Abstract
Precipitation is an important factor that influences the quality of surface water in many regions of the world. The pollution of stormwater runoff from roads and parking lots is an understudied area in water quality research. Therefore, a comprehensive analysis of the physicochemical properties of rainwater flowing from parking lots was carried out, considering heavy metals and organic micropollutants. High concentrations of zinc were observed in rainwater, in addition to alkanes, e.g., tetradecane, hexadecane, octadecane, 2,6,10-trimethyldodecane, 2-methyldodecane; phenolic derivatives, such as 2,6-dimethoxyphenol and 2,4-di-tertbutylphenol; and compounds such as benzothiazole. To remove the contaminants present in rainwater, adsorption using silica carriers of the MCF (Mesostructured Cellular Foams) type was performed. Three groups of modified carriers were prepared, i.e., (1) SH (thiol), (2) NH2 (amino), and (3) NH2/SH (amine and thiol functional groups). The research problem, which is addressed in the presented article, is concerned with the silica carrier influence of the functional group on the adsorption efficiency of micropollutants. The study included an evaluation of the effects of adsorption dose and time on the efficiency of the contaminant removal process, as well as an analysis of adsorption isotherms and reaction kinetics. The colour adsorption from rainwater was 94–95% for MCF-NH2 and MCF-NH2/SH. Zinc adsorbance was at a level of 90% for MCF-NH2, and for MCF-NH2/SH, 52%. Studies have shown the high efficacy (100%) of MCF-NH2 in removing organic micropollutants, especially phenolic compounds and benzothiazole. On the other hand, octadecane was the least susceptible to adsorption in each case. It was found that the highest efficiency of removal of organic micropollutants and zinc ions was obtained through the use of functionalized silica NH2.
1. Introduction
Rainwater is an essential component of the water cycle, playing a key role in maintaining ecosystems and providing water for various uses. However, with increasing urbanization, more and more areas are covered with hard surfaces such as asphalt, increasing the amount of rainwater flowing into the sewer system. Rainwater collects contaminants from hard surfaces, such as car oils, street chemicals, and minerals, before entering the sewer system [1,2]. These substances can be harmful to the aquatic environment and pose a health risk. Rainwater flowing into the sewage system can end up in rivers and lakes, introducing pollutants into them and disrupting natural ecosystem processes. This phenomenon may lead to the deterioration of water quality and a threat to aquatic fauna and flora [3,4]. Sources of pollution from roads and traffic include road surface abrasion (10,000 kg/km per year); tire abrasion (0.12 kg/km per 1000 vehicles per year); brake pad abrasion (15 kg per 106 vehicles per kilometre); organic substances such as fuel, transmission oil, grease, brake fluid, and antifreeze (mainly from droplet losses from vehicles); and corrosion products [5]. Tire abrasion generates contaminants such as rubber, soot, and oxides of heavy metals, including zinc (Zn), lead (Pb), chromium (Cr), copper (Cu), and nickel (Ni). On the other hand, abrasion of brake pads is a particular contributor to the emission of nickel (Ni), chromium (Cr), copper (Cu), and lead (Pb) [5].
As evidenced above, rainwater becomes a carrier of various pollutants that can harm both the environment and human health [6]. These pollutants, divided according to their nature into organic and aromatic compounds, can come from emissions from motor vehicles, the petrochemical industry, and inorganics, including heavy metals. Most of these substances are considered micropollutants due to their low concentrations in water, air, or soil environments. Micropollutants are characterized by the fact that they are present in very small quantities, but can have potentially harmful effects on human health and ecosystems [7]. Their negative impacts on health include, among others, neurodevelopmental defects in children, thyroid disease, bone deformities, and endocrine cancers. Furthermore, these pollutants deteriorate soil conditions, affecting microbes and biogeochemical processes, which can lead to genetic changes in microorganisms [8]. Precipitation is an important factor that influences the quality of surface water in many regions of the world. However, stormwater runoff pollution from roads and parking lots is an understudied area in water quality research. Numerous studies can be found in the literature, most of which have focused on different types of pollutants. In the southwestern United States, atrazine, prometon, simazine, pentachlorophenol, nonylphenol, and PFOA have been detected in rainwater. Average concentrations were significantly higher during the monsoon season than in winter for prometon, simazine, and pentachlorophenol. However, average concentrations of nonylphenol were significantly higher in winter than during the monsoon season [9]. In France, new metals (As, Ti, Sr, and V) and organic substances—such as nonylphenol and octylphenol ethoxylates, some pesticides, and bisphenol A—have been determined in rainwater [10]. However, in rainwater collected from urban areas and agricultural land in northern Thailand, caffeine, 4-nitrophenol, tris(2-butoxyethyl) phosphate, atrazine, phenobucarb, and 2,4-D were determined. In addition, evidence of the presence of acetaminophen, fexofenadine, diphenhydramine, and gabapentin was provided [11]. Extensive studies on surface runoff have been carried out in the centre of Aachen, western Germany, where all polycyclic aromatic hydrocarbons, benzothiazoles, benzotriazoles, and organophosphates were analysed. They are classified as urban and road pollutants. Tris(1-chloro-2-propyl)phosphate (TCPP, 1.6 mg/kg silicone) was also detected, which was believed to have come from car interiors and was found in snow samples near road intersections [12].
However, the concentrations of heavy metals in precipitation waters, both those coming from paved surfaces and those from roofs, are the most widely discussed. For example, the concentration of heavy metals in rainwater from the metropolis and suburbs of Lagos State in Nigeria is as follows: Fe > Zn > Ni > Cu > Cd > Pb. Cd and Pb exceed WHO standards [13]. On the contrary, in Istanbul, metal concentrations in rainwater were found to be higher than those reported by other researchers around the world. The solubility of toxic metals was found to follow the order of Cd > Cu > V > Zn > Ni > Pb > Cr [14]. On a subtropical island off the coast of northern Taiwan, Cu, Zn, Cd, and Pb were detected in rainwater, and the authors confirmed that they come primarily from anthropogenic sources, related to industrial combustion and the emissions from local road traffic [15]. In Lucknow, India, sufficiently high concentrations of chromium, cadmium, and lead have been found to exceed the permissible limit established by the WHO, which can cause various adverse health effects [16]. All these data emphasize that the place (agricultural or industrial areas), the season, the type of surface, and air pollution play a significant role in the type and amount of pollutants present. It is worth noting that the presence of micropollutants highlights the need for additional treatment processes to neutralize potentially negative effects on the environment [17,18]. An adsorption process can be an effective solution due to its ability to efficiently remove contaminants from water [19]. In addition, the process can be adjusted by selecting the appropriate adsorption carrier and optimizing the process conditions—such as adsorbent dose, contact time, and temperature—to achieve high contaminant removal efficiency. Additionally, due to its relatively simple implementation and low operating costs, the adsorption process is often the preferred method of stormwater treatment compared to other technologies [20]. MCF silica carriers were introduced to remove contaminants. These cell foams, made of silica and with a mesoporous structure, are characterized by high adsorption efficiency. Functionalized silica carriers, on the other hand, are materials that have been chemically modified to obtain specific properties, such as chemical reactivity or adsorption capacity aimed at specific substances [21]. Silica carriers are commonly used as adsorption materials to remove a variety of contaminants from a variety of environments, including surface water, groundwater, and gases [22,23,24]. They are minerals composed mainly of silica (silicon dioxide, SiO2) and can have different forms, such as colloidal silica, activated silica, or functionalized silica [25]. Silica can effectively absorb a variety of contaminants, including heavy metals [26,27,28], organic compounds [29], dyes [30,31], and other harmful substances [32]. The silica used in this study has been further functionalized to improve its properties. The functionalization process involves chemical modification of the silica surface by introducing specific functional groups onto it [33]. This modification is intended to improve the ability of silica to adsorb specific contaminants. For example, by adding amino groups, the affinity of silica for the adsorption of dyes or heavy metals can be increased [34,35]. Three groups of modified carriers were prepared—(1) SH (thiol), (2) NH2 (amino), and (3) NH2/SH (amine and thiol functional groups). The research problem, which is addressed in the presented article, is concerned with the silica carrier influence of the functional group on the adsorption efficiency of micropollutants. The modification of MCF using two groups at the same time was aimed at increasing the efficiency of removing both organic and inorganic compounds from water. This is an important aspect, as choosing the right carrier can be crucial for the efficiency of the rainwater removal process. An important element of this article is the comprehensive analysis of the physicochemical properties of rainwater flowing from parking lots, including heavy metals and organic micropollutants. The study also included an evaluation of the effects of adsorption dose and timing on the efficiency of the contaminant removal process, as well as an analysis of adsorption isotherms and reaction kinetics, allowing us to better understand the mechanisms involved in the adsorption process. An additional novelty in the presented article is the fact that the study of the adsorption of micropollutants was carried out on actual rainwater. Until now, many studies on adsorption processes have used laboratory models or artificially prepared contaminant solutions, which can lead to some simplifications and inaccurate representation of real environmental conditions. Real rainwater can contain a variety of chemicals, microorganisms, and other components that can affect the adsorption process in ways that are difficult to predict in laboratory models. The results obtained from such studies may be more reliable and useful for engineering practice, as they reflect the conditions under which adsorption processes are to be used for rainwater treatment in real terms.
2. Materials and Methods
In this section, we will discuss how we collected data, performed analyses, and interpreted the results. To simplify the understanding of this process, we have created a diagram that presents the next steps and stages of our research work. In the description below, we explain the different parts of the diagram and what they mean for a full understanding of our research methodology. Figure 1 schematically shows the different stages of the research.
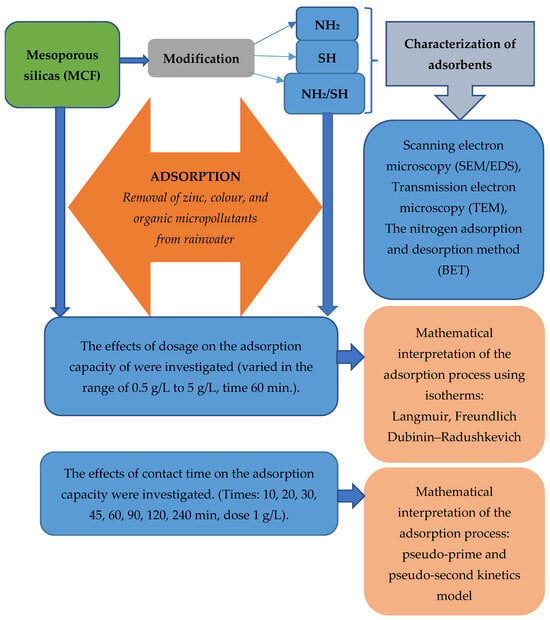
Figure 1.
Stages of the testing of mesoporous silicas, including the assessment of their physicochemical properties and the characteristics of the adsorption process.
2.1. The Subject of the Study
The subject of the study was rainwater from the manhole parking lot of the Silesian University of Technology, located on the campus in Gliwice, Silesian Voivodeship, Poland. Near the place from which the water was collected is the Diameter Road 902. The exact location of the sample collection site is shown in Figure 2. Rainwater was collected in March 2023.
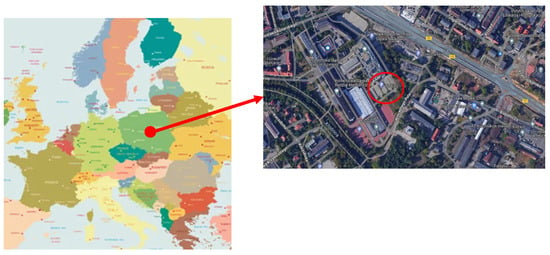
Figure 2.
Location of the rainwater collection site used for the research.
2.2. Analysis of Organic Micropollutants
The chromatographic analysis of the extracts was performed using equipment from Agilent Technologies (Santa Clara, CA, USA). The use of the analytical tool was consistent with the methodology described in a previous article [36]. Compounds were identified by comparing their mass spectra with data from the NIST v17 database. A compound was considered identified if the precision of matching its spectrum to the proposed spectrum in the database (expressed as similarity in %) was greater than or equal to 70%.
2.3. Analysis of Physicochemical Parameters
The efficiency was evaluated by monitoring typical quality parameters (colour, COD, TOC, cooper, nickel, zinc, lead, nitrate nitrogen, phosphate phosphorus, conductivity, pH).
2.4. Synthesis and Modification of MCF Materials
The preparation of MCF was performed as described in [37,38]. In a typical procedure, Pluronic P123 (4 g) was dissolved in 1.6 M HCl (75 mL) at room temperature. Then 1,3,5-trimethylbenzene (5.8 mL) and NH4F (0.023 g) were added by vigorous stirring and the mixture was heated to 40 °C. Following 1 h stirring, TEOS was added (4.7 mL), whereupon the mixture was stirred for 1 h and then stored at 40 °C for 20 h, then at 100 °C for 24 h. After being cooled to room temperature, the precipitate was filtered, dried at room temperature for 4 days, and calcined at 500 °C for 8 h.
Before grafting, MCFs were contacted with water vapour for 5 h and then dried at 200 °C for 2 h. Functional groups were grafted onto the silica surface by reacting the appropriate amounts of organosilanes (3-aminopropyltrimethoxysilane (-NH2) and/or 3-mercaptopropyltrimetoxysilane (-SH)) in toluene under reflux (24 h, 80 °C), with the silanols present on the silica surface to obtain the load of the functional moiety of approximately 1.5 mmol/g of silica. In particular, 50 mL of the solution containing 3-aminopropyltrimethoxysilane (0.269 g for MCF-NH2 or 0.135 g for MCF-NH2/SH) and/or 3-mercaptopropyltrimetoxysilane (0.295 g MCF-SH or 0.147 g MCF-NH2/SH) was stirred under reflux with 1 g of MCF for 24 h, after which the solvent was removed by filtration. Unmodified silicas were designated as MCF, those modified with amine groups as MCF-NH2, those with thiol groups as MCF-SH, and those containing both groups as MCF-NH2/SH.
2.5. Adsorption Process
The influence of contact time and sorbent dosage on the adsorption capacity of zinc and colour from rainwater was investigated. Additionally, an experiment on the adsorption of organic micropollutants was conducted using a fixed adsorbent dosage (1 g/L) and a selected adsorption time (120 min). The adsorption process took place in 100-millilitre glass flasks containing 100 millilitres of the test solution, at room temperature (20 ± 2 °C), in an incubator with a shaker rotating at 300 revolutions per minute. Kinetic experiments were conducted as follows: 1 g/L of sorbent was added to 100 millilitres of feeding water in a glass flask, which was then shaken at 300 revolutions per minute for specific time intervals: 10, 20, 30, 45, 60, 90, 120, 240 min. The pH of the solution was kept constant using a 0.1 mol/L HNO3/NaOH solution. Samples of the adsorbent were separated from the solution at specified time intervals for further analysis. Similarly, an equilibrium adsorption experiment was conducted. The mass of sorbents varied from 0.5 g/L to 5 g/L for 120 min.
To determine the adsorption kinetics, the experimental data were analysed using pseudo-first-order and pseudo-second-order kinetic models, which are given in Equations (1) and (2), respectively [39]:
where q = quantity of adsorbed (mg/g) and t = time, the slope of the linear plot of ln(qe − qt) against t gives –K1, where K2 can be estimated from the linear plot of t/qt vs. t.
The amount adsorbed was calculated from Equation (3) [40],
where Qe (mg/g) is the amount adsorbed, C0 and Ce (mg/L) are the initial and equilibrium concentrations of the adsorbates, respectively, m (g) is the mass of the adsorbent, and V (L) is the volume of the feed water.
Two common adsorption isotherm models were used to fit the experimental data in this study, including the Langmuir model, the Freundlich model, and the Dubinin–Radushkevich model. Each model is briefly described below.
The Langmuir model assumes monolayer adsorption, where molecules interact only with the surface of the sorbent. The Langmuir isotherm is represented by Equation (4) [40],
where Qm (mg/g) is the maximum adsorption capacity and KL (L/mg) is the Langmuir fitting parameter.
The Freundlich model is empirical and adequately describes adsorption on heterogeneous surface energy systems. This model has significant importance in chemisorption and some cases of physisorption, and it can be written as shown in Equation (5) [40],
where KF ((mg/g)(L/mg)n) is the Freundlich adsorption coefficient and n is the number describing surface heterogeneity and sorption intensity.
The Dubinin–Radushkevich model is based on the postulation that the mechanism for adsorption in micropores is that of pore-filling rather than layer-by-layer surface coverage. The Dubinin–Radushkevich equation takes the form shown in Equation (6),
where aDR (mg/g) is the amount of adsorbate that can be adsorbed in micropores (this can be obtained by plotting lnq_e as a function of ε2), E (kJ/mol) is the adsorption energy (which can be read from the slope of that line), and ε is the adsorption potential, which is defined by Equation (7),
where R (8.314 J·mol−1·K−1) is the ideal gas constant, T (K) is the temperature, and C_s (mg/L) is the solubility in water [40].
2.6. Characterization of Adsorbents
The characterization of MCF included the following:
- The surface morphologies of the minerals studied were investigated by scanning electron microscopy (SEM/EDS). The scanning electron microscope used for the JSM 6360LA was manufactured by JEOL-Japan (Tokyo, Japan).
- The textures of the materials were also examined using transmission electron microscopy (TEM, Tecnai G2 apparatus operating at 200 kV).
- The structural properties of the adsorbents were determined by measuring the surface areas and pore size distributions using the low-temperature nitrogen adsorption and desorption technique, according to the Brunauer–Emmett–Teller (BET) method.
- The determination of functional groups and vibrational spectra was performed using FTIR spectroscopy.
2.7. Statistical Analysis of the Results
The results presented in the figures and tables in this paper are the obtained values of the results of the experiment, calculated using the arithmetic method. The removal degree of the compound ions of the MCF studied was calculated using the following Equation (8):
where C0 is the initial concentration of the compound in the solution (mg/L) and C is the concentration of the compound in the solution after adsorption (mg/L).
The experimental errors are presented as confidence intervals, and error bars are given as standard deviation, σ, according to Equation (9):
where x represents the results of a single experiment repetition and n—is the number of repetitions.
3. Results and Discussion
3.1. Characteristics of MCF
Mesoporous cellular foams (MCFs) are characterized by well-defined pores with a uniform size distribution and shape. The pore diameter is about 25 nm and the size of the connecting windows is about 15 nm (Table 1). The SEM image (Figure 3A) shows an extended structure, which affects the size of the specific surface (about 500 m2/g, Table 1, obtained using the BET method). The elemental composition of the materials was confirmed by EDS analysis, showing that the unmodified MCF-type support consists exclusively of silicon and oxygen (Figure 3B). The cellular structure of the MCF was confirmed by the TEM image (Figure 3C).

Table 1.
Physicochemical determinations performed.
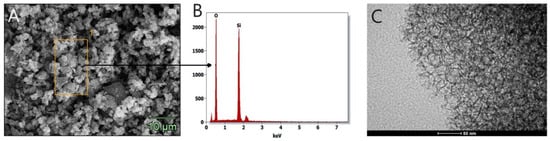
Figure 3.
SEM (A), EDS analysis (B), and TEM (C) images of MCF carrier.
Figure 4 shows the nitrogen adsorption and desorption isotherms of mesoporous cellular foam (MCF) at −196 °C before and after functionalization. These are classified as Type IV isotherms, characterized by a steep H1-type hysteresis in the medium to high relative pressure range typical of mesoporous materials. H1 type hysteresis loops are obtained when the capillaries are shaped as bilaterally open, regular, and irregular cylinders or prisms [41,42].
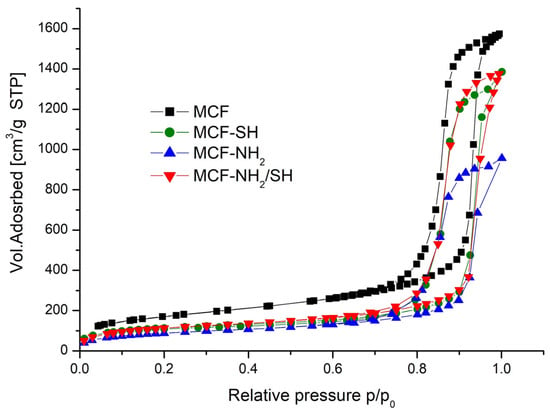
Figure 4.
Nitrogen adsorption and desorption isotherms of silica mesoporous cell foam (−196 °C) before and after its functionalization.
Table 2 shows the texture parameters of cellular foams (MCFs) before and after functionalization with organosilanes. As a result of functionalization, there was a reduction in the volume of adsorbed nitrogen (pore volume), a reduction in the diameters of the pores and the windows connecting them, and a reduction in the specific surface area. This is confirmed by other studies [43,44]. These changes are proportional to the number and size of organic groups introduced.

Table 2.
Structure parameters of pristine and functionalized mesoporous silica carriers.
To confirm the presence of organic functional groups on the surfaces of silica carriers, infrared spectra were analysed (Figure 5).
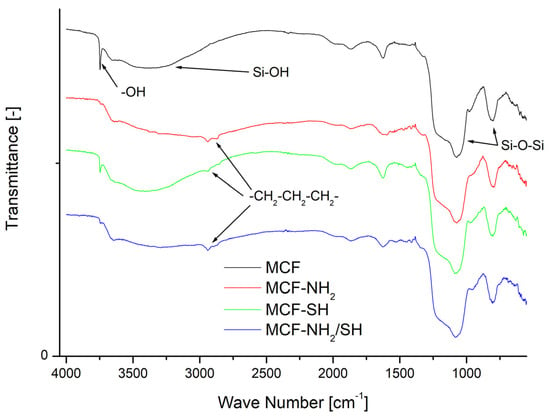
Figure 5.
FTIR spectra of the pristine MCF after functionalization.
Figure 5 shows the FTIR spectra of the MCF material in its pristine form (MCF) and after modification (MCF-NH2, MCF-SH, MCF-NH2/SH). The unmodified silica featured a broad band between 3200 and 3600 cm−1 that could be attributed to Si-OH stretching vibrations; additionally, a sharp and intense band at 3740 cm−1 could be unambiguously assigned to the OH symmetric stretching vibration of isolated silanol groups [43]. The adsorption bands around 1000–1250 cm−1 and 800 cm−1 are attributed to asymmetric and symmetric stretching vibrations of the Si-O-Si framework [45]. Appreciable changes in all of the spectra were observed after modification of the MCF support with organic moieties. First, the intensity of the band at 3740 cm−1 was markedly reduced as a result of the incorporation of organic groups. The presence of –NH2 and –SH groups were identified by the methylene stretching bands of the propyl chain in the region of 2850 to 2950 cm−1, seen in the FTIR spectra of MCF-NH2, MCF-SH, and MCF-NH2/SH, and their deformation bands at 1410 to 1455 cm−1. The N–H absorption bands overlapped with O–H bands at 3200–3600 cm−1 [46].
3.2. Characteristics of Rainwater
The general characteristics of rainwater and the contents of their organic and inorganic compounds are presented below. Table 3 and Table 4 present the physicochemical parameters and the identification of the organic compounds in rainwater, respectively. Figure 6 shows a selected chromatogram from the analysis of the actual rainwater flowing from the parking lot.

Table 3.
Characteristics of rainwater.

Table 4.
Organic compounds identified in rainwater.
Figure 6.
A chromatogram obtained for rainwater before the treatment process.
Based on the results of the physicochemical analysis, it was found that almost all parameters of the tested rainwater did not exceed the standards specified in the Regulation of the Minister of Maritime Economy and Inland Navigation [47]. This regulation establishes the permissible values of substances particularly harmful to the aquatic environment and conditions to be met when discharging wastewater into water or the ground, as well as when discharging rainwater or snowmelt into water or water facilities. In the analysed water, of all the parameters tested, only the concentration of zinc exceeded the standards (2 mg/L) specified in the Regulation [47]. This contaminant may be present in the water due to abrasion from car tires [48,49]. Other researchers have also detected zinc in water from an urbanised catchment of a rainwater collector in Gdansk [50]. These data are also corroborated by researchers who analysed rainwater in Berlin [49].
The tested rainwater also contained alkanes, both simple (e.g., tetradecane, hexadecane, octadecane) and branched (2,6,10-trimethyldodecane, 2-methyldodecane), as well as halogenated alkane derivatives (1-chloroctadecane), phenolic derivatives, and benzotriazole. Higher alkanes are used in the production of fuels and lubricants [51]. Benzothiazole (BT), on the other hand, is an organic chemical and is a heterocyclic compound containing a benzene ring linked to nitrogen and a sulphur atom. There are several potential sources of benzothiazole in rainwater from the street—it can come from vehicle exhaust emissions, especially motor oils; fuels; and materials that wear out during vehicle operation (such as tires or brake pads) [52].
If there are industrial plants in the vicinity of the road that use benzothiazole or benzothiazole derivatives in their production processes, this substance can leach into stormwater [53]. Pollutants generated by human activities, such as vehicle emissions and industrial activities, are contained in road dust. This dust can be released into the atmosphere by re-emitting particles, which contributes to air pollution. Then, during the rainy season, this dust can be flushed into water bodies, leading to pollution of the aquatic environment [54].
Rainwater quality analysis is a very useful tool for understanding air quality and the origin of the pollutants it contains. Contaminants present in rainwater can be chemical, physical, or biological. In addition to atmospheric pollutants, there are a number of pollutants on the road that come from vehicles, such as metals and other materials resulting from the degradation of tyres and other parts, as well as by-products of internal combustion engines and corrosion processes [55]. In Poland, the quality of rainwater is regulated by the Regulation of the Minister of Maritime Economy and Inland Navigation, Journal of Laws 2019, item 1311. In the European Union, on the other hand, the main directives for the management of urban stormwater runoff are the EU Water Framework Directive (WFD) of 2000 and the Environmental Quality Standards Directive (EQSD) of 2008 [56]. In the USA, regulations have been implemented mainly on the basis of arrangements issued by the Environmental Protection Agency (EPA) since 1972 [57]. On the basis of the literature, it has been concluded that rainwater is a service of local responsibility—regulations concerning rainwater are dispersed at all levels of the administrative organization of the state and in all localities [57]. It was found that the general lack of clear guidelines and regulations for the management of stormwater quality and its potential risks to public health may hinder the implementation of stormwater programmes [58]. On the other hand, it is believed that identifying sources of pollution in catchments is crucial for accurately predicting rainwater quality.
3.3. Efficiency of Adsorption of Micropollutants Using Silica
Figure 7 shows the degree of removal of each organic micropollutant through the adsorption process. The degrees of removal were determined based on the peak areas of the compounds present in rainwater before and after the adsorption process.
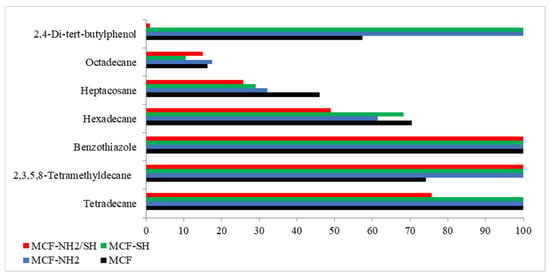
Figure 7.
Degrees of removal of micropollutants for each adsorbent used.
There are a limited number of studies in the scientific literature on the adsorption of organic micropollutants using silica, as well as studies using real aqueous solutions. Our analysis showed that all silica tested achieved 100% benzothiazole removal. The highest efficiency of removal of all considered organic micropollutants was found for the silica MCF-NH2. Studies by Hafezian et al. [59] confirm the efficacy of the NH2 group for sulforaphane adsorption. It was noted that in the case of phenolic impurities, the thiol group showed the same adsorption efficiency as the amino group. Octadecan was the least susceptible to adsorption. Alkanes typically do not adsorb strongly on adsorption surfaces compared to more polar compounds such as alkenes, alcohols, or carboxylic acids. This is due to their low polarity and weak intermolecular interactions [60]. In addition, a number of various other chemical compounds result in a lack of available adsorption sites [61].
Batch adsorption studies show that the adsorption capacity of contaminants in functionalized silica is greater than in pure MCF. It was also found that functionalization of MCF with two thiol and amine groups reduced the efficiency of adsorption.
3.4. Effects of Process Time and Adsorbent Dose on the Efficiency of Zinc and Colour Adsorption Efficiency from Rainwater
To meet the water regulatory standards imposed by governments and other regulators, many advances are being made in improving the water purification process. One such advancement involves functionalized mesoporous silica. This study proved that the large surface areas of mesoporous materials and the possibility of their functionalization with different functional groups make these materials ideal for use in the adsorption area, for example, for the removal of heavy metals and organic pollutants from rainwater. Studies were carried out on the effect of the dose of the adsorbent on zinc and colour adsorption from actual precipitation water (Figure 8 and Figure 9). It was found that the functionalization of MCF with an amino group gave positive results, as the concentration of zinc ions in rainwater was 49% lower than in the case of pure MCF used at a dose of 1.0 g/L. Additionally, an increase in dye removal of 53% was noted. In the context of the adsorption of heavy metals, in this case zinc, a significant performance advantage of amino group-functionalized MCF over thiol group-functionalized MCF was found. A high efficiency of zinc adsorption by amine-functionalized silica carriers was also achieved by Kazuma Nakanishi et al. [62]. In terms of colour removal, MCF-NH2 also achieved the highest efficiency. However, the colour adsorption capacities of MCF-SH and MCF-NH2/SH were higher than their zinc adsorption capacities. It is assumed that silica carriers with a thiol group will remove organic compounds or colour more effectively, and carriers with an amino group will be dedicated to the adsorption of heavy metals from rainwater. Therefore, we decided to combine both functional groups with a silica carrier. Some authors suggest that hydrogen bonds moderated by a water molecule may form between the groups that make up the colour of water and amino groups [63].
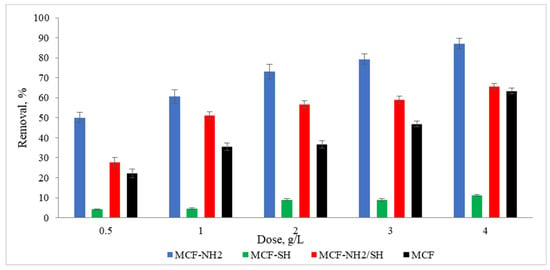
Figure 8.
Effect of adsorbent dose on the concentration of zinc in rainwater (C0 = 4.5 mg/L, time of processes = 120 min, pH rainwater = 6.7).
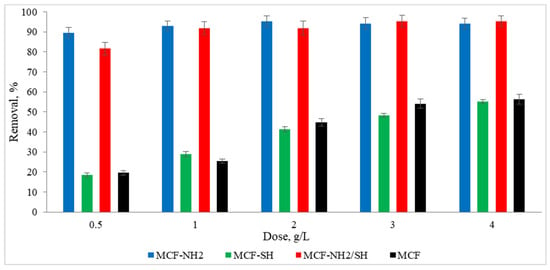
Figure 9.
Effect of adsorbent dose on colour in rainwater (C0 = 87 mg/L, time of processes = 120 min, pH rainwater = 6.7).
The next stage of the research was to determine the effect of the process duration on the adsorption efficiency. The tests were carried out at a constant room temperature. A real rainwater solution was used, in which the zinc concentration was 5 mg/L and the colour was 87 mg Pt/L. Studies were carried out using a dose of 2 g/L and different adsorption times in the range of 10–240 min. The results are shown in Figure 10 and Figure 11.
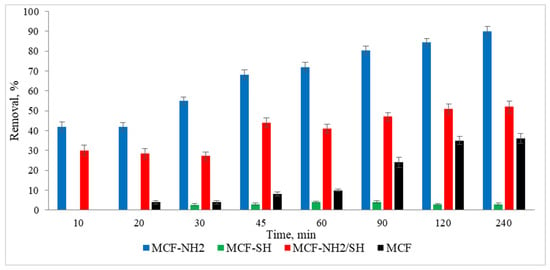
Figure 10.
Effect of processing time on the effectiveness of the removal of colour from rainwater (C0 = 5 mg/L, dose of adsorbents = 1 g/L, pH rainwater = 6.7).
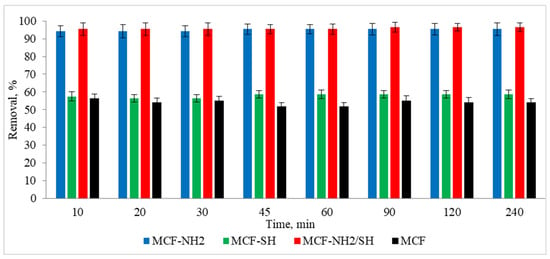
Figure 11.
Effect of processing time on the effectiveness of the removal of zinc from rainwater (C0 = 87 mg/L, dose of adsorbents = 1 g/L, pH rainwater = 6.7).
In the case of colour, rapid and consistent adsorption was recorded after just 10 min of the process. The efficiency was 94–95% for MCF-NH2 and MCF-NH2/SH, while for MCF-SH it was 56–58%, and for pure MCF it was 51–54%. On the other hand, zinc adsorption increased with the duration of the process and for 240 min it was removed at a level of 90% for MCF-NH2; 52% for MCF-NH2/SH; for MCF-SH, only 3%; and for pure MCF, 36%. The effectiveness of silica carriers functionalized with amine groups has been confirmed by numerous studies—for example, they were used for the adsorption of chromium from water [64], as well as phytic substances [65]; copper, nickel, zinc, cadmium, and lead [66]; uranium (VI) [67]; fufic acids [68]; nitrates [69]; and dyes [70]. Other applications of silica were determined by Flores et al., who conducted research on the use of various types of mesoporous silica and showed that among the materials studied, mesoporous silica nanoparticles (MSNPs) turned out to be a highly efficient and versatile adsorbent, showing exceptional efficiency in the removal of both metal ions (iron, nickium, and copper) and organic dyes (MB and MG) [71]. In addition, magnetic mesoporous silica (NSMSiO2) showed a high adsorption capacity for humic substances and cationic dyes [72]. Yet another study has shown that hexagonal (MCM-41) mesoporous materials can be highly efficient in adsorbing trichloroethylene and tetrachloroethylene from water [73]. Wang et al. compared toluene adsorption by macroporous and mesoporous silica and proved that mesoporous silica exhibited a toluene adsorption capacity much higher than macroporous silica due to its very large surface area [74]. In summary, mesoporous silica materials, especially those functionalized with specific groups such as amines, exhibit excellent adsorption properties for various contaminants, including metals, dyes, and organic compounds. Their large surface areas and functionalization potential make them versatile and effective for environmental cleaning applications.
3.5. Zinc Adsorption and Colour Isotherms and Kinetics for MCF-NH2
Adsorption isotherms are very important for describing the interaction between the adsorbate and the adsorbent at equilibrium. Figure 12A–C show the Freundlich, Langmuir, and Dubinin–Radushkevich isotherms of Zn (II) and colour.
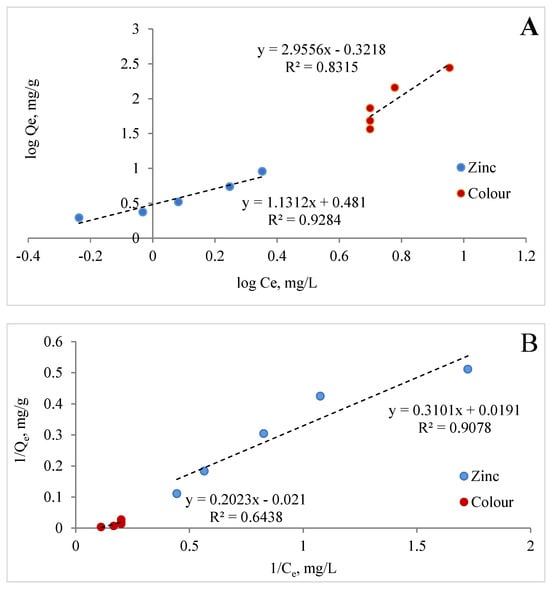
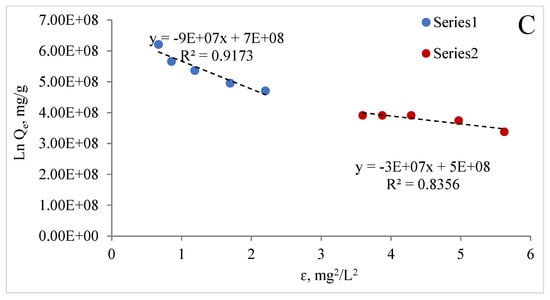
Figure 12.
Freundlich (A), Langmuir (B), and Dubinin-Radushkevich (C) isotherms of zinc and colour for MCF-NH2.
It is known that the magnitude of the apparent adsorption energy E is useful in estimating the type of adsorption, and if this value is less than 8 kJ/mol the type of adsorption can be explained by the physical adsorption; when E is between 8 and 16 kJ/mol, the type of adsorption can be explained by ion exchange; and when the value is over 16 kJ/mol, the adsorption type can be explained by stronger chemical adsorption than ion exchange [75]. The energy value obtained from Dubinin–Raduszkiewicz is less than 8 kJ/mol, suggesting the physical adsorption of zinc and colour by MCF-NH2. It was found that the correlation coefficients of the Langmuir graph model are 0.91 and 0.64, respectively; the Freundlich graph model is 0.93 and 0.34; and the Dubinin–Raduszkiewicz correlation coefficients are 0.917 and 0.836, respectively, for zinc and colour. Table 5 presents the parameters estimated from linear isotherm data plots. The data show that the Langmuir isotherm model provides a much better fit than the Freundlich isotherm model for colour adsorption. However, in the case of the adsorption of zinc and colour described by the Freundlich model, the values of n were greater than 0 and less than 1, which also suggests favourable adsorption [76,77]. Thus, the adsorption of zinc ions and colour by MCF-NH2 can be consistent with the Langmuir and Freundlich isotherm models. In other words, the surface of MCF-NH2 can typically be homogeneous, with metal ions adsorbing through surface amino groups. In addition, the R2 calculated from all models and the E < 8 kJ/mol suggest that adsorption may be compatible with the pore-filling mechanism. On the other hand, Hongjie WANG et al., in their studies on the functionalization of silica carriers and their application to copper adsorption, found that adsorption by binding metal to organic functional groups may be involved [78]. The assumption that types of functional groups have a significant effect on the removal capacity and selectivity of target heavy metal ions is supported by a review by Peng Zhang et al. [79]. These functional groups on the surface of ordered mesoporous materials tend to form stable coordination compounds with heavy metals, so heavy metal ions can be removed from the aqueous solution. In summary, adsorption involves the mechanisms of both chemisorption and pore-filling.

Table 5.
Parameters of the Freundlich, Langmuir, and Dubinin–Radushkevich equations, and the correlation coefficients for the adsorption of zinc and colour on the sorbents.
In the present study, a batch experiment investigated the effect of contact time on the interactions between MCF particles and zinc ions and rainwater colour. The maximum adsorption capacities Qe, K1, and K2 and the correlation coefficient R2 calculated from the pseudo-first- and second-order models are shown in Table 6. In the case of R2, the values of the pseudo-second-order model are larger than in the pseudo-first-order model. This suggests that the adsorption proceeded according to second-order kinetics and that the model used can be used to determine the relevant kinetic parameters. The pseudo-second-order model (Figure 13) describes the zinc and colour adsorption of the samples very well. The adsorption of the colour was faster than that of the zinc ions.

Table 6.
Parameters of the pseudo-first- and second-order kinetic models for the adsorption of zinc and colour.
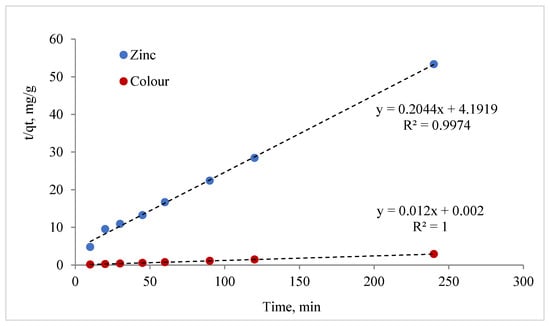
Figure 13.
The pseudo-second-order kinetic model for the adsorption of zinc and colour by MCF-NH2.
4. Conclusions
In light of the obtained results, it is confirmed that the selection of the appropriate functional group should be taken into account during the adsorption process. However, the assumption of equivalent effectiveness of the two functional groups seems to be accurate only in the context of the elimination of the natural colour of rainwater. This colour can be the result of both organic and inorganic pollutants generated by dust and exhaust fumes from vehicles. In addition, the study confirms that the analysis of the adsorption process using real solutions may lead to different conclusions than the analysis of the removal of individual contaminants. Continued research on the use of silica as an adsorbent carrier may contribute to the development of effective and environmentally friendly solutions for the removal of micropollutants from rainwater.
Based on the research carried out, the following conclusions can be drawn:
- The results of the analysis of rainwater by the applicable Polish Regulation on the quality of rainwater show that the permissible concentration of zinc was exceeded. In addition, various compounds such as alkanes, phenol derivatives, and benzothiazole were found.
- MCF-NH2 was found to be effective in the adsorption of zinc ions and colour.
- On the other hand, MCF-NH2/SH showed high colour removal efficiency, while in the case of zinc removal it was less effective than MCF-NH2. Studies confirm the effectiveness of modified MCF in the removal of organic micropollutants, especially phenolic compounds and benzothiazole. However, the alkanes were not adsorbed, probably due to their low polarity and weak intermolecular interactions.
- It was found that the adsorption of zinc and colour by MCF-NH2 can be consistent with the Langmuir and Freundlich isotherm models. Research suggests that functionalization of silica carriers may affect their ability to remove heavy metals. In conclusion, adsorption is affected by the mechanisms of both chemisorption and pore-filling, and functional groups on the surfaces of porous materials form stable compounds with heavy metals.
- It was found that the adsorption was carried out according to second-order kinetics—the model used can be used to determine the relevant kinetic parameters.
The obtained results confirm the practicality of taking into account the selection of the appropriate functional group in the adsorption process, and that attention should be paid to the limited accuracy of the assumption of equivalent effectiveness of two functional groups. In addition, it is important to note that analysis of the adsorption process using real solutions can provide new insights, suggesting the need for further research in this area. The development of methods for rainwater treatment and the elimination of micropollutants should be treated as a continuous process, requiring constant monitoring and improvement.
This article shows that contaminants found in parking lot water are often similar in many locations. These pollutants come from sources such as tire abrasion and diesel fuel residues. Literature analysis has confirmed that zinc is a common pollutant in road rainwater, which has also been shown in studies conducted in various cities around the world. Pollution concentrations may vary depending on the intensity of rainfall and the type of road surface, along with other factors, such as the proximity of industrial plants.
It was shown that the proposed process and adsorbent effectively removed many pollutants, although there were difficulties with the adsorption of alkanes. Our paper makes an important contribution to the development of this field by suggesting that this solution can be applied in various locations.
Author Contributions
Conceptualization, A.M., K.S. and E.P.; methodology, A.M., K.S., E.P. and E.K.; validation, A.M., K.S. and A.G.; formal analysis, A.M., K.S. and E.P.; investigation, A.M., E.P., K.S., E.K. and M.S.; data curation, A.M., K.S. and E.K.; writing—original draft preparation, A.M.; writing—review and editing, A.M., K.S., M.S., E.P. and A.G.; visualization, A.M., K.S. and M.S.; supervision, A.M.; funding, M.S., E.P. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research reported in this paper was co-financed by the European Union through the European Social Fund, in the framework of the project “Silesian University of Technology as a Center of Modern Education based on research and innovation”, POWR.03.05.00-00-Z098/17, and by the Polish Ministry of Science and Higher Education (BK–214/RIE-4/2024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gabr, M.E.; El Shorbagy, A.M.; Faheem, H.B. Assessment of Stormwater Quality in the Context of Traffic Congestion: A Case Study in Egypt. Sustainability 2023, 15, 13927. [Google Scholar] [CrossRef]
- Generowicz, A.; Gronba-Chyła, A.; Kulczycka, J.; Harazin, P.; Gaska, K.; Ciuła, J.; Ocłoń, P. Life Cycle Assessment for the environmental impact assessment of a city’ cleaning system. The case of Cracow (Poland). J. Clean. Prod. 2023, 382, 135184. [Google Scholar] [CrossRef]
- Emmanuel, E.; Balthazard-Accou, K.; Osnick, J. Impact of Urban Wastewater on Biodiversity of Aquatic Ecosystems. In Environmental Management, Sustainable Development and Human Health; CRC Press/Balkema: Boca Raton, FL, USA, 2009; Chapter 29. [Google Scholar]
- Gronba-Chyła, A.; Generowicz, A.; Kwaśnicki, P.; Cycoń, D.; Kwaśny, J.; Grąz, K.; Gaska, K.; Ciuła, J. Determining the Effectiveness of Street Cleaning with the Use of Decision Analysis and Research on the Reduction in Chloride in Waste. Energies 2022, 15, 3538. [Google Scholar] [CrossRef]
- Göbel, P.; Dierkes, C.; Coldewey, W.G. Storm water runoff concentration matrix for urban areas. J. Contam. Hydrol. 2007, 91, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Q.; Gersberg, R.; Ng, J.; Tan, S. Conventional and decentralized urban stormwater management: A comparison through case studies of Singapore and Berlin, Germany. Urban Water J. 2015, 14, 113–124. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Jiang, J.; Han, J.; Li, W.; Li, X.; Leung, K.M.Y.; Snyder, S.A.; Alvarez, P.J.J. Which Micropollutants in Water Environments Deserve More Attention Globally? Environ. Sci. Technol. 2022, 56, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Deepika; Tyagi, D.; Tarkeshwar; Kapinder. Organic Micropollutants and Their Effects on the Environment and Human Health. In Organic Micropollutants in Aquatic and Terrestrial Environments; Bhadouria, R., Tripathi, S., Singh, P., Singh, R., Singh, H.P., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Villagómez-Márquez, N.; Abrell, L.; Foley, T.; Ramírez-Andreotta, M.D. Organic micropollutants measured in roof-harvested rainwater from rural and urban environmental justice communities in Arizona. Sci. Total Environ. 2023, 876, 162662. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Sebastian, C.; Ruban, V.; Delamain, M.; Percot, S.; Wiest, L.; Mirande, C.; Caupos, E.; Demare, D.; Kessoo, M.D.K.; et al. Micropollutants in urban stormwater: Occurrence, concentrations, and atmospheric contributions for a wide range of contaminants in three French catchments. Environ. Sci. Pollut. Res. 2014, 21, 5267–5281. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.Y.; Srinuansom, K.; Snyder, S.A.; Ziegler, A.D. Atmosphere-Transported Emerging and Persistent Contaminants (EPCs) in Rainfall and Throughfall: Insights from a Rural Site in Northern Thailand. Atmosphere 2023, 14, 1603. [Google Scholar] [CrossRef]
- Fuchte, H.E.; Beck, N.; Bieg, E.; Bayer, V.J.; Achten, C.; Krauss, M.; Schäffer, A.; Smith, K.E.C. A look down the drain: Identification of dissolved and particle bound organic pollutants in urban runoff waters and sediments. Environ. Pollut. 2022, 302, 119047. [Google Scholar] [CrossRef] [PubMed]
- Iroegbulema, I.U.; Egereonu, U.U.; Ogukwea, C.E.; Egereonub, J.C.; Okorocand, N.J.; Nwoko, C.I.A. Assessment of Heavy Metals in Rainwater from Metropolis and Suburbs, Lagos State, Nigeria. Int. J. Environ. Clim. Chang. 2023, 13, 831–857. [Google Scholar] [CrossRef]
- Başak, B.; Alagha, O. Trace metals solubility in rainwater: Evaluation of rainwater quality at a watershed area, Istanbul. Env. Monit. Assess. 2010, 167, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; You, C.-F.; Lin, F.-J.; Huang, K.-F.; Chung, C.-H. Sources of Cu, Zn, Cd and Pb in rainwater at a subtropical islet offshore northern Taiwan. Atmos. Environ. 2011, 45, 1919–1928. [Google Scholar] [CrossRef]
- Sharma, P.; Rai, V. Assessment of rain water chemistry in the Lucknow metropolitan city. Appl. Water Sci. 2018, 8, 67. [Google Scholar] [CrossRef]
- Hatt, B.E.; Deletic, A.; Fletcher, T.D. Integrated treatment and recycling of stormwater: A review of Australian practice. J. Environ. Manag. 2006, 79, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Ciuła, J.; Gaska, K.; Siedlarz, D.; Koval, V. Management of sewage sludge energy use with the application of bi-functional bioreactor as an element of pure production in industry. E3S Web Conf. 2019, 123, 01016. [Google Scholar] [CrossRef]
- Ewis, D.; Ba-Abbad, M.M.; Benamor, A.; El-Naas, M.H. Adsorption of organic water pollutants by clays and clay minerals composites: A comprehensive review. Appl. Clay Sci. 2022, 229, 106686. [Google Scholar] [CrossRef]
- Azha, S.F.; Shahadat, M.; Ismail, S.; Ali, S.W.; Ahammad, S.Z. Prospect of clay-based flexible adsorbent coatings as cleaner production technique in wastewater treatment, challenges, and issues: A review. J. Taiwan Inst. Chem. Eng. 2021, 120, 178–206. [Google Scholar] [CrossRef]
- Tehrani, N.H.M.H.; Ardjmand, M.; Bazmi, M.; Rashidi, A.; Zadeh, M.R.M. Polydopamine-modified mesoporous silica materials as a novel adsorbent for superior CO2 adsorption: Experimental and DFT study. J. Environ. Chem. Eng. 2023, 11, 110451. [Google Scholar] [CrossRef]
- Addy, M.; Losey, B.; Mohseni, R.; Zlotnikov, E.; Vasiliev, A. Adsorption of heavy metal ions on mesoporous silica-modified montmorillonite containing a grafted chelate ligand. Appl. Clay Sci. 2012, 59–60, 115–120. [Google Scholar] [CrossRef]
- Ciuła, J.; Kowalski, S.; Wiewiórska, I. Pollution Indicator of a Megawatt Hour Produced in Cogeneration—The Efficiency of Biogas Purification Process as an Energy Source for Wastewater Treatment Plants. J. Ecol. Eng. 2023, 24, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Ueta, I.; Hayashibe, M.; Sumiya, K.; Ariizumi, Y.; Fujimura, K.; Saito, Y. Polydimethylsiloxane-coated macroporous silica adsorbent in thermal desorption gas chromatography. J. Chromatogr. Open 2023, 3, 100084. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized silica nanoparticles: Classification, synthetic approaches and recent advances in adsorption applications. Nanoscale 2021, 13, 5998–16016. [Google Scholar] [CrossRef] [PubMed]
- Wieszczycka, K.; Wojciechowska, I.; Filipowiak, K.; Buchwald, T.; Nowicki, M.; Dudzinska, P.; Strzemiecka, B.; Voelkel, A. Novel iminepyridinium-modified silicas as super-adsorbents for metals ions. Appl. Surf. Sci. 2022, 596, 153555. [Google Scholar] [CrossRef]
- Angotzi, M.S.; Mameli, V.; Cara, C.; Borchert, K.B.L.; Steinbach, C.; Boldt, R.; Schwarz, D.; Canna, C. Meso- and macroporous silica-based arsenic adsorbents: Effect of pore size, nature of the active phase, and silicon release. Nanoscale Adv. 2021, 3, 6100–6113. [Google Scholar] [CrossRef]
- Wang, P.; Du, M.; Zhu, H.; Bao, S.; Yang, T.; Zou, M. Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J. Hazard. Mat. 2015, 286, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, L.; Nikolantonaki, M.; Weber, G.; Bezverkhyy, I.; Chassagnon, R.; Assifaoui, A.; Bouyer, F. Functionalization of SBA-15 mesoporous silica for highly efficient adsorption of glutathione: Characterization and modeling studies. J. Taiwan Inst. Chem. Eng. 2023, 152, 105169. [Google Scholar] [CrossRef]
- Antony, J.; Gonzalez, S.V.; Bandyopadhyay, S.; Yang, J.; Rønning, M. Silica-modified bismutite nanoparticles for enhanced adsorption and faster solar photocatalytic degradation of methylene blue. Catal. Today 2023, 413–415, 113986. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, X.; Li, Q.; Yang, J.; Wang, R.; Sun, X.; Wei, L. Construction of C8/threonine-functionalized mesoporous silica aerogel based on mixed-mode and its adsorption properties for both anionic and cationic dyes. Powder Technol. 2024, 434, 119360. [Google Scholar] [CrossRef]
- Shan, W.; Zhang, Y.; Shu, Y.; Zhang, D.; Xing, C.; Xiong, Y. Enhanced adsorption and separation of Re(VII) using organic-inorganic hybrid silica adsorbent. Microporous Mesoporous Mater. 2021, 317, 110996. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Cao, Y.; Malekshah, R.E.; Heidari, Z.; Pelalak, R.; Marjani, A.; Shirazian, S. Molecular dynamic simulations and quantum chemical calculations of adsorption process using amino-functionalized silica. J. Mol. Liq. 2021, 330, 115544. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Torres, R.B.; Vareda, J.P.; Lopes, D.; Ferreira, M.; Valente, V.; Girão, A.V.; Valente, A.J.M.; Durães, L. Amine Modification of Silica Aerogels/Xerogels for Removal of Relevant Environmental Pollutants. Molecules 2019, 24, 3701. [Google Scholar] [CrossRef] [PubMed]
- Kudlek, E. Decomposition of contaminants of emerging concern in advanced oxidation processes. Water 2018, 10, 955. [Google Scholar] [CrossRef]
- Jarzębski, A.B.; Szymańska, K.; Bryjak, J.; Mrowiec-Białoń, J. Covalent immobilization of trypsin on to siliceous mesostructured cellular foams to obtain effective biocatalysts. Catal. Today 2007, 124, 2–10. [Google Scholar] [CrossRef]
- Szymańska, K.; Bryjak, J.; Jarzębski, A.B. Immobilization of Invertase on Mesoporous Silicas to Obtain Hyper Active Biocatalysts. Top. Catal. 2009, 52, 1030–1036. [Google Scholar] [CrossRef]
- Kamińska, G.; Bohdziewicz, J. Potential of various materials for adsorption of micropollutants from wastewater. Environ. Prot. Eng. 2016, 42, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, A.; Kamińska, G.; Fathy Abdel Salam, N. Simultaneous adsorption of organic and inorganic micropollutants from rainwater by bentonite and bentonite-carbon nanotubes composites. J. Water Process Eng. 2022, 46, 102550. [Google Scholar] [CrossRef]
- Maury, S.; Buisson, P.; Pierre, A.C. Porous texture modification of silica aerogels in liquid media and its effect on the activity of a lipase. Langmuir 2001, 17, 6443–6446. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, R.-F.; Wu, D.-Q.; Chan, K.-Y. Enzyme immobilization on amino-functionalized mesostructured cellular foam surfaces, characterization and catalytic properties. J. Mol. Catal. B Enzym. 2005, 33, 43–50. [Google Scholar] [CrossRef]
- Karbowiak, T.; Saada, M.A.; Rigolet, S.; Ballandras, A.; Weber, G.; Bezverkhyy, I.; Soulard, M.; Patarin, J.; Bellat, J.-P. New insights in the formation of silanol defects in silicalite-1 by water intrusion under high pressure. Phys. Chem. Chem. Phys. 2010, 12, 11454–11466. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska, A.; Derylo-Marczewska, A.; Wasilewska, M. Mesocellular silica foams (MCFs) with tunable pore size as a support for lysozyme immobilization: Adsorption equilibrium and kinetics, biocomposite properties. Int. J. Mol. Sci. 2020, 21, 5479. [Google Scholar] [CrossRef] [PubMed]
- Ciemięga, A.; Maresz, K.; Mrowiec-Białoń, J. Meervein-Ponndorf-Vereley reduction of carbonyl compounds in monolithic siliceous microreactors doped with Lewis acid centres. Appl. Catal. A Gen. 2018, 560, 111–118. [Google Scholar] [CrossRef]
- Szymańska, K.; Bryjak, J.; Mrowiec-Białoń, J.; Jarzębski, A.B. Application and properties of siliceous mesostructured cellular foams as enzymes carriers to obtain efficient biocatalysts. Microporous Mesoporous Mater. 2007, 99, 167–175. [Google Scholar] [CrossRef]
- Regulation of the Minister of Maritime Economy and Inland Navigation. Journal of Laws 2019, item 1311. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20190001311 (accessed on 2 May 2024).
- Sieber, G.; Beisser, D.; Rothenberger, J.; Shah, M.; Schumann, M.; Sures, B.; Boenigk, J. Microbial community shifts induced by plastic and zinc as substitutes of tire abrasion. Sci. Rep. 2022, 12, 18684. [Google Scholar] [CrossRef] [PubMed]
- Wicke, D.; Matzinger, A.; Sonnenberg, H.; Caradot, N.; Schubert, R.-L.; Dick, R.; Heinzmann, B.; Dünnbier, U.; von Seggern, D.; Rouault, P. Micropollutants in Urban Stormwater Runoff of Different Land Uses. Water 2021, 13, 1312. [Google Scholar] [CrossRef]
- Jakubowicz, P.; Fitobór, K.; Gajewska, M.; Drewnowska, M. Detection and Removal of Priority Substances and Emerging Pollutants from Stormwater: Case Study of the Kołobrzeska Collector, Gdańsk, Poland. Sustainability 2022, 14, 1105. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Pitz, W.J.; Herbinet, O.; Curran, H.J.; Silke, E.J. A comprehensive detailed chemical kinetic reaction mechanism for combustion of n-alkane hydrocarbons from n-octane to n-hexadecane. Combust. Flame 2009, 156, 181–199. [Google Scholar] [CrossRef]
- Feltracco, M.; Mazzi, G.; Barbaro, E.; Rosso, B.; Sambo, F.; Biondi, S.; Gambaro, A. Occurrence and phase distribution of benzothiazoles in untreated highway stormwater runoff and road dust. Environ. Sci. Pollut. Res. 2023, 30, 107878–107886. [Google Scholar] [CrossRef] [PubMed]
- Struk-Sokołowska, J.; Gwoździej-Mazur, J.; Jurczyk, Ł.; Jadwiszczak, P.; Kotowska, U.; Piekutin, J.; Canales, F.A.; Kaźmierczak, B. Environmental risk assessment of low molecule benzotriazoles in urban road rainwaters in Poland. Sci. Total Environ. 2022, 839, 156246. [Google Scholar] [CrossRef] [PubMed]
- Men, C.; Liu, R.; Xu, F.; Wang, Q.; Guo, L.; Shen, Z. Pollution characteristics, risk assessment, and source apportionment of heavy metals in road dust in Beijing, China. Sci. Total Environ. 2018, 612, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.-H.; Kayhanian, M.; Zoh, K.-D.; Stenstrom, M.K. Modeling of highway stormwater runoff. Sci. Total Environ. 2005, 348, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.R.; Thomsen, A.T.H.; Larsen, T.; Egemose, S.; Mikkelsen, P.S. From EU Directives to Local Stormwater Discharge Permits: A Study of Regulatory Uncertainty and Practice Gaps in Denmark. Sustainability 2020, 12, 6317. [Google Scholar] [CrossRef]
- Novaes, C.A.F.O.; Marques, R.C. Regulation of urban stormwater management is not a matter of choice, but performance. Water Policy 2022, 24, 1325–1342. [Google Scholar] [CrossRef]
- Bichai, F.; Ashbolt, N. Public health and water quality management in low-exposure stormwater schemes: A critical review of regulatory frameworks and path forward. Sustain. Cities Soc. 2017, 28, 453–465. [Google Scholar] [CrossRef]
- Hafezian, S.M.; Biparva, P.; Bekhradnia, A.; Azizi, S.N. Amine and thiol functionalization of SBA-15 nanoparticles for highly efficient adsorption of sulforaphane. Adv. Powder Technol. 2021, 32, 779–790. [Google Scholar] [CrossRef]
- Xie, Y.; Lyu, S.; Zhang, Y.; Cai, C. Adsorption and Degradation of Volatile Organic Compounds by Metal–Organic Frameworks (MOFs): A Review. Materials 2022, 15, 7727. [Google Scholar] [CrossRef] [PubMed]
- Pourhakkak, P.; Taghizadeh, M.; Taghizadeh, A.; Ghaedi, M. Adsorbent. In Adsorption: Fundamental Processes and Applications, Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 71–210. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tomita, M.; Kato, K. Synthesis of amino-functionalized mesoporous silica sheets and their application for metal ion capture. J. Asian Ceram. Soc. 2015, 3, 70–76. [Google Scholar] [CrossRef]
- Melnyk, I.V.; Tomina, V.V.; Stolyarchuk, N.V.; Seisenbaeva, G.A.; Kessler, V.G. Organic dyes (acid red, fluorescein, methylene blue) and copper(II) adsorption on amino silica spherical particles with tailored surface hydrophobicity and porosity. J. Mol. Liq. 2021, 336, 116301. [Google Scholar] [CrossRef]
- Li, J.; Miao, X.; Hao, Y.; Zhao, J.; Sun, X.; Wang, L. Synthesis, amino-functionalization of mesoporous silica and its adsorption of Cr(VI). J. Colloid Interface Sci. 2008, 318, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, S.; Liu, J.; Xu, Z. Tannic acid adsorption on amino-functionalized magnetic mesoporous silica. Chem. Eng. J. 2010, 165, 10–16. [Google Scholar] [CrossRef]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A.; Lindo, M.; Gascón, V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Palacio, R.; Allavena, A.; Gallard, H.; Descostes, M.; Mamède, A.-S.; Royer, S.; Tertre, E.; Batonneau-Gener, I. Selective adsorption of U(VI) from real mine water using an NH2-functionalized silica packed column. Chem. Eng. J. 2021, 405, 126912. [Google Scholar] [CrossRef]
- Jayalath, S.; Larsen, S.C.; Grassian, V.H. Surface adsorption of Nordic aquatic fulvic acid on amine-functionalized and non-functionalized mesoporous silica nanoparticles. Environ. Sci. Nano 2018, 5, 2162–2171. [Google Scholar] [CrossRef]
- Ebrahimi-Gatkash, M.; Younesi, H.; Shahbazi, A.; Heidari, A. Amino-functionalized mesoporous MCM-41 silica as an efficient adsorbent for water treatment: Batch and fixed-bed column adsorption of the nitrate anion. Appl. Water Sci. 2017, 7, 1887–1901. [Google Scholar] [CrossRef]
- Nayab, S.; Farrukh, A.; Oluz, Z.; Tuncel, E.; Tariq, S.R.; Rahman, H.; Kirchhoff, K.; Duran, H.; Yameen, B. Design and Fabrication of Branched Polyamine Functionalized Mesoporous Silica: An Efficient Absorbent for Water Remediation. ACS Appl. Mater. Interfaces 2014, 6, 4408–4417. [Google Scholar] [CrossRef]
- Flores, D.; Almeida, C.M.R.; Gomes, C.R.; Balula, S.S.; Granadeiro, C.M. Tailoring of Mesoporous Silica-Based Materials for Enhanced Water Pollutants Removal. Molecules 2023, 28, 4038. [Google Scholar] [CrossRef]
- Brigante, M.; Pecini, E.; Avena, M. Magnetic mesoporous silica for water remediation: Synthesis, characterization and application as adsorbent of molecules and ions of environmental concern. Microporous Mesoporous Mater. 2016, 230, 1–10. [Google Scholar] [CrossRef]
- Zhao, H.; Nagy, K.L.; Waples, J.S.; Vance, G.F. Surfactant-Templated Mesoporous Silicate Materials as Sorbents for Organic Pollutants in Water. Environ. Sci. Technol. 2000, 34, 22. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Wu, J.; Hu, Y. Pivotal role of pH value in the preparation of mesoporous silica with high surface area for toluene adsorption. Mater. Lett. 2024, 364, 136381. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Ebrahimi, M. Thermodynamics and kinetics of adsorption of Cu(II) from aqueous solutions onto multi-walled carbon nanotubes. J. Saudi Chem. Soc. 2014, 18, 792–801. [Google Scholar] [CrossRef]
- Makara, A.; Kowalski, Z.; Sówka, I. Possibility to eliminate emission of odor from pig manure using AMAK filtration method. Desalin. Water Treat. 2016, 57, 1543–1551. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, S.; Yuan, X.; Ding, L.; Ai, T.; Yuan, K.; Wang, W.; Magagnin, L.; Jiang, Z. High-efficiency removal of Pb (II) and Cu (II) by amidoxime functionalized silica aerogels: Preparation, adsorption mechanisms and environmental impacts analysis. Sep. Purif. Technol. 2024, 335, 126079. [Google Scholar] [CrossRef]
- Wang, H.; Kang, J.; Liu, H.; Qu, J. Preparation of organically functionalized silica gel as adsorbent for copper ion adsorption. J. Environ. Sci. 2009, 21, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, M.; Teng, W.; Li, F.; Qiu, X.; Li, K.; Wang, H. Ordered mesoporous materials for water pollution treatment: Adsorption and catalysis. Green Energy Environ. 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).