Optimizing Ultraviolet Illumination for Detecting Fluorescent Orthodontic Adhesive Residues during Debonding Procedures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spectrophotometry
2.1.1. Adhesive Selection
2.1.2. Sample Preparation for Spectrophotometry
2.1.3. Spectrophotometry
2.2. UV Illumination Parameters
2.2.1. LED Light Selection
2.2.2. Sample Preparation
2.2.3. Flashlight UV Intensity Calibration
2.2.4. Digital Image Capture and Conversion
2.2.5. Relative Resin Photointensity According to Work Parameters
2.2.6. Visual Assessment
2.2.7. Statistical Analysis
3. Results
3.1. Spectrophotometry—Excitation Maxima, Emission Maxima, and Fluorescence Intensity
3.2. Digital Image Analysis and Visual Assessment
3.2.1. Intra-Rater Reliability and Measurement Error
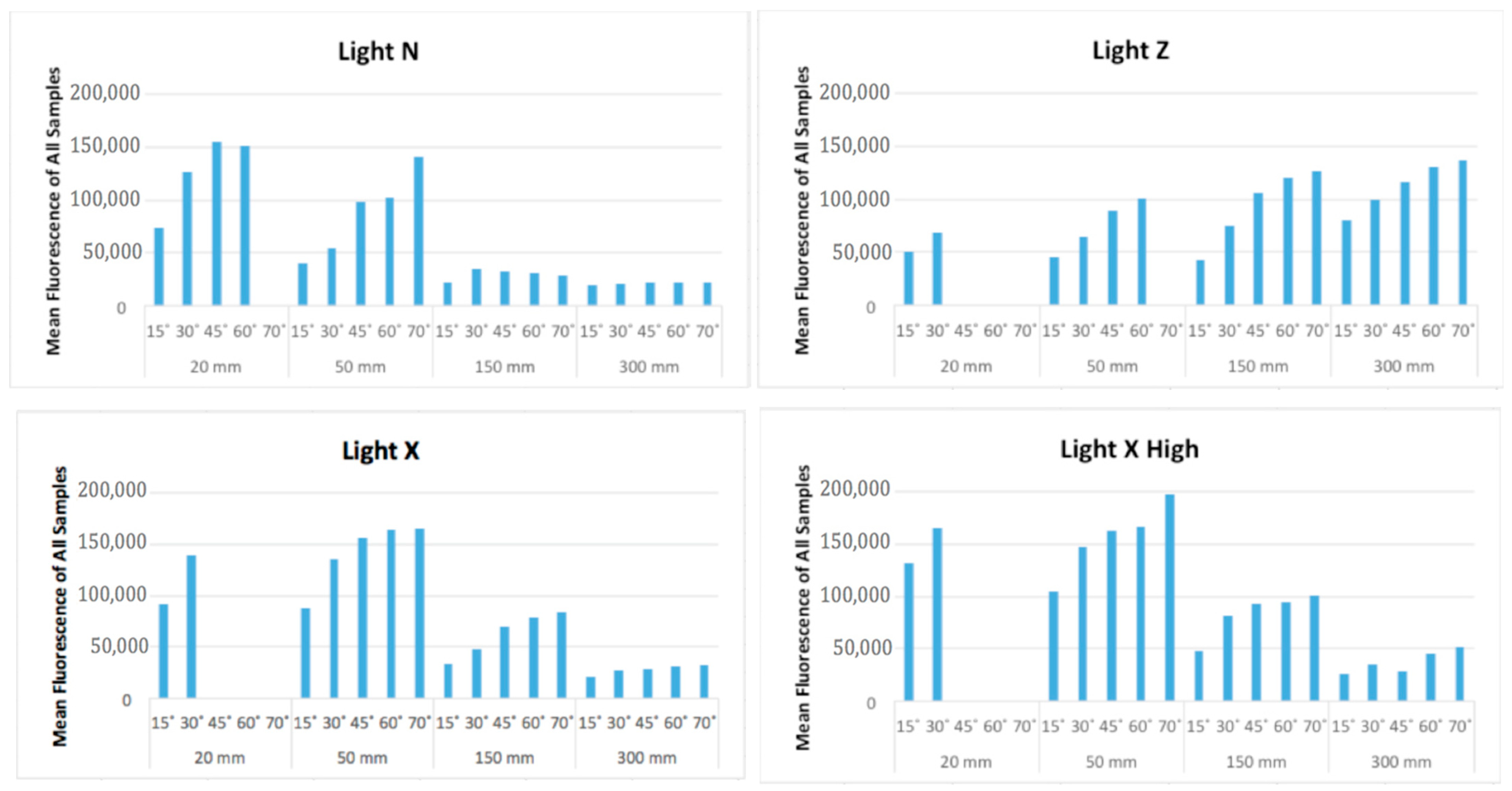
3.2.2. Digital Image Analysis: Fluorescence Intensity
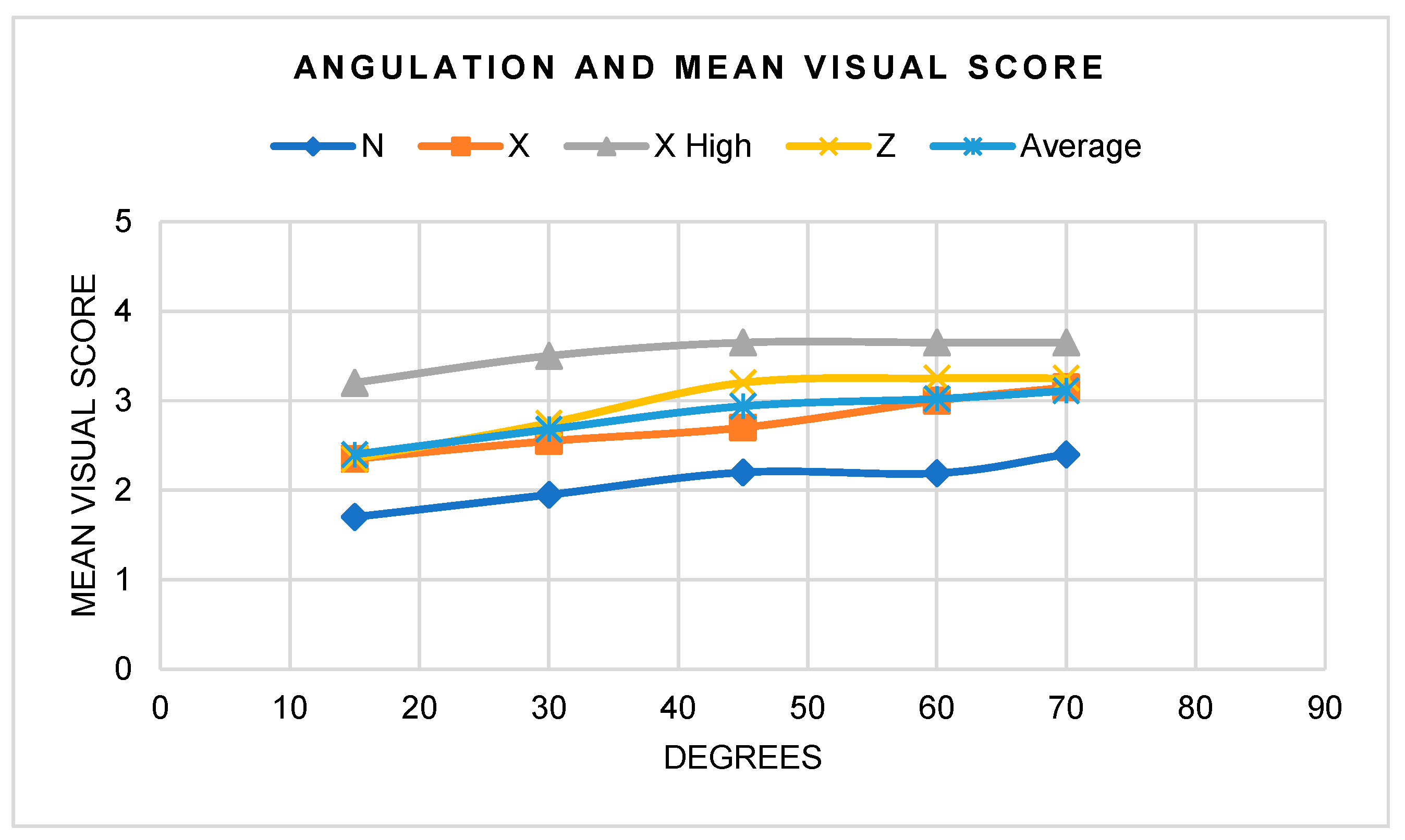
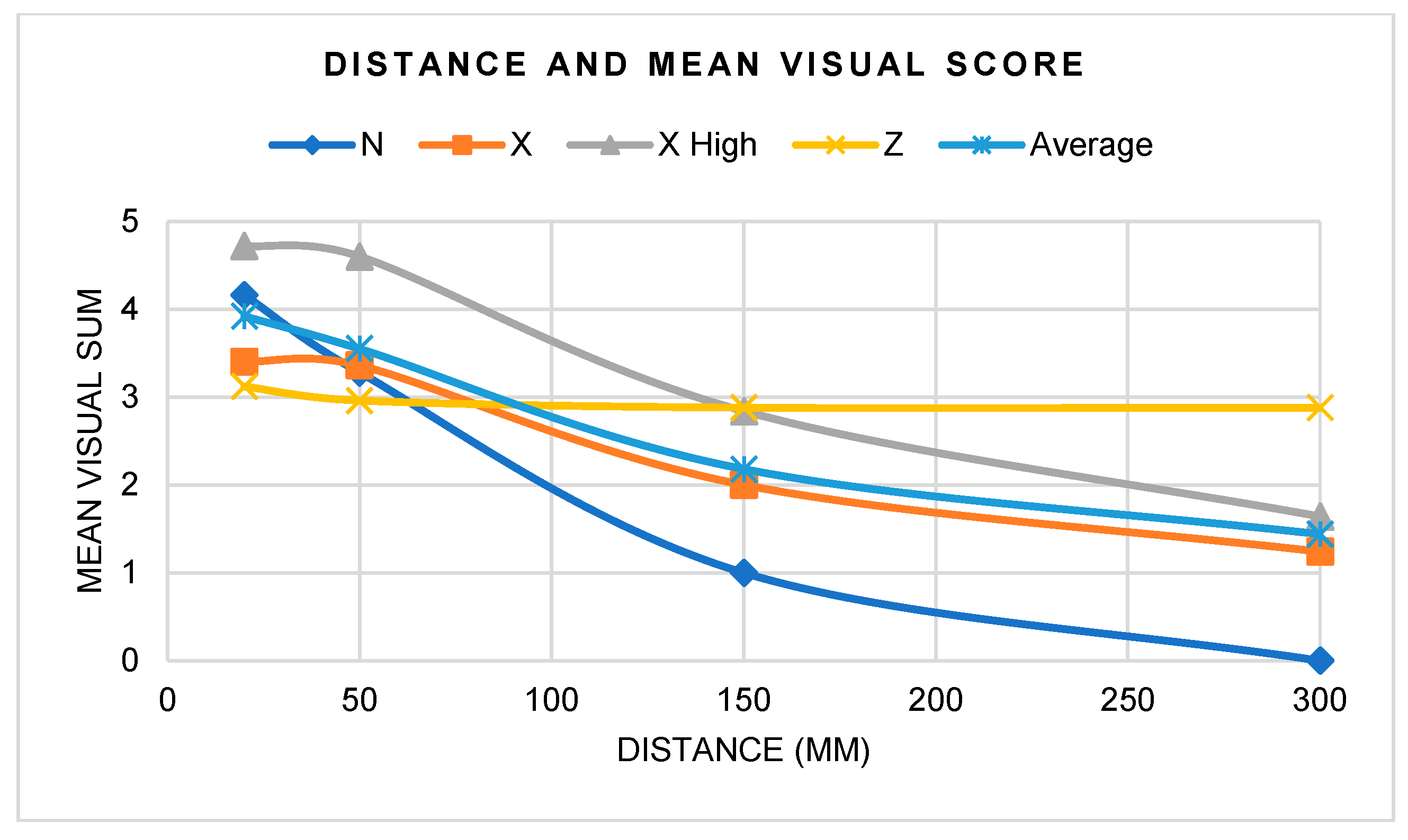
3.2.3. Visual Assessment
4. Discussion
5. Conclusions
- The appropriate wavelengths for FIT light specification are 395–405 nm; wavelengths closer to 395 nm allow for better visualization.
- All resin samples showed different relative fluorescence intensity and anisotropic fluorescence at 25 μm, except for the Transbond XT control.
- There were statistically significant differences between distances, angles, and light sources, as well as interactions when considered as pairs; fluorescence intensity increased with more perpendicular angles and with greater UV intensity, but decreased with increased distance.
- The highest fluorescence was recorded with a combination of Flashlight X High at 50 mm and 70°.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Lai, C.; Bush, P.J.; Warunek, S.; Covell, D.A., Jr.; Al-Jewair, T. An in vitro comparison of ultraviolet versus white light in the detection of adhesive remnants during orthodontic debonding. Angle Orthod. 2019, 89, 438–445. [Google Scholar] [CrossRef]
- Stadler, O.; Dettwiler, C.; Meller, C.; Dalstra, M.; Verna, C.; Connert, T. Evaluation of a Fluorescence-aided Identification Technique (FIT) to assist clean-up after orthodontic bracket debonding. Angle Orthod. 2019, 89, 876–882. [Google Scholar] [CrossRef]
- Janiszewska-Olszowska, J.; Szatkiewicz, T.; Tomkowski, R.; Tandecka, K.; Grocholewicz, K. Effect of orthodontic debonding and adhesive removal on the enamel—Current knowledge and future perspectives—A systematic review. Med. Sci. Monit. 2014, 20, 1991–2001. [Google Scholar] [CrossRef]
- Baumann, D.F.; Brauchli, L.; van Waes, H. The influence of dental loupes on the quality of adhesive removal in orthodontic debonding. J. Orofac. Orthop. 2011, 72, 125–132. [Google Scholar] [CrossRef]
- Ribeiro, A.A.; Almeida, L.F.; Martins, L.P.; Martins, R.P. Assessing adhesive remnant removal and enamel damage with ultraviolet light: An in-vitro study. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 292–296. [Google Scholar] [CrossRef]
- Rocha, R.S.; Salomao, F.M.; Silveira Machado, L.; Sundfeld, R.H.; Fagundes, T.C. Efficacy of auxiliary devices for removal of fluorescent residue after bracket debonding. Angle Orthod. 2017, 87, 440–447. [Google Scholar] [CrossRef]
- Moecke, S.E.; Barros, P.C.A.; Andrade, A.C.M.; Borges, A.B.; Pucci, C.R.; Torres, C.R.G. Efficacy of Bracket Adhesive Remnant Removal by a Fluorescence-Aided Identification Technique with a UV Light Handpiece: In Vitro Study. Int. J. Dent. 2022, 2022, 4821021. [Google Scholar] [CrossRef]
- Ryf, S.; Flury, S.; Palaniappan, S.; Lussi, A.; van Meerbeek, B.; Zimmerli, B. Enamel loss and adhesive remnants following bracket removal and various clean-up procedures in vitro. Eur. J. Orthod. 2012, 34, 25–32. [Google Scholar] [CrossRef]
- Prabhakar, A.R.; Murthy, S.A.; Sugandhan, S. Comparative evaluation of the length of resin tags, viscosity and microleakage of pit and fissure sealants—An in vitro scanning electron microscope study. Contemp. Clin. Dent. 2011, 2, 324–330. [Google Scholar] [CrossRef]
- Zaher, A.R.; Abdalla, E.M.; Abdel Motie, M.A.; Rehman, N.A.; Kassem, H.; Athanasiou, A.E. Enamel colour changes after debonding using various bonding systems. J. Orthod. 2012, 39, 82–88. [Google Scholar] [CrossRef]
- Wu, C.; Mangal, U.; Kim, J.; Lee, K.J.; Cha, J.Y.; Kwon, J.S.; Choi, S.H. Quantitative light-induced fluorescence enables effective detection of orthodontic adhesive residues in diverse environments. Photodiagn. Photodyn. Ther. 2023, 44, 103743. [Google Scholar] [CrossRef]
- Al Shamsi, A.H.; Cunningham, J.L.; Lamey, P.J.; Lynch, E. Three-dimensional measurement of residual adhesive and enamel loss on teeth after debonding of orthodontic brackets: An in-vitro study. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 301.e309–301.e315. [Google Scholar] [CrossRef]
- Salomao, F.M.; Rocha, R.S.; Franco, L.M.; Sundfeld, R.H.; Bresciani, E.; Fagundes, T.C. Auxiliary UV light devices for removal of fluorescent resin residues after bracket debonding. J. Esthet. Restor. Dent. 2019, 31, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Schott, T.C.; Meller, C. A new Fluorescence-aided Identification Technique (FIT) for optimal removal of resin-based bracket bonding remnants after orthodontic debracketing. Quintessence Int. 2018, 49, 809–813. [Google Scholar] [CrossRef]
- Albertini, P.; Albertini, E.; Siciliani, G.; Lombardo, L. Fluorescence-aided composite removal during lingual bracket debonding. J. Esthet. Restor. Dent. 2020, 32, 634–637. [Google Scholar] [CrossRef]
- Kaneshima, E.N.; Berger, S.B.; Fernandes, T.M.F.; Navarro, M.F.L.; Oltramari, P.V.P. Using UV light for adhesive remnant removal after debonding of orthodontic accessories. Braz. Oral Res. 2018, 32, e47. [Google Scholar] [CrossRef]
- Bush, M.A.; Hermanson, A.S.; Yetto, R.J.; Wieczkowski, G., Jr. The use of ultraviolet LED illumination for composite resin removal: An in vitro study. Gen. Dent. 2010, 58, e214–e218. [Google Scholar] [PubMed]
- Hermanson, A.S.; Bush, M.A.; Miller, R.G.; Bush, P.J. Ultraviolet illumination as an adjunctive aid in dental inspection. J. Forensic Sci. 2008, 53, 408–411. [Google Scholar] [CrossRef]
- Meller, C.; Klein, C. Fluorescence properties of commercial composite resin restorative materials in dentistry. Dent. Mater. J. 2012, 31, 916–923. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, J.H.; Ahn, J.S. Influence of the changes in the UV component of illumination on the color of composite resins. J. Prosthet. Dent. 2007, 97, 375–380. [Google Scholar] [CrossRef]
- Meller, C.; Klein, C. Fluorescence of composite resins: A comparison among properties of commercial shades. Dent. Mater. J. 2015, 34, 754–765. [Google Scholar] [CrossRef]
- Lim, Y.K.; Lee, Y.K. Fluorescent emission of varied shades of resin composites. Dent. Mater. 2007, 23, 1262–1268. [Google Scholar] [CrossRef]
- Albertini, P.; Tauro, R.; Barbara, L.; Albertini, E.; Lombardo, L. Fluorescence-aided removal of orthodontic composites: An in vivo comparative study. Prog. Orthod. 2022, 23, 16. [Google Scholar] [CrossRef]
- Kim, G.M.; Kim, B.R.; Lee, E.S.; de Josselin de Jong, E.; Kim, B.I. Detection of residual resin-based orthodontic adhesive based on light-induced fluorescence. Photodiagnosis Photodyn. Ther. 2018, 24, 69–74. [Google Scholar] [CrossRef]
- Yamagata, S.; Hamba, Y.; Nakanishi, K.; Abe, S.; Akasaka, T.; Ushijima, N.; Uo, M.; Iida, J.; Watari, F. Introduction of rare-earth-element-containing ZnO nanoparticles into orthodontic adhesives. Nano Biomed. 2012, 4, 11–17. [Google Scholar]
- Uo, M.; Okamoto, M.; Watari, F.; Tani, K.; Morita, M.; Shintani, A. Rare earth oxide-containing fluorescent glass filler for composite resin. Dent. Mater. J. 2005, 24, 49–52. [Google Scholar] [CrossRef]
- Namura, Y.; Tsuruoka, T.; Ryu, C.; Kaketani, M.; Shimizu, N. Usefulness of orthodontic adhesive-containing fluorescent dye. Eur. J. Orthod. 2010, 32, 620–626. [Google Scholar] [CrossRef]
- Tani, K.; Watari, F.; Uo, M.; Morita, M. Discrimination between composite resin and teeth using fluorescence properties. Dent. Mater. J. 2003, 22, 569–580. [Google Scholar] [CrossRef]
- Kim, B.R.; Kang, S.M.; Kim, G.M.; Kim, B.I. Differences in the intensity of light-induced fluorescence emitted by resin composites. Photodiagnosis Photodyn. Ther. 2016, 13, 114–119. [Google Scholar] [CrossRef]
- Kumar, K.R.; Sundari, K.S.; Venkatesan, A.; Chandrasekar, S. Depth of resin penetration into enamel with 3 types of enamel conditioning methods: A confocal microscopic study. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Volpato, C.A.M.; Pereira, M.R.C.; Silva, F.S. Fluorescence of natural teeth and restorative materials, methods for analysis and quantification: A literature review. J. Esthet. Restor. Dent. 2018, 30, 397–407. [Google Scholar] [CrossRef]
- Garrido, T.M.; Hoshino, L.C.; Hirata, R.; Sato, F.; Neto, A.M.; Guidini, V.H.; Terada, R.S. In vitro evaluation of composite resin fluorescence after natural aging. J. Clin. Exp. Dent. 2020, 12, e461–e467. [Google Scholar] [CrossRef]
- Rattle, C.N.; Bush, M.A. Fluorescence and structural degradation in composite resins as a function of temperature. J. Forensic Sci. 2009, 54, 433–438. [Google Scholar] [CrossRef]
- Klein, C.; Wolff, D.; Ohle, C.V.; Meller, C. The fluorescence of resin-based composites: An analysis after ten years of aging. Dent. Mater. J. 2021, 40, 94–100. [Google Scholar] [CrossRef]
- Takahashi, M.K.; Vieira, S.; Rached, R.N.; de Almeida, J.B.; Aguiar, M.; de Souza, E.M. Fluorescence intensity of resin composites and dental tissues before and after accelerated aging: A comparative study. Oper. Dent. 2008, 33, 189–195. [Google Scholar] [CrossRef]
- Florencio, S., Jr. Systematic Evaluation of Fluorescence in CAD/CAM Ceramic Materials. Ph.D. Thesis, Harvard School of Dental Medicine, Boston, MA, USA, 2017. [Google Scholar]




| Sample | Excitation Maxima (nm) | Emission Maxima (nm) |
|---|---|---|
| Lithium Disilicate | 350 | 545 |
| Transbond™ XT | 370 | 405 |
| GoTo® | 390 | 442 |
| BracePaste® | 370 | 446 |
| Opal™ Bond™ MV | 380 | 465 |
| Pad Lock | 390 | 460 |
| BrackFix® | 390 | 446 |
| Mean of fluorescent resins | 384 | 452 |
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Variable | Mean (a.u.) | Lower Bound | Upper Bound | |
| Resin | Transbond™ XT | 72,309.5 a | 71,124.8 | 73,494.3 |
| GoTo® | 75,379.2 a | 74,194.5 | 76,564.0 | |
| BracePaste® | 85,796.9 a | 84,612.2 | 86,981.7 | |
| Opal™ Bond™ MV | 83,415.8 a | 82,231.1 | 84,600.6 | |
| Pad Lock | 82,068.8 a | 80,884.0 | 83,253.6 | |
| BrackFix® | 88,564.9 a | 87,380.1 | 89,749.6 | |
| Angle | 15° | 57,529.4 | 56,720.8 | 58,338.0 |
| 30° | 82,760.3 | 81,951.7 | 83,569.0 | |
| 45° | 89,041.6 a | 88,144.5 | 89,938.7 | |
| 60° | 95,313.2 a | 94,416.1 | 96,210.3 | |
| 70° | 98,987.8 a | 98,012.6 | 99,963.1 | |
| Distance | 20 mm | 115,470.6 a | 114,447.8 | 116,493.5 |
| 50 mm | 116,972.9 a | 116,230.8 | 117,714.9 | |
| 150 mm | 67,712.1 | 66,988.8 | 68,435.4 | |
| 300 mm | 49,934.1 | 49,210.8 | 50,657.4 | |
| Light | Light X | 82,138.5 a | 81,354.0 | 82,923.0 |
| Light Z | 90,891.3 a | 90,082.6 | 91,699.9 | |
| Light N | 63,080.5 a | 62,338.4 | 63,822.5 | |
| Light X High | 98,880.6 a | 98,096.1 | 99,665.1 | |
| Source | df | Mean Square | F | Sig. * |
|---|---|---|---|---|
| S (Sample) | 4 | 1,688,718,239.1 | 125.1 | 0.000 |
| A (Angle) | 4 | 32,105,679,385.7 | 2378.7 | 0.000 |
| D (Distance) | 3 | 116,964,668,292.3 | 8666.0 | 0.000 |
| Light type | 3 | 31,467,684,346.6 | 2331.5 | 0.000 |
| D * A | 11 | 2,901,121,459.6 | 214.9 | 0.000 |
| A * Light | 12 | 824,333,791.7 | 61.1 | 0.000 |
| D * Light | 9 | 19,285,101,263.8 | 1428.8 | 0.000 |
| D * A * Light | 26 | 604,203,700.0 | 44.8 | 0.000 |
| Angle | Distance | Light Type | Mean | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| 15° | 20 mm | Light X | 92,219.0 | 88,984.4 | 95,453.6 |
| Light Z | 50,971.0 | 47,736.4 | 54,205.6 | ||
| Light N | 73,483.0 | 70,248.4 | 76,717.6 | ||
| Light X High | 132,090.6 | 128,856.0 | 135,325.2 | ||
| 50 mm | Light X | 87,551.2 | 84,316.6 | 90,785.8 | |
| Light Z | 45,390.8 | 42,156.2 | 48,625.4 | ||
| Light N | 39,913.6 | 36,679.0 | 43,148.2 | ||
| Light X High | 104,626.8 | 101,392.2 | 107,861.4 | ||
| 150 mm | Light X | 33,586.6 | 30,352.0 | 36,821.2 | |
| Light Z | 42,913.6 | 39,679.0 | 46,148.2 | ||
| Light N | 22,211.4 | 18,976.8 | 25,446.0 | ||
| Light X High | 48,250.4 | 45,015.8 | 51,485.0 | ||
| 300 mm | Light X | 20,721.6 | 17,487.0 | 23,956.2 | |
| Light Z | 79,919.6 | 76,685.0 | 83,154.2 | ||
| Light N | 20,073.8 | 16,839.2 | 23,308.4 | ||
| Light X High | 26,547.4 | 23,312.8 | 29,782.0 | ||
| 30° | 20 mm | Light X | 139,171.8 | 135,937.2 | 142,406.4 |
| Light Z | 68,022.8 | 64,788.2 | 71,257.4 | ||
| Light N | 126,755.0 | 123,520.4 | 129,989.6 | ||
| Light X High | 165,535.6 | 162,301.0 | 168,770.2 | ||
| 50 mm | Light X | 135,170.0 | 131,935.4 | 138,404.6 | |
| Light Z | 64,459.2 | 61,224.6 | 67,693.8 | ||
| Light N | 54,583.6 | 51,349.0 | 57,818.2 | ||
| Light X High | 147,866.4 | 144,631.8 | 151,101.0 | ||
| 150 mm | Light X | 48,188.2 | 44,953.6 | 51,422.8 | |
| Light Z | 75,228.8 | 71,994.2 | 78,463.4 | ||
| Light N | 35,644.8 | 32,410.2 | 38,879.4 | ||
| Light X High | 81,123.6 | 77,889.0 | 84,358.2 | ||
| 300 mm | Light X | 27,274.6 | 24,040.0 | 30,509.2 | |
| Light Z | 99,632.4 | 96,397.8 | 102,867.0 | ||
| Light N | 20,865.4 | 17,630.8 | 24,100.0 | ||
| Light X High | 34,643.0 | 31,408.4 | 37,877.6 | ||
| 45° | 20 mm | Light X | . a | . | . |
| Light Z | . a | . | . | ||
| Light N | 154,800.8 | 151,566.2 | 158,035.4 | ||
| Light X High | . a | . | . | ||
| 50 mm | Light X | 156,086.4 | 152,851.8 | 159,321.0 | |
| Light Z | 88,884.2 | 85,649.6 | 92,118.8 | ||
| Light N | 97,757.4 | 94,522.8 | 100,992.0 | ||
| Light X High | 162,382.6 | 159,148.0 | 165,617.2 | ||
| 150 mm | Light X | 69,465.6 | 66,231.0 | 72,700.2 | |
| Light Z | 106,261.6 | 103,027.0 | 109,496.2 | ||
| Light N | 33,041.0 | 29,806.4 | 36,275.6 | ||
| Light X High | 93,562.0 | 90,327.4 | 96,796.6 | ||
| 300 mm | Light X | 28,220.8 | 24,986.2 | 31,455.4 | |
| Light Z | 116,845.6 | 113,611.0 | 120,080.2 | ||
| Light N | 22,201.4 | 18,966.8 | 25,436.0 | ||
| Light X High | 28,031.8 | 24,797.2 | 31,266.4 | ||
| 60° | 20 mm | Light X | . a | . | . |
| Light Z | . a | . | . | ||
| Light N | 151,656.8 | 148,422.2 | 15,4891.4 | ||
| Light X High | . a | . | . | ||
| 50 mm | Light X | 164,408.2 | 161,173.6 | 167,642.8 | |
| Light Z | 101,201.2 | 97,966.6 | 104,435.8 | ||
| Light N | 101,890.4 | 98,655.8 | 105,125.0 | ||
| Light X High | 166,721.2 | 163,486.6 | 169,955.8 | ||
| 150 mm | Light X | 79,018.2 | 75,783.6 | 82,252.8 | |
| Light Z | 119,993.2 | 116,758.6 | 123,227.8 | ||
| Light N | 30,985.6 | 27,751.0 | 34,220.2 | ||
| Light X High | 93,858.8 | 90,624.2 | 97,093.4 | ||
| 300 mm | Light X | 31,758.6 | 28,524.0 | 34,993.2 | |
| Light Z | 130,085.4 | 126,850.8 | 133,320.0 | ||
| Light N | 21,760.2 | 18,525.6 | 24,994.8 | ||
| Light X High | 45,733.6 | 42,499.0 | 48,968.2 | ||
| 70° | 20 mm | Light X | . a | . | . |
| Light Z | . a | . | . | ||
| Light N | .a | . | . | ||
| Light X High | . a | . | . | ||
| 50 mm | Light X | 166,051.6 | 162,817.0 | 169,286.2 | |
| Light Z | . a | . | . | ||
| Light N | 140,271.2 | 137,036.6 | 143,505.8 | ||
| Light X High | 197,268.2 | 194,033.6 | 200,502.8 | ||
| 150 mm | Light X | 84,667.6 | 81,433.0 | 87,902.2 | |
| Light Z | 126,852.4 | 123,617.8 | 130,087.0 | ||
| Light N | 28,986.2 | 25,751.6 | 32,220.8 | ||
| Light X High | 100,402.4 | 97,167.8 | 103,637.0 | ||
| 300 mm | Light X | 32,795.0 | 29,560.4 | 36,029.6 | |
| Light Z | 137,598.4 | 134,363.8 | 140,833.0 | ||
| Light N | 21,647.6 | 18,413.0 | 24,882.2 | ||
| Light X High | 52,325.6 | 49,091.0 | 55,560.2 | ||
| N | X | X High | Z | Average | |
|---|---|---|---|---|---|
| 20 mm | 4.2 | 3.4 | 4.7 | 3.1 | 3.9 |
| 50 mm | 3.3 | 3.4 | 4.6 | 3.0 | 3.6 |
| 150 mm | 1.0 | 2.0 | 2.8 | 2.9 | 2.2 |
| 300 mm | 0.0 | 1.2 | 1.6 | 2.9 | 1.4 |
| Average | 2.1 | 2.5 | 3.5 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, G.; Makowka, S.; Warunek, S.; Al-Jewair, T. Optimizing Ultraviolet Illumination for Detecting Fluorescent Orthodontic Adhesive Residues during Debonding Procedures. Materials 2024, 17, 2961. https://doi.org/10.3390/ma17122961
Chung G, Makowka S, Warunek S, Al-Jewair T. Optimizing Ultraviolet Illumination for Detecting Fluorescent Orthodontic Adhesive Residues during Debonding Procedures. Materials. 2024; 17(12):2961. https://doi.org/10.3390/ma17122961
Chicago/Turabian StyleChung, Grace, Steven Makowka, Stephen Warunek, and Thikriat Al-Jewair. 2024. "Optimizing Ultraviolet Illumination for Detecting Fluorescent Orthodontic Adhesive Residues during Debonding Procedures" Materials 17, no. 12: 2961. https://doi.org/10.3390/ma17122961
APA StyleChung, G., Makowka, S., Warunek, S., & Al-Jewair, T. (2024). Optimizing Ultraviolet Illumination for Detecting Fluorescent Orthodontic Adhesive Residues during Debonding Procedures. Materials, 17(12), 2961. https://doi.org/10.3390/ma17122961






