The Effects of Different Zn Forms on Sintering Basic Characteristics of Iron Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Micro-Sintering Experiment
- 1.
- Assimilation performance
- 2.
- Liquid Phase Fluidity
- 3.
- Silico-Ferrite of Calcium and Aluminum (SFCA) Formation Characteristics
- 4.
- Compressive strength
2.2.2. Microhardness Detection
2.2.3. TG Experiment
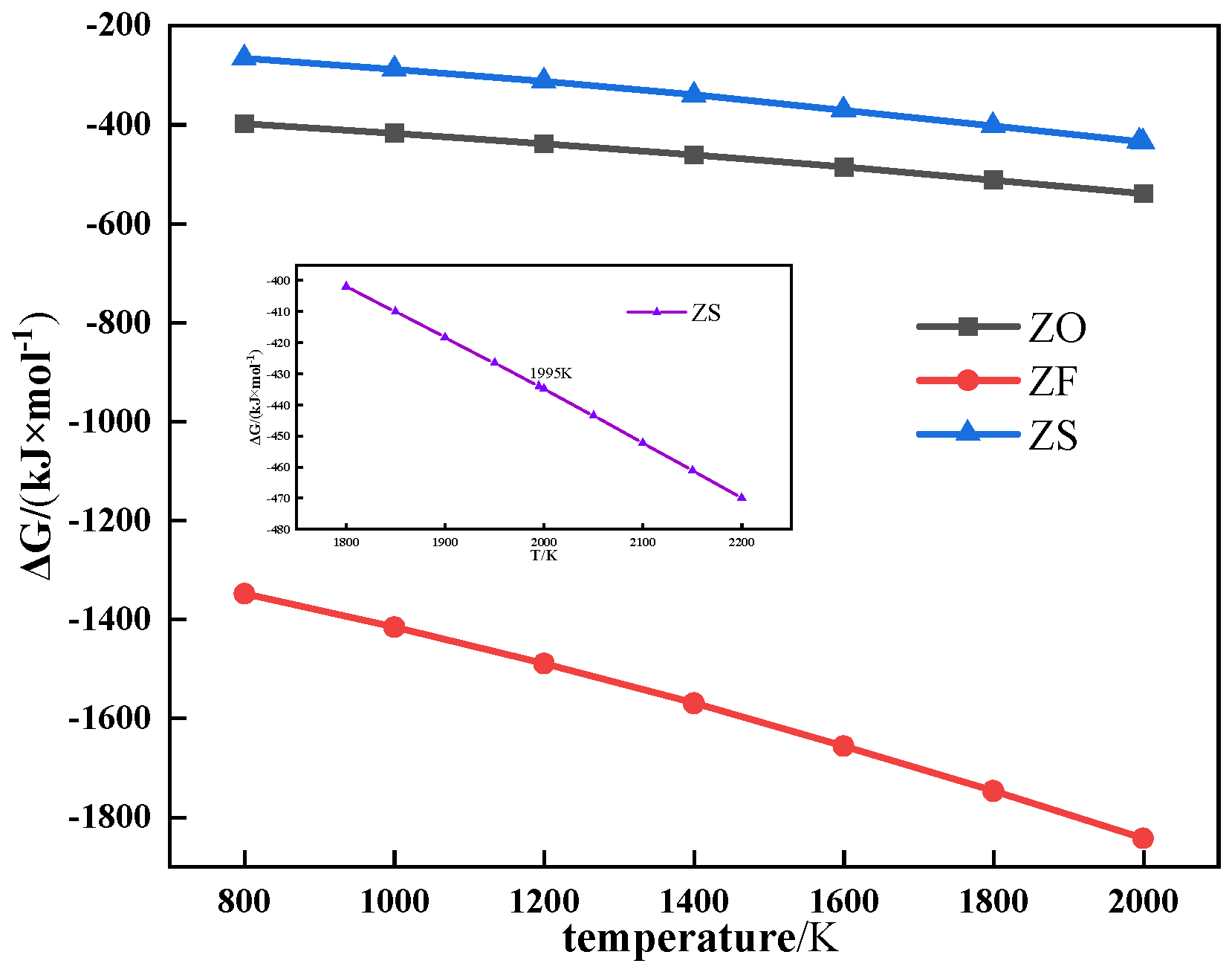
2.2.4. Thermodynamic Calculation
3. Results and Discussion
3.1. The Effects of Different Forms of Zn on Assimilation Performance
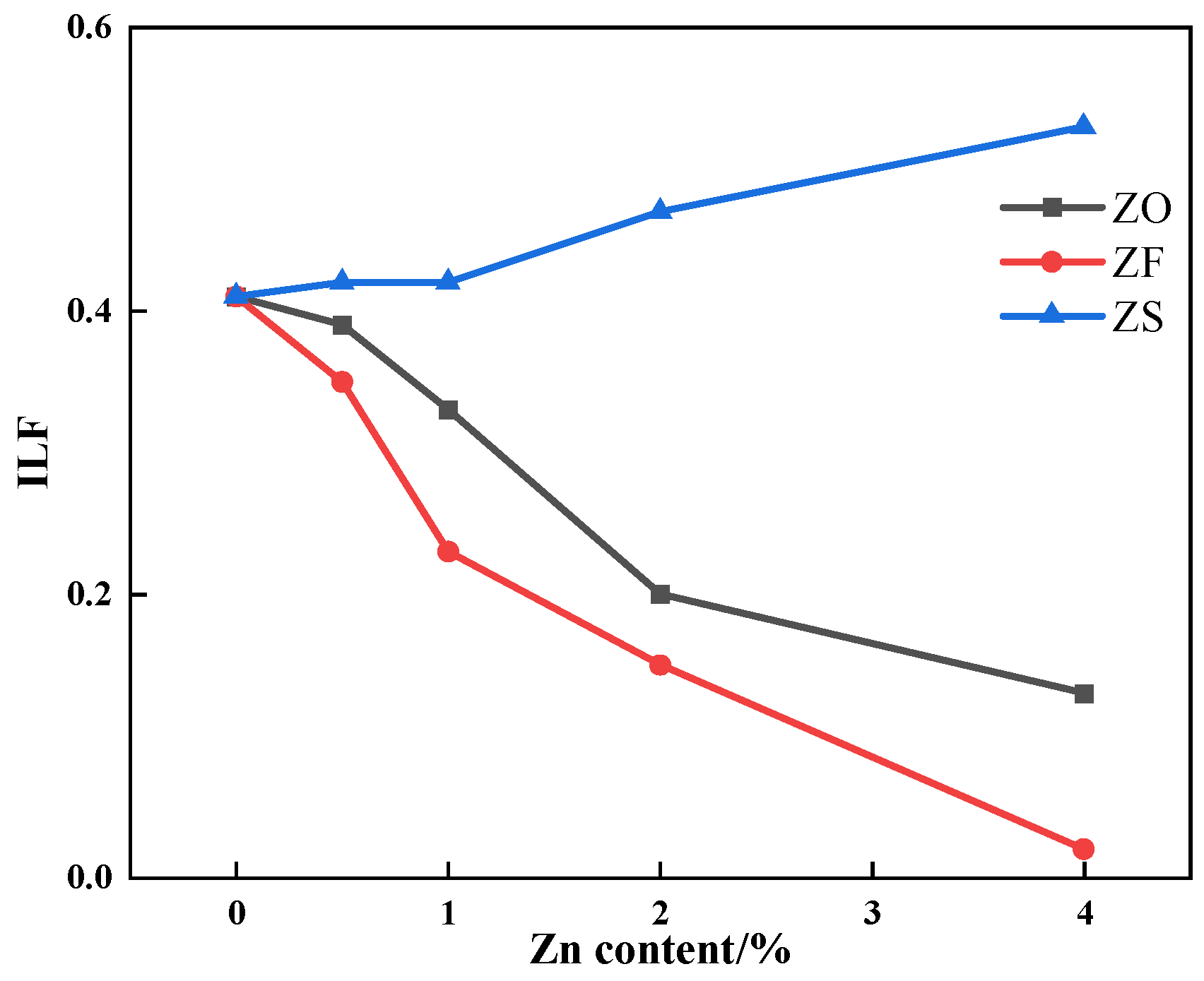
3.2. The Effects of Different Forms of Zn on Liquid Phase Fluidity
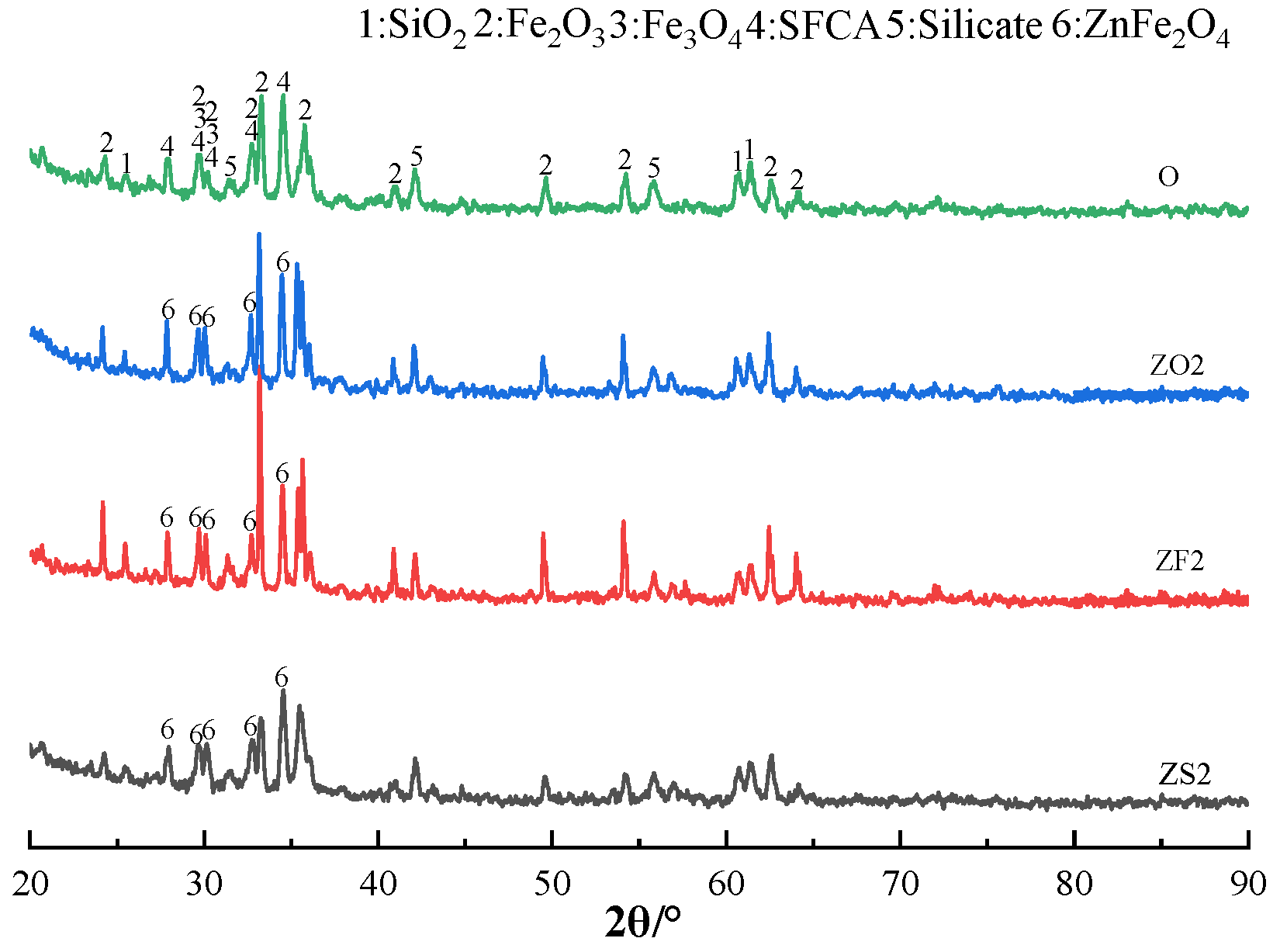
3.3. The Effects of Different Forms of Zn on SFCA Formation Characteristics
3.4. The Effects of Different Forms of Zn on Bonding Phase Strength
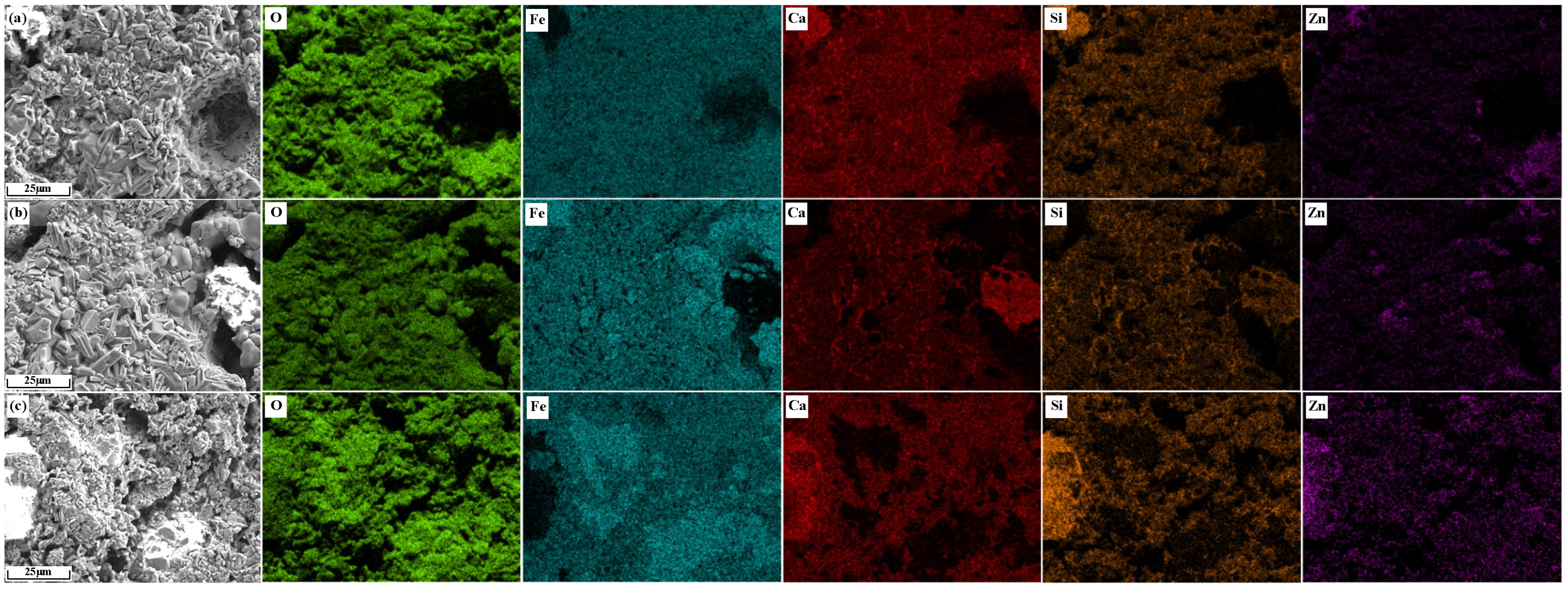
3.4.1. The Distribution Characteristics of Zn and Microhardness
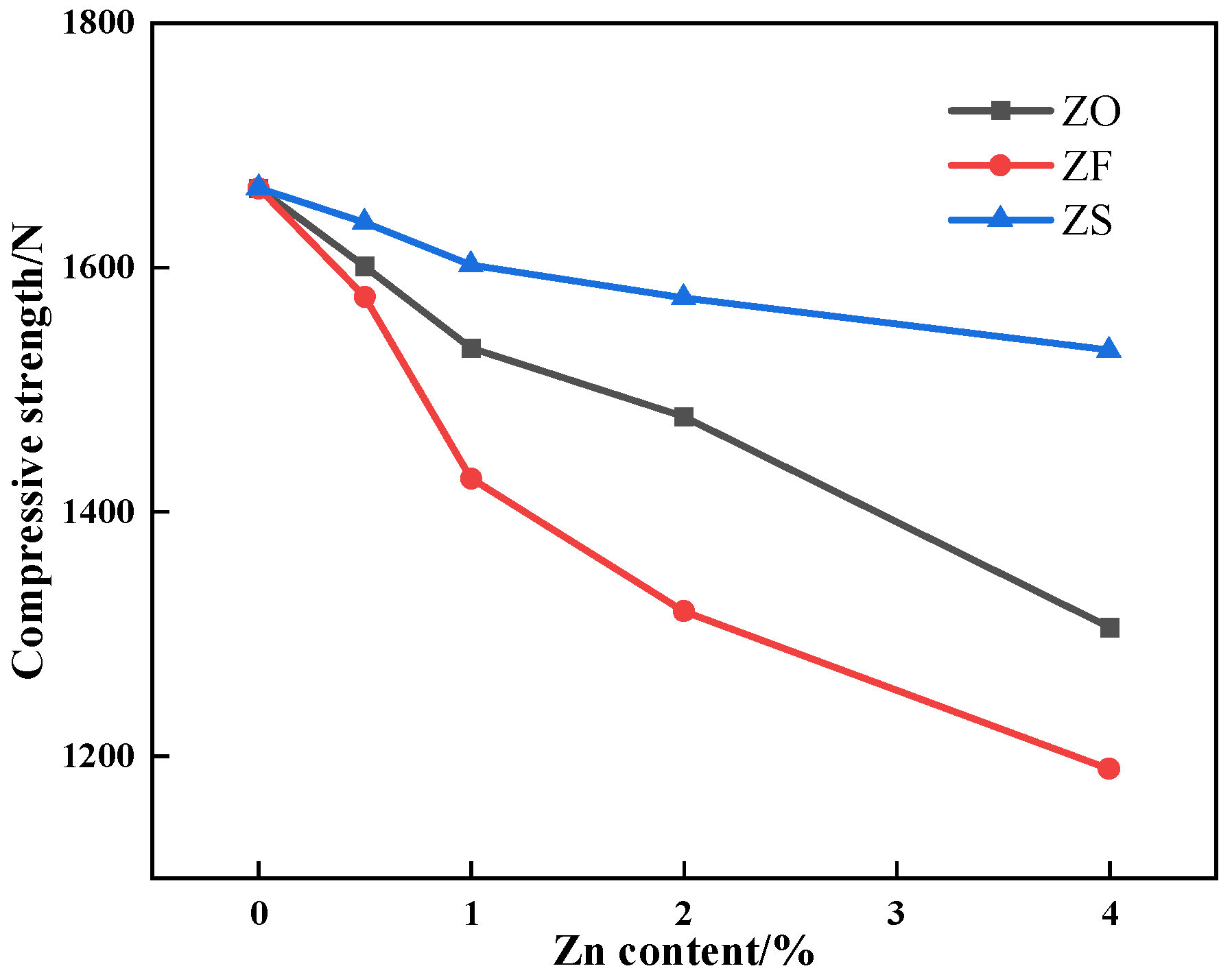
3.4.2. Compression Strength
4. Conclusions
- After the addition of ZnO and ZnFe2O4, the LAT of iron ore increased while the ILF of iron ore decreased. The addition of ZnFe2O4 consumed limited CaO, so the LAT and ILF of iron ore containing ZnFe2O4 had a greater variation. As the ZnS content increased, the LAT of iron ore had no obvious change, while the ILF of iron ore increased;
- The addition of ZnO and ZnFe2O4 resulted in the formation of ZnFe2O4 and Ca2Fe2O5, respectively; the content of reactants that participated in the formation of SFCA decreased. Hence, the presence of ZnO and ZnFe2O4 had a serious impact on SFCA formation during sintering. The addition of ZnS promoted the decomposition of Fe2O3; however, the process was limited by temperature. Hence, the presence of ZnS had a slight influence on SFCA formation during sintering;
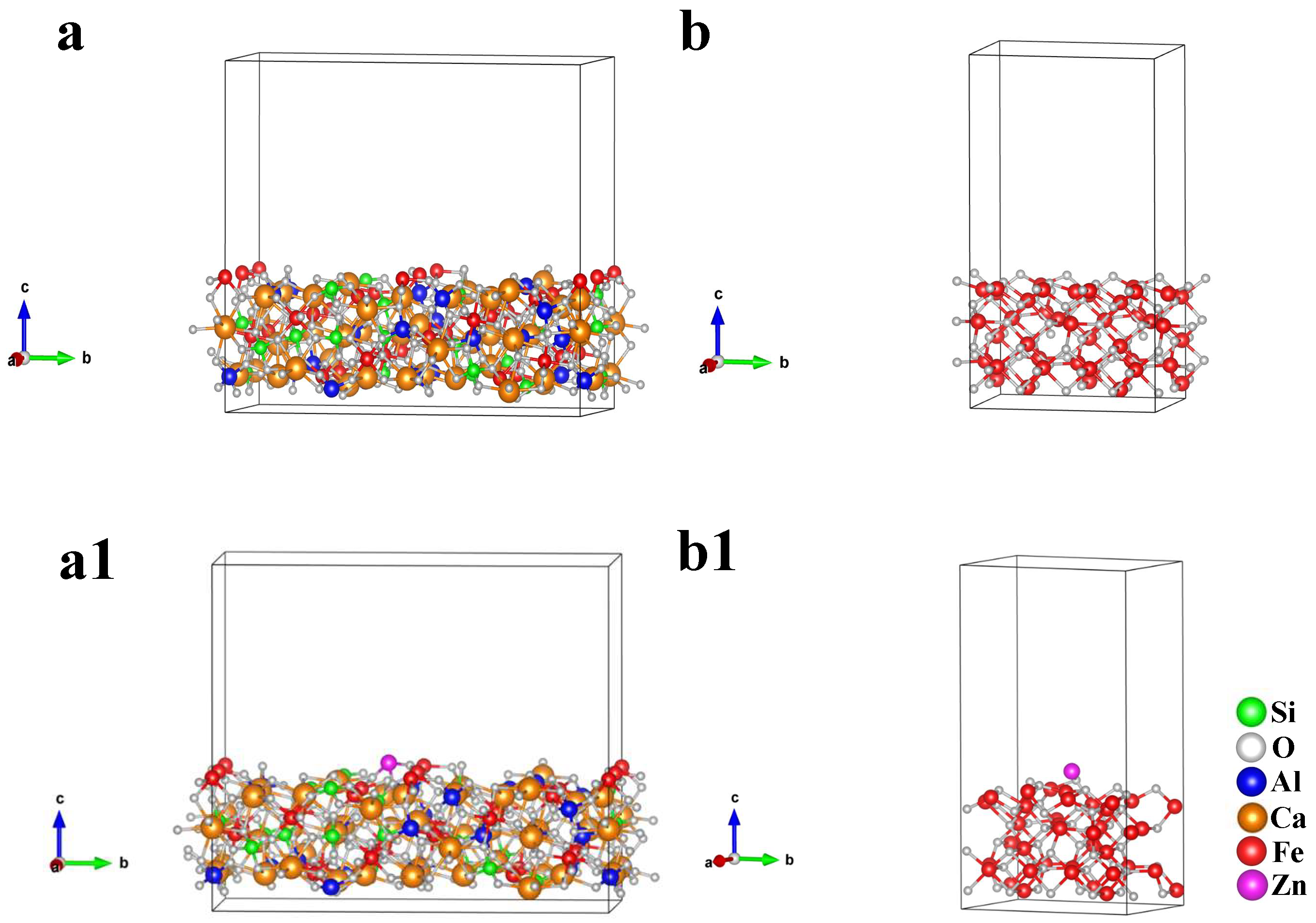
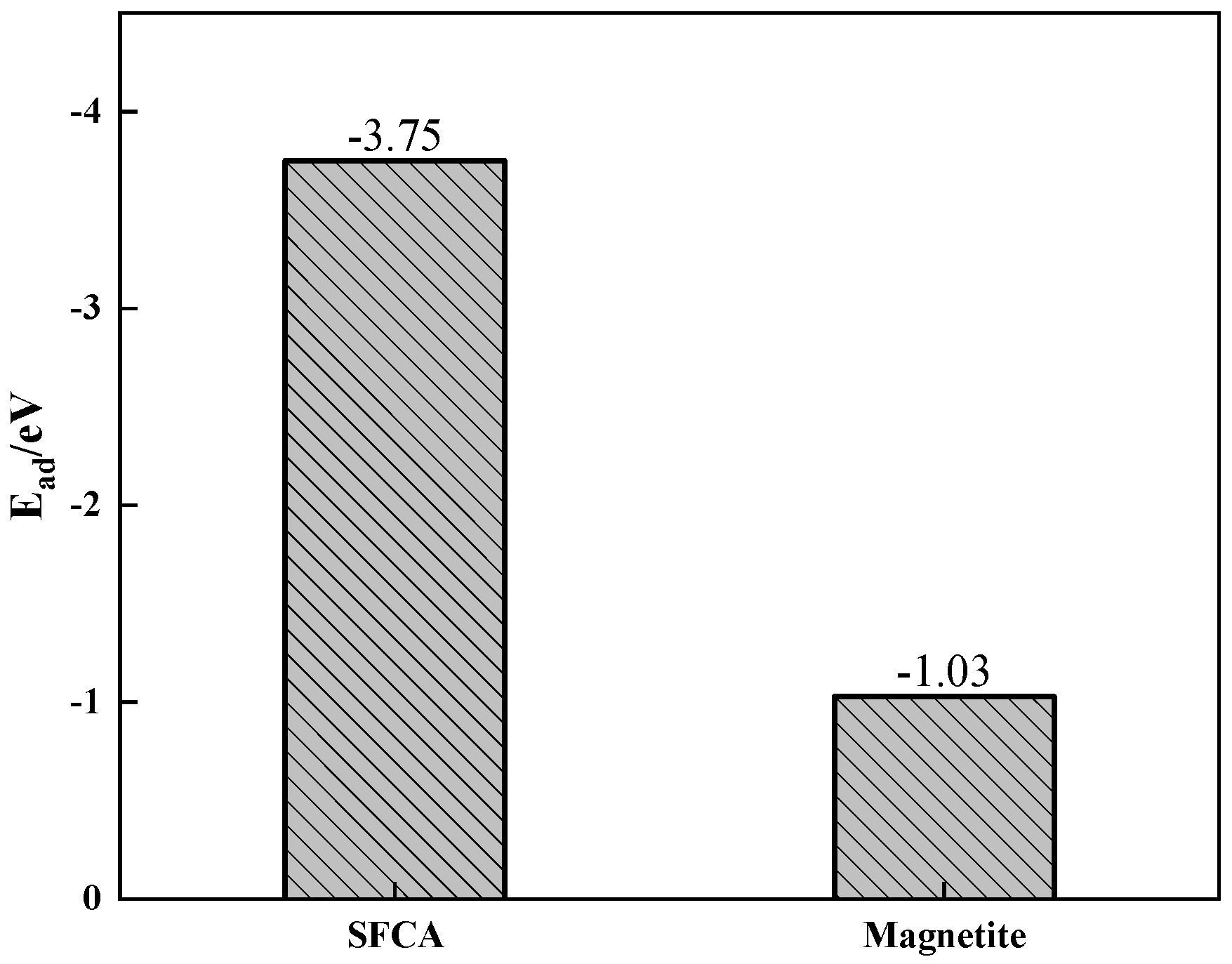
- The Zn is mainly concentrated in hematite or silicate, less distributed in SFCA and magnetite in the form of a solid solution. Among SFCA and magnetite, the adsorption energy of Zn on the SFCA crystal surface was lower, so the SFCA bonding phase contained more Zn-containing solid solutions, which decreased the microhardness of the mineral phase. The decrease in microhardness and content of the SFCA bonding phase decreased the bonding phase strength;
- The iron ore with high Zn content presented poor sintering basic characteristics during the sintering process. Hence, both removing the Zn element in dust and ore blending optimization based on sintering basic characteristics are not only good approaches to recovering metallurgical dust and producing high-quality sinter but are also future research directions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkogatse, T.; Garbers-Craig, A. Evaluation of Iron Ore Concentrate and Micropellets as Potential Feed for Sinter Production. Miner. Process. Extr. Metall. Rev. 2022, 43, 300–312. [Google Scholar] [CrossRef]
- Ju, J.T.; Ma, K.; Xing, X.D.; Zhao, G.Q.; Li, Q.D. Investigations on the Barium-Bearing Magnetite Concentrate Roasted in Air. Metall. Mater. Trans. B 2022, 53, 1495–1503. [Google Scholar] [CrossRef]
- Gan, M.; Li, Q.; Ji, Z.Y.; Fan, X.H.; Lv, W.; Chen, X.L.; Tian, Y.; Jiang, T. Influence of Surface Modification on Combustion Characteristics of Charcoal and Its Performance on Emissions Reduction in Iron Ore Sintering. ISIJ Int. 2017, 57, 420–428. [Google Scholar] [CrossRef]
- Maiorova, A.V.; Kulikova, T.V.; Shubin, A.B. Extraction of Zinc and Arsenic from Metallurgical Furnace Dust. JOM 2021, 73, 3588–3596. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Zhang, J.L.; Yang, X.T.; Xu, J.; Zhang, G.H. Formation Mechanism of the Brittle Layer in Carbon Brick of Blast Furnace Hearth. Steel Res. Int. 2023, 94, 2300168. [Google Scholar] [CrossRef]
- Trinkel, V.; Aschenbrenner, P.; Thaler, C.; Rechberger, H.; Mallow, O.; Fellner, J. Distribution of Zn, Pb, K, and Cl in Blast Furnace Lining. Steel Res. Int. 2017, 88, 1600153. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Zhang, J.L.; Ji, C.K.; Wang, Z.Y.; Zhang, C.F. Erosion mechanism of harmful elements K and Zn on carbon brick in a 4000 m3 industrial blast furnace hearth. Ironmak. Steelmak. 2024, 51, 224–235. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhang, Y.Z.; Long, Y.; Du, P.P.; Tian, T.L.; Ren, Q.Q. Multi-Source Ferrous Metallurgical Dust and Sludge Recycling: Present Situation and Future Prospects. Crystals 2024, 14, 273. [Google Scholar] [CrossRef]
- Pal, J. Innovative Development on Agglomeration of Iron Ore Fines and Iron Oxide Wastes. Miner. Process. Extr. Metall. Rev. 2019, 40, 248–264. [Google Scholar] [CrossRef]
- Sasiain, A.; Thallner, S.; Habermaier, C.; Spiess, S.; Birklbauer, L.; Wallner, M.; Haberbauer, M. Bioleaching of Zinc from Blast Furnace Cast House Dust. Minerals 2023, 13, 1007. [Google Scholar] [CrossRef]
- Deng, X.; Huang, R.; Lv, X.D.; Yang, J.P.; Yang, J. Separation and recovery of metallic zinc and iron concentrate from blast furnace dust by vacuum carbothermal reduction. Process Saf. Environ. 2022, 162, 746–751. [Google Scholar] [CrossRef]
- Wang, W.A.; Li, X.M.; He, Y.; Xi, H.D.; Wang, J.L.; Qiu, G.X. Effect of CaCO3 on volatilization of self-reduced zinc from blast furnace dust. J. Iron Steel Res. Int. 2022, 29, 1404–1411. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, Z.M.; Hu, D.S.; Zhang, L.G.; Wang, Z.Y.; Wang, X.L. Balance analysis and thermodynamic behavior of zinc in Anling blast furnace. J. Iron Steel Res. 2015, 27, 32–36. [Google Scholar]
- Wang, L.H.; Lu, X.M.; Wei, X.J.; Jiang, Z.; Gu, S.Q.; Gao, Q.; Huang, Y.Y. Quantitative Zn speciation in zinc-containing steelmaking wastes by X-ray absorption spectroscopy. J. Anal. At. Spectrom. 2012, 27, 1667–1673. [Google Scholar] [CrossRef]
- Wang, Y.N.; Gan, M.; Fan, X.H.; Lv, W.; Ji, Z.Y. Phase Transformation and Enhanced Zn Removal Technology During the Iron Ore Sintering Process. JOM 2023, 75, 310–320. [Google Scholar] [CrossRef]
- Lv, W.; Gan, M.; Fan, X.H.; Ji, Z.Y.; Chen, X.L.; Yao, G.W.; Jiang, T. Recycling Utilization of Zinc-Bearing Metallurgical Dust by Reductive Sintering: Reaction Behavior of Zinc Oxide. JOM 2019, 71, 3173–3180. [Google Scholar] [CrossRef]
- Guo, H.; Guo, X.M. Effect of Alumina on Liquid Phase Formation in Sintering Process of Iron Ore Fines. Steel Res. Int. 2019, 90, 1900138. [Google Scholar] [CrossRef]
- Wang, W.; Yang, D.W.; Ou-Yang, Z.L.; Xu, R.S.; Song, M.M. Effect of SiO2 on the Formation of Acicular Calcium Ferrite in Sinter. Metall. Mater. Trans. B 2019, 50, 678–687. [Google Scholar] [CrossRef]
- Wu, S.L.; Zhang, G.L.; Chen, S.G.; Su, B. Influencing Factors and Effects of Assimilation Characteristic of Iron Ores in Sintering Process. ISIJ Int. 2014, 54, 582–588. [Google Scholar] [CrossRef]
- Yang, S.P.; Liu, H.J.; Sun, H.X.; Zhang, T.T.; Liu, S.M. Study on Influencing Factors of High-Temperature Basic Characteristics of Iron Ore Powder and Optimization of Ore Blending. Materials 2022, 15, 3329. [Google Scholar] [CrossRef]
- Chen, X.H.; Wang, W.; Yang, D.W.; Zheng, H.; Wang, L.; Chen, S.J. Influence Mechanism of Zn on the Iron Ore-Sintering Mineralization Process. Metall. Mater. Trans. B 2023, 54, 550–561. [Google Scholar] [CrossRef]
- Zhou, M.; Jiang, T.; Yang, S.T.; Xue, X.X. Vanadium-titanium magnetite ore blend optimization for sinter strength based on iron ore basic sintering characteristics. Int. J. Miner. Process. 2015, 142, 125–133. [Google Scholar] [CrossRef]
- Wang, X.Z.; Liu, D.H.; Zhang, J.L.; Liu, Z.J. Research status and prospects of the iron ore’s high temperature behaviors in the sintering process. Metall. Res. Technol. 2018, 115, 604. [Google Scholar] [CrossRef]
- Alfahed, B.; Alayad, A. The Effect of Sintering Temperature on Vickers Microhardness and Flexural Strength of Translucent Multi-Layered Zirconia Dental Materials. Coatings 2023, 13, 688. [Google Scholar] [CrossRef]
- Huang, M.; Li, M.R.; Tian, L.L.; Huang, Y.; Ma, B.D.; Liu, K. Effect of Ferrous Ion on Reducing the Viscosity of Oil Flooding Polymer Solution and Its Mechanism. Chin. J. Appl. Chem. 2013, 30, 1399–1403. [Google Scholar]
- Nicol, S.; Cheng, S.Y.; Jak, E.; Hayes, P.C. Mechanisms of Phase and Microstructure Formation during the Cooling of “Fe2O3”-CaO-SiO2-Al2O3 Melts in Air and Implications for Iron Ore Sintering. ISIJ Int. 2020, 60, 2659–2668. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.X.; Li, L.S.; Yu, Y.; Tian, X.F. First-principles calculations of phase transition, elasticity, phonon spectra, and thermodynamic properties for ThO2 polymorphs. J. Solid State Chem. 2023, 326, 124221. [Google Scholar] [CrossRef]
- Zhu, X.T.; Li, T.Y.; Wu, J.; Wang, L.J.; Yu, Y.; Tang, H.G.; Qiao, Z.H.; Zhang, S.X. Electronic and optical properties of Be, Ca, Ba and Eu adsorbed on β-Si3N4 (200) surface based on first-principles calculations. Mater. Sci. Semicond. Process. 2023, 160, 107406. [Google Scholar] [CrossRef]
- Torres, E.; Kaloni, T.P. Projector augmented-wave pseudopotentials for uranium-based compounds. Comp. Mater. Sci. 2020, 171, 109237. [Google Scholar] [CrossRef]
- Yang, D.W.; Wang, W.; Li, J.X.; Chen, X.H.; Chen, P.F. Effect of Al2O3/SiO2 ratio on morphology of complex calcium ferrite. J. Iron Steel Res. Int. 2023, 30, 1921–1928. [Google Scholar] [CrossRef]
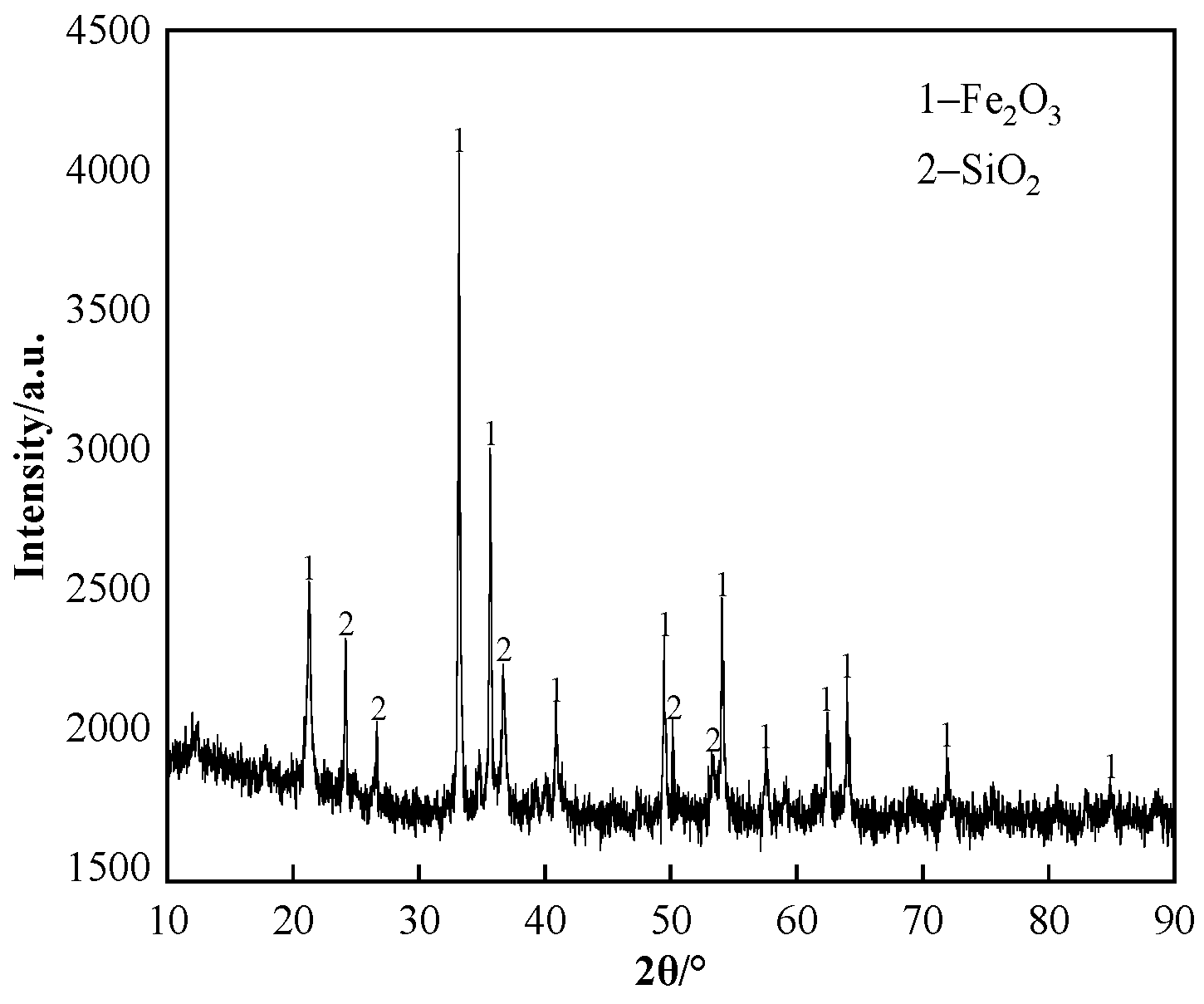


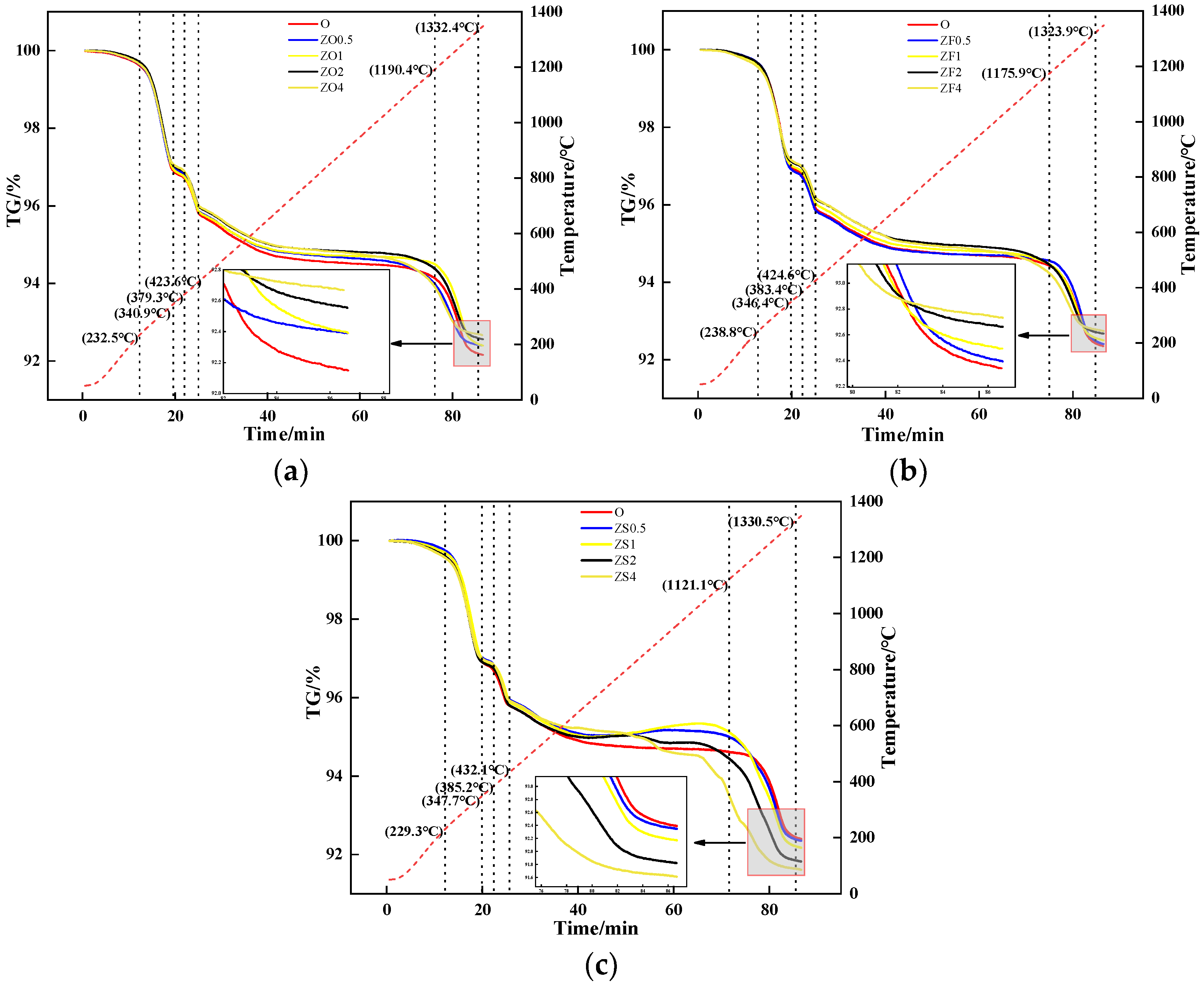
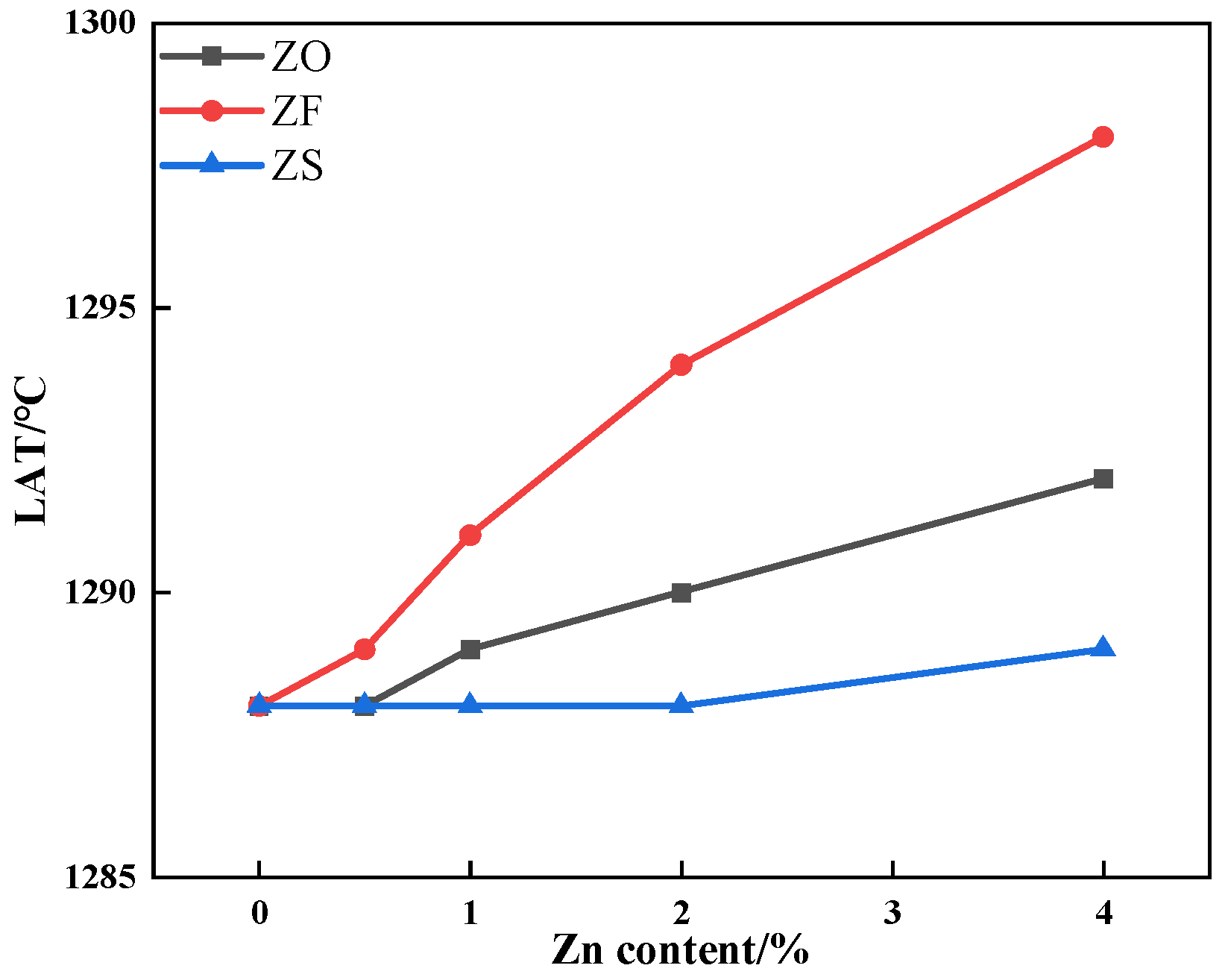

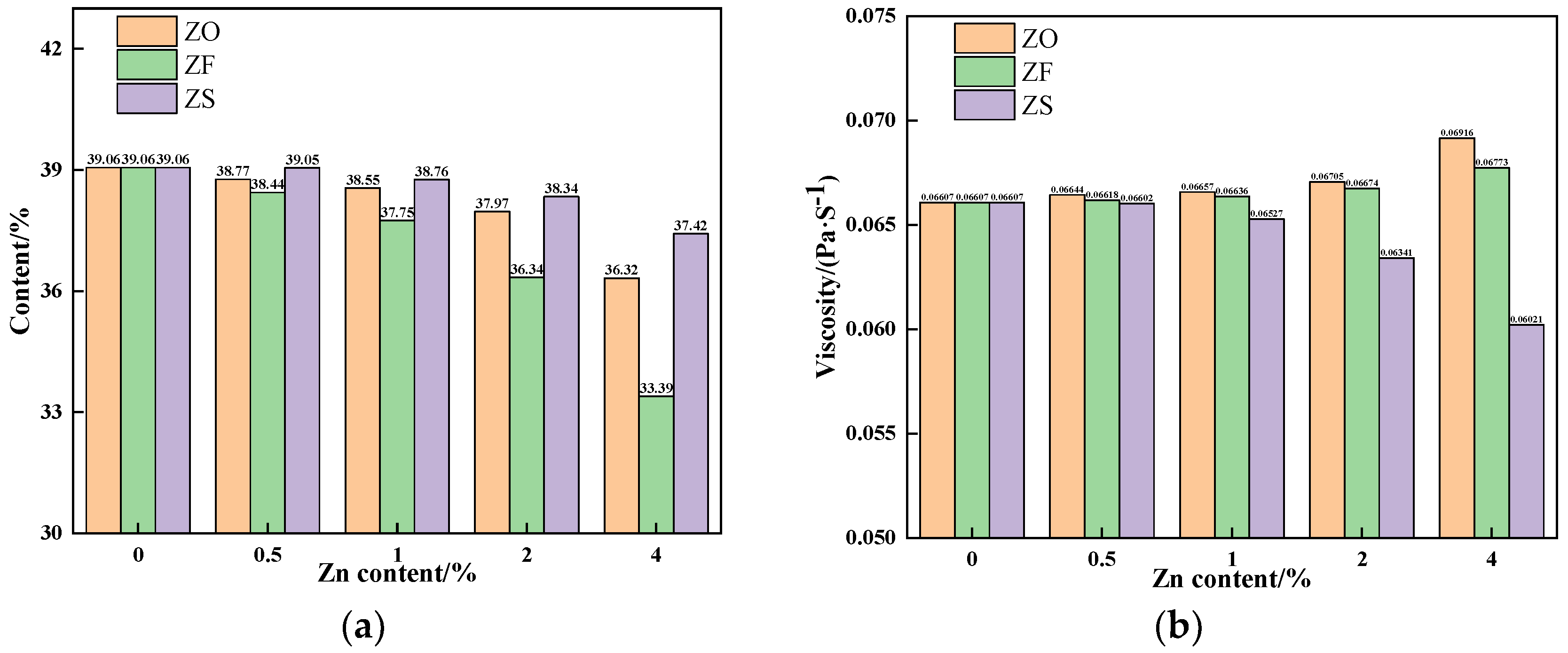

| TFe | CaO | SiO2 | Al2O3 | MgO | ZnO |
|---|---|---|---|---|---|
| 61.28 | 0.17 | 5.27 | 2.69 | 0.26 | ≤0.01 |
| Samples | TFe | CaO | SiO2 | Al2O3 | MgO | ZnO | ZnFe2O4 | ZnS |
|---|---|---|---|---|---|---|---|---|
| O | 61.28 | 0.17 | 5.27 | 2.69 | 0.26 | -- | -- | -- |
| ZO0.5 | 60.89 | 0.17 | 5.24 | 2.67 | 0.26 | 0.63 | -- | -- |
| ZO1 | 60.51 | 0.17 | 5.20 | 2.66 | 0.26 | 1.25 | -- | -- |
| ZO2 | 59.75 | 0.17 | 5.14 | 2.62 | 0.25 | 2.49 | -- | -- |
| ZO4 | 58.23 | 0.16 | 5.01 | 2.56 | 0.25 | 4.98 | -- | -- |
| ZF0.5 | 60.15 | 0.17 | 5.17 | 2.64 | 0.26 | -- | 1.85 | -- |
| ZF1 | 59.01 | 0.16 | 5.08 | 2.59 | 0.25 | -- | 3.70 | -- |
| ZF2 | 56.76 | 0.16 | 4.88 | 2.49 | 0.24 | -- | 7.38 | -- |
| ZF4 | 52.24 | 0.14 | 4.49 | 2.29 | 0.22 | -- | 14.76 | -- |
| ZS0.5 | 60.82 | 0.17 | 5.23 | 2.67 | 0.26 | -- | -- | 0.75 |
| ZS1 | 60.37 | 0.17 | 5.19 | 2.65 | 0.26 | -- | -- | 1.49 |
| ZS2 | 59.45 | 0.16 | 5.11 | 2.61 | 0.25 | -- | -- | 2.98 |
| ZS4 | 57.63 | 0.16 | 4.96 | 2.53 | 0.24 | -- | -- | 5.96 |
| Samples | Fe2O3 | Al2O3 | SiO2 | CaO | FeO | MgO | ZnO |
|---|---|---|---|---|---|---|---|
| O | 57.839 | 4.085 | 11.064 | 22.152 | 4.730 | 0.131 | 0.000 |
| ZO0.5 | 57.692 | 4.067 | 11.106 | 22.213 | 4.510 | 0.088 | 0.323 |
| ZO1 | 57.652 | 4.054 | 11.106 | 22.236 | 4.351 | 0.068 | 0.531 |
| ZO2 | 57.509 | 4.003 | 11.167 | 22.334 | 4.134 | 0.047 | 0.806 |
| ZO4 | 56.676 | 3.912 | 11.426 | 22.858 | 3.887 | 0.029 | 1.211 |
| ZF0.5 | 57.768 | 4.051 | 11.071 | 22.187 | 4.513 | 0.089 | 0.321 |
| ZF1 | 57.682 | 3.993 | 11.102 | 22.252 | 4.371 | 0.068 | 0.532 |
| ZF2 | 57.532 | 3.899 | 11.170 | 22.371 | 4.173 | 0.045 | 0.810 |
| ZF4 | 57.103 | 3.677 | 11.364 | 22.719 | 3.953 | 0.025 | 1.158 |
| ZS0.5 | 57.714 | 4.052 | 11.046 | 22.124 | 4.740 | 0.069 | 0.255 |
| ZS1 | 57.535 | 4.020 | 11.071 | 22.145 | 4.812 | 0.047 | 0.370 |
| ZS2 | 53.829 | 4.015 | 12.122 | 24.255 | 5.084 | 0.038 | 0.657 |
| ZS4 | 51.132 | 3.992 | 12.379 | 26.134 | 5.476 | 0.021 | 0.866 |
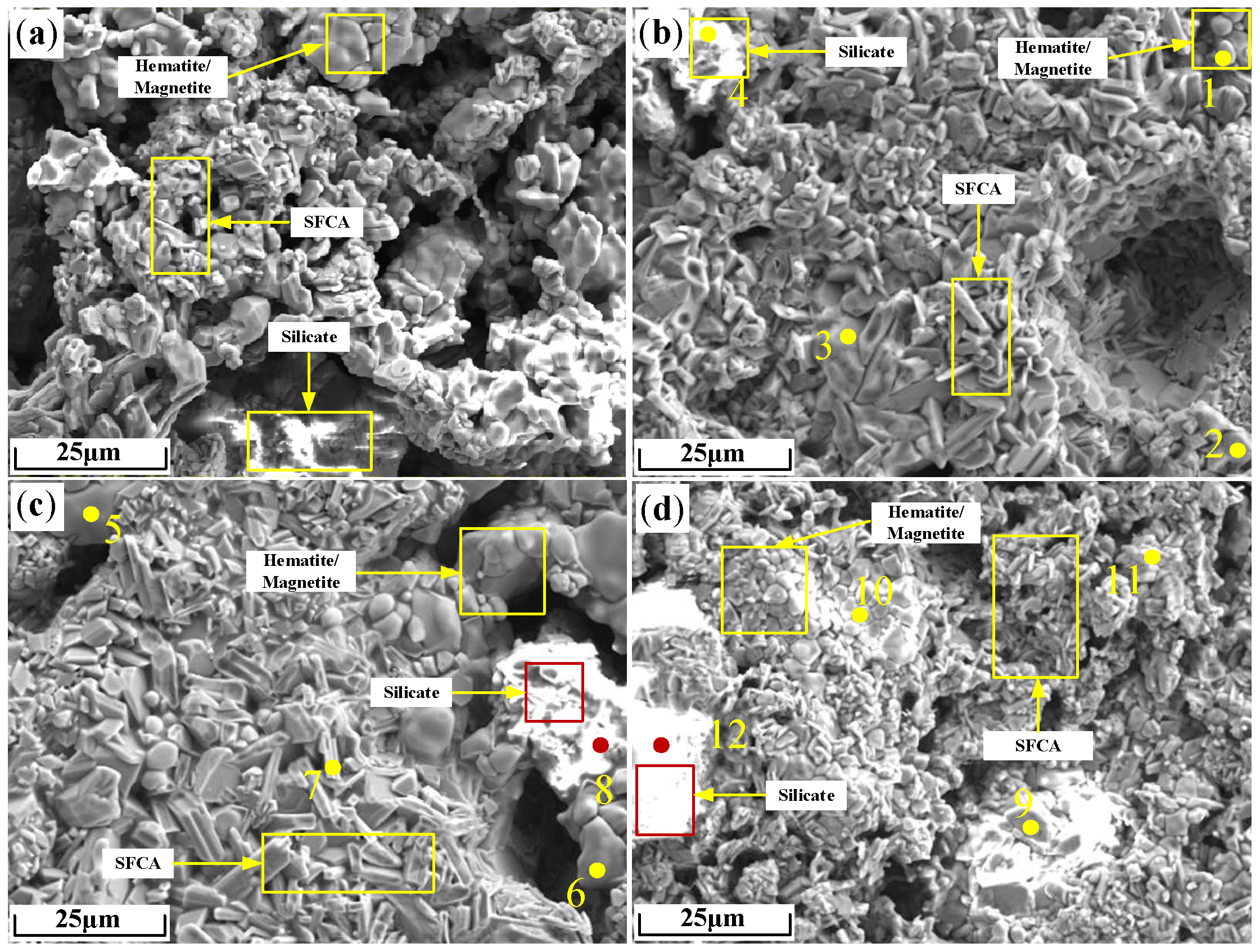
| Points | Fe | Ca | Si | Al | Mg | O | Zn | Minerals |
|---|---|---|---|---|---|---|---|---|
| 1 | 47.39 | 1.40 | 0.12 | 0.64 | 0.15 | 50.04 | 0.26 | magnetite |
| 2 | 38.95 | 0.23 | 0.08 | 0.42 | 0.16 | 59.01 | 1.15 | hematite |
| 3 | 24.17 | 6.46 | 3.02 | 2.09 | 0.22 | 63.64 | 0.40 | SFCA |
| 4 | 4.41 | 14.82 | 9.59 | 8.04 | 1.02 | 61.99 | 0.13 | silicate |
| 5 | 48.80 | 0.38 | 0.16 | 0.50 | 0.11 | 49.90 | 0.15 | magnetite |
| 6 | 36.26 | 0.79 | 0.10 | 0.57 | 0.21 | 60.50 | 1.57 | hematite |
| 7 | 26.80 | 8.55 | 4.25 | 2.03 | 0.13 | 57.82 | 0.42 | SFCA |
| 8 | 6.93 | 23.04 | 12.78 | 0.34 | 0.16 | 56.66 | 0.09 | silicate |
| 9 | 46.19 | 0.24 | 0.16 | 0.42 | 0.14 | 52.77 | 0.08 | magnetite |
| 10 | 39.07 | 0.21 | 0.07 | 0.41 | 0.02 | 59.66 | 0.56 | hematite |
| 11 | 23.43 | 6.90 | 3.69 | 2.09 | 0.06 | 63.71 | 0.12 | SFCA |
| 12 | 2.30 | 18.42 | 12.94 | 5.32 | 0.09 | 59.64 | 1.29 | silicate |
| Crystals | a | b | c | |
|---|---|---|---|---|
| SFCA | Calc | 10.080 | 10.660 | 9.110 |
| Exp | 10.050 | 10.558 | 9.069 | |
| Error | 0.30% | 0.96% | 0.45% | |
| Fe3O4 | Calc | 8.399 | 8.399 | 8.399 |
| Exp | 8.389 | 8.389 | 8.389 | |
| Error | 0.12% | 0.12% | 0.12% |
| Samples | Silicate | SFCA | Hematite | Magnetite |
|---|---|---|---|---|
| O | 404.3 | 891.4 | 1056.5 | 775.2 |
| ZO0.5 | 409.5 | 862.2 | 1041.3 | 768.6 |
| ZO1 | 411.2 | 815.4 | 1033.2 | 762.5 |
| ZO2 | 401.3 | 723.1 | 1035.6 | 743.1 |
| ZO4 | 406.3 | 652.4 | 1029.7 | 730.2 |
| ZF0.5 | 383.2 | 877.4 | 1047.4 | 760.3 |
| ZF1 | 401.3 | 832.6 | 1042.3 | 755.6 |
| ZF2 | 397.5 | 756.7 | 1039.5 | 740.9 |
| ZF4 | 407.4 | 676.5 | 1040.3 | 720.8 |
| ZS0.5 | 417.4 | 867.2 | 1054.1 | 770.7 |
| ZS1 | 390.6 | 857.4 | 1051.7 | 767.5 |
| ZS2 | 410.3 | 830.5 | 1052.8 | 764.2 |
| ZS4 | 402.7 | 812.2 | 1040.9 | 757.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, J.; Zu, J.; Xing, X.; Yang, L.; Xiang, X. The Effects of Different Zn Forms on Sintering Basic Characteristics of Iron Ore. Materials 2024, 17, 2919. https://doi.org/10.3390/ma17122919
Ju J, Zu J, Xing X, Yang L, Xiang X. The Effects of Different Zn Forms on Sintering Basic Characteristics of Iron Ore. Materials. 2024; 17(12):2919. https://doi.org/10.3390/ma17122919
Chicago/Turabian StyleJu, Jiantao, Jian Zu, Xiangdong Xing, Lei Yang, and Xinru Xiang. 2024. "The Effects of Different Zn Forms on Sintering Basic Characteristics of Iron Ore" Materials 17, no. 12: 2919. https://doi.org/10.3390/ma17122919




