Regulation of the Degradation Properties of Tyrosinase-Catalyzed Crosslinking Silk Membranes for Superficial Wound Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of c-SF Membrane
2.3. Evaluation of Crosslinking Degree
2.4. Physical Properties of c-SF Membrane
2.5. Cells Growth and Metabolism Assay on c-SF Membrane
2.6. Secretion of Collagenase in L929 Cell Culture Medium
2.7. Residual Mass of c-SF Membranes after Degradation
2.8. The Analyses of the Residues after Degradation
2.9. Statistical Analysis
3. Results
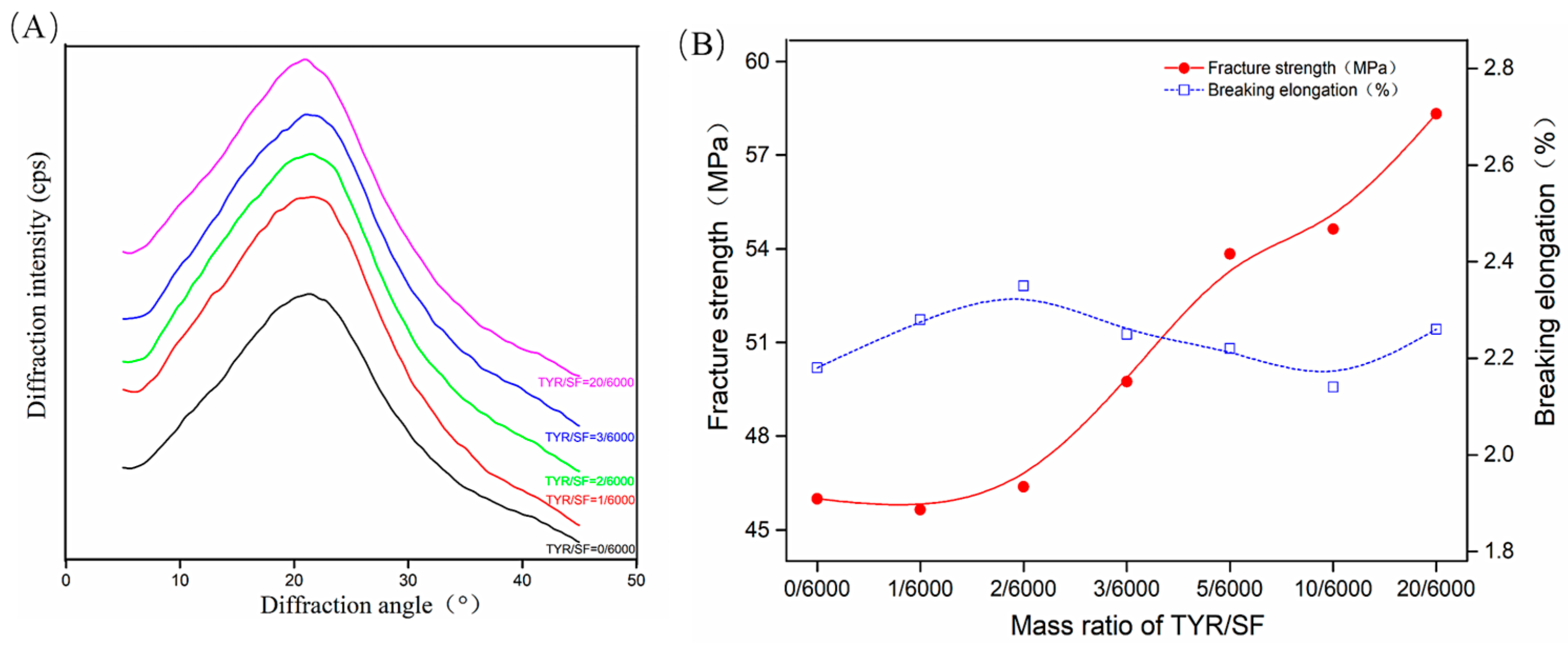
3.1. Crosslinking of the c-SF Membrane
3.2. Characterization of c-SF Membranes
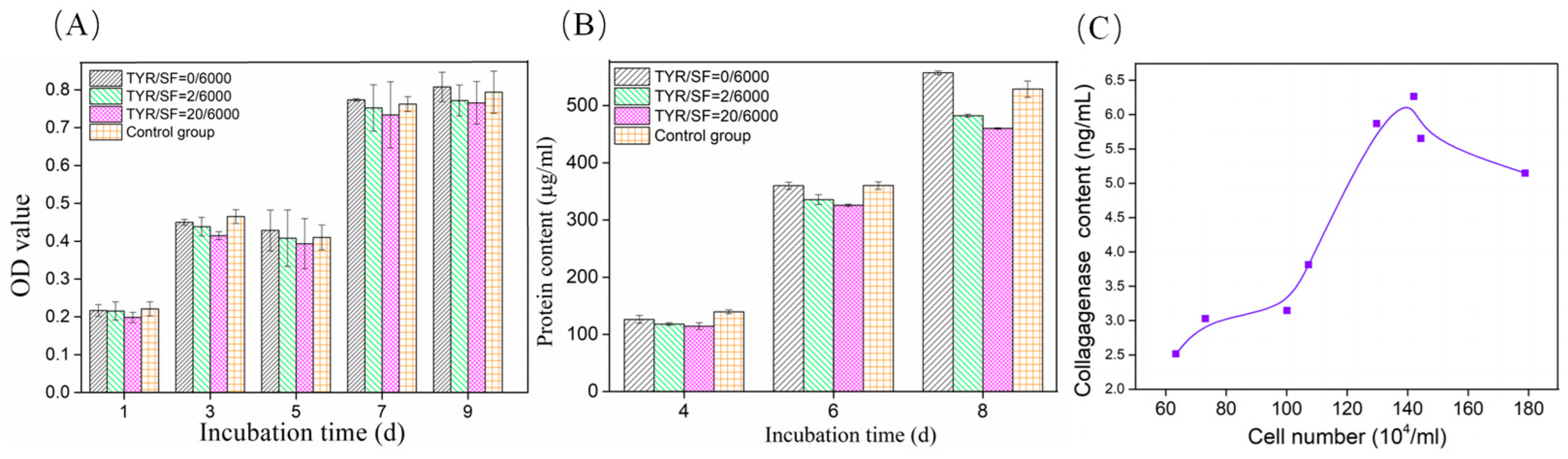
3.3. Cell Viability and Collagenase Secretion on c-SF Membrane
3.4. Degradation of c-SF Membrane In Vitro
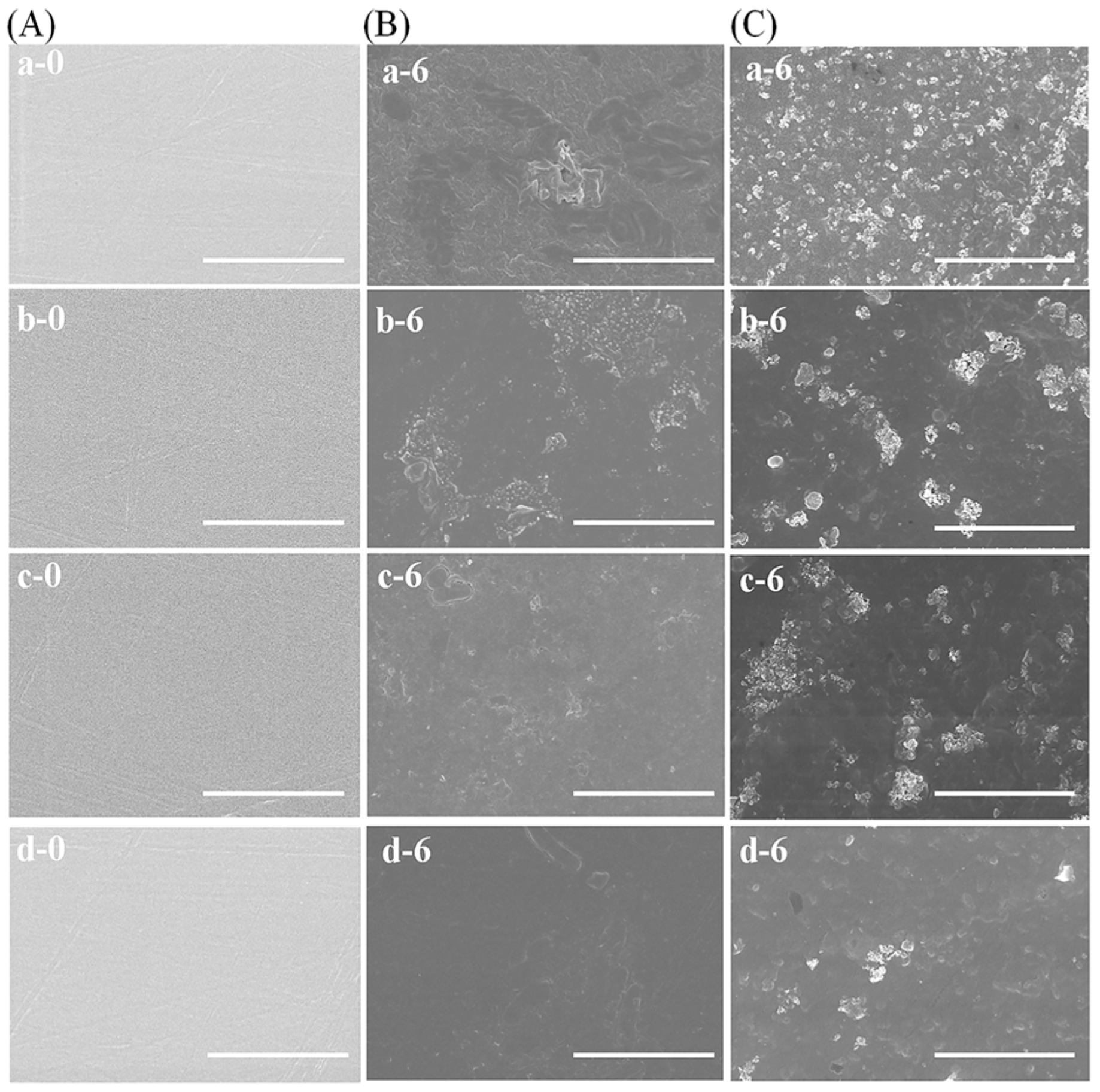
3.5. Morphological Changes during Degradation
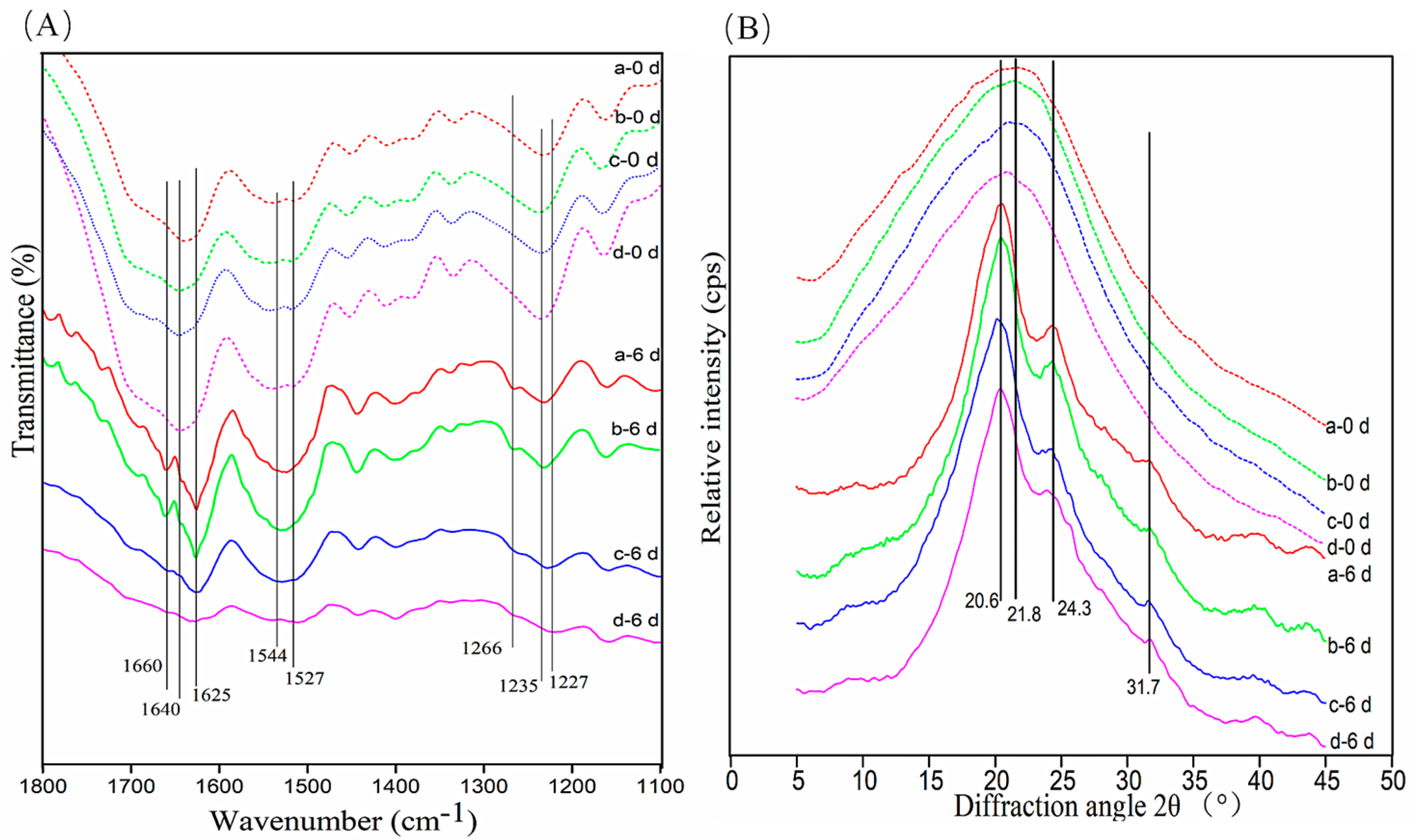
3.6. Conformational Changes in c-SF Membrane
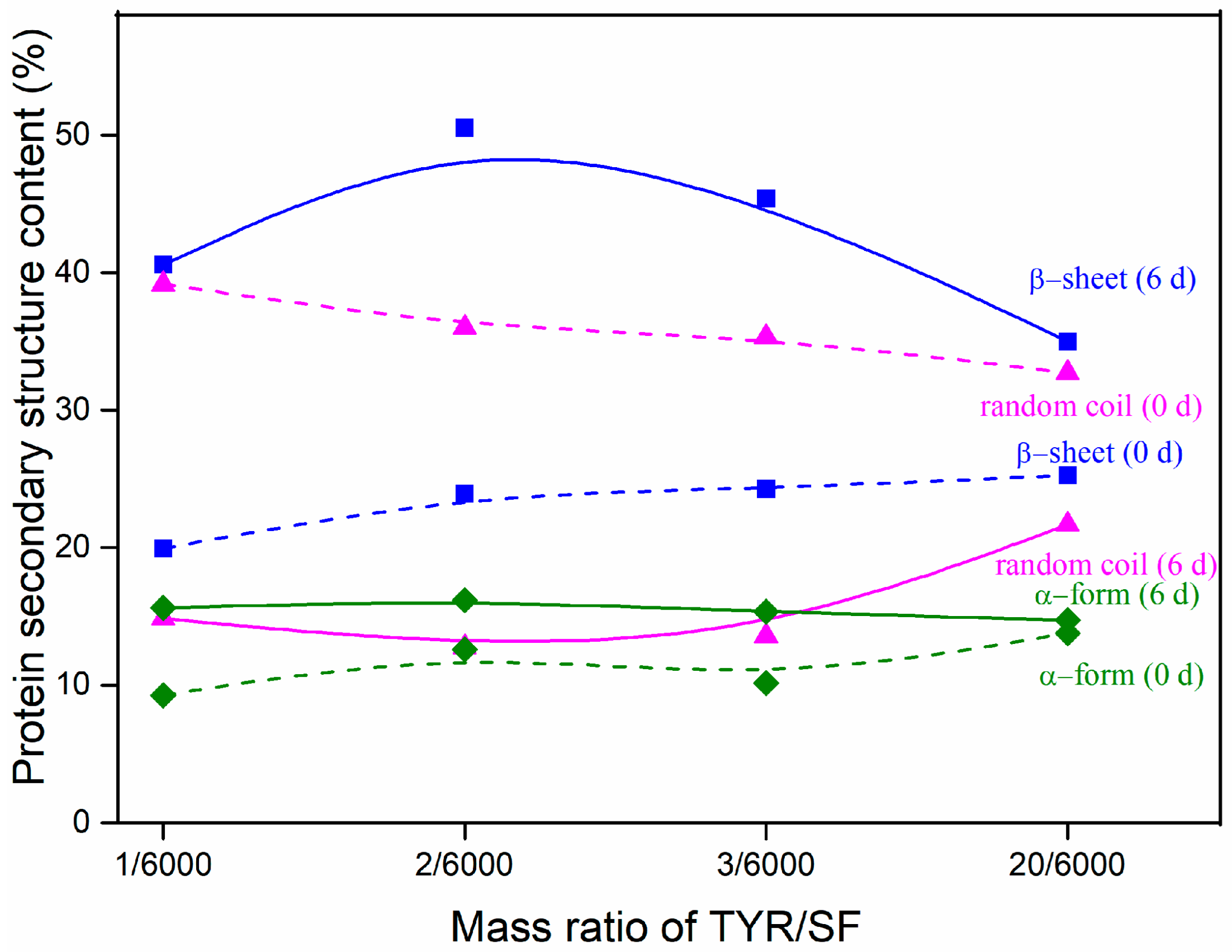
3.7. Protein Secondary Structure Content after Degradation
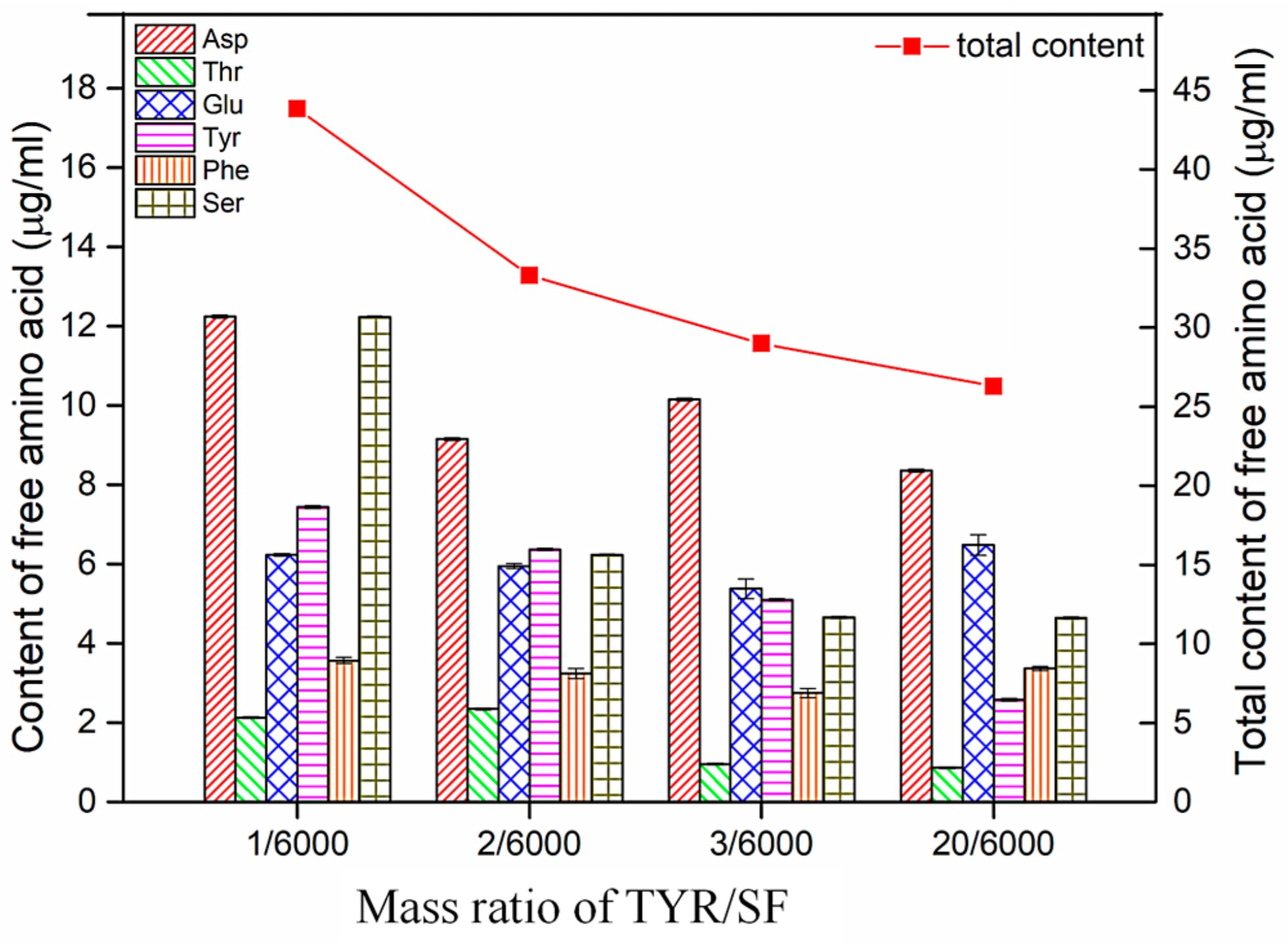
3.8. Changes in Free Amino Acid Content after Degradation
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narita, C.; Okahisa, Y.; Wataoka, I.; Yamada, K. Characterization of ground silk fibroin through comparison of nanofibroin and higher order structures. ACS Omega 2020, 5, 22786–22792. [Google Scholar] [CrossRef]
- De Giorgio, G.; Matera, B.; Vurro, D.; Manfredi, E.; Galstyan, V.; Tarabella, G.; Ghezzi, B.; D’Angelo, P. Silk fibroin materials: Biomedical applications and perspectives. Bioengineering 2024, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Koczoń, P.; Dąbrowska, A.; Laskowska, E.; Łabuz, M.; Maj, K.; Masztakowski, J.; Bartyzel, B.J.; Brys, A.; Brys, J.; Gruczyńska-Sękowska, E. Applications of silk fibroin in human and veterinary medicine. Materials 2023, 16, 7128. [Google Scholar] [CrossRef]
- Murchio, S.; Benedetti, M.; Berto, A.; Agostinacchio, F.; Zappini, G.; Maniglio, D. Hybrid Ti6Al4V/silk fibroin composite for load-bearing implants: A hierarchical multifunctional cellular scaffold. Materials 2022, 15, 6156. [Google Scholar] [CrossRef]
- Madappura, A.P.; Madduri, S. A comprehensive review of silk-fibroin hydrogels for cell and drug delivery applications in tissue engineering and regenerative medicine. Comput. Struct Biotechnol. J. 2023, 21, 4868–4886. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Sahoo, J.K.; Cebe, P.; Kaplan, D.L. Photo-crosslinked silk fibroin for 3D printing. Polymers 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Zhang, H.; Zhao, W.W.; Qu, L.J.; Chen, S.J.; Wu, S.H. Electrospun strong, bioactive, and bioabsorbable silk fibroin/poly (L-lactic-acid) nanoyarns for constructing advanced nanotextile tissue scaffolds. Mater. Today Bio 2022, 14, 100243. [Google Scholar] [CrossRef]
- Fu, X.H.; Wang, J.; Qian, D.J.; Chen, Z.W.; Chen, L.; Cui, W.G.; Wang, Y. Living electrospun short fibrous sponge via engineered nanofat for wound healing. Adv. Fiber Mater. 2022, 5, 979–993. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Liu, J.; Sun, W.; Li, W.; Shen, H.; Wang, L.; Li, G. Advances in fiber-based wearable sensors for personal digital health monitoring. Materials 2023, 16, 7428. [Google Scholar] [CrossRef]
- Sideratou, Z.; Biagiotti, M.; Tsiourvas, D.; Panagiotaki, K.N.; Zucca, M.V.; Freddi, G.; Lovati, A.B.; Bottagisio, M. Antibiotic-loaded hyperbranched polyester embedded into peptide-enriched silk fibroin for the treatment of orthopedic or dental infections. Nanomaterials 2022, 12, 3182. [Google Scholar] [CrossRef]
- Tan, G.; Jia, T.; Qi, Z.; Lu, S. Regenerated fiber’s ideal target: Comparable to natural fiber. Materials 2024, 17, 1834. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.; Queiroz, H.; Aguiar, R.; Lima, R.; Cavalcanti, D.; Banea, M.D. A review of recent advances in hybrid natural fiber reinforced polymer composites. J. Renew. Mater. 2022, 10, 561–589. [Google Scholar] [CrossRef]
- Metalssi, O.; Ragoug, R.; Barberon, F.; De Lacaillerie, J.; Roussel, N.; Divet, L.; Torrenti, J. Effect of an early-age exposure on the degradation mechanisms of cement paste under external sulfate attack. Materials 2023, 16, 6013. [Google Scholar] [CrossRef] [PubMed]
- Pirsa, S.; Mahmudi, M.; Ehsani, A. Biodegradable film based on cress seed mucilage, modified with lutein, maltodextrin and alumina nanoparticles: Physicochemical properties and lutein controlled release. Int. J. Biol. Macromol. 2023, 224, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Channab, B.-E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, modification, biodegradation and applications in agriculture as slow/controlled release fertilizer, superabsorbent, and crop protection: A review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ma, L.; Fu, Y.; Chen, H.; Dai, H.; Zhu, H.; Wang, H.; Yu, Y.; Zhang, Y. Transglutaminase modified type a gelatin gel: The influence of intra-molecular and inter-molecular crosslinking on structure-properties. Food Chem. 2022, 395, 133578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, X.; Zhu, H.; Xin, J.H.; Zhang, Q.; Zhu, S. Reversible water transportation diode: Temperature-adaptive smart janus textile for moisture/thermal management. Adv. Funct. Mater. 2020, 30, 1907851. [Google Scholar] [CrossRef]
- Augustine, T.Z.; Zeina, B.; Cao, Y.T.; Sun, H.; Doyoon, K.; Liu, M.C.; Eugene, J.L.; Benedetto, M. Degradation of regenerated silk fibroin in soil and marine environments. ACS Sustain. Chem. Eng. 2022, 10, 11088–11097. [Google Scholar] [CrossRef]
- Zhao, M.; Qi, Z.; Tao, X.; Newkirk, C.; Hu, X.; Lu, S. Chemical, thermal, time, and enzymatic stability of silk materials with silk i structure. Int. J. Mol. Sci. 2021, 22, 4136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, S.; Chae, K.J. Discharge of microplastics fibres from wet wipes in aquatic and solid environments under different release conditions. Sci. Total Environ. 2021, 784, 147144. [Google Scholar] [CrossRef]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, E.R.; Lakshminarayanan, R.; Chellappan, V.; Verma, N.K.; Chinnappan, A.; Neisiany, R.E.; Amuthavalli, K.; Poh, Z.S.; Wong, B.H.S.; Dubey, N. Electrospun aligned PCL/gelatin scaffolds mimicking the skin ECM for effective antimicrobial wound dressings. Adv. Fiber Mater. 2022, 5, 235–251. [Google Scholar] [CrossRef]
- Kim, H.S.; Kumbar, S.G.; Nukavarapu, S.P. Biomaterial-directed cell behavior for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100260. [Google Scholar] [CrossRef]
- Bhat, R.; Dogra, A.; Chib, S.; Kumar, M.; Khan, I.A.; Nandi, U.; Saran, S. Development of mupirocin-impregnated bacterial cellulosic transdermal patches for the management of skin infection. ACS Omega 2024, 9, 5496–5508. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.; Zulfiqar, S.; Anjum, M.A.; Bullock, A.J.; MacNeil, S.; Yar, M. An alginate-based tube gel delivering 2-deoxy-D-ribose for stimulation of wound healing. J. Biomater. Appl. 2023, 38, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Teixeira, I.; Carmo, L.; Campos, M.; Garcia, M. The use of a polylactic membrane in pediatric burns proves to be successful even after late application. J. Burn. Care Res. 2023, 7, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Hu, N.; Wang, H. Isolation, purification and tyrosinase inhibitory activity of anthocyanins and their novel degradation compounds from solanum tuberosum. Molecules 2024, 29, 1492. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Filip, N.; Radu, I.; Veliceasa, B.; Filip, C.; Pertea, M.; Clim, A.; Pinzariu, A.C.; Drochioi, I.C.; Hilitanu, R.L.; Serban, I.L. Biomaterials in orthopedic devices: Current issues and future perspectives. Coatings 2022, 12, 1544. [Google Scholar] [CrossRef]
- Wang, L.; Ding, X.; Feng, W.; Gao, Y.; Zhao, S.; Fan, Y. Biomechanical study on implantable and interventional medical devices. Acta Mech. Sin. 2021, 37, 875–894. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.; Kim, B.S.; An, Y.H.; Lee, U.J.; Lee, S.H.; Kim, S.L.; Kim, B.G.; Hwang, N.S. Fabrication of polyphenol-incorporated anti-inflammatory hydrogel via high-affinity enzymatic crosslinking for wet tissue adhesion. Biomaterials 2020, 26, 119905. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Li, Z.; Xu, L.; Qazi, I.M.; Luo, C.; Gao, X.; Khan, M.U.; Iqbal, A.; Guo, Y.; et al. Tyrosinase/caffeic acid cross-linking alleviated shrimp (Metapenaeus ensis) tropomyosin-induced allergic responses by modulating the Th1/Th2 immunobalance. Food Chem. 2021, 15, 127948. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Tyrosinase immobilization strategies for the development of electrochemical biosensors-a review. Nanomaterials 2023, 17, 760. [Google Scholar] [CrossRef]
- Wang, M.M.; Wang, Y.; Pan, P.; Liu, X.P.; Zhang, W.J.; Hu, C.; Li, M.Z. A high molecular weight silk fibroin scaffold that resists degradation and promotes cell proliferation. Biopolymers 2023, 114, e23554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, F.; Jiang, X.; Cao, M.; Wang, S.; Zou, H.; Cao, Y.; Xian, M.; Liu, H. Microbial production of amino acid-modified spider dragline silk protein with intensively improved mechanical properties. Prep. Biochem. Biotechnol. 2016, 46, 552–558. [Google Scholar] [CrossRef] [PubMed]
- ASTM D3039/D3039M-00; Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Mattson, N.M.; Chan, A.K.N.; Miyashita, K.; Mukhaleva, E.; Chang, W.H.; Yang, L.; Ma, N.; Wang, Y. A novel class of inhibitors that disrupts the stability of integrin heterodimers identified by CRISPR-tiling-instructed genetic screens. Nat. Struct. Mol. Biol. 2024, 31, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Yu, Y.; Jiang, S.S.; Li, J.W.; Wang, S.; Chen, S.J.; Ma, J.W. Biocompatible gradient chitosan fibers with controllable swelling and antibacterial properties. Fibers Polym. 2022, 23, 1–9. [Google Scholar] [CrossRef]
- Janowicz, M.; Galus, S.; Szulc, K.; Ciurzynska, A.; Nowacka, M. Investigation of the structure forming potential of protein components in the reformulation of the composition of edible films. Materials 2024, 17, 937. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Honda, T.; Kato, K.; Hori, R.; Ujike, N.; Uemura, A.; Murakami, T.; Kitpipatkun, P.; Nakazawa, Y.; Tanaka, R. Silk fibroin-based vascular repairing sheet with angiogenic-promoting activity of SVVYGLR peptide regenerated the damaged vascular in rats. J. Biomater. Appl. 2022, 37, 3–11. [Google Scholar] [CrossRef]
- Mester, L.; Govyadinov, A.A.; Chen, S.; Goikoetxea, M.; Hillenbrand, R. Subsurface chemical nanoidentification by nano-FTIR spectroscopy. Nat. Commun. 2020, 11, 3359. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, X.; Jiao, Y.; Li, M. Regulation of the Degradation Properties of Tyrosinase-Catalyzed Crosslinking Silk Membranes for Superficial Wound Repair. Materials 2024, 17, 2839. https://doi.org/10.3390/ma17122839
Liu Y, Liu X, Jiao Y, Li M. Regulation of the Degradation Properties of Tyrosinase-Catalyzed Crosslinking Silk Membranes for Superficial Wound Repair. Materials. 2024; 17(12):2839. https://doi.org/10.3390/ma17122839
Chicago/Turabian StyleLiu, Yu, Xuping Liu, Yuhong Jiao, and Mingzhong Li. 2024. "Regulation of the Degradation Properties of Tyrosinase-Catalyzed Crosslinking Silk Membranes for Superficial Wound Repair" Materials 17, no. 12: 2839. https://doi.org/10.3390/ma17122839





