397. [Google Scholar] [CrossRef]
- Bräuer, G.; Szyszka, B.; Vergöhl, M.; Bandorf, R. Magnetron Sputtering—Milestones of 30 Years. Vacuum 2010, 84, 1354–1359. [Google Scholar] [CrossRef]
- Kaiser, N. Review of the Fundamentals of Thin-Film Growth. Appl. Opt. AO 2002, 41, 3053–3060. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.P.; Wang, G.J.; Ma, Y.J.; Cao, Z.H.; Meng, X.K. High Hardness Dual-Phase High Entropy Alloy Thin Films Produced by Interface Alloying. Scr. Mater. 2019, 162, 281–285. [Google Scholar] [CrossRef]
- Xu, Y.X.; Chen, L.; Pei, F.; Chang, K.K.; Du, Y. Effect of the Modulation Ratio on the Interface Structure of TiAlN/TiN and TiAlN/ZrN Multilayers: First-Principles and Experimental Investigations. Acta Mater. 2017, 130, 281–288. [Google Scholar] [CrossRef]
- El Garah, M.; Touaibia, D.E.; Achache, S.; Michau, A.; Sviridova, E.; Postnikov, P.S.; Chehimi, M.M.; Schuster, F.; Sanchette, F. Effect of Nitrogen Content on Structural and Mechanical Properties of AlTiZrTaHf(-N) High Entropy Films Deposited by Reactive Magnetron Sputtering. Surf. Coat. Technol. 2022, 432, 128051. [Google Scholar] [CrossRef]
- El Garah, M.; Touaibia, D.E.; Achache, S.; Michau, A.; Sviridova, E.; Postnikov, P.S.; Chehimi, M.M.; Schuster, F.; Sanchette, F. Data on Nitridation Effect of AlTiTaZrHf(-N) High Entropy Films by X-Ray Photoelectron Spectroscopy. Data Brief 2022, 42, 108241. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Muders, C.M.; Kumar, A.; Jiang, X.; Pei, Z.L.; Gong, J.; Sun, C. Study on Nanocomposite Ti–Al–Si–Cu–N Films with Various Si Contents Deposited by Cathodic Vacuum Arc Ion Plating. Appl. Surf. Sci. 2012, 258, 9642–9649. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chang, S.-Y.; Huang, Y.-C.; Shieu, F.-S.; Yeh, J.-W. Mechanical Performance and Nanoindenting Deformation of (AlCrTaTiZr)NCy Multi-Component Coatings Co-Sputtered with Bias. Surf. Coat. Technol. 2012, 206, 5096–5102. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Lin, S.-Y.; Huang, Y.-C.; Wu, C.-L. Mechanical Properties, Deformation Behaviors and Interface Adhesion of (AlCrTaTiZr)Nx Multi-Component Coatings. Surf. Coat. Technol. 2010, 204, 3307–3314. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lin, S.-J.; Yeh, J.-W.; Chang, S.-Y. Preparation and Characterization of AlCrTaTiZr Multi-Element Nitride Coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Chang, H.-W.; Huang, P.-K.; Davison, A.; Yeh, J.-W.; Tsau, C.-H.; Yang, C.-C. Nitride Films Deposited from an Equimolar Al–Cr–Mo–Si–Ti Alloy Target by Reactive Direct Current Magnetron Sputtering. Thin Solid Film. 2008, 516, 6402–6408. [Google Scholar] [CrossRef]
- Liang, S.-C.; Tsai, D.-C.; Chang, Z.-C.; Sung, H.-S.; Lin, Y.-C.; Yeh, Y.-J.; Deng, M.-J.; Shieu, F.-S. Structural and Mechanical Properties of Multi-Element (TiVCrZrHf)N Coatings by Reactive Magnetron Sputtering. Appl. Surf. Sci. 2011, 258, 399–403. [Google Scholar] [CrossRef]
- Bouissil, A.; Achache, S.; Touaibia, D.E.; Panicaud, B.; Yazdi, M.A.P.; Sanchette, F.; El Garah, M. Properties of a New TiTaZrHfW(N) Refractory High Entropy Film Deposited by Reactive DC Pulsed Magnetron Sputtering. Surf. Coat. Technol. 2023, 462, 129503. [Google Scholar] [CrossRef]
- Vepřek, S.; Reiprich, S.; Shizhi, L. Superhard Nanocrystalline Composite Materials: The TiN/Si3N4 System. Appl. Phys. Lett. 1995, 66, 2640–2642. [Google Scholar] [CrossRef]
- Yashar, P.C.; Sproul, W.D. Nanometer Scale Multilayered Hard Coatings. Vacuum 1999, 55, 179–190. [Google Scholar] [CrossRef]
- Sandu, C.S.; Sanjinés, R.; Benkahoul, M.; Medjani, F.; Lévy, F. Formation of Composite Ternary Nitride Thin Films by Magnetron Sputtering Co-Deposition. Surf. Coat. Technol. 2006, 201, 4083–4089. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.A.R.; Morilla dos Santos, C.; Sasaki, J.M. The Scherrer Equation and the Dynamical Theory of X-Ray Diffraction. Acta Crystallogr. Sect. A 2016, 72, 385–390. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Chang, Z.-C.; Kuo, B.-H.; Shiao, M.-H.; Chang, S.-Y.; Shieu, F.-S. Structural Morphology and Characterization of (AlCrMoTaTi)N Coating Deposited via Magnetron Sputtering. Appl. Surf. Sci. 2013, 282, 789–797. [Google Scholar] [CrossRef]
- Nieborek, M.; Jastrzębski, C.; Płociński, T.; Wróbel, P.; Seweryn, A.; Judek, J. Optimization of the Plasmonic Properties of Titanium Nitride Films Sputtered at Room Temperature through Microstructure and Thickness Control. Sci. Rep. 2024, 14, 5762. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Patscheider, J.; Zehnder, T.; Diserens, M. Structure–Performance Relations in Nanocomposite Coatings. Surf. Coat. Technol. 2001, 146–147, 201–208. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lee, K.W.; Chiou, W.-A.; Chung, Y.-W.; Keer, L.M. Synthesis and Structure of Smooth, Superhard TiN/SiNx Multilayer Coatings with an Equiaxed Microstructure. Surf. Coat. Technol. 2001, 146–147, 209–214. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Dai, J.; Li, G.; Gu, M. Study on the Superhardness Mechanism of Ti–Si–N Nanocomposite Films: Influence of the Thickness of the Si3N4 Interfacial Phase. J. Vac. Sci. Technol. A 2004, 23, 114–117. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, W.; Yue, J.; Li, G. Crystallization of Si3N4 Layers and Its Influences on the Microstructure and Mechanical Properties of ZrN/Si3N4 Nanomultilayers. Appl. Phys. Lett. 2006, 89, 121916. [Google Scholar] [CrossRef]
- Xu, J.; Hattori, K.; Seino, Y.; Kojima, I. Microstructure and Properties of CrN/Si3N4 Nano-Structured Multilayer Films. Thin Solid Film. 2002, 414, 239–245. [Google Scholar] [CrossRef]
- Jeong, J.J.; Hwang, S.K.; Lee, C. Hardness and Adhesion Properties of HfN/Si3N4 and NbN/Si3N4 Multilayer Coatings. Mater. Chem. Phys. 2003, 77, 27–33. [Google Scholar] [CrossRef]
- Jeong, J.J.; Lee, C.M. Effects of Post-Deposition Annealing on the Mechanical and Chemical Properties of the Si3N4/NbN Multilayer Coatings. Appl. Surf. Sci. 2003, 214, 11–19. [Google Scholar] [CrossRef]
- Parry, V. Corrosion Sèche Des Métaux—Méthodes D’étude; Techniques de l’Ingénieur: Saint-Denis, France, 2015. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.-J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High Temperature Oxidation Behavior of an Equimolar Refractory Metal-Based Alloy 20Nb20Mo20Cr20Ti20Al with and without Si Addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef]
- Antoni, L.; Galerie, A. Corrosion sèche des métaux—Mécanismes. Corros. Vieil. 2003. [Google Scholar] [CrossRef]
- Hung, S.-B.; Wang, C.-J.; Chen, Y.-Y.; Lee, J.-W.; Li, C.-L. Thermal and Corrosion Properties of V-Nb-Mo-Ta-W and V-Nb-Mo-Ta-W-Cr-B High Entropy Alloy Coatings. Surf. Coat. Technol. 2019, 375, 802–809. [Google Scholar] [CrossRef]
- Gorr, B.; Schellert, S.; Müller, F.; Christ, H.-J.; Kauffmann, A.; Heilmaier, M. Current Status of Research on the Oxidation Behavior of Refractory High Entropy Alloys. Adv. Eng. Mater. 2021, 23, 2001047. [Google Scholar] [CrossRef]
- Li, S.; Yamaguchi, T. High-Temperature Oxidation Behavior of Laser-Cladded Refractory NiSi0.5CrCoMoNb0.75 High-Entropy Coating. J. Mater. Res. Technol. 2022, 17, 1616–1627. [Google Scholar] [CrossRef]
- Logan, S.R. The Origin and Status of the Arrhenius Equation. J. Chem. Educ. 1982, 59, 279. [Google Scholar] [CrossRef]
- Schmitt, T. Mécanismes de Dégradation de Revêtements Base CrN Élaborés Par Arc-PVD: Intérêt D’une Nano-Architecture. Ph.D. Thesis, Ecole Centrale de Lyon, Écully, France, 2010. [Google Scholar]
- Kumar, P.; Avasthi, S. Diffusion Barrier with 30-Fold Improved Performance Using AlCrTaTiZrN High-Entropy Alloy. J. Alloys Compd. 2020, 814, 151755. [Google Scholar] [CrossRef]
- Chu, T.L.; Lee, C.H.; Gruber, G.A. The Preparation and Properties of Amorphous Silicon Nitride Films. J. Electrochem. Soc. 1967, 114, 717. [Google Scholar] [CrossRef]
- Tien, T.Y.; Bonnel, D.A. Preparation and Properties of Silicon Nitride Based Materials; Trans Tech Publications Ltd.: Baech, Switzerland, 1989; ISBN 978-3-0357-0445-7. [Google Scholar]
- Hovsepian, P.E.; Lewis, D.B.; Münz, W.-D. Recent Progress in Large Scale Manufacturing of Multilayer/Superlattice Hard Coatings. Surf. Coat. Technol. 2000, 133–134, 166–175. [Google Scholar] [CrossRef]
- Tomlinson, M.; Lyon, S.B.; Hovsepian, P.; Munz, W.-D. Corrosion Performance of CrN/NbN Superlattice Coatings Deposited by the Combined Cathodic Arc/Unbalanced Magnetron Technique. Vacuum 1999, 53, 117–121. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, J.J.; Yang, Z.B.; Qiu, S.Y.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Improved Corrosion Resistance of Reactive Gas Pulse Sputtered (TiTaNbZrNi)N High Entropy Alloy Coatings with a Hybrid Architecture of Multilayered and Compositionally Graded Structures. J. Nucl. Mater. 2021, 543, 152558. [Google Scholar] [CrossRef]
- Steyer, P.; Pilloud, D.; Pierson, J.F.; Millet, J.-P.; Charnay, M.; Stauder, B.; Jacquot, P. Oxidation Resistance Improvement of Arc-Evaporated TiN Hard Coatings by Silicon Addition. Surf. Coat. Technol. 2006, 201, 4158–4162. [Google Scholar] [CrossRef]


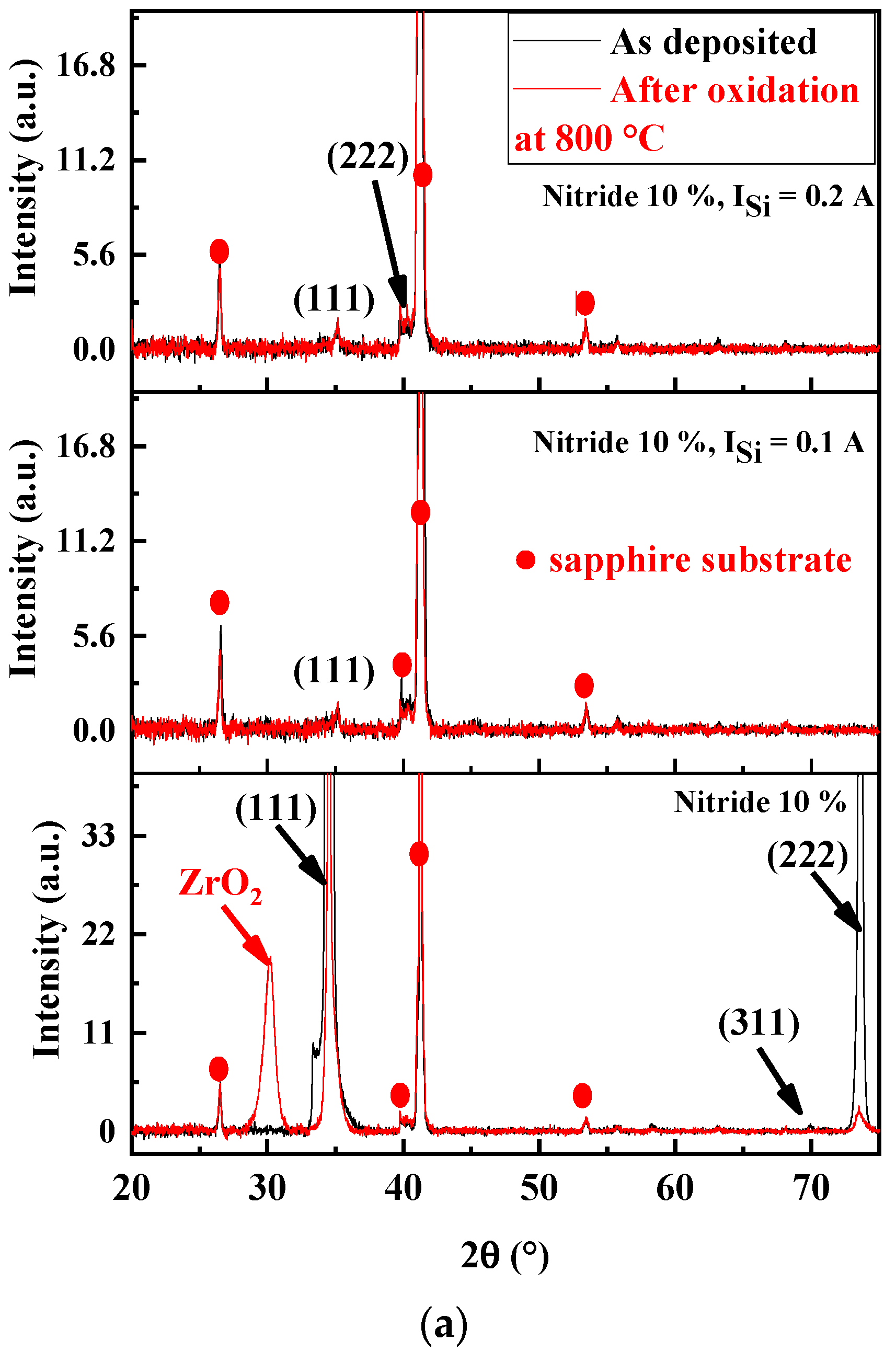
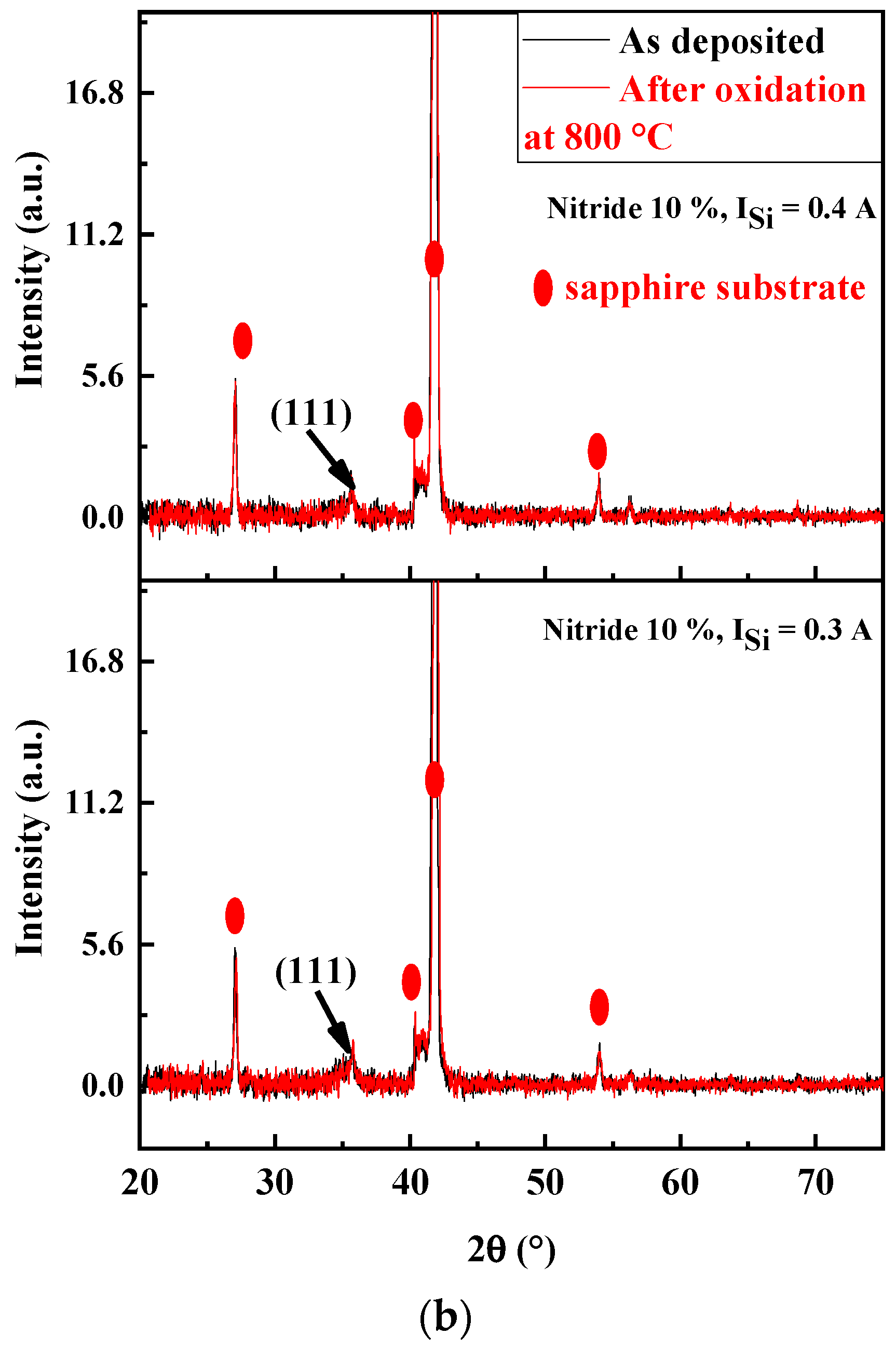
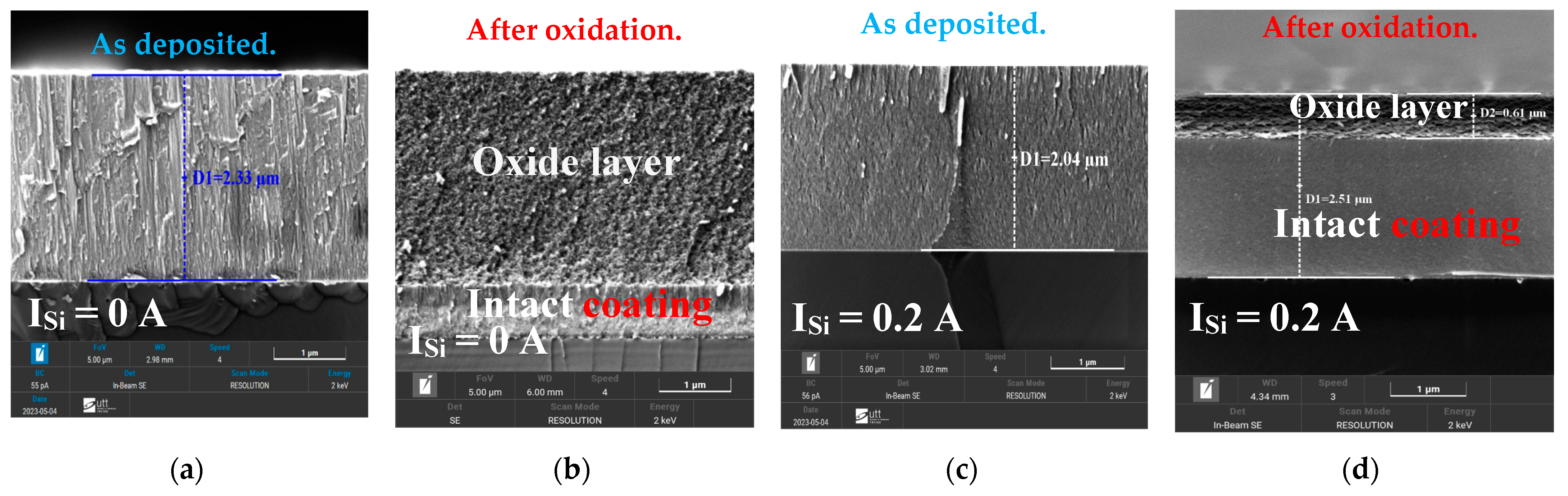
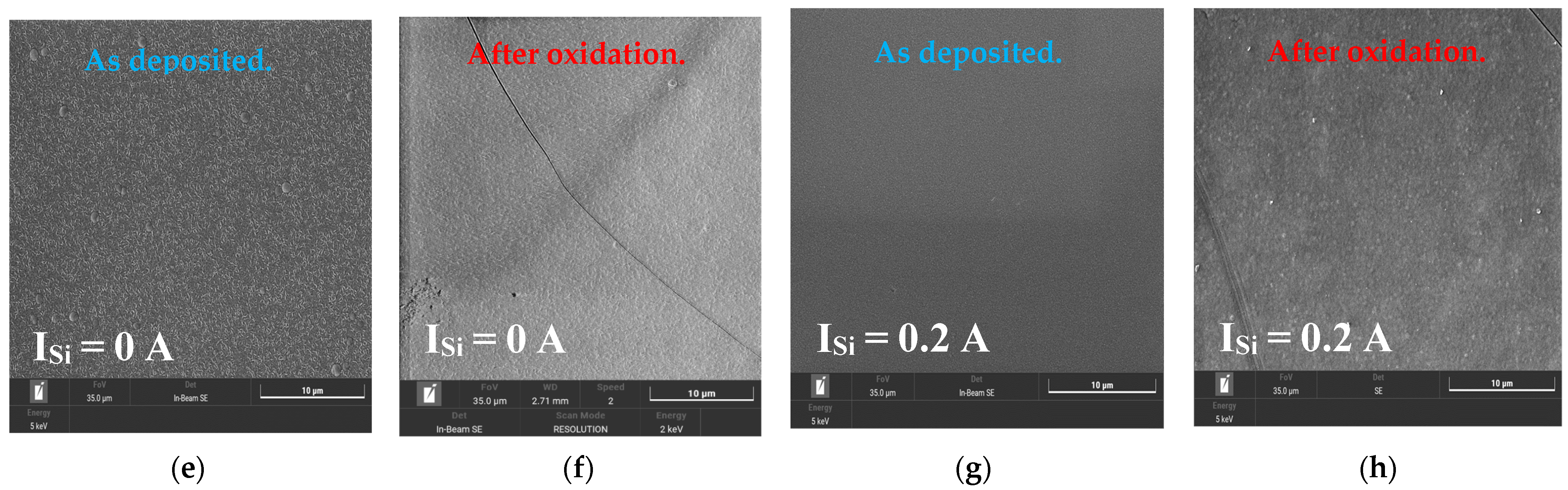


| Coating | ISi (A) | Average Grain Size, Ø (nm) |
|---|---|---|
| (AlTiZrHfTa)N (RN2 = 10%) | 0 | 45.92 |
| (AlTiZrHfTa)Nx/SiNx (RN2 = 10%) | 0.1 | 2.1 |
| (AlTiZrHfTa)Nx/SiNx (RN2 = 10%) | 0.2 | 2 |
| (AlTiZrHfTa)Nx/SiNx (RN2 = 10%) | 0.3 | 2 |
| (AlTiZrHfTa)Nx/SiNx (RN2 = 10%) | 0.4 | 2.2 |
| ISi (A) | Corresponding Si Atomic Percentage (at.%) |
|---|---|
| 0 | 0 |
| 0.1 | 5.3 |
| 0.2 | 12 |
| 0.3 | 16.4 |
| 0.4 | 21.5 |
| Coating | Ea (Activation Energy) (kJ·mol−1) | Ea Error | Reference |
|---|---|---|---|
| (AlTiZrHfTa)Nx (RN2 = 10%) | 90.8 | 0.05 | [42] |
| (AlTiZrHfTa)Nx/SiNx (RN2 = 10%, ISi = 0.2 A) | 126.52 | 0.02 | This study |
| CrN | 243 | - | [77] |
| CrAlN | 280 | - | [77] |
| TiN | 193 | - | [77] |
| TiSiN | 260 | - | [77] |
| (AlCrTaTiZr) N | 208.6 | - | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touaibia, D.E.; Achache, S.; Bouissil, A.; Parent, F.; Ghanbaja, J.; Gorbunova, A.; Postnikov, P.S.; Chehimi, M.M.; Schuster, F.; Sanchette, F.; et al. Oxidation Performance of Nano-Layered (AlTiZrHfTa)Nx/SiNx Coatings Deposited by Reactive Magnetron Sputtering. Materials 2024, 17, 2799. https://doi.org/10.3390/ma17122799
Touaibia DE, Achache S, Bouissil A, Parent F, Ghanbaja J, Gorbunova A, Postnikov PS, Chehimi MM, Schuster F, Sanchette F, et al. Oxidation Performance of Nano-Layered (AlTiZrHfTa)Nx/SiNx Coatings Deposited by Reactive Magnetron Sputtering. Materials. 2024; 17(12):2799. https://doi.org/10.3390/ma17122799
Chicago/Turabian StyleTouaibia, Djallel Eddine, Sofiane Achache, Abdelhakim Bouissil, Fabrice Parent, Jaafar Ghanbaja, Alina Gorbunova, Pavel S. Postnikov, Mohamed Mehdi Chehimi, Frederic Schuster, Frederic Sanchette, and et al. 2024. "Oxidation Performance of Nano-Layered (AlTiZrHfTa)Nx/SiNx Coatings Deposited by Reactive Magnetron Sputtering" Materials 17, no. 12: 2799. https://doi.org/10.3390/ma17122799
APA StyleTouaibia, D. E., Achache, S., Bouissil, A., Parent, F., Ghanbaja, J., Gorbunova, A., Postnikov, P. S., Chehimi, M. M., Schuster, F., Sanchette, F., & El Garah, M. (2024). Oxidation Performance of Nano-Layered (AlTiZrHfTa)Nx/SiNx Coatings Deposited by Reactive Magnetron Sputtering. Materials, 17(12), 2799. https://doi.org/10.3390/ma17122799









