Kinetics of Alkali–Silica Reaction: Application to Sandstone
Abstract
:1. Introduction
1.1. Chemistry of Alkali–Silica Reaction
- (1)
- The formation of Q3 sites due to siloxane bonds first breaking up by hydroxide ion attack, as follows:
- (2)
- The dissolution of silica due to continued hydroxide ion attack on the Q3 sites to form silica ions, as follows:
1.2. Effect of Aggregate Properties
2. Materials and Methods
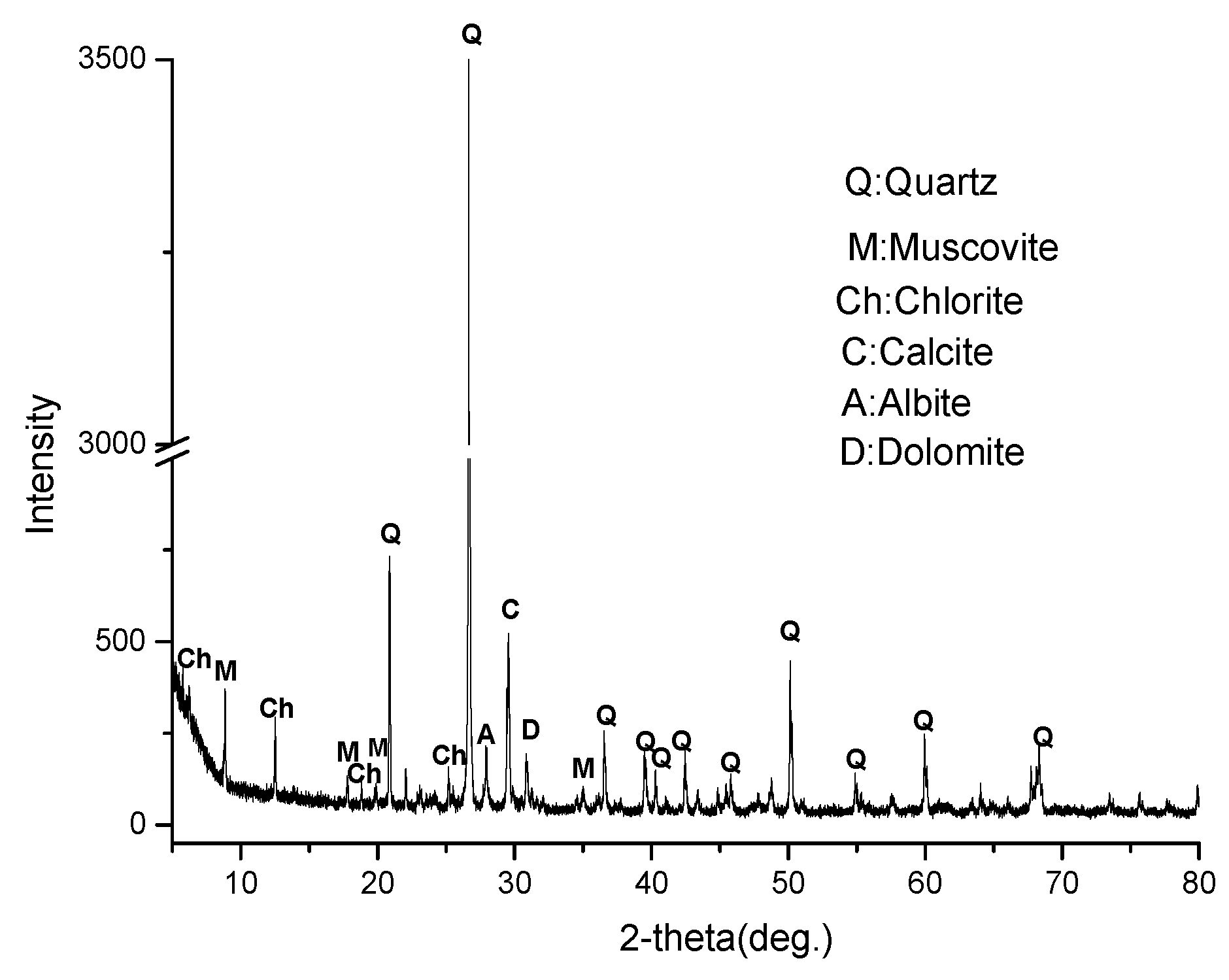
2.1. Materials
2.2. Experimental Methods
2.2.1. Kinetics of Silica Dissolution
2.2.2. Expansion of Rock Prism
3. Results and Discussion
3.1. Kinetics of ASR in Sandstone
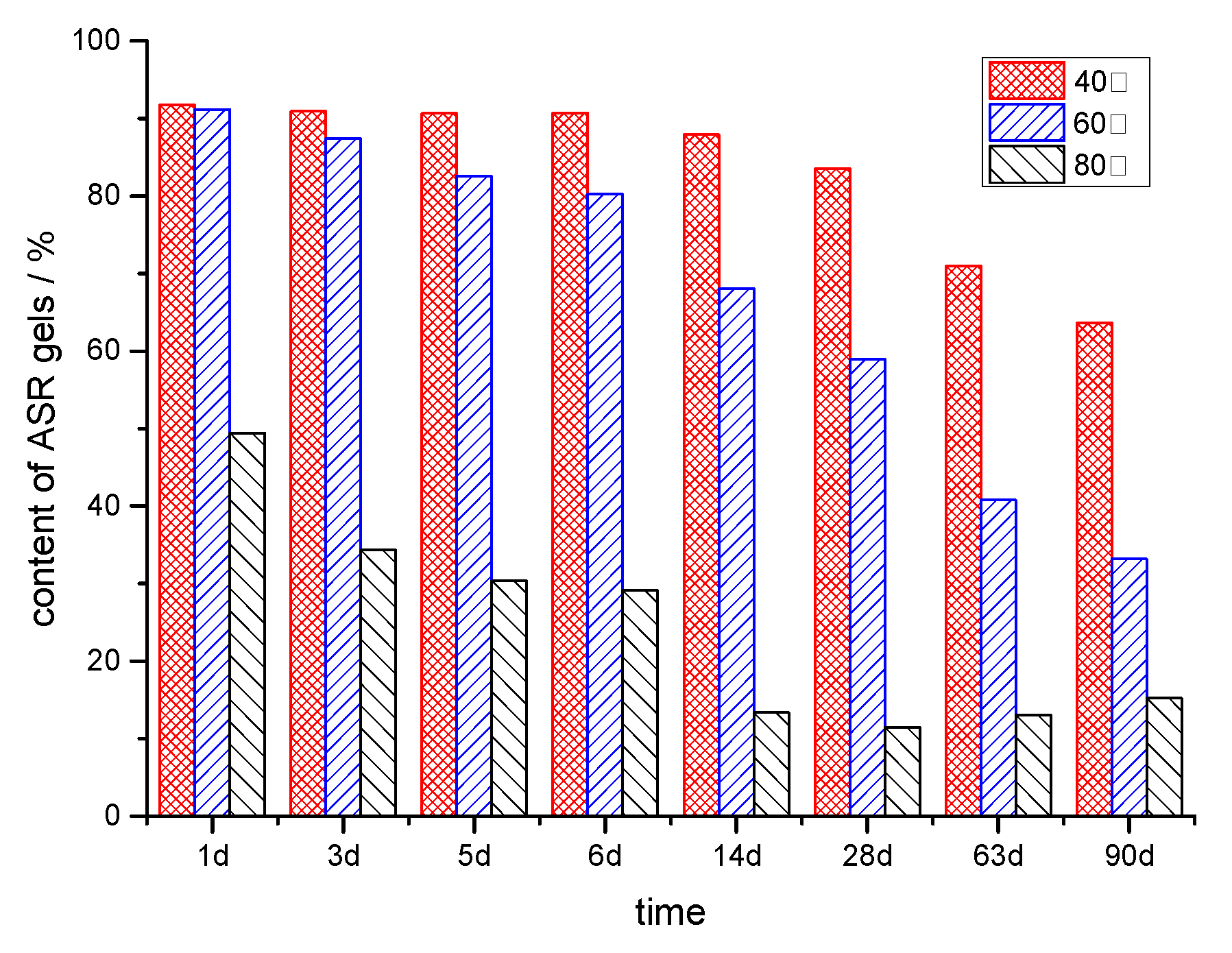
3.1.1. Reaction Degree of Silica in Sandstone
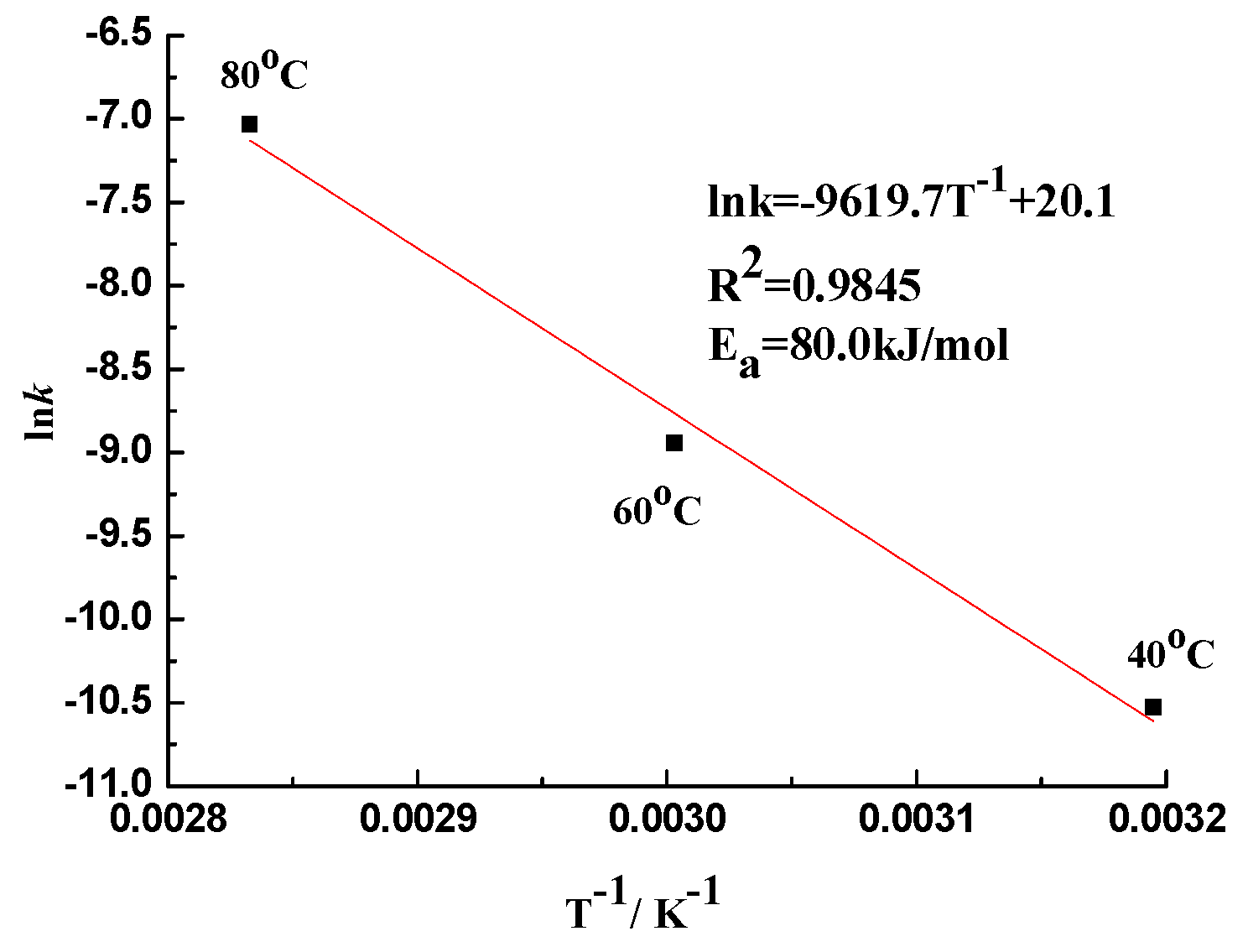
3.1.2. Dissolution Rate Constant
3.1.3. Reaction Order
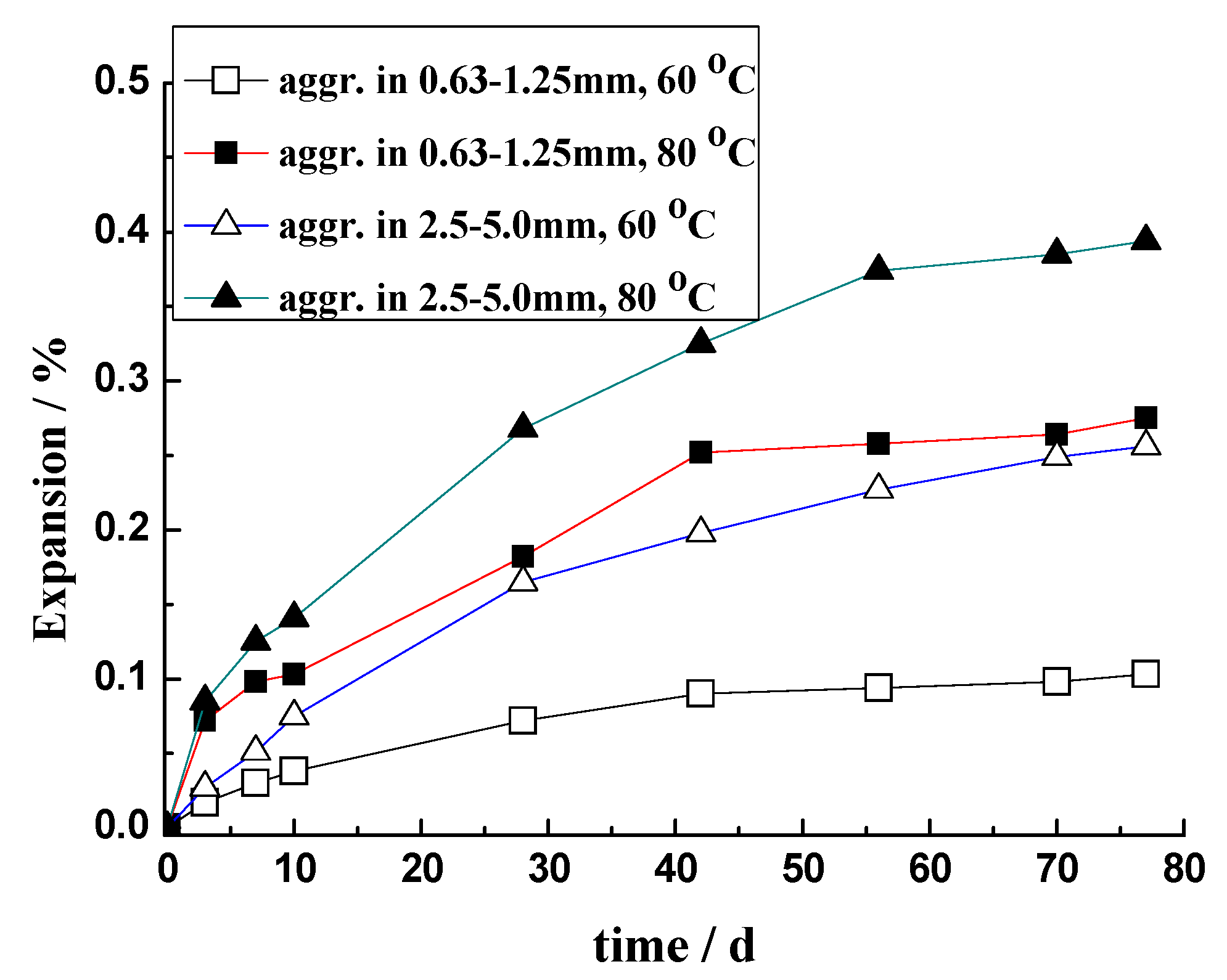
3.2. Expansion of Rock Prism
3.3. Discussion
3.3.1. Effect of the Temperature
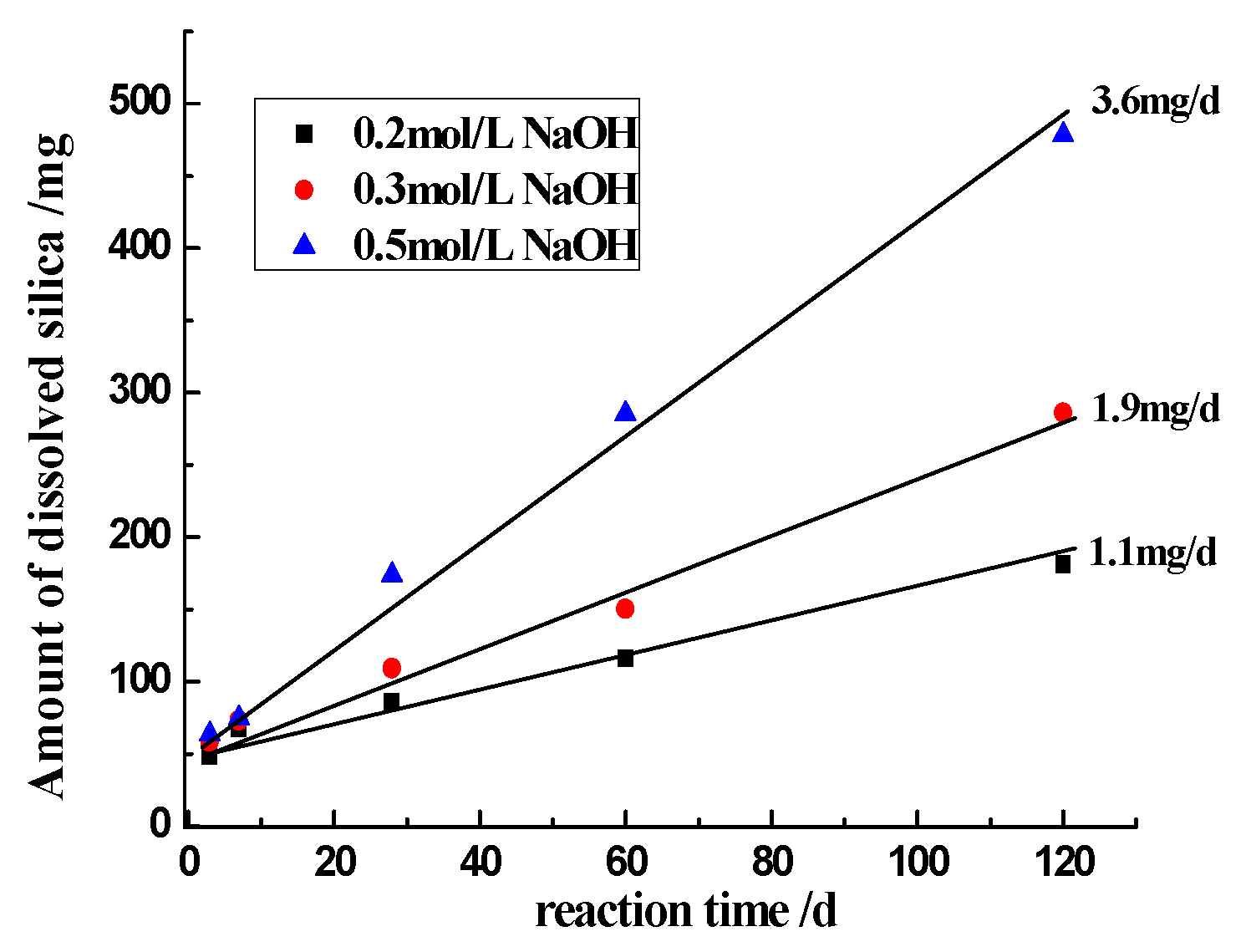
3.3.2. Effect of Hydroxyl Ions
3.3.3. Effect of Particle Size
4. Conclusions
- (1)
- The ASR of sandstone is a first-order reaction. The alkali–silica reaction in sandstone conforms to a first-order kinetic model. The reaction rate is directly proportional to the concentration of hydroxide ions ([OH−]) and decreases exponentially over time as the OH− ions are consumed. This indicates that without the introduction of new hydroxide ions, the silica dissolution rate will progressively diminish. The reaction kinetics adhere to the principles outlined by Arrhenius’ law, demonstrating that temperature is a critical factor in the reaction rate.
- (2)
- There is a temperature influence on ASR dynamics. Elevated temperatures significantly accelerate the ASR process, increasing the rate of silica dissolution and subsequent gel formation. However, higher temperatures also reduce the retention of ASR gels within the aggregate particles, which mitigates the overall expansion caused by the reaction. This dual effect underscores the complexity of predicting ASR behavior solely based on temperature. Larger aggregates tend to retain more ASR gels, leading to greater expansion, which is a crucial consideration for understanding and modeling ASR in real-world scenarios.
- (3)
- The implications for predictive modeling are as follows: The study highlights the necessity of incorporating various parameters, such as temperature and aggregate size, into predictive models for ASR. These factors must be meticulously controlled in experimental setups to ensure the accuracy and applicability of the models. The relationship between ASR expansion at different temperatures and the structural properties of the aggregate provides a basis for developing more reliable predictive tools for concrete deterioration due to ASR.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sims, I.; Poole, A. Alkali-Aggregate Reaction in Concrete: A World Review; CRC Press: London, UK, 2017. [Google Scholar]
- Chatterji, S. Chemistry of alkali-silica reaction and testing of aggregates. Cem. Concr. Res. 2005, 27, 788–795. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ogawa, S.; Tanaka, Y.; Maekawa, K. Scale-dependent ASR expansion of concrete and its prediction coupled with silica gel generation and migration. J. Adv. Concr. Technol. 2016, 14, 444–463. [Google Scholar] [CrossRef]
- Allahyari, H.; Heidarpour, A.; Shayan, A.; Nguyen, V.P. A robust time-dependent model of alkali-silica reaction at different temperatures. Cem. Concr. Compos. 2020, 106, 103460. [Google Scholar] [CrossRef]
- Shi, Z.; Park, S.; Lothenbach, B.; Leemann, A. Formation of shlykovite and ASR-P1 in concrete under accelerated alkali–silica reaction at 60 and 80 °C. Cem. Concr. Res. 2020, 137, 106213. [Google Scholar] [CrossRef]
- Leemann, A.; Saout, G.; Winnefeld, F.; Rentsch, D.; Lothenbach, B. Alkali–silica reaction: The influence of calcium on silica dissolution and the formation of reaction products. J. Am. Ceram. Soc. 2011, 94, 1243–1249. [Google Scholar] [CrossRef]
- Strack, C.; Barnes, E.; Ramsey, M.; Williams, R.K.; Klaus, K.L.; Moser, R.D. Impact of aggregate mineralogy and exposure solution on alkali-silica reaction product composition and structure within accelerated test conditions. Constr. Build. Mater. 2020, 240, 117929. [Google Scholar] [CrossRef]
- Broekmans, M. The Alkali–Silica Reaction: Mineralogical and Geochemical Aspects of Some Dutch Concretes and Norwegian Mylonites. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2002. [Google Scholar]
- Bleszynski, R.; Thomas, M. Microstructural studies of alkali-silica reaction in fly ash concrete immersed in alkaline solutions. Adv. Cem. Base Mater. 1998, 7, 66–78. [Google Scholar] [CrossRef]
- Giorla, A.; Scrivener, K.; Dunant, C. Influence of visco-elasticity on the stress development induced by alkali-silica reaction. Cem. Concr. Res. 2015, 70, 1–8. [Google Scholar] [CrossRef]
- Giorla, A.; Dunant, C. Microstructural effects in the simulation of creep of concrete. Cem. Concr. Res. 2018, 105, 44–53. [Google Scholar] [CrossRef]
- Miura, T.; Multon, M.; Kawabata, Y. Influence of the distribution of expansive sites in aggregates on microscopic damage caused by alkali-silica reaction: Insights into the mechanical origin of expansion. Cem. Concr. Res. 2021, 142, 106355. [Google Scholar] [CrossRef]
- Vayghan, A.; Rajabipour, F.; Rosenberger, J. Composition–rheology relationships in alkali–silica reaction gels and the impact on the Gel’s deleterious behavior. Cem. Concr. Res. 2016, 83, 45–56. [Google Scholar] [CrossRef]
- Glasser, L.; Kataoka, N. The chemistry of alkali-aggregate reaction. Cem. Concr. Res. 1981, 11, 1–9. [Google Scholar] [CrossRef]
- Bulteel, D.; Garcia-Diaz, E.; Vernet, C.; Zanni, H. Alkali–silica reaction: A method to quantify the reaction degree. Cem. Concr. Res. 2002, 32, 1199–1206. [Google Scholar] [CrossRef]
- Dron, R.; Brivot, F.; Chaussadent, T. The mechanism of the alkali-silica reaction. Bull. Lab. Ponts Chaussées 1998, 214, 61–68. [Google Scholar]
- Balachandran, C.; Munoz, J.; Peethamparan, S.; Arnold, T.S. Alkali -silica reaction and its dynamic relationship with cement pore solution in highly reactive systems. Constr. Build. Mater. 2023, 362, 129702. [Google Scholar] [CrossRef]
- Broekmans, M.; Jansen, J. Silica dissolution in impure sandstone: Application to concrete. J. Geochem. Explor. 1998, 62, 311–318. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Chemical sequence and kinetics of alkali-silica reaction Part I. experiments. J. Am. Ceram. Soc. 2014, 97, 2195–2203. [Google Scholar] [CrossRef]
- Dron, R.; Brivot, F. Thermodynamic and kinetic approach to the alkali-silica reaction. Part 2: Experiment. Cem. Concr. Res. 1993, 23, 93–103. [Google Scholar] [CrossRef]
- ASTM C289; Standard Test Method for Potential Alkali-Silica Reactivity of Aggregates (Chemical Method). ASTM: West Conshohocken, PA, USA, 2003.
- Verstraete, J.; Khouchaf, L.; Bulteel, D.; Garcia-Diaz, E.; Flank, A.M.; Tuilier, M.H. Amorphisation mechanism of a flint aggregate during the alkali–silica reaction: X-ray diffraction and X-ray absorption XANES contributions. Cem. Concr. Res. 2004, 34, 581–586. [Google Scholar] [CrossRef]
- Maraghechi, H. Development and Assessment of Alkali-Activated Recycled Glass Based Concretes for Civil Infrastructure; The Pennsylvania State University: State College, PA, USA, 2014. [Google Scholar]
- Kim, T.; Olek, J.; Jeong, H. Alkali-silica reaction: Kinetics of chemistry of pore solution and calcium hydroxide content in cementitious system. Cem. Concr. Res. 2015, 71, 36–45. [Google Scholar] [CrossRef]
- ASTM C1260; Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). ASTM: West Conshohocken, PA, USA, 1994.
- ASTM C1293; Standard Test Method for Determination of Length Change of Concrete due to Alkali Silica Reaction. ASTM: West Conshohocken, PA, USA, 2009.
- Ichikawa, T.; Miura, M. Modified model of alkali-silica reaction. Cem. Concr. Res. 2007, 37, 1291–1297. [Google Scholar] [CrossRef]
- Kawabata, Y.; Dunant, C.; Yamada, K.; Scrivener, K. Impact of temperature on expansive behavior of concrete with a highly reactive andesite due to the alkali–silica reaction. Cem. Concr. Res. 2019, 125, 105888. [Google Scholar] [CrossRef]
- Ponce, J.; Batic, O. Different manifestations of the alkali-silica reaction in concrete according to the reaction kinetics of the reactive aggregate. Cem. Concr. Res. 2006, 36, 1148–1156. [Google Scholar] [CrossRef]
- Ben, H.; Gallucci, E.; Guidoum, A.; Scrivener, K.L. Relation of expansion due to alkali silica reaction to the degree of reaction measured by SEM image analysis. Cem. Concr. Res. 2007, 37, 1206–1214. [Google Scholar]
- Dunant, C.; Scrivener, K. Micro-mechanical modeling of alkali-silica-reaction-induced degradation using the AMIE framework. Cem. Concr. Res. 2010, 40, 517–525. [Google Scholar] [CrossRef]
- Joo, H.; Takahashi, Y. Analytical and experimental studies on alkali–silica reaction mechanism: Aggregate cracking and chemical composition change of gel. Cem. Concr. Compos. 2023, 139, 105003. [Google Scholar] [CrossRef]
- Gao, X. Contribution to the Requalification of Alkali Silica Reaction (ASR) Damaged Structures: Assessment of the ASR Advancement in Aggregates. Ph.D. Thesis, Toulouse University, Toulouse, France, 2010. [Google Scholar]
- DL/T 5151; Test Code for Aggregates of Hydraulic Concrete. Standardization Administration of China: Beijing, China, 2014. (In Chinese)
- Yang, W.; Deng, M.; Huang, B.; Chen, W.; Wang, Z. Effects of NaOH solution concentration on expansion of rock-prisms of siliceous and dolomitic aggregates. J. Nanjing Tech. Univ. 2018, 3, 18–23. (In Chinese) [Google Scholar]
- Iler, R. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry; Wiley-Interscience: Hoboken, NJ, USA, 1979. [Google Scholar]
- Anthony, R. Basic Solid State Chemistry; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Vail, J. Soluble Silicates—Their Properties and Uses; Chemistry; Reinhold: New York, NY, USA, 1952. [Google Scholar]
- Mbetmi, G.; Multon, S.; Larrard, T.; Duprat, F.; Tieudjo, D. Sensitivity of an alkali-silica reaction kinetics model to diffusion and reactive mechanisms parameters. Constr. Buld. Mater. 2021, 299, 123913. [Google Scholar] [CrossRef]
- Chatterji, S. The role of Ca(OH)2 in the breakdown of Portland cement concrete due to alkali–silica reaction. Cem. Concr. Res. 1979, 9, 185–188. [Google Scholar] [CrossRef]
- Struble, L.; Diamond, S. Swelling properties of synthetic alkali silica gels. J. Am. Ceram. Soc. 1981, 64, 652–655. [Google Scholar] [CrossRef]
- Struble, L.; Diamond, S. Unstable swelling behaviour of alkali silica gels. Cem. Concr. Res. 1981, 11, 611–617. [Google Scholar] [CrossRef]
- Ke, J. Kinetics of Expansion due to Alkali-Aggregate Reaction and Expansion Prediction; Nanjing Tech University: Nanjing, China, 2008. (In Chinese) [Google Scholar]






| Time/Days | 40 °C | 60 °C | 80 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| α | α | α | |||||||
| 1 | 0.015 | 5.071 × 10−3 | 7.842 × 10−5 | 0.024 | 7.932 × 10−3 | 1.902 × 10−4 | 0.050 | 1.690 × 10−2 | 8.526 × 10−4 |
| 3 | 0.018 | 2.054 × 10−3 | 3.844 × 10−5 | 0.035 | 3.907 × 10−3 | 1.375 × 10−4 | 0.085 | 9.724 × 10−3 | 8.375 × 10−4 |
| 5 | 0.020 | 1.347 × 10−3 | 2.749 × 10−5 | 0.042 | 2.827 × 10−3 | 1.196 × 10−4 | 0.114 | 7.887 × 10−3 | 9.110 × 10−4 |
| 6 | 0.022 | 1.257 × 10−3 | 2.869 × 10−5 | 0.046 | 2.620 × 10−3 | 1.230 × 10−4 | 0.125 | 7.268 × 10−3 | 9.253 × 10−4 |
| 14 | 0.033 | 7.882 × 10−4 | 2.614 × 10−5 | 0.072 | 1.768 × 10−3 | 1.296 × 10−4 | 0.282 | 7.462 × 10−3 | 2.178 × 10−3 |
| 28 | 0.047 | 5.657 × 10−4 | 2.676 × 10−5 | 0.105 | 1.298 × 10−3 | 1.384 × 10−4 | 0.359 | 4.918 × 10−3 | 1.847 × 10−3 |
| 63 | 0.068 | 3.693 × 10−4 | 2.549 × 10−5 | 0.150 | 8.384 × 10−4 | 1.284 × 10−4 | 0.366 | 2.240 × 10−3 | 8.598 × 10−4 |
| 90 | 0.082 | 3.141 × 10−4 | 2.623 × 10−5 | 0.184 | 7.291 × 10−4 | 1.375 × 10−4 | 0.377 | 1.623 × 10−3 | 6.421 × 10−4 |
| SD | |||
|---|---|---|---|
| 40 °C | 60 °C | 80 °C | |
| kr | 0.00157 | 0.00236 | 0.00481 |
| kd | 1.8 × 10−5 | 2.2 × 10−5 | 5.5 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Deng, M.; Mo, L.; Li, W. Kinetics of Alkali–Silica Reaction: Application to Sandstone. Materials 2024, 17, 2956. https://doi.org/10.3390/ma17122956
Yang Y, Deng M, Mo L, Li W. Kinetics of Alkali–Silica Reaction: Application to Sandstone. Materials. 2024; 17(12):2956. https://doi.org/10.3390/ma17122956
Chicago/Turabian StyleYang, Yongfu, Min Deng, Liwu Mo, and Wei Li. 2024. "Kinetics of Alkali–Silica Reaction: Application to Sandstone" Materials 17, no. 12: 2956. https://doi.org/10.3390/ma17122956
APA StyleYang, Y., Deng, M., Mo, L., & Li, W. (2024). Kinetics of Alkali–Silica Reaction: Application to Sandstone. Materials, 17(12), 2956. https://doi.org/10.3390/ma17122956






